Abstract
Stroke remains a primary cause of morbidity throughout the world mainly because of its effect on cognition. Individuals can recover from physical disability resulting from stroke, but might be unable to return to their previous occupations or independent life because of cognitive impairments. Cognitive dysfunction ranges from focal deficits, resulting directly from an area of infarction or from hypoperfusion in adjacent tissue, to more global cognitive dysfunction. Global dysfunction is likely to be related to other underlying subclinical cerebrovascular disease, such as white-matter disease or subclinical infarcts. Study of cognitive dysfunction after stroke is complicated by varying definitions and lack of measurement of cognition before stroke. Additionally, stroke can affect white-matter connectivity, so newer imaging techniques, such as diffusion-tensor imaging and magnetisation transfer imaging, that can be used to assess this subclinical injury are important tools in the assessment of cognitive dysfunction after stroke. As research is increasingly focused on the role of preventable risk factors in the development of dementia, the role of stroke in the development of cognitive impairment and dementia could be another target for prevention.
Introduction
Stroke is a leading cause of morbidity worldwide, not only because of its effect on motor function, but also because of the cognitive dysfunction that commonly results from stroke. The most common cognitive deficits after stroke are aphasia (language impairment) and hemispatial neglect (failure to attend or respond to stimuli on the side contralateral to the stroke). Other deficits that result directly from a stroke or from adjacent areas of hypoperfusion include impairments in working memory, attention, learning, calculation, visual perception, or executive function (ie, decision making, organisation, and problem solving). Ideomotor apraxia—an impairment in skilled movements in the absence of motor weakness or incoordination—is a cognitive impairment that affects motor planning and usually occurs after left hemisphere stroke. Aphasia occurs in anywhere from 15%1 to a third of patients with stroke,2 depending on the population studied, the way language is tested, and when it is tested, and also typically occurs after left hemisphere stroke. Similar frequencies of occurrence have been reported for hemispatial neglect, with rates above 40% among patients with right hemisphere stroke.3
Cognition is rarely assessed in detail in the acute setting, but studies that have assessed cortical function at the time of stroke have shown how quickly cognition can fluctuate, for example in response to changes in cerebral perfusion.4–7 In the subacute and chronic time periods after stroke, studies of cognitive deficits after stroke are limited by a lack of standardised definitions of what constitutes stroke and what determines cognitive impairment. For example, studies of strokes that are clinically apparent might underestimate the extent of cerebrovascular damage, as many patients with or without clinical strokes have had other subclinical strokes or white-matter injury. Conversely, many studies of vascular dementia involve neuropathological identification of strokes, which might or might not have been clinically apparent.8 Stroke is an important cause of dementia that could be preventable, so the mechanisms and consequences of the relationship between stroke and dementia need to be understood.
In this Review, we will summarise the literature on cognitive deficits resulting from clinically apparent stroke. We aim to provide an understanding of the factors, ranging from demographic to radiographic, that have been associated with higher risk of cognitive dysfunction after stroke. We will also outline the methodological limitations of the assessment of cognitive impairment resulting from stroke, to allow better critical review of the literature. Finally, we will discuss recent developments in imaging, particularly in microstructural assessment of the white matter, that could provide new insights into the substrates of cognitive dysfunction after stroke, and provide suggestions for future areas of research in this field.
Cognition in acute stroke
Infarction of brain tissue will typically lead to aphasia, neglect, apraxia, hemisensory loss, visual field loss, or hemiparesis, depending on the function of the infarcted tissue. This core infarcted region is usually visualized with MRI diffusion-weighted imaging (DWI). Because the primary language and spatial processing centres are in the cortex, cardioembolic strokes—which are more likely to embolise to the grey–white junctions or to the inferior division of the middle cerebral artery territory—are more likely to result in problems with language (aphasia) or with spatial processing (neglect) than are other types of strokes.2,9 The type of language deficit (eg, impairment of comprehension, grammatical sentence production, speech articulation, reading, spelling, or naming) or type of hemispatial neglect (whether a patient neglects the left half of objects or the left half of space) depends on the location of ischaemia.10–12
Acute stroke results in cognitive dysfunction not only because of the location of infarcted tissue, but also because of adjacent regions of inadequate perfusion. The concept of “misery perfusion” was first described in the early 1980s to describe a mismatch between reduced cerebral blood flow and an increase in oxygen extraction fraction in an area distal to a large-vessel occlusion.13 Other studies have provided further evidence that brain tissue that receives inadequate perfusion might become dysfunctional, and that restoration of flow (eg, with intravenous alteplase) can improve perfusion and cognitive performance in acute ischaemic stroke.14 Specifically, individuals with hemispatial neglect could have hypoperfusion of right-hemispheric regions even in the absence of cortical infarction.4 Similarly, individuals with left-hemispheric hypoperfusion could have aphasia, which then improves when perfusion is restored.15 These results have been found with both CT and MRI perfusion techniques (figure 1).16,17 Additionally, degree of hypoperfusion, defined by delay in time-to-peak measures from MRI perfusion-weighted imaging (PWI), seems to correlate with severity of neglect or aphasia, further supporting a causative association between hypoperfusion and cognitive dysfunction.5,18 These studies, along with further evidence that restoration of perfusion to these brain regions is associated with improvement in function,6,7 support the hypothesis that an area of hypoperfusion on PWI also represents dysfunctional tissue, and not only benign oligaemia.
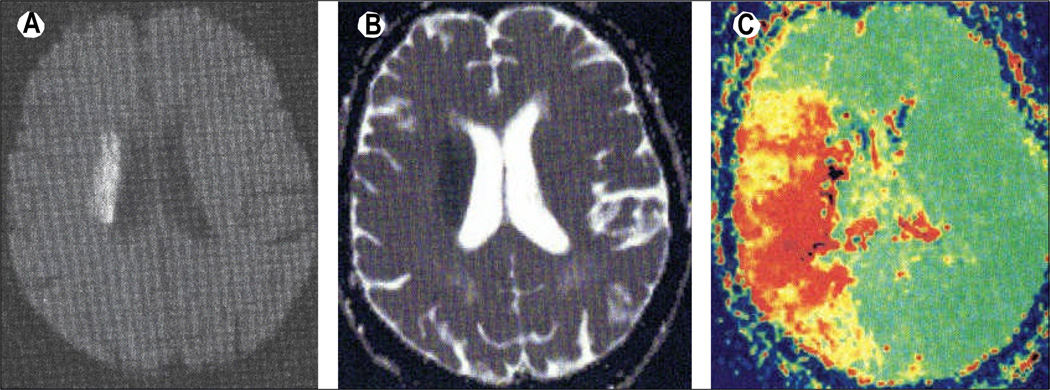
Figure 1. Cognitive deficits can be associated with areas of hypoperfusion that extend beyond the stroke lesion, as shown by the diffusion-perfusion mismatch on MRI.
MRI sequences from an 85-year-old woman with stroke shows a relatively small right-sided subcortical infarct, but a large area of hypoperfusion. (A) DWI, with area of acute infarction. (B) Apparent diffusion coefficient map, obtained from DWI, showing the same region. (C) PWI time-to-peak map; the larger region (red) shows the area of hypoperfusion, and compared with DWI (A) shows a diffusion–perfusion mismatch, because the infarct size (on DWI) is much smaller than the area of abnormality on PWI. The right side of the brain is shown on the left side of the images, as per radiological convention. This patient had impaired performance on tests of hemispatial neglect, corresponding to the cortical hypoperfusion shown on PWI. DWI=diffusion-weighted imaging. PWI=perfusion-weighted imaging.
Beyond focal hypoperfusion or infarction and resulting cognitive problems, global cognitive dysfunction has been reported in stroke.19 Global cognitive dysfunction can involve impairment in memory, attention, executive functioning, or visuoconstruction, among other cognitive domains.20,21 This pattern of deficits is similar to the pattern described in vascular cognitive impairment, suggesting that many of these early deficits are important in the subsequent development of cognitive impairment and dementia. The frequency of any cognitive impairment in the first few weeks after a stroke has been cited in multiple studies to be above 70%.20,21 However, many of the early cognitive problems in acute stroke improve in the weeks or months after onset.
Cognition in subacute stroke
In the subacute time frame, which we define as within 3 months after a stroke, the estimated proportion of patients having cognitive impairment ranges from below 50% to over 90%.22,23 These differences in frequency might be partly explained by differences in the study populations and stroke subtypes. For example, Jaillard and colleagues23 included primarily large-vessel and cardioembolic strokes in their study, with fewer lacunar infarcts, and used a fairly liberal definition of cognitive dysfunction. Nys and colleagues20 classified cognitive impairment separately depending on the stroke subtype and reported that fewer than 50% of patients with subcortical or infratentorial strokes had cognitive impairment compared with 74% of individuals with cortical strokes.
