Abstract
Normal aging is associated with impairments in cognitive function, including memory, and with specific and relatively subtle synaptic alterations in the hippocampus and prefrontal cortex. The authors describe these structural changes reported in monkeys and rodents, how they might affect age-associated cognitive decline and potential strategies to limit their impact.
Preface
Normal aging is associated with impairments in cognitive function, including memory. These impairments are linked, not to a loss of neurons in the forebrain, but to specific and relatively subtle synaptic alterations in the hippocampus and prefrontal cortex. Here we review studies that have shed light on the cellular and synaptic changes observed in these brain structures during aging that can be directly related to cognitive decline in young and aged animals. We also discuss the influence of the hormonal status on these age-related alterations and recent progress in the development of therapeutic strategies to limit the impact of aging on memory and cognition in humans.
Introduction
Humans, like other mammals, are vulnerable to age-related cognitive decline in the absence of Alzheimer’s disease (AD) or other forms of neurodegenerative disease (Box 1). The cognitive processes mediated by the hippocampus (e.g., declarative memory) and the dorsolateral prefrontal cortex (dlPFC) (e.g., working memory) are those that are most vulnerable to aging. Studies in humans and animal models suggest that age-related cognitive decline is more likely to be associated with alterations in synaptic connectivity than with neuronal loss. This article will review the cellular and synaptic changes observed in the hippocampus and the PFC during aging that can be directly related to cognitive performance and decline, highlighting important differences in the nature of synaptic aging between these regions. The hormonal status also influences these age-related alterations, suggesting that neural and cognitive aging must be viewed from a broad physiological perspective rather than as processes that are isolated from other organ systems. Finally, although the synaptic alterations that underlie age-related cognitive decline differ from the extensive neuron loss that leads to dementia in AD, they may render neurons more vulnerable to degeneration induced by disease processes, reinforcing the importance of early intervention to maintain synaptic health.
Box 1. “Successful aging” and individual differences.
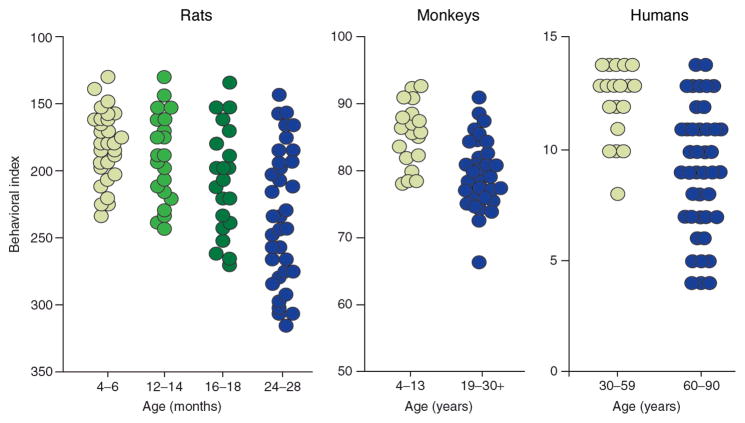
Even in the absence of pathological conditions such as AD, some elderly individuals are cognitively-impaired relative to adults, whereas others show cognitive abilities well within the range of healthy adult performance. This phenomenon can be observed in mice, rats, rhesus monkeys, and humans: see Figure on spatial memory (rats), object recognition memory (rhesus monkeys), and delayed recall (humans) performance in adult and elderly individuals (adapted from 154). Elderly individuals that experience a reduction in cognitive ability that is not a consequence of neurodegenerative disease can be said to be suffering from age-related cognitive impairment (ARCI), which is distinct from amnestic mild cognitive impairment (MCI) — associated with markers of brain atrophy and often a prelude to AD. A major goal of research in the neurobiology of cognitive aging has been to define the extent to which age-related biological changes represent correlates of impaired or maintained cognitive ability, rather than being associated simply with advanced chronological age (like gray hair or wrinkled skin). For example, within a group of individuals of similar chronological age, a loss of a particular neuromodulator in cognitively-impaired individuals relative to cognitively-intact may represent a target for intervention. Conversely, a neurobiological change seen in cognitively-intact elderly individuals relative to cognitively-impaired (or young) individuals may indicate an adaptive change associated with preserved cognition in old age. The discovery of the basis for this change could also lead to novel interventions.
The notion that cognitive ability and chronological age are not necessarily directly coupled within individuals has led to the concept of “mindspan”155, similar to “lifespan” and “healthspan”, which defines the period of time during which intact cognitive ability is maintained. The goal of research on the neurobiology of cognitive aging is to discover the means by which mindspan can be maximized, maintaining the quality of life that is associated with intact cognitive ability.
Synaptic Aging in the Prefrontal Cortex
Studies of nonhuman primates (NHPs), rhesus monkeys in particular, have shown that the dlPFC is required for cognitive tasks reliant on working memory (WM), and related tasks such as executive function and implementation of goal-directed behavior. This important role in cognition has been demonstrated most clearly in area 46 of the rhesus monkey brain1–4. Importantly, WM as mediated by area 46 is not dependent on current sensory perception of the outside world, nor is it directly mediating motor responses, but it is required to integrate these neural representations to guide goal-directed behavior. WM has been referred to as “the ability to keep events in mind”2, and as such it is constantly updated and does not represent long-term memory of events or places. The cognitive domain of dlPFC has been defined as the capacity to “acquire and implement the ‘rules of the game’ needed to achieve a given goal in a given situation” which is further complicated by the ongoing requirement to modify such rules3. There is also an element of timing to the cognitive domain mediated by area 46 as the process must be organized and executed in an appropriate sequence if the goal is to be achieved4. These are perhaps the most complex cognitive tasks performed by the cerebral cortex, and it is no surprise that dlPFC is greatly expanded in humans and NHPs compared to other mammals. Such complex tasks require complex circuitries that have been shown to be highly vulnerable to aging5–10. In both humans and NHPs, these cognitive functions are among the first to be affected by aging, suggesting that in primates the dlPFC may be more vulnerable to aging than the hippocampus5–10. Such functions — particularly establishing ever-changing rules to guide goal-directed behavior — may require an extraordinary level of synaptic plasticity, perhaps more than any other brain region. Such short-term memory frameworks have been proposed to be mediated by recurrent activity of modules of interconnected pyramidal neurons in layer 3 of area 46 modulated by both GABAergic and dopaminergic inputs2, a concept that has recently been further elucidated in the context of neuroplasticity11. The requirement for extensive synaptic plasticity might explain why the cognitive functions mediated by area 46 in monkeys are so vulnerable to aging.
Efforts to reveal the neurobiological underpinnings of age-related cognitive decline in area 46 of rhesus monkeys have focused on the synapse for several reasons. First, there does not appear to be any significant neuron loss in dlPFC12, although age-related neuron loss has been reported in the frontal eye fields13. There is an age-related decrease in volume of dlPFC that is not apparent in other regions of PFC14,15. EM studies have shown that there is substantial synapse loss in area 46, particularly in layers 1 (30–60%) and 3 (30–35%) (16,17), suggesting that a potential loss of neuropil rather than actual neuron loss may be responsible for apparent shrinkage of area 46. However, not all synapses are equally vulnerable; glutamatergic axospinous synapses account for the vast majority of lost synapses16,17. Furthermore, the extent of axospinous synapse loss in layer 3 correlates with the degree of cognitive impairment16,17, although this is not the case for layer 516, suggesting that the extensive corticocortical convergence that occurs in layer 3 is more affected by aging than the downstream projections that emanate from layer 5. This conclusion is supported by the demonstration of the loss of spines in neurons known to furnish corticocortical projections between the temporal and prefrontal cortex18. In addition, an age-related disconnection of dlPFC from other cortical regions is supported by extensive loss of myelinated fibers and myelin defects in area 46 and related white matter tracts19–21. Regression analyses demonstrated that these myelin defects correlate with impaired cognitive performance19,21. A key role for age-related alterations in layer 3 in cognitive aging is also supported by electrophysiological analyses that implicate increased excitability of layer 2/3, but not layer 5, pyramidal neurons in age-related cognitive impairments22–24. The interactions between structural and functional changes in layer 3 of dlPFC, for example whether spine loss and excitability changes counteract or exacerbate each other, have not been elucidated.
