Abstract
Background
FoxP3+/CD4+/CD25+ regulatory T cells (Treg) play an important role in maintaining peripheral tolerance and are potent suppressors of T cell activation. In the present studies we evaluated the role Treg might play in peripheral tolerance to composite tissue allotransplants (CTA).
Methods
Mixed allogeneic chimeric rats were prepared by pre-conditioning recipients with anti-αβ-TCR monoclonal antibody (mAb) followed by total body irradiation. Animals received T cell-depleted ACI bone marrow cells followed by anti-lymphocyte serum and FK-506. A modified osteomyocutaneous hind-limb flap composed of bone and all limb tissue components was placed at 29 days in animals with chimerism ≥ 1% on day 28. Recipients with CTA surviving ≥ 6 months were evaluated for Treg. Skin from tolerant long-term allogeneic transplanted, syngeneic transplanted, rejected and naïve animals were immunostained with fluorochrome conjugated anti-FoxP3 and anti-CD4 mAb and visualized under a laser confocal microscope.
Results
Significant CD4+/FoxP3+ Treg infiltrates were observed in tolerant donor-allograft skin samples. No graft infiltrating FoxP3+ cells were observed in rejector, naïve, or syngeneic CTA transplanted skin. In parallel experiments, mixed leukocyte reactions assays were performed to investigate the suppressor function of Treg cells. Splenocytes from tolerant, rejected, and naïve rats were sorted by flow cytometry for CD4+/CD25+ T cells. Treg demonstrated similar suppressive levels between the three groups.
Conclusions
These data suggest that Treg may play an important role in maintenance of tolerance and promoting graft acceptance in long-term CTA acceptors and may explain the favorable outcomes observed in clinical CTA recipients.
Keywords: composite tissue allotransplant, T regulatory cells, tolerance, bone marrow transplantation
INTRODUCTION
Composite tissue allotransplantation (CTA) is among the most immunologically complex transplant fields. Widespread application of CTA procedures is limited due to the need for lifelong immunosuppressive therapy and their associated toxicity (1). Tolerance induction to transplanted CTA would significantly broaden the clinical feasibility of this procedure by preventing the complications associated with long-term immunosuppression. In 1985, Ildstad et al. reported that donor-specific tolerance was achieved in mixed allogeneic chimeras (2). These chimeras exhibited superior immunocompetence due to the presence of recipient antigen presenting cells (APC) (3). Mixed chimerism was first attained through myeloablative conditioning followed by transplantation of a mixture of syngeneic and allogeneic bone marrow stem cells. The development of nonmyeloablative conditioning protocols has substantially reduced the toxicity of conditioning while still retaining a portion of the recipient’s native immune system (4-6). Under some conditions, high levels of donor chimerism may not be established or maintained. Under these circumstances, regulation of immune responsiveness can be attained through peripheral mechanisms such as CD4+/CD25+/FoxP3+ regulatory T cells (Treg) (7).
Treg play an important role regulating immune responses to self- and allogeneic-antigens (8). Treg cells express FoxP3, a member of the forkhead/winged-helix transcription factors shown to be important in the regulation of both Treg development and function (9). Bashuda et al. showed that adoptively transferred anergic T cells into non-human primate recipients promoted tolerance to renal allografts in 3 of 6 recipients treated with cyclophosphamide and cyclosporine A (10). Dubernard showed that skin biopsies contain graft-infiltrating cells that express FoxP3 and increased IL-10 and TGF-β mRNA levels 6 years post bilateral hand-allograft transplantation (11). These cells entirely inhibited the anti-donor T cell responses in in vitro suppressor cell assays.
In addition to their role in suppressing conventional CD4+ and CD8+ T cells, Treg can suppress natural killer (NK) cell-mediated bone marrow (BM) rejection and support the development of mixed chimerism (12-15). In the present study, we show that significantly more CD4+/FoxP3+ Treg infiltrate the donor skin of long-term CTA recipients compared to skin samples from naïve, syngeneic CTA transplanted or rejector animals. These results suggest that hind-limb allotransplant acceptance is associated with infiltration of FoxP3+ Treg into the transplanted donor skin. Infiltrating Treg may promote tolerance induction and long-term allograft survival by means of local suppression. As a cautionary note, these findings may influence the scoring of rejection if it is based solely upon cells infiltrating CTA allografts. The phenotype of the cellular infiltrates may assist in tailored immunosuppressive management (16).