Because many cognitive deficits resulting from stroke improve in the first few weeks to months after stroke, studies that use cognitive performance a few months after stroke as a baseline assessment might not accurately measure the true baseline frequency of cognitive dysfunction after stroke. Some of this recovery is spontaneous, due to recanalisation, with other recovery occurring secondary to cerebral plasticity and as a result of adjacent or contralesional brain regions taking over cognitive tasks previously performed by ischaemic regions.24 Most areas of hypoperfusion in tissue beyond the core infarct—the so-called diffusion-perfusion mismatch—are salvaged by 3 months after stroke, with fixed infarct size and less fluctuation in cognitive symptoms related to perfusion abnormalities by this stage.25
Although treatments aimed at reducing cognitive dysfunction after stroke are not the focus of this Review, rehabilitation is an important component of cognitive recovery from the acute to subacute time period. In a recent study by Lazar and colleagues,26 even minimum speech rehabilitation in individuals with aphasia after stroke was associated with recovery of language to 70% of the individual’s maximum possible level.
Predictors of cognitive dysfunction in acute and subacute stroke
Tables 1 and 2 summarise some of the characteristics reported in the literature that might influence the development of cognitive dysfunction in the acute and subacute period—ie, the first weeks to months after stroke. Differences in some of these characteristics in a particular study cohort and between studies might explain the differences in prevalence estimates of cognitive impairment after stroke.
Table 1.
Medical, demographic, and cerebrovascular predictors of global cognitive dysfunction in the first 3 months after stroke
| Country Patients (n) | Study design and population |
Primary results | Comments | ||
|---|---|---|---|---|---|
| Age | |||||
| Nys et al20 cohort, | Netherlands | 168 | Prospective hospital | Mean age 60 years for cognitively intact vs 65·2 years for cognitively impaired patients (p=0·02, univariate) | “Cognitively impaired” could involve just one or multiple domains |
| Klimkowicz-Mrowiec et al27 | Poland cohort, | 220 | Prospective hospital no prestroke dementia |
Age predicted dementia at 3 months after stroke, in multivariate model | Dementia defined according to standard DSM-IV criteria, including informant interview and neuropsychological testing |
| Sex | |||||
| Nys et al20 cohort, | Netherlands | 168 | Prospective hospital first-ever stroke |
41% of cognitively intact vs 60% of cognitively impaired patients were female (p=0·01) | “Cognitively impaired” could involve just one or multiple domains |
| Fever or recent infection | |||||
| Nys et al20 cohort, | Netherlands | 168 | Prospective hospital first-ever stroke |
Temperature >38°C: present in 22% of cognitively impaired vs 8% of cognitively intact patients (p=0·01) | Relationship did not persist in multivariate analysis |
| Hoffmann9 for | South Africa | 1000 | Hospital-based registry | Infection within previous month: OR 5·75 (95% CI 2·02–16·39) developing “higher cortical function deficits” |
Infection defined by presence of clinical, laboratory, and/or radiographic support |
| Hyperglycaemia | |||||
| Kruyt et al28 cohort, | Netherlands | 113 | Prospective hospital no prestroke dementia |
Hyperglycaemia was associated with impaired executive function, in patients with cortical infarcts only; no effect on long-term cognitive performance | Hyperglycaemia defined as glucose >7 mmol/L on admission |
| Klimkowicz-Mrowiec et al27 | 220 | Prospective hospital cohort, no prestroke dementia | Diabetes was associated with 2·67 times higher risk of post-stroke dementia at 3 months | 21·5% of overall study population had diabetes | |
| Seizures (early after stroke) | |||||
| Cordonnier et al29 cohort, | France | 169 | Prospective hospital no prestroke dementia |
Presence of post-stroke seizures within 7 days of stroke had increased hazard of dementia (hazard ratio 3·81, 95% CI 1·13–12·82) | Dementia defined at 3 years after stroke |
| Previous stroke | |||||
| Mok et al30 cohort, | Hong Kong | 75 | Prospective hospital Lacunar stroke |
Previous stroke was associated with 11 times increased odds of cognitive impairment after stroke, in multivariate model | Selected for individuals with stroke from small-vessel disease only |
| Prestroke cognition | |||||
| Mok et al30 cohort, | Hong Kong | 75 | Prospective hospital lacunar stroke |
Prestroke IQCODE was strongly associated with odds of post-stroke cognitive dysfunction | IQCODE was retrospectively determined (after stroke) |
| APOE ε4 status | |||||
| Wagle et al31 | Norway | 152 | Prospective rehabilitation cohort, ischaemic or haemorrhagic stroke | Presence of APOE ε4 allele was independently associated with post-stroke cognitive impairment at 2 weeks after stroke (OR 3·7, 95% CI 1·2–11·6) | Exclusion of individuals with prestroke cognitive impairment (by retrospective IQCODE) yielded similar results |
| Ballard et al32 stroke | UK | 137 | Prospective cohort of =survivors aged >75 |
Risk of cognitive impairment at 3 and 15 months was increased in the presence of APOE ε4 allele | Cognitive decline from early cognitive impairment was steeper in the presence of the APOE ε4 allele |
DSM-IV=diagnostic and statistical manual of mental disorders, 4th edition. OR=odds ratio. IQCODE=informant questionnaire on cognitive decline in the elderly.
Table 2.
Imaging characteristics associated with increased risk of cognitive dysfunction in the first 3 months after stroke
| Country | Study population and design |
Primary results | Comments | ||
|---|---|---|---|---|---|
| Size of stroke |
Patient s (n) |
||||
| Nys et al20 cohort, | Netherlands | 168 | Prospective hospital first-ever stroke |
Strokes in individuals with cognitive dysfunction after stroke were three times larger than those in individuals with intact cognition | Relationship did not persist in multivariate model |
| White-matter disease | |||||
| Jokinen et al33 cohort | Finland | 323 | Prospective hospital study (Helsinki Stroke Aging Memory Study), age 55–85 years |
Degree of white-matter hyperintensity was independently related to post-stroke executive function and motor speed Individuals with lacunes with more white-matter disease had worse executive function performance |
White matter was rated categorically from MRI done at the time of admission for stroke; cognitive assessment at 3 months Association with white-matter disease did not persist in multivariate modelling |
| Wen et al34 cohort, | Hong Kong | 94 | Prospective hospital acute lacunar infarcts |
||
| Regional atrophy | |||||
| Sachdev et al35 cohort | Australia | 165 | Prospective hospital (Sydney Stroke Study), stroke and TIA |
Amygdala volume was smaller in stroke/TIA patients with cognitive impairment | No differences were found in hippocampal volumes at 6 months |
| Stebbins et al36 cohort, | USA | 91 | Prospective hospital age ≥50 years |
Voxel-based analysis showed grey-matter atrophy in the thalamus and other regions in individuals with cognitive impairment after stroke Atrophy was present in fronto-striato-thalamo-frontal circuits in stroke survivors with cognitive impairment |
Two independent neuropsychologists determined whether patients were cognitively impaired on the basis of multiple domain scores |
| Burton et al37 cohort, | UK | 50 | Prospective hospital age >75 years, no prestroke dementia |
White-matter hyperintensities in similar regions were also associatedwith cognitive impairment | |
| Stroke location | |||||
| Tay et al38 cohort, | Singapore | 169 | Prospective hospital first-ever stroke |
Individuals with anterior infarcts (total more than partial) had lower global cognitive scores than those with other stroke types | 81% of participants had lacunar strokes, with only 11% having total or partial anterior circulation infarcts |
| Nys et al20 cohort, | Netherlands | 168 | Prospective hospital first-ever stroke | Cortical location of stroke was associated with having cognitive impairment (OR 3·6, 95% CI 1·3–9·9) | Cortical location remained a strong predictor in multivariate models |
| Jaillard et al39 cohort, | France | 177 | Prospective hospital first-ever ischaemic stroke |
In multivariate models, stroke in the middle cerebralartery territory was associated with higher risk of cognitive impairment soon after stroke(OR 2·96, 95% CI 1·30–6·73) | On the basis of multiple tests, cognitive functioning was classified into normal, mild, or moderate impairment |
| Supratentorial vs infratentorial location | |||||
| Nys et al20 cohort, | Netherlands | 168 | Prospective hospital first-ever stroke | Fewer individuals with infratentorial stroke had cognitive impairment (43% vs 74% in overall group) | Relationship did not persist in multivariate models |
| Hoffman et al40 | USA | 199 | Prospective hospital registry, subset with brainstem or cerebellar stroke | Similar frequencies of cognitive impairment after infratentorial stroke as reported after supratentorial stroke; 47% with impairment on frontal tasks | Compared with reported cognitive impairment rates in other studies |
| Haemorrhagic stroke | |||||
| Nys et al20 | Netherlands | 168 | Prospective hospital cohort, first-ever stroke | Haemorrhagic stroke was independently associated with cognitive impairment (adjusted OR, 5·6 95% CI | Only 17 individuals in the cohort had haemorrhagic stroke |
| Stroke recurrence | |||||
| Srikanth et al41 | Australia | 99 | Prospective hospital cohort, first-ever stroke | Among individuals with first-ever stroke, subsequent recurrent stroke was associated with higher rates of dementia (p=0·02) | Follow-up started at 3 months, but dementia was not assessed until 1–2 years after stroke; no relationship found with cognitive |
| Cortical hypoperfusion | |||||
| Hillis et al4 | USA | 115 | Prospective hospital cohort, no prestroke dementia | Hypoperfusion of cortical regions was more strongly associated with impaired aphasia or neglect than was cortical infarction (p<0·0001) | MRI diffusion-weighted imaging and perfusion-weighted imaging and cognitive testing were done within 24 h of symptom |
TIA=transient ischaemic attack. OR=odds ratio.
