Abstract
A possible strategy to promote the wound-healing cascade in both soft and hard tissues is the preparation of an autologous platelet-rich plasma (PRP) to encourage the release of growth factors from activated platelets. In this process, PRP combines the advantage of an autologous fibrin clot that will aid in hemostasis as well as provide growth factors in high concentrations to the site of a tissue defect. The PRP preparation can be used as a biological enhancer in the healing of fractures and lumbar fusions. The local application of growth factors seems to promote initiation and early maturation of bone formation. Autologous bone or bone substitutes can be added to this mixture to increase the volume of grafting material. A simplified technique utilizing a commercially available separation system (GPS—Gravitational Platelet Separation System) is described. This system provides a less costly alternative to other previously described augmentation techniques and also presents a patient-friendly and operator-safe alternative. Further experimental studies of the actual concentrations of the growth factors in the PRP samples are necessary in order to validate the platelet concentration and growth-factor activation by laboratory evidence. In further prospective clinical trials, the safety and efficacy of PRP, in combination with autologous bone or bone graft substitutes, must be evaluated.
Keywords: Platelet concentrate, Growth factors, Bone healing, Platelet-rich plasma, GPS
Introduction
Autologous platelet-rich plasma (PRP) is increasingly used in almost all fields of surgery for the clinical treatment of a variety of soft and hard tissue applications, most notably in accelerating bone formation and in the management of chronic nonhealing wounds [1, 13, 15, 24]. The use of PRP combines the advantage of an autologous fibrin clot that will aid in hemostasis as well as the provision of growth factors in high concentrations to the site of a bone defect or a region requiring augmentation after platelet release.
Bone formation at the site of a fracture or at the site of grafting is initiated by a process of fibrin clot formation, platelet aggregation, and degranulation. Platelet granules contain a variety of physiologically active substances, such as catecholamines, serotonin, calcium ions, adenosine triphosphate (ATP), albumin, fibrinogen, osteonectin, osteocalcin, various clotting factors, and the locally active growth factors, such as PDGF, TGF-β, IGF, FGF, and EGF [12]. With a better understanding of the various biological factors that initiate, maintain, and regulate the complex process of osteogenesis, new strategies can be followed to manipulate the biological environment of the site of bone formation and to enhance bone fusion.
For bone defects, PRP can be mixed with autologous bone or with bone graft materials [8, 9]. PRP is then delivered to the recipient bed along with autologous or bovine thrombin and placed in the graft site. The fibrinogen present in the PRP is activated and becomes cross-linked to form a fibrin network [16]. Thus, the graft is solidified and adheres within the defect.
The extraction of platelet concentrates through plasmapheresis is a process by which PRP is taken from the patient, and the remaining components of blood are delivered back into the body. With this technique, PRP can be produced at a concentration of 300% of normal blood levels [15, 22]. For practical and economic reasons, this procedure is generally only suitable within larger clinics or hospitals. For a broader and individual use of PRP, different platelet-concentration preparation systems are now commercially available. We describe a simple and disposable system for the preparation of PRP using the Gravitational Platelet Separation System (GPS).