The focus on axospinous synapses and layer 3 led to the analysis of the spines of layer 3 pyramidal neurons in area 46. Spines have been divided into three major categories25,26: mushroom, thin, and stubby. The role of stubby spines remains elusive. By contrast, mushroom spines, which have relatively large spine heads, a high concentration of AMPA receptors, and a high degree of structural stability, have been shown to mediate strong synaptic currents. Thin spines, which emerge from the dendrite over a short time frame and can either stabilize or retract, are associated with a high degree of plasticity compared to mushroom spines27–31. It has been hypothesized that the large, stable mushroom spines mediate long term memories while small, thin spines are more closely associated with learning new information28,31,32. Thin spines predominantly have small, nonperforated synapses of which only ~60% have detectable AMPA receptor immunoreactivity27,33–35 and may represent the location of immature ‘silent synapses’ that bear NMDA but not AMPA receptors (but see 36,37). To determine whether a given spine class was more vulnerable to ageing, the dendritic arbor, spine density, spine size and morphology were analysed in rodent and NHP pyramidal neurons filled with Lucifer yellow38–42 (Figure 1). This approach revealed that 33% of spines on layer 3 pyramidal neurons in area 46 are lost with aging, which is remarkably close to the 32% loss demonstrated with EM counts of axospinous synapses. Perhaps more importantly, virtually all spine loss is accounted for by the loss of thin spines, in that nearly half (46%) of the thin spines are lost with aging, whereas there is no loss of mushroom or stubby spines with age17. If a significant proportion of the thin spines contain silent synapses then there may also be an age-related loss of this class of highly plastic synapses. In addition, while axospinous synapse density as determined by EM counts correlates with the capacity to learn delayed nonmatching-to-sample (DNMS), the relationship is stronger when behavior is correlated with thin spine density17. Furthermore, the mean size of the spine head in thin spines correlates with the capacity to learn DNMS for a given monkey, with a Pearson’s correlation (r) value of 0.97, the strongest correlation ever reported between a morphologic measurement and cognitive performance. Neither the density nor size of mushroom spines correlate with performance (Figure 2). These data suggest that one of the strongest contributors to age-related cognitive decline in area 46 is the loss of the most plastic thin spines, which reflects a decreased capacity for both plasticity and dynamic shifts in the glutamatergic inputs to layer 3 pyramidal neurons in area 46. It is possible that the thin spines and their capacity for dynamic plasticity play a critically important role in the ’synaptic strategy’ employed by area 46 to meet the demands of WM and accomplish tasks that require a high degree of cognitive flexibility, such as executive function, learning and planning. As discussed below, there is likely to be a class of thin spines that is highly stable as well. Interestingly, the relative stability of the mushroom spines, which mediate more stable circuits, may be related to the maintenance of expertise with aging17, which has been demonstrated in human studies43,44.
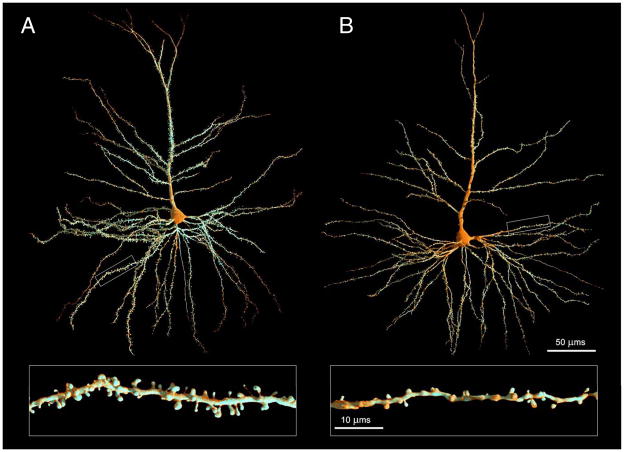
Figure 1. Cortical neuron spines in young and aged non-human primates.
Representative examples of Lucifer Yellow-filled prefrontal cortical neurons from young (A) and aged (B) non-human primates. Z-stack tiles of the two neurons were imaged using a Zeiss 510 confocal laser-scanning microscope (CLSM) and rendered into the complete neuronal reconstructions by tiling the entire set of z-stacks. The rectangle identifying a basal dendritic segment in each neuron is shown at higher magnification below each neuron. Note that while the overall dendritic arborization does not diminish with age, the robust spine density present in the young animal is significantly reduced with age, particularly the thin spines (C). Scale bar (low magnification) = 50 μm, scale bar (high magnification) = 10 μm. Image courtesy of theVisualMD.
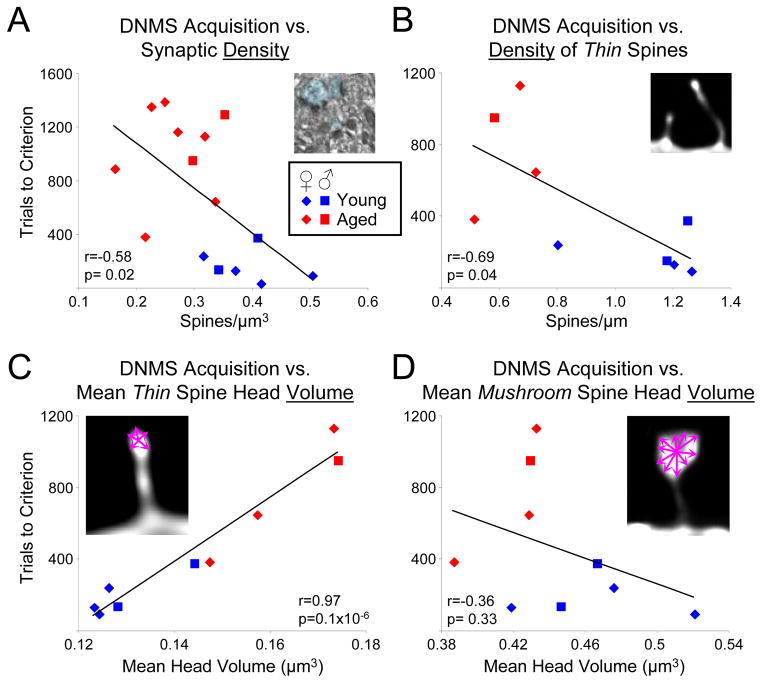
Figure 2. DNMS acquisition correlates with synaptic indices.
A) The scatter plot of DNMS acquisition (trials required to reach 90% accuracy with a 10 second delay) versus EM synaptic density shows a moderate though significant inverse correlation, meaning that higher synaptic density is predictive of faster learning. B) This inverse correlation is somewhat strengthened when the density of only thin spines is used on the subset of animals for which spine density analysis was performed (r=−0.58 in A and r=−0.69 in B; p<0.05 for both). C) DNMS acquisition correlates most strongly with the mean volume of thin spines, where smaller volumes are predictive of faster learning. D) In contrast, no correlation is seen between learning of the DNMS task and mean mushroom head volume. R = Pearson correlation coefficient. (Taken from Figure 8, ref. 16,17)
Other electrophysiological and pharmacological studies of area 46 in aged rhesus monkeys have also highlighted the possible interaction between channels bound to dendrites and spines, including thin spines, as a key factor in the maintenance of youthful cognitive performance. Neurons that fire preferentially during the delay period in a delayed response (DR) task (thought to be the essential electrophysiological component of WM2) display a loss of persistent firing across the delay by middle age that is further exacerbated in aged monkeys45. (Figure 3) Neurons that preferentially fire during the cue phase of the DR task (i.e. respond to the sensory input), displayed no such decrease with age. It has previously been shown that in young monkeys the capacity for persistent firing during the delay phase is dependent on key signaling events in spines. For example, signals that inhibit cAMP levels within the spine, such as α2A-adrenoreceptor (α2A AR) activation, enhance firing during the delay phase46. This is mediated by decreasing cAMP-mediated activation of potassium channels in dendrites, such as the hyperpolarization-activated cyclic nucleotide-gated channel (HCN), which enhances persistent firing during the delay. Conversely, pharmacological manipulations that increase cAMP-HCN activity decrease persistent firing across the delay in DR11,46,47. EM studies have shown that all the essential protein machinery for such signaling cascades is present in thin spines of neurons in layer 3 of area 46 11, suggesting that the thin spines play a crucial role in mediating WM. Presumably, the thin spines that are critically involved in WM represent a stable class of thin spines that maintain their morphologic characteristics over time. Given the dramatic loss of these spines with age17 one would predict that these signaling cascades would be profoundly affected by age. Indeed, the age-related decrease in firing during the delay phase described above was reversed by inhibiting cAMP signaling or by directly blocking HCN channels45. Together, these data have led to a potential drug target for protection against age-related decline in WM. Guanfacine, an α2A AR agonist, has been shown to enhance WM in rhesus monkeys48, presumably through activation of α2A AR on thin spines11, although this is unlikely to be the only site of action. As the unique molecular attributes of both stable and dynamic thin spines (and their synapses) are characterized, additional therapeutic strategies for protection against age-related decline in prefrontal functions should emerge.
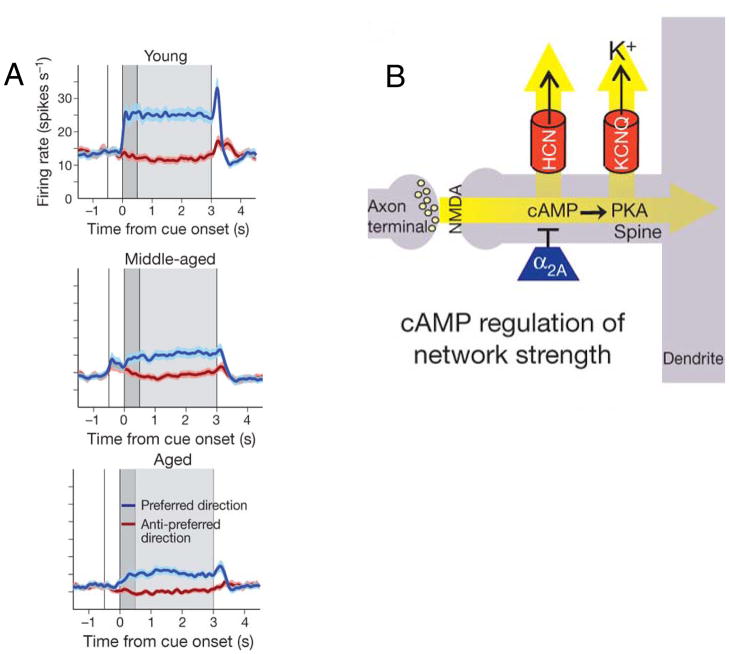
Figure 3. Age-related changes in the response properties of neurons within area 46 of rhesus monkey during a DR task.