Individual-level characteristics
Because individuals with stroke often have other comorbidities, the manifestation of cognitive symptoms after stroke is not always as straightforward as might be expected from the location of the lesion or even the location of an area of hypoperfusion. Demographic and medical factors have been shown to influence the extent to which cognitive deficits after stroke are present (table 1).
In general, older age is an important predictor of worse functional and cognitive outcome soon after stroke.20,27,42,43 Age has also been shown to be an important predictor of the development of cognitive impairment or dementia in the chronic period after stroke.44 Specific cognitive deficits might also be more common in older individuals acutely after stroke. For instance, although not consistently reported,45 several studies have shown that hemispatial neglect is more common in older individuals than in younger individuals who experience stroke,3,46 and this association seems to be independent of infarct size and stroke severity.47 Aphasia after stroke seems to be more common with older age, with a 3% increase in risk per additional year of age.2 Study of age as a predictor of cognitive dysfunction after stroke is primarily confounded by higher likelihood of prestroke cognitive dysfunction, as well as by a higher frequency of cardioembolic stroke, such as from atrial fibrillation, which is more likely to lead to cortical ischaemia and thus to language dysfunction or neglect.
Sex differences in the distribution of cognitive dysfunction after stroke might be attributable to differences in stroke mechanisms between men and women. Women tend to have more cardioembolic strokes, whereas men have more lacunar strokes, which might explain the higher frequency of cognitive dysfunction in women than in men.20 Additionally, women tend to experience strokes at an older age than men,48 so might have more prestroke cognitive dysfunction that is not fully accounted for in age-adjusted analyses.
Other markers, such as fever and hyperglycaemia, have been associated with worse cognitive performance after stroke, but most assessments of these markers are confounded by the fact that these might both occur in response to stroke in the acute phase, or might be present in the setting of a larger stroke. Additionally, data on hyperglycaemia are conflicting.20,28 Haemoglobin level also seems to be an important factor in determining degree of specific cognitive impairment at the time of stroke. Both low and high haemoglobin levels are associated with worse performance on tests of neglect and with more frequent neglect, independent of infarct size and stroke severity.49
Other important individual-level characteristics that have been associated with higher risk of early post-stroke cognitive impairment include seizures, previous history of stroke, and cognitive difficulties before the onset of stroke.29,30 Unfortunately, baseline cognition, which is probably a very important factor, is virtually impossible to measure. Retrospective (post-stroke) assessment of prestroke cognition is likely to be biased,50 but many individuals with risk factors for stroke are likely to have some cognitive impairment before onset of stroke. The observed association between apolipoprotein E (APOE) ε4 status and cognitive impairment after stroke might also be attributable to differences in baseline cognition associated with this allele, although these associations were found even among individuals without baseline cognitive problems in a study that used retrospective measurement with the informant questionnaire on cognitive decline in the elderly.31
Another important factor in assessment of cognitive impairment acutely after stroke is the effect of medications. Some inpatients experience agitation or delirium, and might even be on sedating medications, which are likely to worsen cognitive performance. Other non-psychoactive medications often have sedating side-effects when used in combination, and especially in elderly patients.51 Furthermore, individuals who are most at risk for delirium or cognitive worsening are those who have dementia at baseline,52 which further complicates measurement of post-stroke cognition. In a meta-analysis of cognitive dysfunction before stroke, up to 14% of individuals were found to have dementia before the development of stroke.53
Imaging features
Features of the strokes themselves, as well as the health of the underlying white matter and cortex, seem to affect the risk of developing cognitive deficits after stroke (table 2). Larger strokes seem to be associated with higher likelihood of cognitive dysfunction in some studies,20 but whether this is a feature of the actual size or just the fact that larger strokes are more likely to involve the cortex and other regions that support cognition remains unclear, because size and location are confounding variables in stroke. Some studies have shown no association between cognitive dysfunction (ie, aphasia) and larger strokes,54 and others have reported higher rates of cognitive impairment in individuals with middle cerebral artery territory strokes or supratentorial stroke, thus supporting this latter hypothesis.20,39
Although lacunar infarcts were previously not thought to have any effect on cognition in individuals without dementia,55 more recent research does suggest that even a single lacunar infarct might adversely affect long-term cognition,56 particularly in the presence of concurrent leukoaraiosis.34,57 Some individuals are so impaired after lacunar infarction that they have symptoms consistent with vascular cognitive impairment in the subacute period, although the prevalence of cognitive impairment does not seem to continue to increase in the years after stroke.58 Other studies do not support this association, instead reporting no increase in risk for mild cognitive impairment (MCI) at 1 year after a single lacunar stroke.59 One potential explanation for the conflicting findings is that cognitive impairment in some patients with lacunar stroke is due to concurrent cortical hypoperfusion that might not have been apparent on imaging obtained acutely. Patients with lacunar strokes in other studies might not have had cortical hypoperfusion and would therefore not necessarily have the same cognitive complications.4
The role of pre-existing pathological changes in the brain on the development of cognitive deficits acutely after stroke is uncertain. In some reports, localisable regions of atrophy,36 including both middle temporal gyri and more extensive white-matter hyperintensities,33,60 have been reported in individuals with cognitive impairment after stroke, but not in those with intact post-stroke cognition. However, others have reported no significant differences in presence or absence of cerebral atrophy, silent infarcts, or white-matter lesions in those with and without cognitive dysfunction after stroke,20 or have not found that these associations persist after multivariable adjustment.39 Analyses of these associations might also be confounded by lack of cognitive assessment before stroke: individuals with more white-matter disease and more atrophy are likely to have worse cognition at baseline, and this might be the reason for apparent associations with cognitive function after stroke. These differences in baseline cognition might also explain differences in the frequency of cognitive impairment in individuals with haemorrhagic as opposed to ischaemic stroke.20 Individuals with haemorrhagic stroke include those with cerebral amyloid angiopathy, many of whom have baseline cognitive dysfunction, either because of neurodegenerative changes or concurrent infarcts, white-matter disease, or microbleeds, all of which are often found in such patients.61
Newer imaging techniques, such as diffusion-tensor imaging (DTI) and magnetisation transfer imaging (MTI), are showing promise in assisting in the identification of individuals with white-matter micro-structural abnormalities who might be at higher risk for cognitive impairment.62,63 However, these techniques are still in development; we will discuss these techniques below in the context of the assessment of cognitive decline after stroke.
Acute interventions
Although we will not focus specifically on interventions aimed at cognitive dysfunction, many interventions used acutely in stroke might affect cognitive performance. For example, intravenous alteplase has been shown to reduce disability after stroke,64 but has not been shown to similarly improve cognitive outcomes in the months after stroke,65 although might be beneficial for cognition at the acute stage in some patients.14,15 Cortical reperfusion with temporary blood pressure increase for patients with large-vessel stenosis and no significant cardiac risk factors in the acute stage did result in improved cognitive function in a small group of patients both acutely and in follow-up, illustrating that cortical reperfusion can have long-term positive effects on cognition.66
Dementia and other long-term cognitive effects
Most cognitive impairments after stroke resolve beyond the subacute time period, if not earlier. In one report, over 83% of 36 patients with initial deficits in verbal memory and 78% of 18 patients with deficits in visuospatial construction and visual memory had shown recovery by 6 months, with somewhat less frequent recovery in other domains.22 In another cohort, 43 (54%) of 80 patients still had deficits in attention at 1 year, but fewer had deficits in executive dysfunction, language, and long-term memory compared with the acute period.21 However, some individuals with stroke can have further progression of cognitive dysfunction, even in the absence of new clinically apparent ischaemic events.67
As the time after stroke increases, so does the likelihood that an individual might have other cerebrovascular injuries (either clinical or subclinical stroke) that could further contribute to impaired cognition (table 2). Individuals with multiple strokes seem to be at higher risk for cognitive deficits than are individuals with single strokes.41,53 Additionally, more subclinical lesions are likely to be present in individuals with multiple clinical strokes. Performance on tests of processing speed, memory, and executive function is worse in individuals with multiple infarcts.68
Stroke has been found to be a strong predictor of dementia (not specific to type of dementia, but based on cognitive decline and functional impairment) in an epidemiological study and an autopsy series.69,70 Again, the associations seem to be present both for clinically apparent strokes as well as for subclinical strokes,71 and the risk of dementia associated with single infarctions (odds ratio 1·69, 95% CI 0·70–4·09) is less than that with multiple infarctions (2·67, 1·08–6·61).72 The pathway from stroke to dementia usually passes through vascular MCI. Individuals with vascular MCI after stroke progress to dementia at a rate of about 8% per year.73
Pendlebury and Rothwell53 did a systematic review on the development of dementia at least 3 months after stroke. They compiled 73 papers with original data, including not only hospital-based series but also some population-based studies, and pooled estimates varied depending on the population included. For studies in which only individuals with first-ever stroke were included and participants with previous dementia were excluded, pooled mean prevalence estimates were 7·4–12·0%. When dementia after either first or recurrent stroke was examined, pooled mean estimates were even higher at 26·5% (95% CI 24·3–28·7) and were 20·3% (18·2–22·5) when prestroke dementia was excluded. Individuals with recurrent stroke had pooled estimates of 41·3% (95% CI 29·6–53·1).53 The primary significant predictors of dementia after stroke identified in this systematic review included older age, female sex, lower educational attainment, non-white race, as well as diabetes and atrial fibrillation. Factors specific to the strokes that significantly predicted subsequent dementia included having had a haemorrhagic stroke, dysphasia, left-hemispheric involvement, and whether this was a recurrent stroke. Similar to some of the reports on predictors of acute and subacute cognitive dysfunction, seizures at the time of stroke were associated with higher risk of post-stroke dementia, as was incontinence or an acute confusional episode, among other factors. Leukoaraiosis and atrophy (particularly of the medial temporal lobe) were also highly associated with the development of post-stroke dementia.53 Many of these factors point to ongoing underlying cerebrovascular injury, which in many cases is subclinical.