RESULTS
Establishment of multilineage donor chimerism in nonmyeloablatively conditioned animals
We previously reported that when recipient WF rats were preconditioned with 600 cGy total body irradiation (TBI), transplanted with 100 × 106 T cell-depleted (TCD) ACI donor BMC, and treated with a short course of FK-506 and a single dose of ALS, 100% engrafted with donor chimerism of ~40% at 1 month (17). We therefore examined whether myelotoxic conditioning could further be reduced, thereby promoting mixed chimerism. Recipient WF rats were nonmyeloablatively conditioned as described (Figure 1). 95% of WF recipients conditioned with anti-αβ-TCR mAb plus a 400 cGy TBI and transplanted with BMC and CTA engrafted (Figure 2A). Engraftment was associated with over 30% donor chimerism (Figure 2B). Enhancement of engraftment and donor chimerism was not a result of the CTA, as WF recipients conditioned similarly that received BMC but no CTA displayed similar engraftment patterns and donor chimerism levels. Composite tissue recipients who were conditioned but not reconstituted with donor BMC did not develop chimerism (Figure 2A). Multilineage analysis demonstrated the presence of both lymphoid and myeloid lineages using donor class I major histocompatibility complex markers (Figure 2C).
Figure 1. Nonmyeloablative conditioning approach.
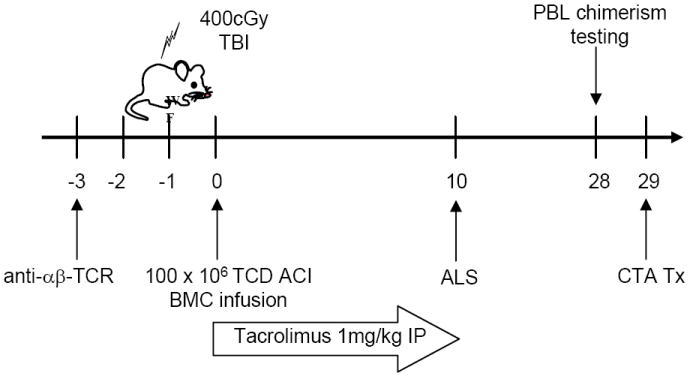
WF rats were preconditioned with anti-αβ-TCR mAb on day -3, 400 cGy TBI on day -1 and transplanted with 100 × 106 T cell depleted ACI donor BMC on day 0. Recipients were treated with 1 mg/kg/day tacrolimus on days 0-10 and received a single dose of ALS on day 10. After PBL testing on day 28, CTA was performed the following day.
Figure 2. Establishment of multilineage donor chimerism.
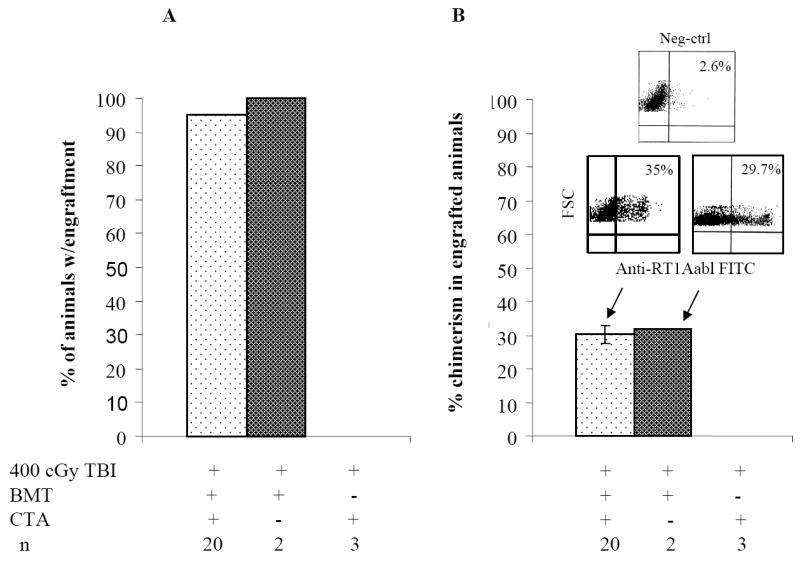
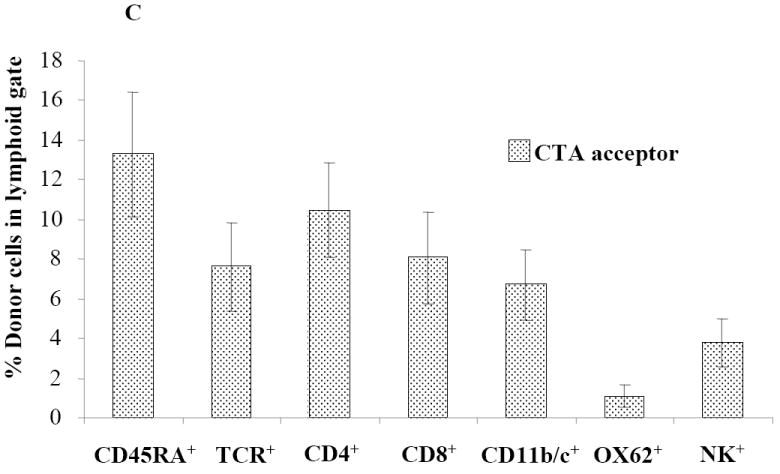
(A-B) WF rats were preconditioned with anti-αβ-TCR mAb on day -3. Recipients were treated with 1 mg/kg/day tacrolimus on days 0-10 and received a single dose of ALS on day 10. On day 0, some recipients were transplanted with 100 × 106 T cell depleted ACI BMC 4 to 6 h after the 400 cGy TBI dose followed by CTA 1 month after BM infusion (n = 20). On day 0, some recipients were transplanted with 100 × 106 T cell depleted ACI BMC 4 