A) The average firing rate of delay neurons in area 46 recorded in young, middle-aged, and aged monkeys. Blue indicates the firing rate for the preferred direction, and red for the anti-preferred direction. Dark grey background coincides with the time during which the cue is visible and the light grey background designates the delay period. Note that the activity during the delay is decreased in both middle-aged and aged monkeys. B) A schematic of the spines thought to play a key role in WM where the strength of the synapse is modulated by c-AMP-PKA signaling that regulates the activity of HCN and KCNQ channels. This signaling pathway in select synapses is disrupted by aging, and this is thought to occur primarily in thin spines. (A and C taken from Figure 1, ref. 45).
The data from rat PFC are far less extensive than from the NHP model, perhaps because the anatomical homology between rodent and primate prefrontal cortex is controversial49,50. However, studies of the infralimbic (IL) and prelimbic (PL) areas within rat medial PFC (mPFC), the most likely functional homologue of dlPFC in NHP and human, largely support the results from rhesus monkey. First, aged rats show impairments in the same attentional set-shifting task that has been shown to be reliant on mPFC in young rats51. They are also impaired on delayed alternation in a T maze52, a test of WM similar to the spatial delayed response task in monkeys. As in the NHP studies described above, dysregulation of cAMP has been implicated in age-related cognitive decline mediated by mPFC52,53. In addition, rat pyramidal neurons in layer 3 of PL display age-related spine loss that is evident by middle age, and as with rhesus monkeys, the small, thin spines are most vulnerable to aging42. Neuron numbers in the PL/IL of aged rats are the same as those in young rats54. These studies indicate that the same spine class and related signaling mechanisms that lead to age-related cognitive decline linked to dlPFC in monkeys may be responsible for loss of cognitive functions mediated by the mPFC in rats. Future studies of synaptic aging in the rat PFC should use behavioral assessments that are sensitive to PFC function, such as attentional set-shifting, as a behavioral correlate to identify age-related changes that are related to impairment of cognitive abilities that depend on the PFC.
Recent studies on stress and aging in rat mPFC have suggested that the effects of stress and aging may converge in this brain region. Interestingly, attentional set shifting is also impaired following chronic stress55 in rats, and the same chronic stress paradigm leads to dendritic shrinkage and loss of spines in young rats56–58. The synaptic effects of stress are both structurally59 and functionally60 reversible, which indicates that there is a high level of behaviorally induced synaptic plasticity during both damage and recovery. Recent studies that examined the interactive effects of stress and aging on dendritic arbors and spines of neurons in the PL area showed that although layer 3 neurons recover their full dendritic arbor during a rest period following chronic stress in young rats, this capacity for recovery is compromised in middle-aged rats and completely lacking in aged rats41. Interestingly, although chronic stress reduces spine density on the same neurons that exhibit dendritic retraction in young rats, there is no effect on spine density in middle aged or aged rats42. In fact, the middle-aged and aged rats displayed extensive spine loss (35%), and the small thin spines were particularly vulnerable, suggesting that the remaining spines are resistant to experience-dependent plasticity. Although the experience that failed to induce plasticity in this paradigm was stress, it is possible that the age-related loss of plasticity in these neurons reflects a general inability to adapt that would negatively impact cognitive tasks that require a high degree of synaptic flexibility.
Synaptic Aging in the Hippocampus
The hippocampus is the focal point of a network of cortical areas (the medial temporal lobe, MTL) that are associated with declarative or explicit memory function. Generally, this refers to memory in the everyday sense of the word, for facts and events, that (in humans) can be consciously accessed and verbalized. The MTL is critical for memories of personal experience and events, but many forms of enduring behavioral change as a consequence of experience can occur in the absence of the MTL. Many different behavioral tests have been devised to measure the functional integrity of the MTL in animals. Tests of stimulus recognition are impaired by damage to the MTL, particularly the entorhinal and perirhinal cortex61,62, with the hippocampus potentially playing an indirect role63. Spatial navigation tasks are commonly used in rats as measures of the functional integrity of the hippocampus64,65.
Some investigators have identified the hippocampus more specifically with episodic memory, that is, memory for events occurring at specific places and times. Human episodic memory is defined in terms of mental states and linguistic ability (e.g.66), which has created challenges in terms of developing animal models. A number of creative behavioral tasks have been developed to address this problem, some of which have been applied to the study of cognitive impairment in aged rats (e.g.67). For the purposes of this review, we will consider cellular and synaptic changes in the context of general impairments in hippocampal-dependent memory abilities, usually in tests of spatial learning. This provides a basis for defining functional impairment (or preservation) of the hippocampus. The advent of more specific tests that may serve as behavioral probes of particular cortical regions (e.g.68,69) and hippocampal subregions promise a finer-grained analysis of the relationship between neurobiological and behavioral changes in aging.
Analyses of the functional neurobiology of aging in the dlPFC have focused on the domain of spatial working memory and the neurophysiological and neuroanatomical correlates related to WM-dependent tasks. This represents an ideal model system for investigating the synaptic strategy of the prefrontal cortex, which is associated with extensive and rapid plasticity to allow for rapid updating of information that is relevant to immediate behavior. This contrasts with the hippocampus and the MTL, which are associated with the formation of long-term memories of facts and events, dictating a different synaptic strategy with different neuroanatomical and neurophysiological correlates. Because more kinds of behavioral tasks have been employed to probe hippocampal and MTL function in aging, a greater number of different anatomical and physiological correlates have been explored. The synaptic basis of memory processes in the hippocampus and the MTL is less clearly defined relative to that of working memory in the primate PFC.
A number of recent studies in rats and monkeys, using modern quantitative neuroanatomical methods that include unbiased stereological estimation, have demonstrated that aging is not associated with neurodegeneration in MTL structures. Aged rats have no loss of principal hippocampal neurons relative to young rats70, or neurons in cortical areas of the MTL71,72. Aging in monkeys is not associated with loss of hippocampal volume73 or with loss of neurons in entorhinal cortex74,75, though both of these changes are associated with pathological memory decline (and progression to Alzheimer’s disease) in elderly humans76–78. Hippocampal synapse number seems to be preserved in aged rodents in some regions, and decreased in other regions. For example, stereological quantification of spinophilin-immunoreactive spines79 and electron micrographic analyses of synapses have revealed that there is no age-related synapse loss in hippocampal area CA180. Loss of CA1 synapses is characteristic of AD and, to a lesser extent, mild cognitive impairment in humans81. Collectively, these data highlight the differences between normal aging, characterized by structural preservation in the MTL, and AD, which is associated with neuronal and synaptic loss in the MTL and in the hippocampus in particular.
Against this background of substantial structural preservation in CA1, subtle and region-specific alterations in synapses have been identified that are associated with impairment in hippocampal-dependent memory in aging. For example, a reduction in the size of postsynaptic densities of perforated synapses in hippocampal CA1 has been reported in aged rats with spatial learning impairment82 in the absence of synapse loss. A decrease in synaptophysin immunoreactivity in the lacunosum-moleculare layer of hippocampal CA3 was noted in aged, spatial-learning impaired rats83. Additionally, levels of synaptophysin staining in the outer and middle portions of the dentate gyrus molecular layer, in the absence of an age-related decrease, correlated with spatial learning ability in aged rats. A recent electron microscopic analysis confirmed that there is an age-related loss of synapses in the lacunosum-moleculare layer84, which is particularly interesting because this is the layer in CA3 that receives input from the entorhinal cortex. EM studies of dentate gyrus have revealed an age-related loss of both perforated and nonperforated synapses in the portion of the molecular layer that receives input from the entorhinal cortex as well as other portions of the molecular layer85, however it is the loss of perforated synapses in the sublayer of the dentate gyrus that receives input from the entorhinal cortex that correlates with memory impairments86 in aged rats, further emphasizing the importance of entorhinal/hippocampal connections to cognitive aging87. In contrast, in aged monkeys the density of perforated synapses in the dentate gyrus is preserved relative to young monkeys, although this is modulated by ovarian status in aged female monkeys88. Instead, a reduction in synaptic contacts per axonal bouton was associated with cognitive impairment89, suggestive of a failure of complex synaptic inputs to the dentate gyrus in aged monkeys rather than a reduction of synaptic strength in the dentate gyrus of aged rats (Figure 4). In addition, there is an age-related increase in nonsynaptic boutons in the portion of the molecular layer that receives input from the entorhinal cortex in aged monkeys89, suggesting that there is a decreased capacity to sustain this critically important circuit that negatively impacts cognition. The potential for age-related synaptic changes in monkey CA1 that are associated with cognitive performance requires further analysis. There have been reports of age-related decreases in NMDA receptors and AMPA receptors in hippocampus (reviewed in90). Although these studies lack the resolution to equate precise levels and subunit profiles of these receptors in axospinous synapses to age-related memory impairment, we hypothesize that altered glutamate receptor profiles in intact complex axospinous synapses might also contribute to age-related decline in tasks mediated by hippocampus.
Figure 4. Age-related changes in the synaptic characteristics of monkey dentate gyrus axonal boutons.