Extraction of PRP
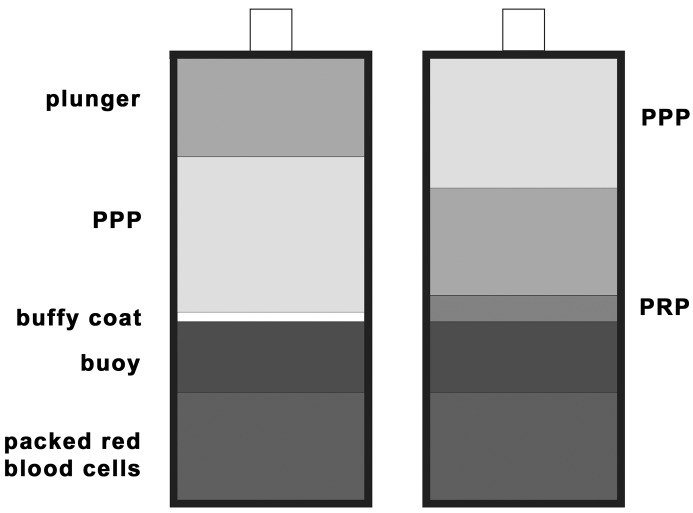
The Gravitational Platelet Separation System (GPS System, Biomet Merck Biomaterials, Darmstadt, Germany) is a simplified, commercially available technique for the extraction of PRP. The system consists of a disposable GPS tube that is used together with a bench-top centrifuge. The preparation can be performed in the operating theater during the actual surgical intervention and takes about 30 min. First, 6 ml (1 ml=1 cm3=1 cc) of Anticoagulant Citrate Dextrose Solution (ACD-A) is drawn into a 60 ml syringe, followed by 54 ml of whole blood. The sample is gently agitated to mix the anticoagulant thoroughly with the blood. Then, the blood is injected into the GPS disposable unit and centrifuged for 12 min at 3200 rpm using a standard electronically controlled bench-top centrifuge (Thermo, IEC International Equipment Company, Needham Heights, Mass.). Following centrifugation, the blood sample is separated in different blood fractions: the red blood cells form a red-colored fraction on the bottom of the tube separated through a buoy from the buffy coat and a whitish layer rich in white blood cells and platelets and the platelet-poor plasma (PPP), which contains autologous fibrinogen and is poor in platelets (Fig. 1). In the next step, a plunger is manually pushed down and separates the platelet-poor from the platelet-rich plasma (Fig. 2). The PPP is extracted through a separate luer-lock connection using a 30-ml syringe. Then the tube is vigorously shaken for 30 s, and the platelets are suspended in the remaining plasma. Through a second luer-lock connection the PRP is separately extracted in a 10-ml syringe. For sufficient clot formation, the PRP can now be used with autologous or bovine thrombin solution at a 10:1 ratio. Once the PRP is produced, it can be mixed with the preferred augmentation material, such as autologous bone or different bone substitutes (Fig. 3, Fig. 4).
Fig. 1A, B.
Schematic view of the disposable GPS tube after centrifugation at 3,200 rpm for 12 min. The red blood cell fraction on the bottom of the tube is separated through a buoy from the buffy coat and the platelet-poor from the platelet-rich plasma (A). A plunger is manually pushed down and separates the platelet-poor from the platelet-rich plasma (B)
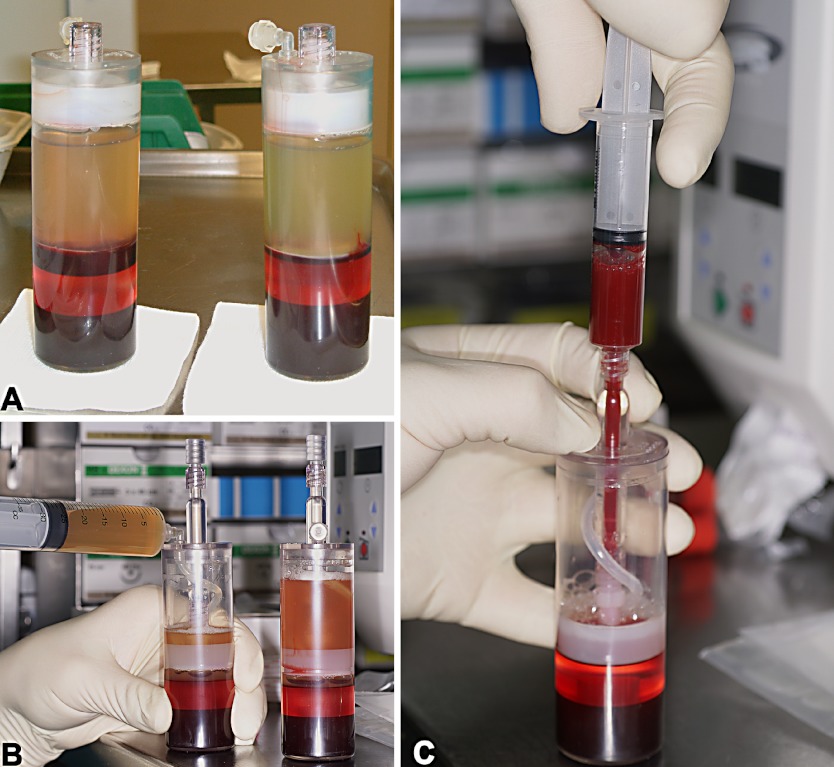
Fig. 2A-C.
