Abstract
Orthopaedic patients frequently require blood transfusions to treat peri-operative anemia. Research in the area of hemoglobin substitutes has been of great interest since it holds the promise of reducing the reliance on allogeneic blood transfusions. The three categories of hemoglobin substitutes are (1) cell-free, extracellular hemoglobin preparations made from human or bovine hemoglobin (hemoglobin-based oxygen carriers or HBOCs); (2) fluorine-substituted linear or cyclic carbon chains with a high oxygen-carrying capacity (perfluorocarbons); and (3) liposome-encapsulated hemoglobin. Of the three, HBOCs have been the most extensively studied and tested in preclinical and clinical trials that have shown success in diminishing the number of blood transfusions as well as an overall favorable side-effect profile. This has been demonstrated in vascular, cardiothoracic, and orthopaedic patients. HBOC-201, which is a preparation of cell-free bovine hemoglobin, has been approved for clinical use in South Africa. These products may well become an important tool for physicians treating peri-operative anemia in orthopaedic patients.
Keywords: Hemoglobin substitute, HBOC, HBOC-201, Perfluorocarbons
Introduction
Considerable progress has been made in recent years in understanding the biochemical properties and clinical efficacy of hemoglobin substitutes. Cell-free bovine hemoglobin was approved for clinical use in South Africa in 2001, and active clinical trials using the same product are well underway internationally, including in the United States. This technology will likely prove to be a useful adjunct in treating anemia following major orthopaedic surgery.
Blood requirements in orthopaedic surgery
The impetus to develop hemoglobin substitutes stems from the fact that allogeneic blood transfusions are frequently required for surgical patients. Approximately two-thirds of all transfusions in the United States are related to surgical procedures [12]. The average hemoglobin drop is 3.85±1.4 g/dl for single total knee replacement, 4.07±1.74 g/dl for total hip replacement, and 5.42±1.8 g/dl for bilateral knee replacement [13]. Bierbaum et al. prospectively evaluated 9,482 patients undergoing hip or knee arthroplasty and found that 30% received autologous blood and 16% received allogeneic blood during the post-operative period [3]. The risk factors associated with the need for allogeneic transfusion were a lack of autologous blood and a pre-operative hemoglobin below 13 g/dl. Of 8,561 patients whose pre-operative hemoglobin was known, 3,020 (35%) had a level of 13 g/dl or below. Of these 3,020 patients, 29% required allogeneic blood transfusion. It bears mention that the incidence of anemia is correlated with increasing age [2], so that older patients undergoing orthopaedic procedures are more likely to need allogeneic transfusions.
Complications of allogeneic and autologous blood transfusion
Both allogeneic and autologous blood transfusion have inherent complications that make it desirable to avoid them if possible. While allogeneic blood is safer than it has ever been, the risk of acquiring an infectious agent from a transfusion is still a concern for patients and their physicians. All donated blood undergoes testing for ABO group, Rh type, antibody screen, hepatitis B surface and core antigens, hepatitis C, HIV-1 and HIV-2, human T-cell lymphotropic virus (HTLV) -1 and HTLV-2, and syphilis [7]. Despite this testing, there is a small incidence of viral transmission with an allogeneic blood transfusion [18]. This is estimated at 1:180,000 for Hepatitis B, 1:1.6 million for Hepatitis C, and 1:1.9 million for HIV. In addition to viral transmission, bacterial contamination of blood products is possible. It is estimated that septic complications caused 16% of the 182 transfusion-associated fatalities reported to the US Federal Drug Administration (FDA) between 1986 and 1991 [18]. Only 28% of these fatalities followed red blood cell (RBC) transfusions; the rest occurred after platelet transfusion. With regard to prion-mediated diseases such as Creutzfeldt-Jakob disease, no documented case of transfusion-related prion infection has ever been reported in animals or humans. However, there recently was a case of variant Creutzfeldt-Jakob disease (vCJD) that had a strong possibility of being the result of a blood transfusion, based on probability analysis [1, 17]. There are non-infectious risks of allogeneic transfusion as well [11]. These include transfusion-related, acute lung injury, graft-versus-host disease, anaphylaxis, hemolysis, and post-transfusion purpura. Transfusion-related acute lung injury is a syndrome of dyspnea, hypotension, and pulmonary edema that develops between the beginning of transfusion and up to 4 h afterwards [14]. It appears to be associated with the presence of human leukocyte antigens (HLA) class I and II antibodies, and is fatal in 5–10% of cases. Therefore, allogeneic blood transfusion is associated with several important risks and it remains true that minimizing allogeneic transfusions would be a worthwhile advantage of a hemoglobin substitute.