to 6 h after 400 cGy TBI dose but did not receive a CTA 1 month after BM infusion (n = 2). Some WF recipients were not infused with 100 × 106 T cell-depleted ACI BMC after 400 cGy TBI dose but did receive a CTA (n = 3). Recipients were monitored for engraftment 30 days after BMC transplantation. (C) Multilineage typing of representative WF recipients treated with anti-αβ-TCR mAb, tacrolimus, ALS, 100 × 106 TCDACI BMC and 400 cGy TBI dose. Multilineage data are representative of 4 independent determinations from PB 1 months after BM transplantation and analyzed using the lymphoid gate. Data represents the means ± SE (vertical bar) of data from 4 separate experiments.
Chimerism and CTA flap survival
Donor chimerism levels in 10 CTA acceptor animals were durable 5 months post-BMT (Figure 3A). Sixty seven percent (10/15) of animals conditioned with anti-αβ-TCR and 400 cGy TBI and who received a CTA displayed long-term acceptance of their CTA (Figure 3B). The 400 cGy treatment group demonstrated the highest CTA flap survival compared to those treated with 300 or 200 cGy TBI (Figure 3B). None of the 5 animals that received a CTA and were conditioned with 100 cGy TBI displayed long-term CTA flap acceptance. As expected, 100% of syngeneic controls accepted their CTA grafts (Figure 3B).
Figure 3. Donor chimerism and flap survival in CTA recipients.
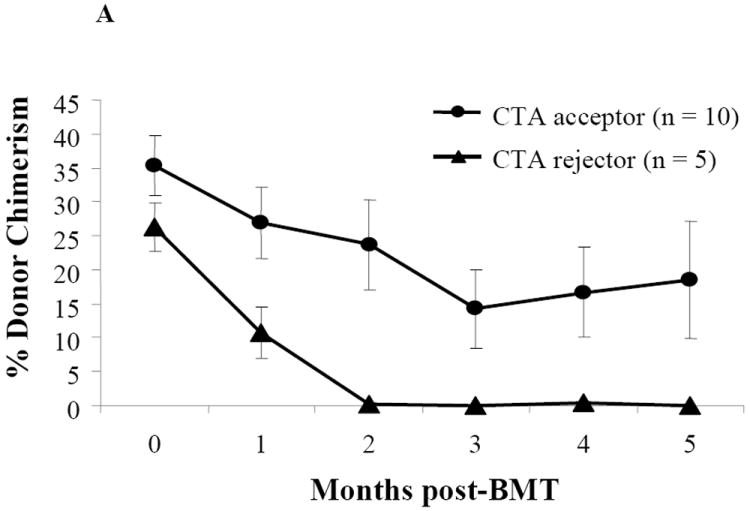
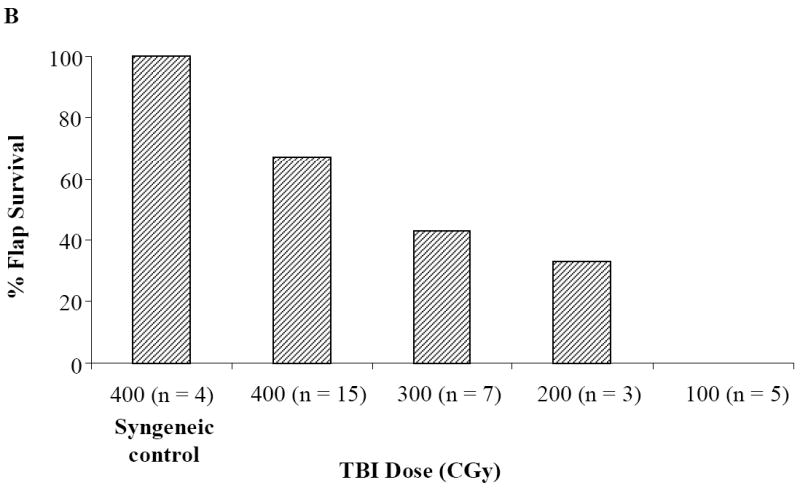
(A) WF recipients were conditioned as previously described. The kinetics of donor-specific chimerism were followed monthly in 10 CTA flap acceptor animals and 5 CTA flap rejector animals following BMT by flow cytometric analysis. The mean chimerism or percentage of donor cells from recipient peripheral blood was evaluated by assessment of donor-specific MHC class I marker (RT1Aabl) staining in the lymphoid gate. (B) Flap survival was assessed over time for clinical signs of rejection. WF recipients were conditioned as previously described (n = 15) and transplanted with an ACI CTA flap. Some WF were conditioned with TBI doses of 300 cGy (n = 7), 200 cGy (n = 3), and 100 cGy (n = 5). Some WF recipients received a 400 cGy TBI dose, but received a syngeneic WF flap (n = 4). Data represents the means ± SE (vertical bar) of combined data.