The GPS tube after centrifugation with the red-colored fraction of red blood cells on the bottom and the buffy coat above the buoy (A). The tube after the separation of the PRP, with a manually pushed down plunger (B) and the extraction of the PRP (C)
Fig. 3A-D.
PRP and autologous thrombin solution on the operation table (A), aspirated in a double syringe in a 10:1 ratio (B), forming a clot after combination (C) and mixed with autologous bone (D)
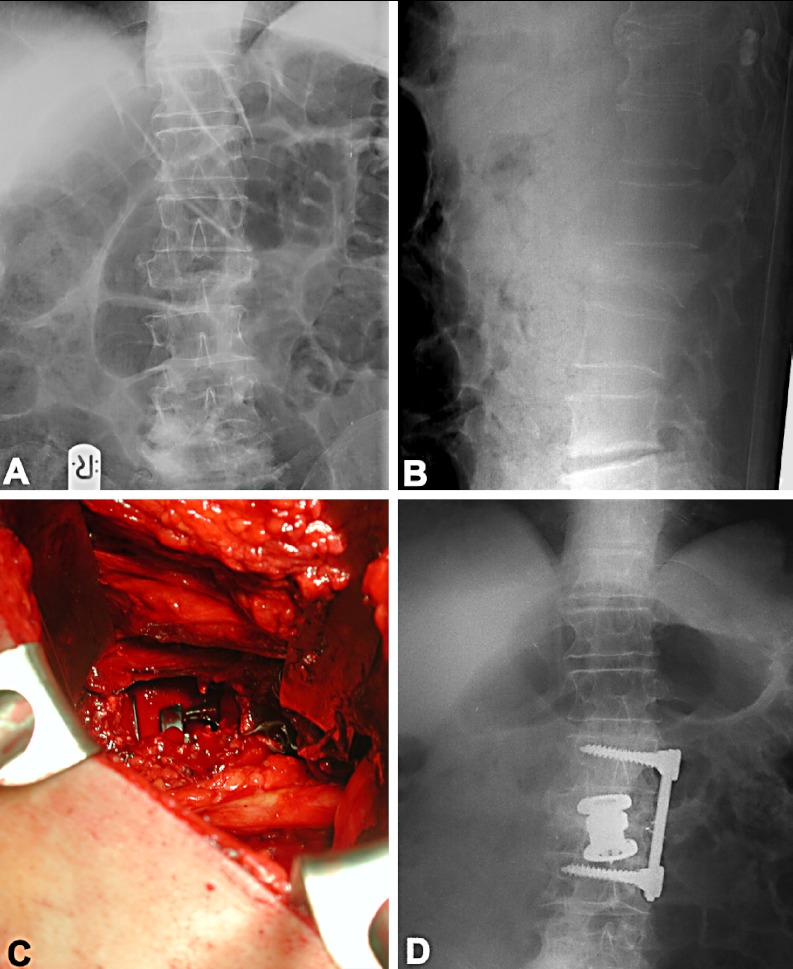
Fig. 4A-D.
Example of a 62-year-old man who presented with a burst fracture of L2, with extensive loss of height in the vertebral body (A, ap view; and B, lateral view). The operation consisted of a retroperitoneal approach, partial resection of the fractured vertebral body, reduction by a distractable cage, and stabilization with a plate system. Autogenous bone was mixed with PRP (C, intraoperative site; and D, postoperative ap X-ray)
Discussion
Platelet concentrates function as a tissue sealant and a drug-delivery system that contains a host of powerful mitogenic and chemotactic growth factors. Hemostasis is achieved through the formation of a fibrin clot that is initiated by the activation and aggregation of platelets. The clot is generated by the polymerization of fibrin from the monomer, fibrinogen, in the presence of calcium and thrombin. Platelet aggregation results in a platelet plug that is held in place by the clot and inhibits blood flow [14]. Beyond maintaining hemostasis, the fibrin clot then provides a matrix for the migration of tissue-forming cells and endothelial cells involved in angiogenesis and the remodeling of the clot into repair tissue.
Platelet α-granules have been shown to contain mitogenic and chemotactic growth factors, such as platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and insulinlike growth factor (IGF) [21]. The effects of these growth factors on cell behavior and bone-healing sequences have been thoroughly studied [4, 5, 7, 11, 15, 20, 21].