Although many adverse events are eliminated with pre-operative autologous donation (PAD), autologous transfusion still has associated risks. First, there is the potential for patients to develop anemia as a result of repeated phlebotomy, particularly if the last unit of donated blood is given within 15 days of the anticipated surgery [7]. Furthermore, since the patients donating blood are frequently elderly or have medical comorbidities, there is a reported 12-fold increase in the number of post-donation adverse reactions, including hospitalizations, compared to allogeneic blood donors. This risk is higher in the elderly [7]. The complications related to fluid overload and bacterial contamination are still present with autologous blood. Finally, there is the cost and logistic effort involved in collecting the blood as well as the frequent wasting of unneeded units.
The rationale for hemoglobin substitutes
With almost half of all patients undergoing hip or knee arthroplasty requiring a blood transfusion, a reliable safe hemoglobin substitute would give the operative team a viable option in avoiding a transfusion. In addition, hemoglobin substitutes are useful in other patients who suffer from acute anemia, including victims of trauma and patients undergoing vascular or cardiothoracic procedures. A hemoglobin substitute that is stable at room temperature and does not require cross-matching may also be a lifesaving measure in ambulances and at the scenes of mass casualties. Finally, a hemoglobin substitute can function as a “bridge,” facilitating oxygen transport during the acute phase of blood loss, which allows the hematopoietic system an opportunity to stimulate its production of reticulocytes.
Research in the field of hemoglobin substitutes has led to the development of several substances that seek to reproduce the function of RBCs. Such a product must satisfy several criteria to be useful. First, it must mimic the oxygen-carrying capacity of hemoglobin; as explained below, no product in clinical trials has the exact affinity for oxygen at different oxygen concentrations as does erythrocyte-encased human hemoglobin. Second, it must be safe with a low side-effect profile compared to allogeneic blood transfusion. Third, it must be retained in plasma for a clinically relevant period of time. Finally, it must be cost-effective and convenient in order to achieve widespread use [24].
The physiology of hemoglobin and red blood cells
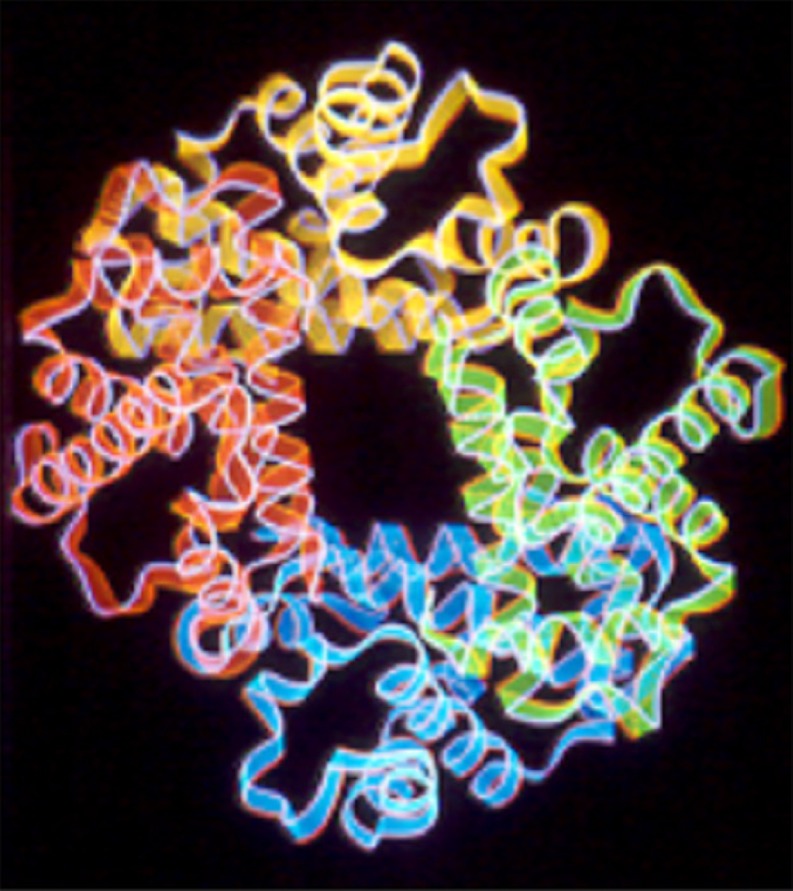
Familiarity with the structure and physiology of hemoglobin is critical to understanding the potential advantages and drawbacks of hemoglobin substitutes. Synthesis of hemoglobin occurs in the proerythrocyte in the bone marrow and continues in the early stage of the reticulocyte as it is released into the bloodstream [8]. First, the heme molecule is synthesized as the combination of protoporphyrin IX and iron (Fe++). Each heme molecule then combines with a long polypeptide chain called a globin, producing a hemoglobin chain. Four hemoglobin chains bind loosely together to form the whole hemoglobin molecule (Fig. 1). There are slight differences between different globin chains (such as alpha, beta, gamma) based on amino acid sequence. The most common form of adult human hemoglobin is hemoglobin A, which is composed of two α and two β polypeptide chains. Therefore, each hemoglobin molecule has four heme groups that are capable of carrying one molecule of O2 each. The molecular weight of the hemoglobin tetramer is 64,458. Hemoglobin is able to combine loosely and reversibly with oxygen. The globin subunits in a hemoglobin molecule that is free of oxygen (deoxyhemoglobin) are held together in a configuration that has a relatively low affinity for oxygen [19]. Once an oxygen molecule binds to a heme group, the electrostatic forces between the individual chains relax, leading to exposure of the other oxygen-binding sites and an increased affinity for oxygen. This explains the sigmoid shape of the hemoglobin oxygen-saturation curve. This relationship allows hemoglobin to serve its function of binding oxygen in a high-O2 pressure environment (the lungs) and releasing it in the low-O2 pressure peripheral tissues. The affinity of hemoglobin to oxygen is affected by several factors. Hydrogen and carbon dioxide decrease hemoglobin’s affinity to oxygen, allowing more oxygen to be released to the end organs. 2,3-diphosphoglycerate (2,3-DPG) in the red blood cell stabilizes the deoxyhemoglobin configuration and reduces the affinity of hemoglobin to oxygen.
Fig. 1.
The hemoglobin tetramer is composed of four globin molecules that form a complex three-dimensional structure
Hemoglobin is carried in high concentrations by RBCs, which have a lipid bilayer membrane. This membrane protects the hemoglobin tetramers; without it, the tetramers would quickly dissociate into dimers and monomers, which subsequently get cleared by the kidney [24]. The average RBC carries its hemoglobin for an average of 120 days before being destroyed [8]. This points to a major challenge with extracellular hemoglobin substitutes, namely, the relatively short half-life of the compound compared to allogeneic RBCs. The RBC also prevents the release of heme from hemoglobin, which would be damaging to peripheral tissues. Moreover, hemoglobin contained in the RBC does not get oxidized to methemoglobin, a molecule that is not capable of transporting oxygen and that can cause oxidative damage to surrounding tissues [24].
Development of hemoglobin substitutes
With this in mind, it is possible to understand the various materials that have been developed to reproduce the function of RBC. Three types of products are under development as hemoglobin substitutes. The first and most vigorously studied is hemoglobin-based oxygen carriers (HBOC), which are cell-free hemoglobin preparations using hemoglobin from animals or humans. The second approach is the use of perfluorocarbons, which are cyclic or linear carbon molecules substituted with fluorine. Lastly, liposome-encapsulated hemoglobin has been researched as a way to avoid the shorter half-life of extracellular hemoglobin; this approach is still in the preclinical development stage.