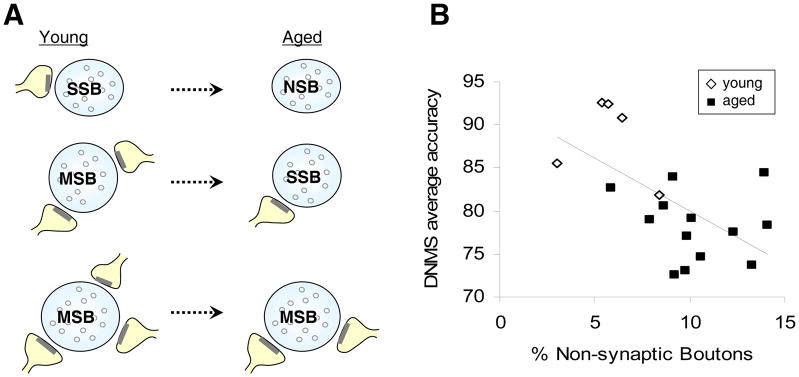
A) Schematic diagrams illustrating the synaptic subtypes in the dentate gyrus that are vulnerable to the effects of aging. Aged monkeys have increased nonsynaptic boutons (NSB), decreased multisynaptic boutons (MSB), and decreased number of synaptic contact per MSB. SSB, single-synaptic bouton. B) A significant inverse correlation is observed between the percentage of NSBs in the monkey dentate gyrus and DNMS average accuracy. Pearson correlation; n=18, r=−0.600, p=0.008.
Gene expression profiling studies have highlighted changes in hippocampal CA3 in aged rats with preserved spatial learning ability, including a downregulation of genes associated with synaptic transmission and plasticity91. Interestingly, age-related changes in place-cell firing properties, associated with spatial coding in the hippocampus, also predominate in CA3 92. These examples indicate that small changes in specific classes of hippocampal synapses may translate into substantial functional impairment, and that these alterations can be found in all subfields of the hippocampus. As in the PFC, stress and aging both affect hippocampal structure and function, as recently reviewed elsewhere93–95.
The role of neurogenesis in the dentate gyrus of adult animals has also been examined with respect to cognitive status in aging. Because neurogenesis in the dentate gyrus declines dramatically with age, this represents another possible cause of age-related impairment in hippocampal-dependent memory. However, the current data are not consistent with respect to a causal link between decreased neurogenesis and age-related cognitive decline. Despite a significant age-related reduction in neurogenesis in the dentate gyrus, the level of birth of new neurons was uncorrelated with spatial learning ability96–98. Survival of new neurons correlated with better spatial learning in one study98 and worse spatial learning in another99. An age-related decline in hippocampal neurogenesis has also been documented in primates100–102, although the relationship of this decline to cognitive impairment is unclear101. Thus, even though the functional role of hippocampal neurogenesis in memory is still not fully understood, the degree to which a decrease in the capacity for generation of new neurons plays a primary role in age-related impairments in hippocampal-dependent memory remains elusive and requires further investigation.
Although we do not focus on age-related neurophysiological changes in this article, we note that many baseline neurophysiological properties of hippocampal neurons are preserved in aging (see review by 103). A notable exception is age-related changes in calcium regulation in aged hippocampal neurons, which have an increased Ca2+ influx through L-type calcium channels. This increased Ca2+ influx in response to an action potential activates a calcium-sensitive potassium current that prolongs hyperpolarization in aged hippocampal neurons (see 104 and 105 for more extensive discussion), and these neurons show a reduced firing rate in response to a tonic current injection. This is an interesting contrast with layer 3 pyramidal cells in dlPFC, which also show an increased afterhyperpolarization, but show increased firing rate after injection with a depolarizing current24.
Differences between synaptic aging in hippocampus and PFC
We have discussed how the hippocampus and PFC employ different synaptic strategies related to their different roles in cognitive function. Synaptic aging and cognitive impairment in PFC is associated primarily with an extensive loss of axospinous synapses, and this loss is highly selective in that nearly 50% of the thin dendritic spines are lost, with other spines classes unaffected by aging. Given that thin spines (potentially the location of silent synapses) are highly plastic and dynamic, compared with the mushroom spines that are stable across time27–31, the cognitive tasks mediated by PFC that decline with age are likely to be associated with dynamic spine turnover and plasticity that may be particularly important for temporary coding of information. By contrast, memory encoding (and successful subsequent retrieval) in the hippocampus is associated with synaptic stability, and the conversion of simple axospinous synapses into complex synapses such as perforated synapses and multisynaptic boutons. Indeed, an increase in the number of perforated synapses has been associated with the induction and maintenance of long-term potentiation (LTP)106,107, and multisynaptic boutons are increased in CA1 following associative learning108, LTP induction109, or estrogen treatment110. There are specific classes of synapses that are vulnerable to aging in the hippocampus. Unlike the PFC, it is the large, complex synapses that appear to be most vulnerable. In this regard, the reduction in length of perforated synapses in hippocampal CA1 (in rats), the loss of perforated synapses in dentate gyrus (of rats), and the reduction in multisynaptic axonal boutons (in aged monkey dentate gyrus) — phenomena all associated with impaired hippocampal and MTL-dependent memory — are likely to reflect a disruption of synaptic complexity and the capacity for encoding and retrieving complex information over time periods that exceed the span of working memory. Notably, in the hippocampus, thin spines, ~40% of which do not have detectable AMPA receptor immunoreactivity and thus may bear silent synapses 33,34, are not lost in aging. Thus, aging in prefrontal cortex may be characterized by a loss of silent synapses, whereas aging in hippocampus may be characterized predominantly by a loss of established, previously-potentiated synapses.
Thus, just as the hippocampus and PFC have very different synaptic strategies to perform quite different cognitive tasks, they have very different synaptic vulnerabilities to aging. These differences suggest that different molecular mechanisms may be responsible for synaptic aging in each region, which has important therapeutic implications. A failure to form thin spines implies that actin dynamics may be compromised in pyramidal cells within PFC. Thus, proteins that regulate actin dynamics such as LIM kinase and cofilin111–113 are potential targets for intervention in PFC. However, in hippocampus, perforated synapses are the main class of synapse affected by aging, suggesting that regulation of the expansion of the PSD in existing synapses and related trafficking of AMPA receptors82,114 may be the key cellular process that should be targeted to protect against synaptic aging in this brain region. Much more work is needed to define the cellular and molecular processes in each case, but the synaptic patterns described above strongly suggest that the molecular mechanisms of synaptic aging will display regional heterogeneity and specificity, as do the structural differences.
Reversing synaptic ageing: a role for estrogen
The average age of both menopause and life expectancy for American women in 1900 was slightly over fifty years old. Although the average age of menopause remains the same, life expectancy has risen to the early eighties, such that women can now expect to live one-third of their lives after the menopausal transition, making a compelling case for determining the beneficial effects of the popular combined hormone treatment regimen (HT; estrogens and a progestin) on synaptic health and cognition.
The Women’s Health Initiative Memory Study (WHIMS) concluded that HT did not improve cognitive function115, and “increased the risk for probable dementia in postmenopausal women aged 65 or older”116. However, multiple observational studies have demonstrated a protective effect of HT with respect to onset of AD117–124. This is also perplexing given other data linking ovarian hormones to cognition in women (reviewed in 125). One explanation for this mismatch is that there is a “critical period” for hormone therapy (HT) to have beneficial effects on cognitive function, such that benefits are seen when HT is initiated soon after menopause but not when treatment is delayed118,126–130, a notion that finds some support in studies in rats 122. Whereas women enrolled in the WHIMS were beyond the critical period, and thus did not benefit from HT, women in observational studies reporting beneficial neurocognitive effects of HT began treatment soon after the onset of menopause, and so received the maximal benefit130.
Studies in animal models have attempted to explore the relationship between estrogen and cognitive ageing further. Estradiol treatment induces spines in the CA1 of young ovariectomized (OVX) female rats but fails to do so in aged OVX females131, which is consistent with the lack of estradiol-induced memory enhancement in aged female rats132. Quantitative electron microscopic immunogold analyses of key signaling molecules in axospinous synapses within CA1 have revealed multiple age-related changes in the molecular phenotype of these synapses that may explain the blunted synaptic response to estradiol. First, synaptic ERα is decreased with aging by 50% in CA1 and estradiol no longer affects the synaptic levels of ERα133. Synaptic pLIMK, which is likely involved in regulating spine formation in CA1134 and is responsive to estradiol135, is also decreased in CA1 of aged females and no longer increased by estradiol as it is in young rats136. The rat model of estrogen and aging has been very useful for dissecting the age-related molecular alterations of CA1 synapses that lead to compromised estradiol-induced plasticity, but it has been less valuable for determining direct links to cognitive performance compared with the NHP model. Recent reports that estradiol increases spines in mPFC of female rats137,138 suggest that rat studies targeting mPFC and related cognitive tasks may be feasible and promising.