Caplan has described three sites in bone containing undifferentiated stem cells that are capable of differentiating into osteoblasts: the marrow, periosteum, and the perivascular sheath [6]. PDGF and TGF-β have a chemotactic and mitogenic effect on these cells that causes them to multiply and secrete additional growth factors. In a rat tibial-fracture model, injections of TGF-β (4 and 40 ng) every other day for 40 days resulted in a dose-dependent increase in bone thickness [17]. Further, the 40 ng dose also increased mechanical strength [17]. Under the influence of other cytokines and local environmental conditions, such as pH, oxygen tension, and micromotion, these cells undergo a differentiation into osteoblasts or chondroblasts. Collagen and protein synthesis by osteoblasts is also stimulated by PDGF, but also requires the presence of IGF-I [7]. PDGF probably enhances the secretion of IGF-I by the osteoblasts and mesenchymal stem cells, which then accelerates the formation of a collagenous matrix [11]. PDGF also seems to enhance the activity of BMP in promoting cartilage and bone formation [10].
A major concern in the delivery of the growth factors to the site of bone healing has been their short half-lives in the systemic circulation [18]. PDGF has a half-life of about 2 min if injected intravenously, and TGF-β, in its active form, is also cleared from the bloodstream within a few minutes. Further, TGF-β acts only locally, with no influence on bone formation at sites distant from its application [19]. Furthermore, multiple growth factors are present at the same time at the site of bone formation and have a synergistic effect on each other [11]. A study about growth factor interactions concluded that TGF-β, IGF-II, and FGF modify the activity of other growth factors and cytokines and actually have a synergistic effect in combination [11]. This has led to the philosophy of providing multiple growth factors in the bone-cell microenvironment simultaneously to promote interactions between the factors that could be important for regulation of bone-cell proliferation. The mitogenic and chemotactic growth factors in platelets can provide a combination of multiple factors simultaneously, rather than individual factors, and accelerate the bone-healing sequences in order to mimic the natural process of osteogenesis as physiologically as possible.
Clinical experience in larger case series in the treatment of fractures of long bones is limited, as is experience with spine surgery. Prospective randomized trials comparing PRP with other bone-healing, accelerating substances are missing. Quantitative and qualitative measurements in maxillofacial surgery have shown that autologous bone grafts treated with PRP mature in two-thirds of non-PRP graft time, have a 1.6- to 2.6-fold higher radio-opacity, and are 70% more mature than untreated, naturally occurring bone at the site [15]. In a retrospective study, Lowery reported the use of autologous growth factors (AGF) in lumbar spinal fusions. Posterolateral fusion and instrumentation, with or without intradiscal fusion and laminectomy, was performed in 15 cases. Seven of these 15 patients had concomitant intradiscal fusion with titanium surgical mesh (TSM) or a femoral ring spacer with autograft. Posterolateral fusion was performed using a mixture of autograft and AGF concentrate activated by thrombin and coralline hydroxyapatite. In 4 cases, intradiscal fusion was performed without posterior fusion using HA dowels with autograft and AGF concentrate placed in carbon fiber cages or TSM. After a follow-up of a minimum of 6 months, bony fusion was confirmed intraoperatively in 5 of 19 patients. In the remaining 14 patients, radiographs showed solid or maturing fusion in all cases with no radiological or clinical evidence of pseudoarthrosis.
To our knowledge, no prospective studies have been performed using PRP. To prove the concept of using PRP in the treatment of nonunions in long bones and in lumbar fractures in combination with autologous bone augmentation, prospective pilot studies were performed at our institution. The preliminary results are promising, but further prospective comparative studies are necessary to validate the efficacy. These studies will also include a comparison with recombinant bone morphogenic proteins (BMP), which are used in the biological manipulation of the osteoinductive process [2]. In spine surgery, rhBMP-2 and rhBMP-7 have been used in clinical trials in different fusion models [2]. In a prospective, randomized, mulitcenter trial, posterolateral intertransverse arthrodesis in degenerative spondylolisthesis and lumbar stenosis was used to compare autograft and rhBMP-7 [23]. In other studies in degenerative disc disease and lumbar interbody fusion, autografts were compared with rh-BMP-2 [3]. If BMPs in these studies are effective and safe, further comparative trials with PRP are necessary and useful.