Hemoglobin-based oxygen carriers (HBOC)
Sources and sterilization
HBOCs are produced by lysing human or bovine RBCs, subjecting them to a rigorous sterilization process, and then treating them chemically (e.g., forming polymers) to allow them to be more stable after transfusion. The three potential sources for hemoglobin for hemoglobin substitutes are outdated human RBCs, bovine RBCs and recombinant human Hb. Only 5–10% of donated allogeneic blood becomes outdated and, therefore, the quantity of hemoglobin available from this source may not be sufficient for mass production of hemoglobin substitute [6]. The use of a transgenic pig to produce recombinant human hemoglobin has been reported [25], but this product has not been utilized in clinical trials [24]. Bovine hemoglobin, on the other hand, has no quantity constraints. It is the ingredient in the hemoglobin substitute actively studied in several clinical trials, including one involving orthopaedic surgery patients (Hemopure, Biopure Corporation, Cambridge, MA, USA). Bovine hemoglobin is obtained by lysing bovine RBCs and purifying the hemoglobin. Then, it is subjected to thorough sterilization and viral-inactivation methods that are not possible with whole blood [24].
Modification of hemoglobin structure
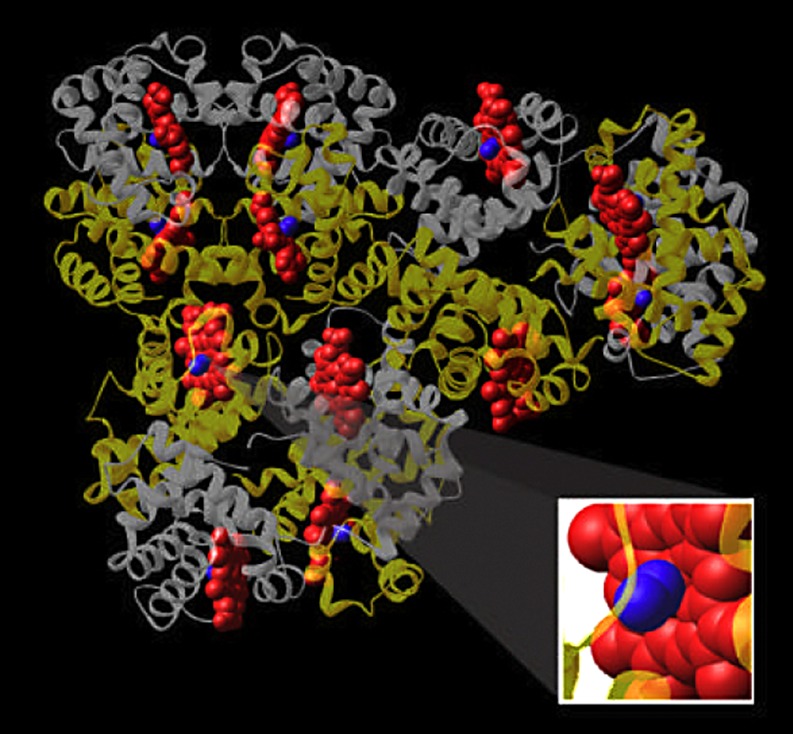
The purified hemoglobin is then modified chemically to prolong its durability in the bloodstream. Various manufacturers have accomplished this in several ways depending on different formulations. As described above, hemoglobin tetramer, if infused into the bloodstream, would rapidly dissociate into dimers and monomers, get filtered by the kidney and potentially damage renal tubular cells. Modification of the chemical structure of the purified hemoglobin prolongs the half-life to 18–58 h [24]. Some formulations stabilize hemoglobin by attaching small molecules such as polyethylene glycol or polyoxyethylene to the lysine residues on the surface of the hemoglobin molecule [5]. This increases the viscosity of the solution and prolongs its half-life. The hemoglobin tetramers can also be induced to form a large polymer of tetramers by reacting with glutaraldehyde [9]. Glutaraldehyde has a free aldehyde group on both ends, allowing it to bond two hemoglobin tetramers and producing a chain of tetramers (Fig. 2). It also forms intramolecular cross-links within each tetramer. Two products, Hemopure (Biopure) and Polyheme (Northfield, Evanston, NJ, USA) are made by polymerizing bovine and human hemoglobin, respectively, using glutaraldehyde. Finally, the α globin units within the hemoglobin tetramer can be cross-linked to each other by molecules such as bis-(3,4-dibromosalicyl) fumarate, producing a tetramer that is more stable in plasma than untreated hemoglobin [4].