Similar studies comparing young and aged OVX monkeys with and without estradiol have attracted interest as they tend to include a cognitive assessment of the subjects. Estradiol also appears to increase spine density in CA1 of young OVX female rhesus monkeys139,140, and interestingly, this effect may be retained in aged monkeys139, although this has not been confirmed with electron microscopic analyses of synapse density. In addition, estradiol treatment improves performance on a cognitive task (DNMS) linked to the integrity of the medial temporal lobe115. The aged vehicle-treated monkeys displayed only a modest decline in performance on DNMS when compared to estradiol-treated monkeys, but a dramatic decline on DR performance was seen compared to both young treatment groups and the aged estradiol-treated group. The effect on DR pointed to dlPFC as a potential target of estradiol, which is consistent with some of the clinical reports on women141. With respect to spine density and size the age and treatment effects were striking; age decreased the number of thin spines in layer 3 of area 46, whereas estradiol induced their formation such that aged untreated monkeys experience a “double hit” of age and depletion of gonadal estradiol that results in aged vehicle-treated monkeys having one third as many thin spines (head diameter <0.4 μm) as young estradiol-treated monkeys. As with the studies of gonadally-intact aged NHPs described above, the thin spine class emerges as the key to both age-related cognitive decline and estradiol-induced protection against such decline (Figure 5). Importantly, these findings indicate that the loss of thin spines and related cognitive decline is not inevitable, and both can be prevented or reversed with intervention. Like guanfacine, the findings with estradiol illustrate the power of studies of synaptic aging to point towards potential therapeutic mechanisms. Although the utility of hormones themselves as therapeutic agents may be problematic, alternative strategies – based either on more selective estrogen receptor modulators or drugs designed to promote the formation and stability of thin spines directly – may have greater clinical utility.
Figure 5. A schematic representation of the effects of age and estradiol treatment on small spines and cognitive performance in monkeys.
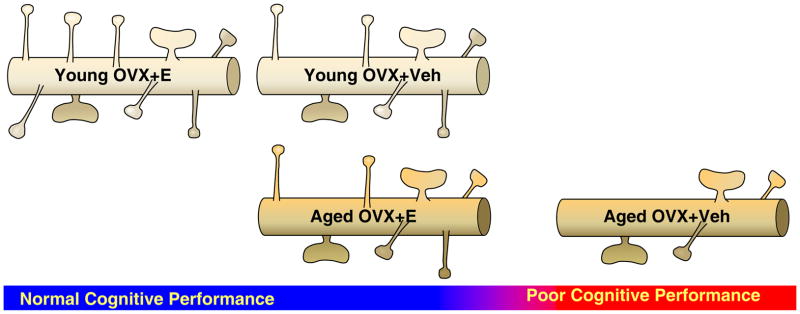
The “double hit” of aging and lack of estradiol leads to a dramatic decrease in small spines that leads to cognitive decline. The intermediate spine levels observed in the young ovariectomized (OVX) monkeys treated with vehicle (Veh) and aged OVX monkeys treated with estradiol, along with their sustained performance on the delayed response task, suggests that in the aged OVX+Veh monkeys the number of small spines may fall below the threshold needed to sustain cognitive performance.
Future Directions
The relative vulnerability of thin spines in the PFC of aged rhesus monkey compared with the stability of mushroom spines needs to be examined at the molecular level. These spine classes probably differ in their glutamate receptor profile28, however, such differences will likely be subtle and unlikely to definitively differentiate spine classes. Future studies should aim to shed light on the synaptic proteins that are relatively specific to each class of spine, and mechanistically linked to the structural plasticity of thin spines or to the GluR trafficking and complexity of mushroom spines. Such studies will not only reveal targets that have spine class specificity, which will likely be required for optimal interventions, but they will also be important if protecting hippocampal synapses in the face of aging requires different approaches than those that are successful in protecting pyramidal neurons in PFC from losing their thin spines. Future efforts must be guided by both the recognition that different spine classes have differential vulnerability to aging, and that there is regional selectivity to the differential vulnerability of axospinous synapses.
One of the most important areas for future investigation is to determine the degree to which synaptic alterations leave a neuron vulnerable to neurodegeneration, and the conditions that promote such vulnerability. This is particularly important with respect to AD. Though more work needs to be done at the synaptic level in brains from elderly humans without AD, there is a high degree of synapse loss early in AD142,143 and it may precede significant tangle formation and neuron loss. There are data that link synapse loss to degeneration in animal models (reviewed in 144). Mouse models of AD with high levels of circulating Aβ and plaques do not have significant neuron loss, though they do have spine loss145, and soluble Aβ causes synaptic pathology146,147. Specific effects of Aβ oligomers on hippocampal synaptic plasticity have been identified, which correlate with behavioral impairment (reviewed in 148). Thus, the effects of Aβ in AD may actually be primarily synaptic and occur much earlier than neuron death attributed to neurofibrillary tangle formation. These effects of Aβ may interact with the synaptic changes that occur with normal aging, which may increase the neurons’ vulnerability to the disruptive effects of Aβ oligomers on synaptic plasticity and neuronal health. If so, maintaining synaptic health in the face of aging may be important to prevent AD (see also 149).
HT for women has traditionally consisted of replacing estradiol, decreased through surgical or natural menopause, with a mixture of estrogenic compounds alone or in combination with P or other progestins. However, as we learn more about the molecular pathways activated by estradiol, we may be able to target key pathways and synapses from the broader context of preventing age-related cognitive decline in both postmenopausal women and men as they age. Although the studies in rats provide insights into age-related alterations in important signaling molecules that may influence therapeutic strategies, the NHP model will be particularly powerful for delineating the synaptic molecular profiles that correlate with cognitive performance. One obvious target for such analyses is synaptic ERα. ERα is present in axospinous synapses in area 46, but unlike the case for rat CA1, it does not decline with age, nor is it altered by OVX or estradiol150. However, the abundance of synaptic ERα does correlate with performance on DR, but only in the young VEH group. This suggests that in the absence of gonadal estradiol, young monkeys have the capacity to activate synaptic ERα in area 46 through alternative mechanisms, such as locally synthesized estradiol that can modulate synaptic plasticity, as has been described in pyramidal cells in rat CA1151,152, or perhaps alternatively by ERα agonists that are of adrenal origin153. Importantly, this suggests that synaptic ERα in area 46 may be linked to the cognitive resilience demonstrated by young female monkeys in the absence of gonadal estradiol. If so, it appears that such an alternate mechanism is attenuated with age. Future studies should be aimed at delineating these mechanisms and promoting their resilience with age.
The data on the effects of stress and sex steroids on the aging brain and cognition, particularly in the PFC, make it clear that the brain can not be viewed in isolation with respect to the neurobiological basis of cognitive decline. Given the impact of menopause on women’s health, as well as the requirements to maintain optimal executive function in the face of stressful conditions, future work should target the nature of these interactions with an eye toward interventions that might be behavioral as well as pharmaceutical. We are at the very early stages of understanding these (and other) endocrine influences on PFC and related cognitive functions in the context of aging, but clearly this needs to be a major focus going forward.
Figure.
Online ‘at-a-glance’ summary.
Individual differences are a hallmark of cognitive and synaptic aging. Neurobiological differences between individuals of the same chronological age may underlie the preservation of cognitive abilities in advanced age, versus cognitive impairment.
In general, age-related cognitive impairments that occur in the absence of neurodegenerative diseases are not associated with loss of cortical neurons. Instead, they seem to be associated with subtle synaptic alterations.
The prefrontal cortex controls higher-order, complex behaviors. A hallmark of cognitive aging is impaired prefrontal function, including impairments in spatial working memory.
One synaptic correlate of age-related impairments in working memory that has been identified in monkeys is a loss of thin spines in layer 3 of the dorsolateral prefrontal cortex.
The medial temporal lobe, including the hippocampus, is responsible for memories of everyday events. Mild impairments in medial temporal lobe function are also observed in cognitive aging.
A variety of synaptic alterations in hippocampal function have been described that correlate with age-related memory impairments. These have been observed in all subfields of the hippocampus, and differ between subfields.
A notable synaptic alteration in the aged monkey hippocampus is the loss of multisynaptic boutons in the dentate gyrus, which correlates with cognitive impairments.
Cyclical estradiol treatment of aged, surgically-menopausal monkeys increases the density of thin dendritic spines in prefrontal cortex and improves working memory. This illustrates the potential of synaptic and cognitive changes in aging to be reversible.
Loss of synapses may predispose neurons to degeneration in disease states. Thus a better understanding of mechanisms that promote stability of synapses in aging should lead not only to amelioration of age-related cognitive impairments, but may also impact vulnerability to neurodegenerative diseases.
Acknowledgments
We thank Drs. Yuko Hara, Graham Ellis-Davies, and Erik Bloss for helpful comments on the manuscript. We also thank Dr. Yuko Hara, Dr. Dani Dumitriu, and Bill Janssen for assistance with the figures. Reconstruction and visualization of Figure 1 was done by our colleagues from theVisualMD. The authors’ research is supported by NIH grants P01-AG016765, R37-AG06647, and R01-AG010606.