It is not clear whether the effects of strokes on dementia are due to vascular disease causing direct increases in the neuropathological changes associated with Alzheimer’s disease, or whether Alzheimer’s neuropathology and vascular neuropathology act synergistically to lead to worse cognition. Evidence exists to support both these theories. In an autopsy study, infarcts in the watershed regions were identified in 32·4% of individuals with Alzheimer’s neuropathological changes, compared with only 2·5% of controls.74 By contrast, in the Nun Study, among individuals meeting neuropathological evidence of Alzheimer’s disease, presence of lacunar infarcts at autopsy (in the basal ganglia, thalamus, or deep white matter) was associated with a 20 times increased odds of having dementia compared with individuals without infarcts,75 suggesting that vascular disease affected cognitive performance in the presence of Alzheimer’s neuropathological changes, but did not directly cause those changes. However, in the Religious Orders Study, there was no interaction between cerebral infarcts and Alzheimer’s neuropathological changes in the development of dementia, and no association between cerebral infarcts and the presence of neuropathological changes.76 Whether these conflicting results can be accounted for by differences in the genetics of the populations or environmental factors remains to be clarified.
Assessment of cognitive impairment Cognitive testing
The frequency of cognitive impairment after stroke will vary greatly depending on the definition of cognitive impairment used. In a study in which patients were required to be impaired on any four or more cognitive domains for inclusion, frequency of cognitive dysfunction at 3 months after stroke was 35%.77 By contrast, when cognitive impairment was defined as a Z score of −1 or lower on at least one cognitive domain, 90% of individuals were identified to have cognitive impairment at 2 weeks after stroke.23 No standardised definition exists for cognitive impairment, and there are no standardised assessment tools for testing cognition after stroke.
Because patients with stroke might have specific cognitive deficits (ie, aphasia or neglect) as well as more global cognitive dysfunction, testing should include assessment in each of these areas. Hoffmann and colleagues78 have proposed the comprehensive cognitive neurological assessment in stroke, which includes testing of language, neglect, and praxis, as well as memory and emotional responses, and screens for a range of specific cognitive syndromes in patients with stroke. This test was found to be highly sensitive (91%), although not very specific (35%), for stroke (defined using MRI).78 However, such detailed assessments might not be practical in inpatients with acute stroke. Other shorter tests have been assessed in patients with stroke in an attempt to improve efficiency. These include Cognistat and the screening instrument for neuropsychologic impairments in stroke, which have sensitivities of 82% and 71%, respectively, in detecting deficits in any cognitive domain when compared with complete neuropsychological assessment.79
The National Institutes of Health stroke scale (NIHSS) was developed in conjunction with the US National Institute of Neurological Disorders and Stroke intravenous alteplase trial,64 and was not designed to test global cognitive function. The cognitive component of the NIHSS primarily includes assessment of language, with more points allocated for aphasia than for neglect, and more points associated with a stroke in the left hemisphere than with a comparably sized stroke in the right hemisphere.80 We have found that the addition of two simple tests of neglect (ie, line cancellation and visual extinction) to the NIHSS improves prediction of infarct volume on DWI, and thus expands the cognitive assessment of the NIHSS.81 Others have reported that a limited version of four components of the NIHSS can be used as an accurate predictor of dementia, when performed at 18 months after stroke, and if compared with a full assessment for dementia as the gold standard (area under the curve of 0·78).82
Although a standardised assessment of cognition in stroke has not been established, in our experience, testing of language and neglect are warranted in all patients with supratentorial anterior circulation stroke, even in individuals with purely subcortical infarctions. To gather complete information on global cognitive function, testing should be accompanied by tests of motor speed, executive function, and attention, although a shorter, more focused examination is more practical in patients with acute stroke. Further study and unified recommendations are needed for accurate estimates of the prevalence of cognitive impairment after stroke.
Imaging techniques
DWI MRI is used to identify a core area of infarct, which is often associated with impairment of a corresponding cognitive function, depending on the location of the infarct. Because deficits are not only caused by this core infarct but also by surrounding areas of hypoperfusion, further imaging is needed to assess the functional status of surrounding tissue. Use of DWI and PWI MRI has allowed the detailed study of transient alterations in perfusion and their association with cognitive function, such as with measurement of a diffusion-perfusion mismatch.4 Additionally, MRI scans can be analysed on a voxel-by-voxel basis and compared with age-appropriate atlases to look for regional variations associated with cognitive symptoms and, with serial scans, to look for long-term structural changes associated with cognitive impairment in patients with stroke. Reduction in volume of grey matter, particularly in the thalamus, has been identified in individuals with stroke and cognitive impairment in at least one domain, compared with individuals with stroke who have intact cognition.36 Another study that used similar voxel-based morphometric techniques reported reductions in hippocampal volumes in individuals with cognitive impairment after lacunar infarction.60
Another important role of imaging in the assessment of cognitive impairment after stroke is in the identification of individuals in whom subclinical brain injury has also occurred. Subclinical cerebrovascular disease includes silent infarcts, white-matter disease (or leukoaraiosis), atrophy, and microbleeds. These changes can be seen with brain MRI, with inclusion of fluid-attenuated inversion recovery sequences for detection of silent infarcts and white-matter hyperintensities and gradient-echo or susceptibility-weighted imaging sequences for detection of microbleeds. So-called “silent” infarcts are strongly associated with dementia and cognitive decline in patients without stroke,71,83 and the combination of a clinical stroke with subclinical strokes further increases risk of cognitive deficits.
Both white-matter disease and regional atrophy are associated with worse cognition among individuals with stroke (table 2). Microbleeds, particularly in the cortex, have been associated with cerebral amyloid angiopathy, but more subcortical microbleeds can be seen in individuals with chronic hypertensive disease. Progression from MCI to Alzheimer’s disease has been associated with the presence of both lacunes and microbleeds,84 as has cognitive decline in individuals with cerebral autosomal dominant arteriopathy subcortical infarcts and leukoencephalopathy (CADASIL).85 Because of these associations, and the likelihood that all of these factors act synergistically in the development of cognitive dysfunction after stroke, the radiographic study of patients with stroke, both clinically and in research, needs to involve some measurement of the subclinical cerebrovascular burden.