Fig. 2.
Schematic representation of HBOC-201. Three bovine hemoglobin molecules are cross-linked to produce the hemoglobin-based oxygen carrier. Each globin subunit carries a blue heme molecule. In turn, each heme molecule is shown as carrying an oxygen molecule (Courtesy of Biopure)
Properties of HBOC-201
We would like to describe in more detail the properties of HBOC-201, since it is the only preparation that is approved for use in humans in South Africa, and since it has been tested in international clinical trials with orthopaedic patients. HBOC-201 is a sterile, ultra-purified, glutaraldehyde polymerized bovine hemoglobin in a balanced saline solution. Only cattle from the United States that are 30 months of age or less and that have been certified as disease-free are used as donors. The hemoglobin is sterilized extensively to eliminate RBC stromata, bacterial endotoxins, viruses, and the prions responsible for vCJD and bovine spongiform encephalopathy. The product has an average molecular weight of 250 kD [9]. Its shelf life is at least 2 years when stored at a temperature of 2–30°C. It does not need cross-matching or typing and can be infused directly without reconstitution. Transfusing such a product gives the post-operative anemic patient a large infusion of iron (which is naturally bound to heme), which would help offset the iron lost from surgical bleeding. HBOC-201’s oxygen-dissociation curve is right-shifted, with a P50 (O2 pressure at which 50% of oxygen-binding sites are saturated with oxygen) of 43 mm Hg, compared with 27 mm Hg for human hemoglobin. This lower affinity for oxygen allows HBOC-201 to release more oxygen in the peripheral tissues. Moreover, unlike human hemoglobin, whose affinity for oxygen is 2,3-DPG-dependent, bovine hemoglobin’s oxygen affinity is chloride-dependent. Since HBOC-201 is an extracellular form of bovine hemoglobin, its affinity for oxygen is in the physiological range because of chloride ions in plasma. Extracellular human hemoglobin, on the other hand, must be modified by pyridoxylation to compensate for the lack of 2,3-DPG in plasma.
Animal and preclinical trials using HBOC
Several investigators have studied HBOCs extensively over the last few years and have shed light on their potential benefits. Hughes et al. [9] drew 15% of the blood volume of 18 healthy volunteers and 23 controls and subsequently transfused different doses of HBOC-201 (test group) or lactated Ringer’s solution (control group). They found that, in the test group, there were dose-dependent increases in oxygen-diffusion capacity calculated from pulmonary function tests. The volunteers receiving the highest doses of HBOC-201 had higher diffusion capacity than they did at baseline. The product had a half-life of about 20 h, and it was not eliminated renally. The authors believed that this increased ability to transport oxygen was secondary to enhanced oxygen diffusion from the extracellular hemoglobin, compared with lipid bilayer-encapsulated hemoglobin. Moreover, the oxygen-dissociation curve for HBOC-201 was shifted to the right relative to human hemoglobin, meaning that HBOC-201 was more able to unbind oxygen in the periphery and release it to the target organs. Standl et al. [22, 23] compared HBOC with autologous blood as resuscitation for dogs after massive blood loss. They found that the increase in skeletal muscle tissue oxygen tension per gram of transfused hemoglobin was higher after HBOC transfusion than after autologous RBC transfusion. They attributed this to better extraction of oxygen in the peripheral tissues because of HBOC’s lower affinity for oxygen. Sampson et al. designed a similar study using a swine model for hemorrhagic shock [20]; they compared HBOC-201 with other low-volume resuscitation fluids, such as hypertonic saline, dextran, and pentastarch. They found that HBOC-201 was able to restore mean arterial pressure from 30 mm to 60 mm with a much smaller volume of infusion than the comparison fluids. They concluded that HBOC-201 could be an effective low-volume resuscitation fluid in the battlefield, rural areas, or other scenarios where efficient transport of fluid products was of prime importance. The same group of investigators evaluated pigs that had received HBOC transfusion, for evidence of end-organ damage [28]. The parameters used were lab values such as blood-gas analyses, urine output, and jejunal oximetry as well as analyses of different tissues at necropsy performed on post-injury day 3. The tissues analyzed were from the stomach, duodenum, ileum, lung, liver, and kidney. There was mild-to-moderate hepatocellular damage in four of six pigs infused with HBOC, accompanied by increases in serum aspartate aminotransferase. These changes were deemed transient, since levels of aspartate aminotransferase (AST) were returning to normal by post-injury day 3. The other tissues showed no histologic abnormalities.