Glossary
- axospinous synapses
Synapses between the axon from one neuron and the dendritic spine of another neuron
- delayed nonmatching-to-sample (DNMS)
A test of recognition memory, commonly used in monkeys. A monkey’s memory for a sample object is tested by offering a choice between the sample and a novel object, and the monkey is rewarded for choosing the novel object (nonmatching). Performance in this task is dependent on an intact medial temporal lobe
- delayed response (DR) task
A test of spatiotemporal working memory, commonly used in monkeys. A monkey is cued to remember a location in space for a brief interval and then is rewarded for selecting that location at the end of the interval. Performance in this task is dependent on an intact prefrontal cortex
- attentional set-shifting task
A test of executive function in which discrimination problems among stimuli with multiple independently-variable relevant characteristics (dimensions), such as shape and color, are presented in succession. Attentional shifting is engaged when the relevant dimension for solving the discrimination problems changes
- spinophilin
A protein that is highly enriched in dendritic spines and that is often used as an immunohistochemical marker of dendritic spines
- perforated synapses
Large synapses implicated in memory-related plasticity. Perforated synapses are characterized by a discontinuity in the postsynaptic density, resulting in a hole, a slit, or a complete segmentation of the postsynaptic density plate
- synaptophysin
A synaptic vesicle protein that is highly enriched in synapses and that is often used as an immunohistochemical marker of synapses
- lacunosum-moleculare layer
The most superficial layer of the CA1–3 fields of the hippocampus. In CA3, it contains synapses from the perforant path (the projection from the entorhinal cortex to the hippocampus)
- multisynaptic bouton
An axonal bouton that forms synaptic contacts with more than one dendritic spine or shaft. Multisynaptic boutons are implicated in hippocampus-dependent learning
Biographies
John H. Morrison is Dean of Basic Sciences and the Graduate School of Biological Sciences, Professor of Neuroscience, and the Willard T. C. Johnson Professor of Geriatrics and Adult Development in Neurobiology of Aging at the Mount Sinai School of Medicine. His laboratory studies synaptic plasticity, the aging brain and the synaptic basis of cognitive decline.
Mark G. Baxter is Associate Professor of Neuroscience, Anesthesiology, and Geriatrics and Palliative Medicine, and director of the Glickenhaus Laboratory of Neuropsychology at the Mount Sinai School of Medicine. His laboratory studies the neurobiology of learning and memory, the neurobiology of aging, and the effects of anesthesia on cognition.
References
- 1.Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- 2.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 3.Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 4.Fuster JM. The Prefrontal Cortex. 4. Elsevier Ltd; 2008. This book provides an authoritiative and comprehensive analysis of the unique role of prefrontal cortex in cognition. [Google Scholar]
- 5.Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. J Neurosci. 1989;9:3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rapp PR. Visual discrimination and reversal learning in the aged monkey (Macaca mulatta) Behav Neurosci. 1990;104:876–884. doi: 10.1037//0735-7044.104.6.876. [DOI] [PubMed] [Google Scholar]
- 7.Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behavioural brain research. 1997;87:25–34. doi: 10.1016/s0166-4328(96)02256-5. This paper reports a cross-sectional study of performance on a comprehensive battery of multiple behavioral tasks across the lifespan of rhesus monkeys. [DOI] [PubMed] [Google Scholar]
- 8.Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiol Aging. 2006;27:1484–1493. doi: 10.1016/j.neurobiolaging.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Voytko ML. Impairments in acquisition and reversals of two-choice discriminations by aged rhesus monkeys. Neurobiology of aging. 1999;20:617–627. doi: 10.1016/s0197-4580(99)00097-4. [DOI] [PubMed] [Google Scholar]
- 10.Lyons-Warren A, Lillie R, Hershey T. Short- and long-term spatial delayed response performance across the lifespan. Developmental neuropsychology. 2004;26:661–678. doi: 10.1207/s15326942dn2603_1. [DOI] [PubMed] [Google Scholar]
- 11.Arnsten AF, Paspalas CD, Gamo NJ, Yang Y, Wang M. Dynamic Network Connectivity: A new form of neuroplasticity. Trends Cogn Sci. 2010;14:365–375. doi: 10.1016/j.tics.2010.05.003. This paper summarizes research on mechanisms of plasticity in prefrontal cortex underlying spatial working memory function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters A, Morrison JH, Rosene DL, Hyman BT. Feature article: are neurons lost from the primate cerebral cortex during normal aging? Cereb Cortex. 1998;8:295–300. doi: 10.1093/cercor/8.4.295. [DOI] [PubMed] [Google Scholar]
- 13.Smith DE, Rapp PR, McKay HM, Roberts JA, Tuszynski MH. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of subcortical neurons. J Neurosci. 2004;24:4373–4381. doi: 10.1523/JNEUROSCI.4289-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander GE, et al. Age-related regional network of magnetic resonance imaging gray matter in the rhesus macaque. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:2710–2718. doi: 10.1523/JNEUROSCI.1852-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shamy JL, et al. Volumetric correlates of spatiotemporal working and recognition memory impairment in aged rhesus monkeys. Cerebral cortex. 2011;21:1559–1573. doi: 10.1093/cercor/bhq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152:970–981. doi: 10.1016/j.neuroscience.2007.07.014. This paper was the first quantitative electron microscopic study to report synapse loss in rhesus monkey prefrontal cortex and to show that the degree of synapse loss in layer 2/3 correlates with cognitive impairment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumitriu D, et al. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. 30/22/7507 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan H, et al. Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cereb Cortex. 2003;13:950–961. doi: 10.1093/cercor/13.9.950. [DOI] [PubMed] [Google Scholar]
- 19.Luebke J, Barbas H, Peters A. Effects of normal aging on prefrontal area 46 in the rhesus monkey. Brain Res Rev. 2010;62:212–232. doi: 10.1016/j.brainresrev.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol. 2002;442:277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- 21.Peters A. In: Brain Aging: Models, Methods, and Mechanisms Frontiers in Neuroscience. Riddle DR, editor. 2007. [PubMed] [Google Scholar]
- 22.Chang YM, Rosene DL, Killiany RJ, Mangiamele LA, Luebke JI. Increased action potential firing rates of layer 2/3 pyramidal cells in the prefrontal cortex are significantly related to cognitive performance in aged monkeys. Cereb Cortex. 2005;15:409–418. doi: 10.1093/cercor/bhh144. 15/4/409 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Luebke JI, Chang YM. Effects of aging on the electrophysiological properties of layer 5 pyramidal cells in the monkey prefrontal cortex. Neuroscience. 2007;150:556–562. doi: 10.1016/j.neuroscience.2007.09.042. S0306-4522(07)01185-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luebke JI, Amatrudo JM. Age-related increase of sI(AHP) in prefrontal pyramidal cells of monkeys: relationship to cognition. Neurobiology of aging. 2010 doi: 10.1016/j.neurobiolaging.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters A, Kaiserman-Abramof IR. The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am J Anat. 1970;127:321–355. doi: 10.1002/aja.1001270402. [DOI] [PubMed] [Google Scholar]
- 26.Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 27.Matsuzaki M, et al. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 29.Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- 31.Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17:381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Kasai H, et al. Learning rules and persistence of dendritic spines. Eur J Neurosci. 2010;32:241–249. doi: 10.1111/j.1460–9568.2010.07344.x. EJN7344 [pii] [DOI] [PubMed] [Google Scholar]
- 33.Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Differences in the expression of AMPA and NMDA receptors between axospinous perforated and nonperforated synapses are related to the configuration and size of postsynaptic densities. J Comp Neurol. 2004;468:86–95. doi: 10.1002/cne.10950. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson DA, et al. Distance-dependent differences in synapse number and AMPA receptor expression in hippocampal CA1 pyramidal neurons. Neuron. 2006;50:431–442. doi: 10.1016/j.neuron.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson DA, Geinisman Y. Axospinous synaptic subtype-specific differences in structure, size, ionotropic receptor expression, and connectivity in apical dendritic regions of rat hippocampal CA1 pyramidal neurons. The Journal of comparative neurology. 2009;512:399–418. doi: 10.1002/cne.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Busetto G, Higley MJ, Sabatini BL. Developmental presence and disappearance of postsynaptically silent synapses on dendritic spines of rat layer 2/3 pyramidal neurons. The Journal of physiology. 2008;586:1519–1527. doi: 10.1113/jphysiol.2007.149336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zito K, Scheuss V, Knott G, Hill T, Svoboda K. Rapid functional maturation of nascent dendritic spines. Neuron. 2009;61:247–258. doi: 10.1016/j.neuron.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dumitriu D, Rodriguez A, Morrison JH. High-throughput, detailed, cell-specific neuroanatomy of dendritic spines using microinjection and confocal microscopy. Nature protocols. 2011;6:1391–1411. doi: 10.1038/nprot.2011.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez A, Ehlenberger DB, Hof PR, Wearne SL. Rayburst sampling, an algorithm for automated three-dimensional shape analysis from laser scanning microscopy images. Nat Protoc. 2006;1:2152–2161. doi: 10.1038/nprot.2006.313. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS ONE. 2008;3:e1997. doi: 10.1371/journal.pone.0001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bloss EB, Janssen WG, McEwen BS, Morrison JH. Interactive effects of stress and aging on structural plasticity in the prefrontal cortex. J Neurosci. 2010;30:6726–6731. doi: 10.1523/JNEUROSCI.0759-10.2010. 30/19/6726 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bloss EB, et al. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:7831–7839. doi: 10.1523/JNEUROSCI.0839-11.2011. Aging in rats is associated with an impairment in recovery of dendritic spines in prefrontal cortex after chronic stress. This supports the view that in addition to age-related changes in spine density and morphology, aging is also associated with a reduction in plasticity of dendritic spines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kramer AF, Bherer L, Colcombe SJ, Dong W, Greenough WT. Environmental influences on cognitive and brain plasticity during aging. J Gerontol A Biol Sci Med Sci. 2004;59:M940–957. doi: 10.1093/gerona/59.9.m940. 59/9/M940 [pii] [DOI] [PubMed] [Google Scholar]