Because the integrity of the white matter is likely to play an important part in an individual’s cognitive abilities after a stroke, newer imaging techniques that allow better estimation of white-matter integrity and connectivity are likely to have important roles in the assessment of cognitive dysfunction after stroke. Two techniques, MTI and DTI, allow better measurement of white-matter function and connectivity. MTI measures exchange of magnetisation between water and macromolecules (in the case of cerebral white matter and myelin). Calculation of the magnetic transfer ratio (MTR) from MTI data can show microstructural abnormalities in white matter: a higher quantity of water in the white matter, as occurs secondary to gliosis or from reactive astrocytes, leads to lower MTR values,86 which are thus indicative of white-matter dysfunction. Some studies have reported strong associations between the MTR and presence of white-matter hyperintensities,62 and also between MTR and cognition among individuals with CADASIL.87 An abnormal MTR has also been shown to precede the development of white-matter lesions in demyelinating diseases such as multiple sclerosis.88
By contrast, DTI is an MRI method that can be used to measure diffusivity in the white matter. It provides a measure of magnitude and direction of the diffusion of water along an axon, for example, and provides information not only about the microstructure of white matter, but also about connections between brain structures. This imaging modality can therefore be used to measure white-matter integrity, and can reveal the downstream effects of stroke.89 Fractional anisotropy, a measure calculated from DTI data, provides an indication of the directionality of white-matter fascicles, with higher anisotropy in highly organised structures, such as the corpus callosum. As white-matter structures lose their integrity, there is less clear directionality and diffusivity, as shown on DTI. Lower anisotropy has been reported in individuals with vascular cognitive impairment, even in regions without visible white-matter abnormalities.90 Additionally, lower fractional anisotropy, particularly in the frontal and parietal regions, has been associated with worse cognitive performance 3–6 months after stroke and in multiple cognitive domains.63 Future studies of vascular cognitive impairment are likely to include DTI and measurement of anisotropy, because this allows measurement of injury to white-matter tracts, which is an important predictor of vascular cognitive impairment and dementia (figure 2).91,92
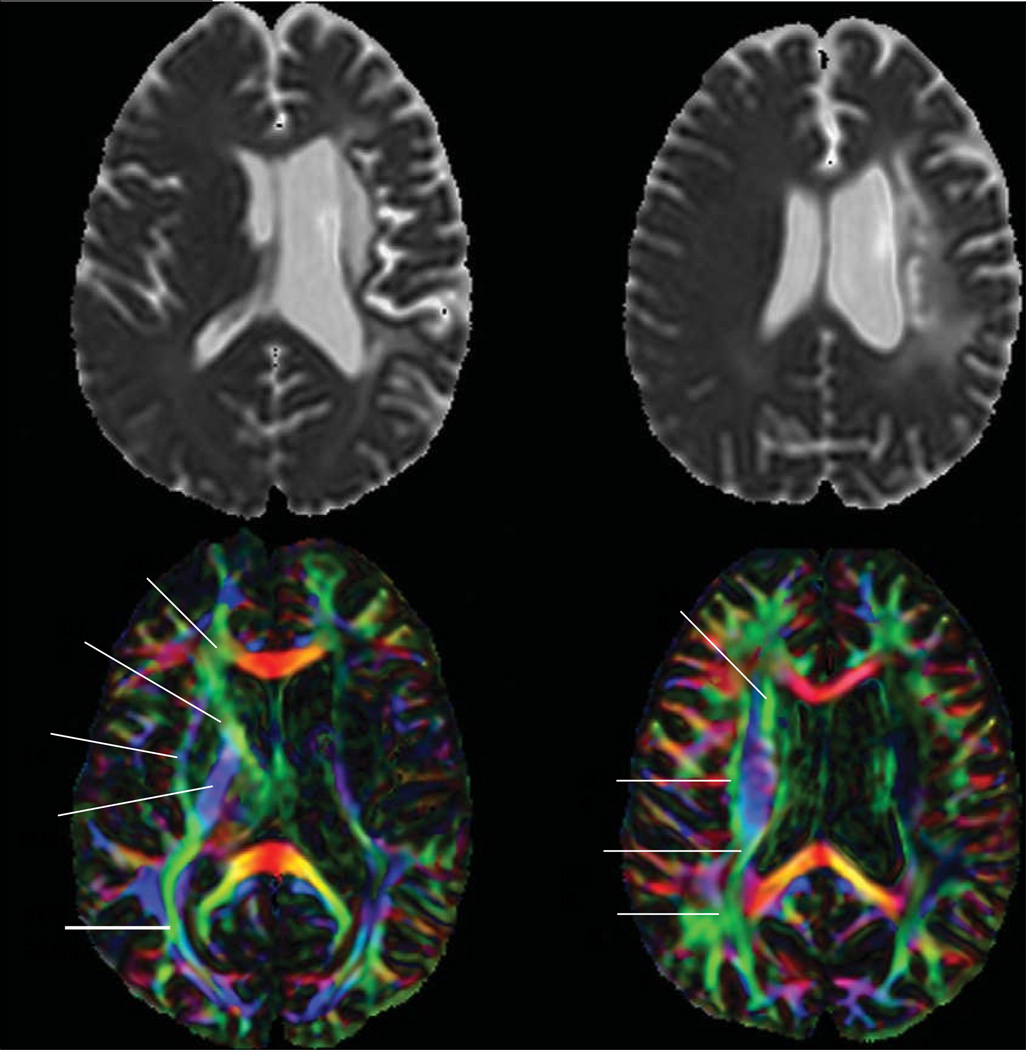
Figure 2. Cognitive deficits can be associated with white-matter tract damage, as shown with DTI.
MRI scans, including apparent diffusion coefficient maps (top) and corresponding DTI scans (bottom), of a 56-year-old man who had a deep left internal capsule and corona radiata infarct 4 years previously. DTI shows degeneration of white-matter tracts, including the PTR, SS, and ILF. The degeneration of white-matter tracts might be responsible for the patient’s language deficits, including impairments in sentence repetition, comprehension of syntactically complex sentences, and production of grammatically complex sentences, which are generally attributable to cortical dysfunction. Alternatively, the language deficits might be due to left frontotemporoparietal cortical hypoperfusion on PWI (not shown), which in turn might have led to the degeneration of white-matter tracts. The right side of the brain is shown on the left side of the image, as per radiological convention. ACR=anterior corona radiata. ALIC=anterior limb of internal capsule. EC=external capsule. PLIC=posterior limb of internal capsule. PTR=posterior thalamic radiation. SS=sagittal striatum. ILF=inferior longitudinal fasciculus. SCR=superior corona radiata. PCR=posterior corona radiata. SLF=superior longitudinal fasciculus. DTI=diffusion tensor imaging.
Future directions
With increasing awareness that stroke can cause long-lasting cognitive impairments, and in some cases contributes to the development of dementia, there has been an increasing need for improved cognitive assessment in studies of stroke, and this remains an important area for future research. Longitudinal studies of cognitive abilities, white-matter integrity, perfusion, and reversal of perfusion abnormalities will be important to establish cause-and-effect relationships between tissue structural abnormalities, tissue dysfunction, and cognitive dysfunction. Moreover, the need to incorporate cognitive assessment into future trials in stroke has been acknowledged in the stroke literature.93,94 Cognitive performance will be an important endpoint in studies of vascular risk-factor modification. The Systolic Blood Pressure Intervention Trial (SPRINT) is a recently launched 9-year study of intensive blood pressure lowering (systolic blood pressure to <140 mm Hg vs <120 mm Hg), in which the SPRINT-MIND (Memory and Cognition in Decreased Hypertension) substudy will include cognitive assessment, dementia ascertainment, and brain imaging with MRI.95 Similarly, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is designed to investigate different glycaemic control targets in patients with diabetes, with an important substudy (ACCORD-MIND)96 to follow cognitive changes related to these interventions in the prevention of vascular outcomes. Similarly, future trials of interventions in acute stroke should include cognitive outcome measures as primary endpoints. Our studies and those of many others suggest that current interventions (ie, thrombolytics, embolectomy, etc) can successfully reperfuse the cortex and improve cognitive function, but often do little to improve recovery of basic motor function (because the internal capsule and basal ganglia are often infarcted early in stroke).4,7,15,97 Therefore, some of the failure of early acute stroke trials might have been due to a mismatch between the outcome measures and the function that was intended to be protected.
In addition to the necessity for improvements and standardisation in the assessment of cognition, future research is needed on interventions to treat cognitive dysfunction after stroke, a topic not covered in this Review but an area in which improved interventions are needed. Although it is highly likely that most reduction in stroke-related cognitive impairment and dementia will result from reduction in the risk factors that contribute to stroke in the first place, well designed clinical trials are needed to assess treatments for cognitive deficits after stroke. A major area of research in neuroprotection is the development of therapeutics that augment cerebral plasticity.98 Any global improvement in cerebral plasticity is likely to improve cognitive recovery.
The study of transcranial magnetic and direct current stimulation are likely to be important areas of focus in rehabilitation of cognitive deficits after stroke. Some evidence suggests that these techniques can help to improve memory formation and motor learning in healthy volunteers.99 Transcranial magnetic stimulation has been shown to be safe in patients with ischaemic stroke,100 has been associated with improved aphasia in small case series,101 and has been shown in small randomised trials to improve motor learning.102,103
Future research on the cognitive effects of stroke and the association between stroke and dementia should include better measurement tools, and perhaps might involve less cognitive burden from individual strokes due to improved treatments of the cognitive effects of stroke. Collaboration between investigators in vascular and cognitive neurology, cardiovascular medicine, and neurorehabilitation will be important to optimise prevention, assessment, and treatment.
Search strategy and selection criteria.
References for this Review were identified through searches of PubMed from 1980 to June, 2010, using search terms including “stroke AND cognition” and “stroke AND dementia”. Additional references were identified through searches of the authors’ own files and from references cited from other papers. Only papers published in English were reviewed.
Acknowledgments
AEH is supported by the National Institutes of Health grants NIH RO1 NS047691 and NIH R01 DC05375. RFG is supported by an American Heart Association Scientist Development Grant.
Footnotes
Contributors
Both authors contributed to the literature search, figures, writing, and revisions of the Review.
Conflicts of interest
We have no conflicts of interest.