Human trials using HBOC
A number of publications describe HBOC trials in different patient populations and several more studies are in the manuscript-preparation stage. LaMuraglia et al. reported on a single-blind multicenter study of 72 patients undergoing elective, infrarenal aortic operations [15]. Patients were assigned to the HBOC-201 group or the allogeneic group at random, when the decision to transfuse was made. Thirty-five of 48 patients in the HBOC-201 group and 24 of 24 patients in the allogeneic blood group required allogeneic blood transfusions. Therefore, the use of HBOC-201 spared 27% of patients (95% confidence interval (CI): 15–42%) from requiring allogeneic blood transfusions. The average number of RBC units needed in the HBOC group, albeit smaller (2.0 vs 2.5) than that of the allogeneic group, did not show a statistically significant difference. The authors explained that, while the use of HBOC did not reduce the overall demand on allogeneic blood, it did allow 27% of patients to avoid the risks of allogeneic blood. There was a 15% increase in mean arterial pressure and a transient increase in blood urea nitrogen (BUN) in the HBOC group. However, the overall complication and mortality rates between the two groups were statistically similar.
Levy et al. [16] performed a double-blinded trial of HBOC-201 in 98 patients undergoing cardiac surgery and in need of a transfusion. HBOC eliminated the need for allogeneic transfusions in 34% (95% CI: 21–49%) of 50 patients in the HBOC group. The number of transfused RBC units was 0.47 units less in the HBOC group (p=0.05). The short half-life of HBOC required the use of as much as 120 g of HBOC hemoglobin (equivalent of 2 units) to spare about one-half unit of allogeneic blood. Furthermore, by post-operative-day 2, about 40% of circulating hemoglobin was in the oxidized methemoglobin form. Similar to the aortic study, there was a slight increase in mean arterial pressure with HBOC infusion. This is hypothesized to be secondary to nitric oxide binding by HBOC or to stimulation of the production of endothelin-1, an endogenous vasoconstrictor. Of 50 patients in the HBOC group, 14 experienced jaundice, but all cases resolved by the time of hospital discharge.
HBOC in orthopaedic patients
The safety and efficacy of HBOC-201 was evaluated in patients undergoing orthopaedic surgery in a Phase III study [10]. In this single-blind, multicenter randomized study of 688 patients who underwent orthopaedic procedures and subsequently needed a blood transfusion, 350 patients received HBOC-201 and 338 received allogeneic blood transfusion. The use of HBOC-201 spared 59.4% of patients in the HBOC group the need for allogeneic transfusion. This was valid at 6 weeks post-operatively. Similar to the studies above, transient increases in blood pressure were observed in the HBOC group, but they were not associated with any clinical sequelae. Adverse events occurring more frequently in the HBOC group also included gastrointestinal and cutaneous events, but these events were mild and self-limited [10]. The two groups had comparable mortality and serious-adverse-event rates. This illustrates that in three different surgical fields, the use of HBOC spared a significant proportion of patients from receiving allogeneic blood. While there were consistently observed associated effects of HBOC transfusion, such as blood pressure elevation, jaundice, and changes in AST and alanine aminotransferase (ALT) levels, there were no increases in renal insufficiency, liver failure, or rates of morbidity and mortality in the HBOC group.