- 44.Horton SBJ, Schorer J. Expertise and aging: maintaining skills through the lifespan. Eur Rev Aging Phys Act. 2008;5:89–96. [Google Scholar]
- 45.Wang M, et al. Neuronal basis of age-related working memory decline. Nature. 2011 doi: 10.1038/nature10243. nature10243 [pii] This paper directly demonstrates a neurophysiological mechanism for age-related impairments in prefrontal-dependent spatial working memory, a reduction in firing rate of cells active during the memory delay. This physiological deficit could be reversed by inhibition of cAMP signaling, or blocking particular subtypes of potassium channels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang M, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 47.Chen S, Wang J, Siegelbaum SA. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J Gen Physiol. 2001;117:491–504. doi: 10.1085/jgp.117.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arnsten AF, Cai JX, Goldman-Rakic PS. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for alpha-2 receptor subtypes. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1988;8:4287–4298. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends in Neurosciences. 2002;25:340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- 50.Preuss T. Do Rats Have Prefrontal Cortex? J Cogn Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 51.Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learning & memory. 2002;9:191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramos BP, et al. Dysregulation of protein kinase a signaling in the aged prefrontal cortex: new strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- 53.Taylor JR, Birnbaum S, Ubriani R, Arnsten AF. Activation of cAMP-dependent protein kinase A in prefrontal cortex impairs working memory performance. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19:RC23. doi: 10.1523/JNEUROSCI.19-18-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stranahan AM, Jiam NT, Stocker AM, Gallagher M. Aging reduces total neuron number in the dorsal component of the rodent prefrontal cortex. J Comp Neurol. 2011 doi: 10.1002/cne.22790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liston C, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radley JJ, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 57.Radley JJ, et al. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. Journal of neurobiology. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 59.Radley JJ, et al. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 60.Goldwater DS, et al. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. S0306-4522(09)01404-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murray EA, Mishkin M. Severe tactual as well as visual memory deficits follow combined removal of the amygdala and hippocampus in monkeys. Journal of Neuroscience. 1984;4:2565–2580. doi: 10.1523/JNEUROSCI.04-10-02565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. Journal of Neuroscience. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baxter MG, Murray EA. Opposite relationship of hippocampal and rhinal cortex damage to delayed nonmatching-to-sample deficits in monkeys. Hippocampus. 2001;11:61–71. doi: 10.1002/1098-1063(2001)11:1<61::AID-HIPO1021>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 64.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 65.Gallagher M, Nicolle MM. Animal models of normal aging: Relationship between cognitive decline and markers in hippocampal circuitry. Behavioural Brain Research. 1993;57:155–162. doi: 10.1016/0166-4328(93)90131-9. [DOI] [PubMed] [Google Scholar]
- 66.Tulving E, Markowitsch HJ. Episodic and declarative memory: role of the hippocampus. Hippocampus. 1998;8:198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 67.Robitsek RJ, Fortin NJ, Koh MT, Gallagher M, Eichenbaum H. Cognitive aging: a common decline of episodic recollection and spatial memory in rats. J Neurosci. 2008;28:8945–8954. doi: 10.1523/JNEUROSCI.1893-08.2008. 28/36/8945 [pii] Estimates of hippocampal-dependent episodic recollection were derived from performance in an olfactory recognition task in young and aged rats. These correlated strongly with spatial memory in the water maze; aged rats with spatial memory impairments demonstrated a selective impairment in episodic recollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baxter MG. “I’ve seen it all before”: explaining age-related impairments in object recognition. Theoretical comment on Burke ., et al. 2010. Behav Neurosci. 2010;124:706–709. doi: 10.1037/a0021029. 2010-20760-015 [pii] [DOI] [PubMed] [Google Scholar]
- 69.Burke SN, Barnes CA. Senescent synapses and hippocampal circuit dynamics. Trends Neurosci. 2010;33:153–161. doi: 10.1016/j.tins.2009.12. 003. S0166-2236(09)00202-1 [pii] This paper provides a comprehensive review of age-related alterations in synaptic physiology in hippocampus and their potential contribution to cognitive decline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci U S A. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. This paper reports rigorous, quantitative evidence that neuron loss does not accompany age-related cognitive decline, reinforcing the search for structural and functional alterations short of neuron death that lead to cognitive decline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rapp PR, Deroche PS, Mao Y, Burwell RD. Neuron number in the parahippocampal region is preserved in aged rats with spatial learning deficits. Cereb Cortex. 2002;12:1171–1179. doi: 10.1093/cercor/12.11.1171. [DOI] [PubMed] [Google Scholar]
- 72.Merrill DA, Chiba AA, Tuszynski MH. Conservation of neuronal number and size in the entorhinal cortex of behaviorally characterized aged rats. J Comp Neurol. 2001;438:445–456. doi: 10.1002/cne.1327. [DOI] [PubMed] [Google Scholar]
- 73.Shamy JL, et al. Hippocampal volume is preserved and fails to predict recognition memory impairment in aged rhesus monkeys (Macaca mulatta) Neurobiol Aging. 2006;27:1405–1415. doi: 10.1016/j.neurobiolaging.2005.07.019. S0197-4580(05)00211-3 [pii] [DOI] [PubMed] [Google Scholar]
- 74.Gazzaley AH, Thakker MM, Hof PR, Morrison JH. Preserved number of entorhinal cortex layer II neurons in aged macaque monkeys. Neurobiol Aging. 1997;18:549–553. doi: 10.1016/s0197-4580(97)00112-7. [DOI] [PubMed] [Google Scholar]
- 75.Merrill DA, Roberts JA, Tuszynski MH. Conservation of neuron number and size in entorhinal cortex layers II, III, and V/VI of aged primates. J Comp Neurol. 2000;422:396–401. doi: 10.1002/1096-9861(20000703)422:3<396::aid-cne6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 76.De Leon MJ, et al. Frequency of hippocampal formation atrophy in normal aging and Alzheimer’s disease. Neurobiology of Aging. 1997;18:1–11. doi: 10.1016/s0197-4580(96)00213-8. [DOI] [PubMed] [Google Scholar]
- 77.Gomez-Isla T, et al. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rusinek H, et al. Regional brain atrophy rate predicts future cognitive decline: 6-year longitudinal MR imaging study of normal aging. Radiology. 2003;229:691–696. doi: 10.1148/radiol.2293021299229/3/691 [pii]. [DOI] [PubMed] [Google Scholar]
- 79.Calhoun ME, et al. Age-related spatial learning impairment is unrelated to spinophilin immunoreactive spine number and protein levels in rat hippocampus. Neurobiol Aging. 2008;29:1256–1264. doi: 10.1016/j.neurobiolaging.2007.02.013. S0197-4580(07)00053-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geinisman Y, et al. Aging, spatial learning, and total synapse number in the rat CA1 stratum radiatum. Neurobiol Aging. 2004;25:407–416. doi: 10.1016/j.neurobiolaging.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 81.Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. 68/18/1501 [pii] [DOI] [PubMed] [Google Scholar]
- 82.Nicholson DA, Yoshida R, Berry RW, Gallagher M, Geinisman Y. Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments. J Neurosci. 2004;24:7648–7653. doi: 10.1523/JNEUROSCI.1725-04.2004. This is one of the more recent papers in an important series of publications highlighting the fact that subtle synaptic alterations revealed by careful quantitative analysis can lead to age-related cognitive decline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adams MM, et al. Age-related synapse loss in hippocampal CA3 is not reversed by caloric restriction. Neuroscience. 2010;171:373–382. doi: 10.1016/j.neuroscience.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Geinisman Y, de Toledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- 86.Geinisman Y, de Toledo-Morrell L, Morrell F. Loss of perforated synapses in the dentate gyrus: morphological substrate of memory deficit in aged rats. Proc Natl Acad Sci U S A. 1986;83:3027–3031. doi: 10.1073/pnas.83.9.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- 88.Hara Y, et al. Synaptic correlates of memory and menopause in the hippocampal dentate gyrus in rhesus monkeys. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.09.014. S0197-4580(10)00394-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hara Y, et al. Synaptic characteristics of dentate gyrus axonal boutons and their relationships with aging, menopause, and memory in female rhesus monkeys. J Neurosci. 2011;31:7737–7744. doi: 10.1523/JNEUROSCI.0822-11.2011. 31/21/7737 [pii] Using serial section electron microscopy, this study demonstrated relationships between a reduction in multiple-synapse boutons and age-related cognitive impairment in rhesus monkeys. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi L, Adams M, Brunso-Bechtold JK. Subtle Alterations in Glutamatergic Synapses Underlie the Aging-Related Decline in Hippocampal Function. 2007 NBK3889 [bookaccession] [PubMed] [Google Scholar]
- 91.Haberman RP, et al. Prominent hippocampal CA3 gene expression profile in neurocognitive aging. Neurobiol Aging. 2011;32:1678–1692. doi: 10.1016/j.neurobiolaging.2009.10.005. S0197-4580(09)00335-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. J Neurosci. 2005;25:6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goosens KA, Sapolsky RM. In: Brain Aging: Models, Methods, and Mechanisms Frontiers in Neuroscience. Riddle DR, editor. 2007. [Google Scholar]
- 94.McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 95.Bloss EB, Morrison JH, McEwen BS. Stress and Aging A Question of resilience with Implications for Disease. In: Conrad Cheryl D., editor. The Handbook of Stress: Neuropsychological Effects on the Brain. 1. Blackwell Publishing Ltd; 2011. [Google Scholar]