References
- 1.Inatomi Y, Yonehara T, Omiya S, Hashimoto Y, Hirano T, Uchino M. Aphasia during the acute phase in ischemic stroke. Cerebrovasc Dis. 2008;25:316–323. doi: 10.1159/000118376. [DOI] [PubMed] [Google Scholar]
- 2.Engelter ST, Gostynski M, Papa S, et al. Epidemiology of aphasia attributable to first ischemic stroke: incidence, severity, fluency, etiology, and thrombolysis. Stroke. 2006;37:1379–1384. doi: 10.1161/01.STR.0000221815.64093.8c. [DOI] [PubMed] [Google Scholar]
- 3.Ringman JM, Saver JL, Woolson RF, Clarke WR, Adams HP. Frequency, risk factors, anatomy, and course of unilateral neglect in an acute stroke cohort. Neurology. 2004;63:468–474. doi: 10.1212/01.wnl.0000133011.10689.ce. [DOI] [PubMed] [Google Scholar]
- 4.Hillis AE, Wityk RJ, Barker PB, et al. Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion. Brain. 2002;125:1094–1104. doi: 10.1093/brain/awf113. [DOI] [PubMed] [Google Scholar]
- 5.Hillis AE, Wityk RJ, Tuffiash E, et al. Hypoperfusion of Wernicke’s area predicts severity of semantic deficit in acute stroke. Ann Neurol. 2001;50:561–566. doi: 10.1002/ana.1265. [DOI] [PubMed] [Google Scholar]
- 6.Hillis AE, Barker PB, Beauchamp NJ, Gordon B, Wityk RJ. MR perfusion imaging reveals regions of hypoperfusion associated with aphasia and neglect. Neurology. 2000;55:782–788. doi: 10.1212/wnl.55.6.782. [DOI] [PubMed] [Google Scholar]
- 7.Hillis AE, Barker PB, Beauchamp NJ, Winters BD, Mirski M, Wityk RJ. Restoring blood pressure reperfused Wernicke’s area and improved language. Neurology. 2001;56:670–672. doi: 10.1212/wnl.56.5.670. [DOI] [PubMed] [Google Scholar]
- 8.Launer LJ, Petrovitch H, Ross GW, Markesbery W, White LR. AD brain pathology: vascular origins? Results from the HAAS autopsy study. Neurobiol Aging. 2008;29:1587–1590. doi: 10.1016/j.neurobiolaging.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann M. Higher cortical function deficits after stroke: an analysis of 1,000 patients from a dedicated cognitive stroke registry. Neurorehabil Neural Repair. 2001;15:113–127. doi: 10.1177/154596830101500205. [DOI] [PubMed] [Google Scholar]
- 10.Hillis AE. Aphasia: progress in the last quarter of a century. Neurology. 2007;69:200–213. doi: 10.1212/01.wnl.0000265600.69385.6f. [DOI] [PubMed] [Google Scholar]
- 11.Verdon V, Schwartz S, Lovblad KO, Hauert CA, Vuilleumier P. Neuroanatomy of hemispatial neglect and its functional components: a study using voxel-based lesion-symptom mapping. Brain. 2010;133:880–894. doi: 10.1093/brain/awp305. [DOI] [PubMed] [Google Scholar]
- 12.Medina J, Kannan V, Pawlak MA, et al. Neural substrates of visuospatial processing in distinct reference frames: evidence from unilateral spatial neglect. J Cogn Neurosci. 2009;21:2073–2084. doi: 10.1162/jocn.2008.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baron JC, Bousser MG, Rey A, Guillard A, Comar D, Castaigne P. Reversal of focal “misery-perfusion syndrome” by extra-intracranial arterial bypass in hemodynamic cerebral ischemia: a case study with 15O positron tomography. Stroke. 1981;12:454–459. doi: 10.1161/01.str.12.4.454. [DOI] [PubMed] [Google Scholar]
- 14.Heiss WD, Grond M, Thiel A, et al. Tissue at risk of infarction rescued by early reperfusion: a positron emission tomography study in systemic recombinant tissue plasminogen activator thrombolysis of acute stroke. J Cereb Blood Flow Metab. 1998;18:1298–1307. doi: 10.1097/00004647-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Hillis AE, Kleinman JT, Newhart M, et al. Restoring cerebral blood flow reveals neural regions critical for naming. J Neurosci. 2006;26:8069–8073. doi: 10.1523/JNEUROSCI.2088-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsen TS, Bruhn P, Oberg RG. Cortical hypoperfusion as a possible cause of ‘subcortical aphasia’. Brain. 1986;109:393–410. doi: 10.1093/brain/109.3.393. [DOI] [PubMed] [Google Scholar]
- 17.Croquelois A, Wintermark M, Reichhart M, Meuli R, Bogousslavsky J. Aphasia in hyperacute stroke: language follows brain penumbra dynamics. Ann Neurol. 2003;54:321–329. doi: 10.1002/ana.10657. [DOI] [PubMed] [Google Scholar]
- 18.Shirani P, Thorn J, Davis C, et al. Severity of hypoperfusion in distinct brain regions predicts severity of hemispatial neglect in different reference frames. Stroke. 2009;40:3563–3566. doi: 10.1161/STROKEAHA.109.561969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee BH, Kim EJ, Ku BD, et al. Cognitive impairments in patients with hemispatial neglect from acute right hemispheric stroke. Cogn Behav Neurol. 2008;21:73–76. doi: 10.1097/WNN.0b013e3181772101. [DOI] [PubMed] [Google Scholar]
- 20.Nys GMS, van Zandvoort MJE, de Kort PLM, Jansen BPW, de Haan EHF, Kappelle LJ. Cognitive disorders in acute stroke: prevalence and clinical determinants. Cerebrovasc Dis. 2007;23:408–416. doi: 10.1159/000101464. [DOI] [PubMed] [Google Scholar]
- 21.Lesniak M, Bak T, Czepiel W, Seniow J, Czlonkowska A. Frequency and prognostic value of cognitive disorders in stroke patients. Dement Geriatr Cogn Disord. 2008;26:356–363. doi: 10.1159/000162262. [DOI] [PubMed] [Google Scholar]
- 22.Nys GMS, van Zandvoort MJE, De Kort PLM, et al. Domain-specific cognitive recovery after first-ever stroke: a follow-up study of 111 cases. J Int Neuropsychol Soc. 2005;11:795–806. doi: 10.1017/s1355617705050952. [DOI] [PubMed] [Google Scholar]
- 23.Jaillard A, Naegele B, Trabucco-Miguel S, LeBas JF, Hommel M. Hidden dysfunctioning in subacute stroke. Stroke. 2009;40:2473–2479. doi: 10.1161/STROKEAHA.108.541144. [DOI] [PubMed] [Google Scholar]
- 24.Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31:656–661. doi: 10.1161/01.str.31.3.656. [DOI] [PubMed] [Google Scholar]
- 25.Koga M, Reutens DC, Wright P, et al. The existence and evolution of diffusion-perfusion mismatched tissue in white and gray matter after acute stroke. Stroke. 2005;36:2132–2137. doi: 10.1161/01.STR.0000181066.23213.8f. [DOI] [PubMed] [Google Scholar]
- 26.Lazar RM, Minzer B, Antoniello D, Festa JR, Krakauer JW, Marshall RS. Improvement in aphasia scores after stroke is well predicted by initial severity. Stroke. 2010;41:1485–1488. doi: 10.1161/STROKEAHA.109.577338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klimkowicz-Mrowiec A, Dziedzic T, Slowik A, Szczudlik A. Predictors of poststroke dementia: results of a hospital-based study in Poland. Dement Geriatr Cogn Disord. 2006;21:328–334. doi: 10.1159/000091788. [DOI] [PubMed] [Google Scholar]
- 28.Kruyt ND, Nys GM, van der Worp HB, van Zandvoort MJ, Kappelle LJ, Biessels GJ. Hyperglycemia and cognitive outcome after ischemic stroke. J Neurol Sci. 2008;270:141–147. doi: 10.1016/j.jns.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Cordonnier C, Hénon H, Derambure P, Pasquier F, Leys D. Early epileptic seizures after stroke are associated with increased risk of new-onset dementia. J Neurol Neurosurg Psychiatry. 2007;78:514–516. doi: 10.1136/jnnp.2006.105080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mok VC, Wong A, Lam WW, et al. Cognitive impairment and functional outcome after stroke associated with small vessel disease. J Neurol Neurosurg Psychiatry. 2004;75:560–566. doi: 10.1136/jnnp.2003.015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagle J, Farner L, Flekkøy K, et al. Association between ApoE ε4 and cognitive impairment after stroke. Dement Geriatr Cogn Disord. 2009;27:525–533. doi: 10.1159/000223230. [DOI] [PubMed] [Google Scholar]
- 32.Ballard CG, Morris CM, Rao H, et al. ApoE ε4 and cognitive decline in older stroke patients with early cognitive impairment. Neurology. 2004;63:1399–1402. doi: 10.1212/01.wnl.0000141851.93193.17. [DOI] [PubMed] [Google Scholar]
- 33.Jokinen H, Kalska H, Mantyla R, et al. White matter hyperintensities as a predictor of neuropsychological deficits post-stroke. J Neurol Neurosurg Psychiatry. 2005;76:1229–1233. doi: 10.1136/jnnp.2004.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen HM, Mok VC, Fan YH, et al. Effect of white matter changes on cognitive impairment in patients with lacunar infarcts. Stroke. 2004;35:1826–1830. doi: 10.1161/01.STR.0000133686.29320.58. [DOI] [PubMed] [Google Scholar]