Other technologies: perfluorocarbons and liposome-encapsulated hemoglobin
Perfluorocarbons (PFCs) are molecules with linear or cyclic carbon backbones that are highly substituted with fluorine and occasionally other halogens [19]. They are immiscible with blood, so they must be prepared as emulsions using a phospholipid as a surfactant [7]. Instead of binding oxygen chemically as hemoglobin does, PFCs simply dissolve oxygen. PFCs have solubility for oxygen that is 20× greater than that of water [26]. Their oxygen-dissociation curve is linear, meaning that the patient needs to be exposed to high fractional inspired oxygen (FiO2) for the PFC to carry meaningful amounts of oxygen. The advantages of PFCs arise from their completely synthetic nature; therefore, they should have no infectious risk. They can be produced on a large scale and with relatively low cost. PFCs, however, have been observed to activate, complement and stimulate the reticuloendothelial system (RES), leading to bronchospasm and thrombocytopenia. Furthermore, they are cleared by the RES relatively quickly, with an intravascular half-life of 12–18 h [26].
Clinical trials have been performed over the last several years using different PFCs. Recently, perflubron (Oxygent; Alliance Pharmaceutical, San Diego, CA, USA) was investigated in a prospective multicenter, single-blind, randomized controlled study on 492 patients undergoing non-cardiac general surgery [21]. The patients in the PFC group underwent acute normovolemic hemodilution (ANH) to a hemoglobin of 8.0 g/dl before incision. Intra-operatively, these patients were transfused with PFC. The autologous blood collected before surgery was transfused to the patients post-operatively. The purpose of this study was to evaluate if ANH/PFC would reduce the need for allogeneic transfusion since the autologous blood collected pre-operatively was returned to the patient after the surgical bleeding had been controlled. There was a statistically significant reduction in the number of allogeneic units transfused in PFC patients who lost >20 ml blood/kg. The overall incidence of adverse events was similar in the two groups. The mortality rate in the PFC group was 4% compared with 2% in the control group, but this was not statistically significant, and the deaths were thought to be related to underlying diseases such as malignancy. The authors concluded that the use of PFC may represent a new alternative for avoiding or minimizing the risks of allogeneic transfusions.
The concept of liposome-encapsulated hemoglobin has been intriguing, since placing the hemoglobin inside a lipid membrane obviates the need to chemically modify it and prolongs its intravascular half-life [24]. It is possible that 2,3-DPG and methemoglobin reductase could be encapsulated with the hemoglobin in the liposome to perform the other important functions of the RBC. However, this is still a concept in development. There will likely be difficulties in preparing liposomes that are uniform in size. There may also be unforeseen effects from the clearance of large amounts of this product by the reticuloendothelial system. This is a novel approach that merits more investigation.
Summary
The past several years have produced a large body of research into red blood cell substitutes. Hemoglobin-based oxygen carriers in particular are currently in use in South Africa and are actively being studied internationally, including in orthopaedic patients. The ability to address intra-operative and post-operative blood loss with a hemoglobin product that is sterile, non-allogeneic and universally accepted would have a significant impact on patient care and orthopaedic surgery. This is particularly important with regard to potential anticipated blood shortages, the reluctance of patients, families, and physicians to accept the risk of allogeneic blood, and the life-saving potential of such a product in the battlefield and other trauma settings.
Acknowledgments
Dr. MacKenzie would like to acknowledge that he is a consultant as well as a site principal investigator for the Biopure Corporation.