- 96.Merrill DA, Karim R, Darraq M, Chiba AA, Tuszynski MH. Hippocampal cell genesis does not correlate with spatial learning ability in aged rats. J Comp Neurol. 2003;459:201–207. doi: 10.1002/cne.10616. [DOI] [PubMed] [Google Scholar]
- 97.Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur J Neurosci. 2003;18:215–219. doi: 10.1046/j.1460-9568.2003.02733.x. 2733 [pii] [DOI] [PubMed] [Google Scholar]
- 98.Drapeau E, et al. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bizon JL, Gallagher M. More is less: neurogenesis and age-related cognitive decline in Long-Evans rats. Sci Aging Knowledge Environ. 2005:re2. doi: 10.1126/sageke.2005.7.re2. 2005/7/re2 [pii] [DOI] [PubMed] [Google Scholar]
- 100.Leuner B, Kozorovitskiy Y, Gross CG, Gould E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17169–17173. doi: 10.1073/pnas. 0708228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aizawa K, Ageyama N, Yokoyama C, Hisatsune T. Age-dependent alteration in hippocampal neurogenesis correlates with learning performance of macaque monkeys. Exp Anim. 2009;58:403–407. doi: 10.1538/expanim.58.403. [DOI] [PubMed] [Google Scholar]
- 102.Aizawa K, Ageyama N, Terao K, Hisatsune T. Primate-specific alterations in neural stem/progenitor cells in the aged hippocampus. Neurobiology of aging. 2011;32:140–150. doi: 10.1016/j.neurobiolaging.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 103.Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- 104.Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell. 2007;6:319–325. doi: 10.1111/j.1474-9726.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 105.Oh MM, Oliveira FA, Disterhoft JF. Learning and aging related changes in intrinsic neuronal excitability. Front Aging Neurosci. 2010;2:2. doi: 10.3389/neuro.24.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Geinisman Y, deToledo-Morrell L, Morrell F. Induction of long-term potentiation is associated with an increase in the number of axospinous synapses with segmented postsynaptic densities. Brain Res. 1991;566:77–88. doi: 10.1016/0006-8993(91)91683-r. [DOI] [PubMed] [Google Scholar]
- 107.Geinisman Y, Detoledo-Morrell L, Morrell F, Persina IS, Beatty MA. Synapse restructuring associated with the maintenance phase of hippocampal long-term potentiation. J Comp Neurol. 1996;368:413–423. doi: 10.1002/(SICI)1096-9861(19960506)368:3<413::AID-CNE7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 108.Geinisman Y, Berry RW, Disterhoft JF, Power JM, Van der Zee EA. Associative learning elicits the formation of multiple-synapse boutons. J Neurosci. 2001;21:5568–5573. doi: 10.1523/JNEUROSCI.21-15-05568.2001. 21/15/5568 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Toni N, et al. Remodeling of synaptic membranes after induction of long-term potentiation. J Neurosci. 2001;21:6245–6251. doi: 10.1523/JNEUROSCI.21-16-06245.2001. 21/16/6245 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Woolley CS, Wenzel HJ, Schwartzkroin PA. Estradiol increases the frequency of multiple synapse boutons in the hippocampal CA1 region of the adult female rat. J Comp Neurol. 1996;373:108–117. doi: 10.1002/(SICI)1096-9861(19960909)373:1<108::AID-CNE9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 111.Arber S, et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- 112.Yang N, et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 113.Maekawa M, et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 114.Desmond NL, Weinberg RJ. Enhanced expression of AMPA receptor protein at perforated axospinous synapses. Neuroreport. 1998;9:857–860. doi: 10.1097/00001756-199803300-00017. [DOI] [PubMed] [Google Scholar]
- 115.Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. This study demonstrated that a cyclic regimen of estradiol replacement to aged, surgically menopausal monkeys was sufficient to preserve their cognitive abilities relative to monkeys that did not receive hormone treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shumaker SA, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. Jama. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 117.Henderson VW. Estrogen-containing hormone therapy and Alzheimer’s disease risk: understanding discrepant inferences from observational and experimental research. Neuroscience. 2006;138:1031–1039. doi: 10.1016/j.neuroscience.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 118.Maki PM. Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience. 2006;138:1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 119.Sherwin BB. Estrogen and memory in women: how can we reconcile the findings? Hormones and behavior. 2005;47:371–375. doi: 10.1016/j.yhbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 120.Turgeon JL, McDonnell DP, Martin KA, Wise PM. Hormone therapy: physiological complexity belies therapeutic simplicity. Science. 2004;304:1269–1273. doi: 10.1126/science.1096725. [DOI] [PubMed] [Google Scholar]
- 121.Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. 2006;26:10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gibbs RB, Gabor R. Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res. 2003;74:637–643. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- 123.Asthana S, et al. Frontiers proposal, National Institute on Aging “bench to bedside: estrogen as a case study”. Age. 2009;31:199–210. doi: 10.1007/s11357-009-9087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009;30:212–222. doi: 10.1016/j.tips.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sherwin BB. Estrogen and cognitive functioning in women: Lessons we have learned. Behavioral neuroscience. 2011 doi: 10.1037/a0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: a critical time. Jama. 2002;288:2170–2172. doi: 10.1001/jama.288.17.2170. [DOI] [PubMed] [Google Scholar]
- 127.Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138:1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 128.Sherwin BB. Estrogen therapy: is time of initiation critical for neuroprotection? Nat Rev Endocrinol. 2009;5:620–627. doi: 10.1038/nrendo.2009.193. nrendo.2009.193 [pii] This article comprehensively reviews studies on estrogen supplementation in which information about the timing of therapy was provided, allowing an appraisal of evidence for and against the “critical period” hypothesis. [DOI] [PubMed] [Google Scholar]
- 129.Zandi PP, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. Jama. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 130.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain research. 2011;1379:188–198. doi: 10.1016/j.brainres.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Adams MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci U S A. 2001;98:8071–8076. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Savonenko AV, Markowska AL. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience. 2003;119:821–830. doi: 10.1016/s0306-4522(03)00213-6. [DOI] [PubMed] [Google Scholar]
- 133.Adams MM, et al. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 135.Spencer JL, Waters EM, Milner TA, McEwen BS. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience. 2008;155:1106–1119. doi: 10.1016/j.neuroscience.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yildirim M, et al. Estrogen and aging affect synaptic distribution of phosphorylated LIM kinase (pLIMK) in CA1 region of female rat hippocampus. Neuroscience. 2008;152:360–370. doi: 10.1016/j.neuroscience.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Luine V, Attalla S, Mohan G, Costa A, Frankfurt M. Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain research. 2006;1126:183–187. doi: 10.1016/j.brainres.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 138.Shansky RM, et al. Estrogen Promotes Stress Sensitivity in a Prefrontal Cortex-Amygdala Pathway. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq003. bhq003 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hao J, et al. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- 140.Leranth C, Shanabrough M, Redmond DE., Jr Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. J Comp Neurol. 2002;447:34–42. doi: 10.1002/cne.10230. [DOI] [PubMed] [Google Scholar]
- 141.Keenan PA, Ezzat WH, Ginsburg K, Moore GJ. Prefrontal cortex as the site of estrogen’s effect on cognition. Psychoneuroendocrinology. 2001;26:577–590. doi: 10.1016/s0306-4530(01)00013-0. [DOI] [PubMed] [Google Scholar]
- 142.Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiology of aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. This paper reports quantitative electron microscopic data from human brain showing that there is extensive synapse loss early in the progression of Alzheimer’s Disease. [DOI] [PubMed] [Google Scholar]
- 143.Akram A, et al. Stereologic estimates of total spinophilin-immunoreactive spine number in area 9 and the CA1 field: relationship with the progression of Alzheimer’s disease. Neurobiol Aging. 2008;29:1296–1307. doi: 10.1016/j.neurobiolaging.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bell KF, Hardingham GE. The influence of synaptic activity on neuronal health. Curr Opin Neurobiol. 2011;21:299–305. doi: 10.1016/j.conb.2011.01.002. S0959–4388(11)00006-7 [pii] This review highlights the relationship between synaptic events and alterations and the risk of neuron death, a relationship that is likely to be critically important in the transition from synaptic alterations to Alzheimer’s Disease in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jacobsen JS, et al. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nature neuroscience. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 147.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 148.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behavioural brain research. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nature reviews. Neuroscience. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang AC, Hara Y, Janssen WG, Rapp PR, Morrison JH. Synaptic estrogen receptor-alpha levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci. 2010;30:12770–12776. doi: 10.1523/JNEUROSCI.3192–10.2010. 30/38/12770 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hojo Y, et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rune GM, Frotscher M. Neurosteroid synthesis in the hippocampus: role in synaptic plasticity. Neuroscience. 2005;136:833–842. doi: 10.1016/j.neuroscience.2005.03.056. [DOI] [PubMed] [Google Scholar]
- 153.Lasley BL, Crawford S, McConnell DS. Adrenal androgens and the menopausal transition. Obstet Gynecol Clin North Am. 2011;38:467–475. doi: 10.1016/j.ogc.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rapp PR. Aging and memory in animals. In: Hof PR, Mobbs CV, editors. Handbook of the Neuroscience of Aging. Academic Press; 2009. pp. 235–242. [Google Scholar]
- 155.Gallagher M, Stocker AM, Koh MT. Mindspan: lessons from rat models of neurocognitive aging. Ilar J. 2011;52:32–40. doi: 10.1093/ilar.52.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]