- 35.Sachdev PS, Chen X, Joscelyne A, Wen W, Altendorf A, Brodaty H. Hippocampal size and dementia in stroke patients: the Sydney Stroke Study. J Neurol Sci. 2007;260:71–77. doi: 10.1016/j.jns.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Stebbins GT, Nyenhuis DL, Wang C, et al. Gray matter atrophy in patients with ischemic stroke with cognitive impairment. Stroke. 2008;39:785–793. doi: 10.1161/STROKEAHA.107.507392. [DOI] [PubMed] [Google Scholar]
- 37.Burton E, Ballard C, Stephens S, et al. Hyperintensities and fronto-subcortical atrophy on MRI are substrates of mild cognitive deficits after stroke. Dement Geriatr Cogn Disord. 2003;16:113–118. doi: 10.1159/000070684. [DOI] [PubMed] [Google Scholar]
- 38.Tay SY, Ampil ER, Chen CPLH, Auchus AP. The relationship between homocysteine, cognition and stroke subtypes in acute stroke. J Neurol Sci. 2006;250:58–61. doi: 10.1016/j.jns.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 39.Jaillard A, Grand S, Le Bas JF, Hommel M. Predicting cognitive dysfunctioning in nondemented patients early after stroke. Cerebrovasc Dis. 2010;29:415–423. doi: 10.1159/000289344. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann M, Schmitt F. Cognitive impairment in isolated subtentorial stroke. Acta Neurol Scand. 2004;109:14–24. doi: 10.1034/j.1600-0404.2003.00169.x. [DOI] [PubMed] [Google Scholar]
- 41.Srikanth VK, Quinn SJ, Donnan GA, Saling MM, Thrift AG. Long-term cognitive transitions, rates of cognitive change, and predictors of incident dementia in a population-based first-ever stroke cohort. Stroke. 2006;37:2479–2483. doi: 10.1161/01.STR.0000239666.46828.d7. [DOI] [PubMed] [Google Scholar]
- 42.Jehkonen M, Ahonen JP, Dastidar P, et al. Visual neglect as a predictor of functional outcome one year after stroke. Acta Neurol Scand. 2000;101:195–201. doi: 10.1034/j.1600-0404.2000.101003195.x. [DOI] [PubMed] [Google Scholar]
- 43.Wade DT, Langton Hewer R. Stroke: associations with age, sex, and side of weakness. Arch Phys Med Rehabil. 1986;67:540–545. [PubMed] [Google Scholar]
- 44.Rasquin SM, Verhey FR, van Oostenbrugge RJ, Lousberg R, Lodder J. Demographic and CT scan features related to cognitive impairment in the first year after stroke. J Neurol Neurosurg Psychiatry. 2004;75:1562–1567. doi: 10.1136/jnnp.2003.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Appelros P, Karlsson GM, Hennerdal S. Anosognosia versus unilateral neglect: coexistence and their relations to age, stroke severity, lesion site and cognition. Eur J Neurol. 2007;14:54–59. doi: 10.1111/j.1468-1331.2006.01544.x. [DOI] [PubMed] [Google Scholar]
- 46.Pedersen PM, Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Hemineglect in acute stroke—incidence and prognostic implications: the Copenhagen Stroke Study. Am J Phys Med Rehabil. 1997;76:122–127. doi: 10.1097/00002060-199703000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Gottesman RF, Kleinman JT, Davis C, et al. Unilateral neglect is more severe and common in older patients with right hemispheric stroke. Neurology. 2008;71:1439–1444. doi: 10.1212/01.wnl.0000327888.48230.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reid JM, Dai D, Gubitz GJ, Kapral MK, Christian C, Phillips SJ. Gender differences in stroke examined in a 10-year cohort of patients admitted to a Canadian teaching hospital. Stroke. 2008;39:1090–1095. doi: 10.1161/STROKEAHA.107.495143. [DOI] [PubMed] [Google Scholar]
- 49.Gottesman RF, Bahrainwala Z, Wityk RJ, Hillis AE. Neglect is more common and severe at extreme hemoglobin levels in right-hemispheric stroke. Stroke. 2010;41:1641–1645. doi: 10.1161/STROKEAHA.110.585265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mok V, Wong A, Tang WK, et al. Determinants of prestroke cognitive impairment in stroke associated with small vessel disease. Dement Geriatr Cogn Disord. 2005;20:225–230. doi: 10.1159/000087310. [DOI] [PubMed] [Google Scholar]
- 51.Wright RM, Roumani YF, Boudreau R, et al. Effect of central nervous system medication use on decline in cognition in community-dwelling older adults: findings from the Health, Aging And Body Composition Study. J Am Geriatr Soc. 2009;57:243–250. doi: 10.1111/j.1532-5415.2008.02127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henon H, Lebert F, Durieu I, et al. Confusional state in stroke: relation to preexisting dementia, patient characteristics, and outcome. Stroke. 1999;30:773–779. doi: 10.1161/01.str.30.4.773. [DOI] [PubMed] [Google Scholar]
- 53.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 54.Lazar RM, Speizer AE, Festa JR, Krakauer JW, Marshall RS. Variability in language recovery after first-time stroke. J Neurol Neurosurg Psychiatry. 2008;79:530–534. doi: 10.1136/jnnp.2007.122457. [DOI] [PubMed] [Google Scholar]
- 55.Fisher CM. Lacunar strokes and infarcts: a review. Neurology. 1982;32:871–876. doi: 10.1212/wnl.32.8.871. [DOI] [PubMed] [Google Scholar]
- 56.van Zandvoort MJ, de Haan EH, Kappelle LJ. Chronic cognitive disturbances after a single supratentorial lacunar infarct. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:98–102. [PubMed] [Google Scholar]
- 57.McMurtray AM, Liao A, Haider J, Licht E, Mendez MF. Cognitive performance after lacunar stroke correlates with leukoaraiosis severity. Cerebrovasc Dis. 2007;24:271–276. doi: 10.1159/000105679. [DOI] [PubMed] [Google Scholar]
- 58.Rasquin SM, van Oostenbrugge RJ, Verhey FR, Lodder J. Vascular mild cognitive impairment is highly prevalent after lacunar stroke but does not increase over time: a 2-year follow-up study. Dement Geriatr Cogn Disord. 2007;24:396–401. doi: 10.1159/000109747. [DOI] [PubMed] [Google Scholar]
- 59.Anderson JF, Saling MM, Srikanth VK, Thrift AG, Donnan GA. Individuals with first-ever clinical presentation of a lacunar infarction syndrome: is there an increased likelihood of developing mild cognitive impairment in the first 12 months after stroke? J Neuropsychol. 2008;2:373–385. doi: 10.1348/174866408x288846. [DOI] [PubMed] [Google Scholar]
- 60.Grau-Olivares M, Bartres-Faz D, Arboix A, et al. Mild cognitive impairment after lacunar infarction: voxel-based morphometry and neuropsychological assessment. Cerebrovasc Dis. 2007;23:353–361. doi: 10.1159/000099134. [DOI] [PubMed] [Google Scholar]
- 61.Pfeifer LA, White LR, Ross GW, Petrovitch H, Launer LJ. Cerebral amyloid angiopathy and cognitive function: the Haas Autopsy Study. Neurology. 2002;58:1629–1634. doi: 10.1212/wnl.58.11.1629. [DOI] [PubMed] [Google Scholar]
- 62.Fazekas F, Ropele S, Enzinger C, et al. MTI of white matter hyperintensities. Brain. 2005;128:2926–2932. doi: 10.1093/brain/awh567. [DOI] [PubMed] [Google Scholar]
- 63.Williamson J, Nyenhuis D, Stebbins GT, et al. Regional differences in relationships between apparent white matter integrity, cognition, and mood in patients with ischemic stroke. J Clin Exp Neuropsychol. 2010;10:1–9. doi: 10.1080/13803390903427406. [DOI] [PubMed] [Google Scholar]
- 64.The NINDS rt-PA Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1588. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 65.Nys GM, van Zandvoort MJ, Algra A, Kappelle LJ, de Haan EH. Cognitive and functional outcome after intravenous recombinant tissue plasminogen activator treatment in patients with a first symptomatic brain infarct. J Neurol. 2006;253:237–241. doi: 10.1007/s00415-005-0966-x. [DOI] [PubMed] [Google Scholar]
- 66.Hillis AE, Ulatowski JA, Barker PB, et al. A pilot randomized trial of induced blood pressure elevation: effects on function and focal perfusion in acute and subacute stroke. Cerebrovasc Dis. 2003;16:236–246. doi: 10.1159/000071122. [DOI] [PubMed] [Google Scholar]
- 67.Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz LM, Koschera A. Progression of cognitive impairment in stroke patients. Neurology. 2004;63:1618–1623. doi: 10.1212/01.wnl.0000142964.83484.de. [DOI] [PubMed] [Google Scholar]