References
- 1.Aguzzi Lancet. 2004;363:411. doi: 10.1016/S0140-6736(04)15520-7. [DOI] [PubMed] [Google Scholar]
- 2.Ania J Am Geriatr Soc. 1997;45:825. doi: 10.1111/j.1532-5415.1997.tb01509.x. [DOI] [PubMed] [Google Scholar]
- 3.BierbaumJ Bone Joint Surg Am 19998129973048 [Google Scholar]
- 4.Chatterjee J Biol Chem. 1986;261:9929. [PubMed] [Google Scholar]
- 5.ConoverJ Investig Med 1996442388763974 [Google Scholar]
- 6.Goodnough N Engl J Med. 1999;340:438. doi: 10.1056/NEJM199902113400606. [DOI] [PubMed] [Google Scholar]
- 7.Greer JP, Foerster J, Lukens JN (eds) (2003) Transfusion medicine. In: Wintrobe’s clinical hematology. Lippincott, Philadelphia
- 8.Guyton AC (2000) Red blood cells, anemia, and polycythemia. In: Textbook of medical physiology, 10th edn. WB Saunders, Philadelphia
- 9.Hughes Crit Care Med. 1996;24:756. doi: 10.1097/00003246-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Jahr JS, Stewart LM, MacKenzie C, Bourke DL, Williams JP (2002) Pivotal phase III study: safety of polymerized bovine hemoglobin (HBOC-201, Hemopure) as compared to RBC in patients undergoing orthopaedic surgery. Anesthesiology Supplement] 97:A243
- 11.Janatpour Curr Hematol Rep. 2002;1:149. [PubMed] [Google Scholar]
- 12.Keating Instr Course Lect. 1999;48:655. [PubMed] [Google Scholar]
- 13.Keating J Am Acad Orthop Surg. 2002;10:393. doi: 10.5435/00124635-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Kopko JAMA. 2002;287:1968. doi: 10.1001/jama.287.15.1968. [DOI] [PubMed] [Google Scholar]
- 15.LaMuraglia GM, O’Hara PJ, Baker WH, Naslund TC, Norris EJ, Li J, Vandermeersch E (2000) The reduction of the allogenic transfusion requirement in aortic surgery with a hemoglobin-based solution J Vasc Surg 31:299–308 [DOI] [PubMed]
- 16.Levy J Thorac Cardiovasc Surg. 2002;124:35. doi: 10.1067/mtc.2002.121505. [DOI] [PubMed] [Google Scholar]
- 17.Llewelyn Lancet. 2004;363:417. doi: 10.1016/S0140-6736(04)15486-X. [DOI] [PubMed] [Google Scholar]
- 18.Menitove JE (2000) Transfusion-transmitted diseases. In: Hoffman R (ed) Hematology: basic principles and practice, 3rd edn. Churchill Livingstone, New York
- 19.Moore J Am Coll Surg. 2003;196:1. doi: 10.1016/S1072-7515(02)01704-0. [DOI] [PubMed] [Google Scholar]
- 20.Sampson J Trauma. 2003;55:747. doi: 10.1097/01.TA.0000084519.47163.77. [DOI] [PubMed] [Google Scholar]
- 21.Spahn Anesthesiology. 2002;97:1338. doi: 10.1097/00000542-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Standl J Vasc Surg. 2003;37:859. doi: 10.1067/mva.2003.127. [DOI] [PubMed] [Google Scholar]
- 23.Standl Can J Anaesth. 1996;43:714. doi: 10.1007/BF03017957. [DOI] [PubMed] [Google Scholar]
- 24.Stowell Transfusion. 2001;41:287. doi: 10.1046/j.1537-2995.2001.41020287.x. [DOI] [PubMed] [Google Scholar]
- 25.SwansonBioTechnology 1992105571368235 [Google Scholar]
- 26.Tremper Anesthesiology. 2002;97:1333. doi: 10.1097/00000542-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Winslow RM (2000) Red cell substitutes. In: Hoffman R (ed) Hematology: basic principles and practice, 3rd edn. Churchill Livingstone, New York
- 28.York J Trauma. 2003;55:873. doi: 10.1097/01.TA.0000092681.17874.6F. [DOI] [PubMed] [Google Scholar]