In conclusion, the GPS is a commercially available separation system for the preparation of PRP using a simplified technique. This system provides an alternative to other previously described augmentation techniques for accelerating bone healing and also presents a patient-friendly and operator-safe alternative. Compared to recombinant proteins and whole BMP extracts, PRP has the advantage that it can also act as a binding medium for bone autografts or granulated bone substitutes, making it easier to handle and place them into the graft site. The most significant benefit of using the PRP is its autologous nature, the fact that it is endogenously derived, and its easy availability. There are no issues about immunogenicity or transmission of infection. There are no known local or systemic side effects or adverse effects. The process is also considerably cost-effective compared to the use of purified or recombinant growth factors.
Acknowledgements
This study was supported by a grant from the Ludwig Boltzmann Society.
References
- 1.Bhanot S, Alex JC (2002) Current applications of platelet gels in facial plastic surgery. Facial Plast Surg;18:27–33 [DOI] [PubMed]
- 2.Boden Spine. 2002;27:S26. doi: 10.1097/00007632-200208151-00007. [DOI] [PubMed] [Google Scholar]
- 3.Boden Spine. 2002;27:2662. doi: 10.1097/00007632-200212010-00005. [DOI] [PubMed] [Google Scholar]
- 4.CanalisClin Orthop 19851932463882294 [Google Scholar]
- 5.Canalis J Cell Physiol. 1989;140:530. doi: 10.1002/jcp.1041400319. [DOI] [PubMed] [Google Scholar]
- 6.Caplan J Orthop Res. 1991;9:641. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 7.Gospodarowicz J Pathol. 1983;141:201. doi: 10.1002/path.1711410304. [DOI] [PubMed] [Google Scholar]
- 8.Hotz Int J Oral Maxillofac Surg. 1991;20:204. doi: 10.1016/s0901-5027(05)80175-4. [DOI] [PubMed] [Google Scholar]
- 9.Hotz Int J Oral Maxillofac Surg. 1991;20:208. doi: 10.1016/s0901-5027(05)80176-6. [DOI] [PubMed] [Google Scholar]
- 10.Howes Calcif Tissue Int. 1988;42:34. doi: 10.1007/BF02555836. [DOI] [PubMed] [Google Scholar]
- 11.Kasperk Growth Factors. 1990;3:147. doi: 10.3109/08977199009108277. [DOI] [PubMed] [Google Scholar]
- 12.Lowery Bone. 1999;25:47S. doi: 10.1016/S8756-3282(99)00132-5. [DOI] [PubMed] [Google Scholar]
- 13.Margolis Diabetes Care. 2001;24:483. doi: 10.2337/diacare.24.3.483. [DOI] [PubMed] [Google Scholar]
- 14.Martinowitz Thromb Haemost. 1997;78:661. [PubMed] [Google Scholar]
- 15.Marx Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 16.Matras J Oral Maxillofac Surg. 1985;43:605. doi: 10.1016/0278-2391(85)90129-6. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen Acta Orthop Scand. 1994;65:37. doi: 10.3109/17453679408993715. [DOI] [PubMed] [Google Scholar]
- 18.Nimni Biomaterials. 1997;18:1201. doi: 10.1016/S0142-9612(97)00050-1. [DOI] [PubMed] [Google Scholar]
- 19.Noda Endocrinology. 1989;124:2991. doi: 10.1210/endo-124-6-2991. [DOI] [PubMed] [Google Scholar]
- 20.Schilephake Int J Oral Maxillofac Surg. 2002;31:469. doi: 10.1054/ijom.2002.0244. [DOI] [PubMed] [Google Scholar]
- 21.SlaterJ Orthop Res 1995136557472743 [Google Scholar]
- 22.Sonnleitner D, Huemer P, Sullivan DY (2000) A simplified technique for producing platelet-rich plasma and platelet concentrate for intraoral bone grafting techniques: a technical note. Int J Oral Maxillofac Implants;15:879–882 [PubMed]
- 23.Vaccaro Spine. 2002;27:S59. doi: 10.1097/00007632-200208151-00013. [DOI] [PubMed] [Google Scholar]
- 24.Whitman J Oral Maxillofac Surg. 1997;55:1294. doi: 10.1016/s0278-2391(97)90187-7. [DOI] [PubMed] [Google Scholar]