- 68.Saczynski JS, Sigurdsson S, Jonsdottir MK, et al. Cerebral infarcts and cognitive performance: importance of location and number of infarcts. Stroke. 2009;40:677–682. doi: 10.1161/STROKEAHA.108.530212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Narasimhalu K, Ang S, De Silva DA, et al. Severity of CIND and MCI predict incidence of dementia in an ischemic stroke cohort. Neurology. 2009;73:1866–1872. doi: 10.1212/WNL.0b013e3181c3fcb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O’Brien RJ. Effects of infarcts on dementia in the Baltimore Longitudinal Study of Aging. Ann Neurol. 2008;64:168–176. doi: 10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 72.Schneider JA, Wilson RS, Cochran EJ, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60:1082–1088. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- 73.Sachdev PS, Chen X, Brodaty H, Thompson C, Altendorf A, Wen W. The determinants and longitudinal course of post-stroke mild cognitive impairment. J Int Neuropsychol Soc. 2009;15:915–923. doi: 10.1017/S1355617709990579. [DOI] [PubMed] [Google Scholar]
- 74.Suter O-C, Sunthorn T, Kraftsik R, et al. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke. 2002;33:1986–1992. doi: 10.1161/01.str.0000024523.82311.77. [DOI] [PubMed] [Google Scholar]
- 75.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease: the Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 76.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 77.Tatemichi TK, Desmond DW, Stern Y, Paik M, Sano M, Bagiella E. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry. 1994;57:202–207. doi: 10.1136/jnnp.57.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoffmann M, Schmitt F, Bromley E. Comprehensive cognitive neurological assessment in stroke. Acta Neurol Scand. 2008;119:162–171. doi: 10.1111/j.1600-0404.2008.01101.x. [DOI] [PubMed] [Google Scholar]
- 79.Nokleby K, Boland E, Bergersen H, et al. Screening for cognitive deficits after stroke: a comparison of three screening tools. Clin Rehabil. 2008;22:1095–1104. doi: 10.1177/0269215508094711. [DOI] [PubMed] [Google Scholar]
- 80.Fink JN, Selim MH, Kumar S, et al. Is the association of National Institutes of Health Stroke Scale scores and acute magnetic resonance imaging stroke volume equal for patients with right- and left-hemisphere ischemic stroke? Stroke. 2002;33:954–958. doi: 10.1161/01.str.0000013069.24300.1d. [DOI] [PubMed] [Google Scholar]
- 81.Gottesman RF, Kleinman JT, Davis C, Heidler-Gary J, Newhart M, Hillis AE. The NIHSS-plus: improving cognitive assessment with the NIHSS. Behav Neurol. 2009/2010;22:11–15. doi: 10.3233/BEN-2009-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cumming TB, Blomstrand C, Bernhardt J, Linden T. The NIH Stroke Scale can establish cognitive function after stroke. Cerebrovasc Dis. 2010;30:7–14. doi: 10.1159/000313438. [DOI] [PubMed] [Google Scholar]
- 83.Carey CL, Kramer JH, Josephson SA, et al. Subcortical lacunes are associated with executive dysfunction in cognitively normal elderly. Stroke. 2008;39:397–402. doi: 10.1161/STROKEAHA.107.491795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Staekenborg SS, Koedam EL, Henneman WJ, et al. Progression of mild cognitive impairment to dementia: contribution of cerebrovascular disease compared with medial temporal lobe atrophy. Stroke. 2009;40:1269–1274. doi: 10.1161/STROKEAHA.108.531343. [DOI] [PubMed] [Google Scholar]
- 85.Liem MK, Lesnik Oberstein SA, Haan J, et al. MRI correlates of cognitive decline in CADASIL: a 7-year follow-up study. Neurology. 2009;72:143–148. doi: 10.1212/01.wnl.0000339038.65508.96. [DOI] [PubMed] [Google Scholar]
- 86.Wong KT, Grossman RI, Boorstein JM, Lexa FJ, McGowan JC. Magnetic transfer imaging of periventricular hyperintense white matter in the elderly. Am J Neuroradiol. 1995;16:253–258. [PMC free article] [PubMed] [Google Scholar]
- 87.Iannucci G, Dichgans M, Rovaris M, et al. Correlations between clinical findings and magnetization transfer imaging metrics of tissue damage in individuals with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke. 2001;32:643–648. doi: 10.1161/01.str.32.3.643. [DOI] [PubMed] [Google Scholar]
- 88.Filippi M, Rocca M, Martino G, Horsfield MA, Comi G. Magnetization transfer changes in the normal appearing white matter precede the appearance of enhancing lesions in patients with multiple sclerosis. Ann Neurol. 1998;43:809–814. doi: 10.1002/ana.410430616. [DOI] [PubMed] [Google Scholar]
- 89.Chen Z, Ni P, Zhang J, et al. Evaluating ischemic stroke with diffusion tensor imaging. Neurol Res. 2008;30:720–726. doi: 10.1179/174313208X297968. [DOI] [PubMed] [Google Scholar]
- 90.Medina D, de Toledo-Morrell L, Urresta F, et al. White matter changes in mild cognitive impairment and AD: a diffusion tensor imaging study. Neurobiol Aging. 2006;27:663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 91.Kuczynski B, Targan E, Madison C, et al. White matter integrity and cortical metabolic associations in aging and dementia. Alzheimers Dement. 2010;6:54–62. doi: 10.1016/j.jalz.2009.04.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chua TC, Wen W, Chen X, et al. Diffusion tensor imaging of the posterior cingulate is a useful biomarker of mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17:602–613. doi: 10.1097/JGP.0b013e3181a76e0b. [DOI] [PubMed] [Google Scholar]
- 93.Croquelois A, Bogousslavsky J. Cognitive deficits in hyperacute stroke. Stroke. 2004;35:25. doi: 10.1161/01.STR.0000115532.05870.B8. [DOI] [PubMed] [Google Scholar]
- 94.Merino JG, Heilman KM. Measurement of cognitive deficits in acute stroke [comment] Stroke. 2003;34:2396–2398. doi: 10.1161/01.STR.0000094661.43891.F9. [DOI] [PubMed] [Google Scholar]
- 95.US National Institutes of Health News. [accessed July 7, 2010];NIH launches multicenter clinical trial to test blood pressure strategy. 2009 Oct 29; http://www.nih.gov/news/health/oct2009/nhlbi-29.htm.
- 96.Williamson JD, Miller ME, Bryan RN, et al. for the ACCORD Study Group. The Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Study (ACCORD-MIND): rationale, design, and methods. Am J Cardiol. 2007;99:112i–122i. doi: 10.1016/j.amjcard.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 97.Olivot JM, Mlynash M, Thijs VN, et al. Relationships between infarct growth, clinical outcome, and early recanalization in diffusion and perfusion imaging for understanding stroke evolution (DEFUSE) Stroke. 2008;39:2257–2263. doi: 10.1161/STROKEAHA.107.511535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gutierrez M, Merino JJ, de Lecinana MA, Diez-Tejedor E. Cerebral protection, brain repair, plasticity and cell therapy in ischemic stroke. Cerebrovasc Dis. 2009;27:177–186. doi: 10.1159/000200457. [DOI] [PubMed] [Google Scholar]
- 99.Reis J, Robertson E, Krakauer JW, et al. Consensus: “Can TDCS and TMS enhance motor learning and memory formation?”. Brain Stimulat. 2008;1:363–369. doi: 10.1016/j.brs.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carey JR, Evans CD, Anderson DC, et al. Safety of 6-Hz primed low-frequency RTMS in stroke. Neurorehabil Neural Repair. 2008;22:185–192. doi: 10.1177/1545968307305458. [DOI] [PubMed] [Google Scholar]
- 101.Kakuda W, Abo M, Kaito N, Watanabe M, Senoo A. Functional MRI-based therapeutic RTMS strategy for aphasic stroke patients: a case series pilot study. Int J Neurosci. 2010;120:60–66. doi: 10.3109/00207450903445628. [DOI] [PubMed] [Google Scholar]
- 102.Takeuchi N, Tada T, Toshima M, Matsuo Y, Ikoma K. Repetitive transcranial magnetic stimulation over bilateral hemispheres enhances motor function and training effect of paretic hand in patients after stroke. J Rehabil Med. 2009;41:1049–1054. doi: 10.2340/16501977-0454. [DOI] [PubMed] [Google Scholar]
- 103.Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36:2681–2686. doi: 10.1161/01.STR.0000189658.51972.34. [DOI] [PubMed] [Google Scholar]