Sorted CD8-/CD4+/CD25+ FoxP3+ Treg from naïve, acceptor, and rejector animals suppress one-way mixed lymphocyte proliferation assays
Sorted CD8-/CD4+/CD25+ Treg cells from naïve WF animals significantly inhibited cell proliferation (compared to alloresponse) when plated in a 1:1 ratio with naïve WF responder/ACI stimulator cells (P≤0.01) or when plated with WF responder/third-party Fisher 344 (F344) stimulators (P≤0.01) (Figure 4A). Similar levels of suppression were observed when sorted Treg cells from CTA acceptor animals were plated with naïve WF responders/ACI stimulator cells (P < 0.01) or with naïve WF responders/third-party F344 stimulator cells (P < 0.05) in 1:1 ratio (Figure 4B). Significant suppression of cell proliferation was also observed when sorted CD8-/CD4+/CD25+ Treg cells from CTA rejector animals were plated in ratios of 1:1 with naïve WF responder cells/ACI stimulator cells (P < 0.05) but not when plated with third-party Fisher stimulator cells (Figure 4C). Treg cells from rejector animals plated with third-party Fisher stimulator cells failed to demonstrate a dose dependent suppression of cell proliferation (Figure 4C).
Figure 4. Mixed lymphocyte reaction suppression assay.
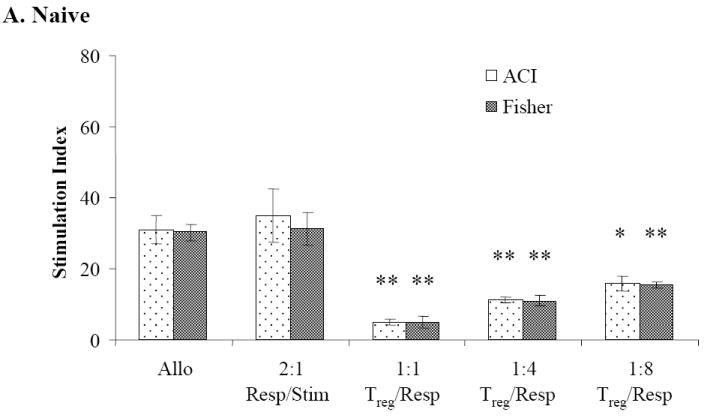
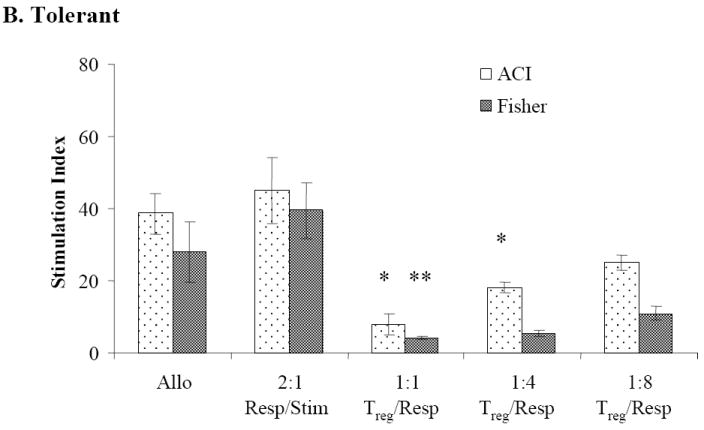
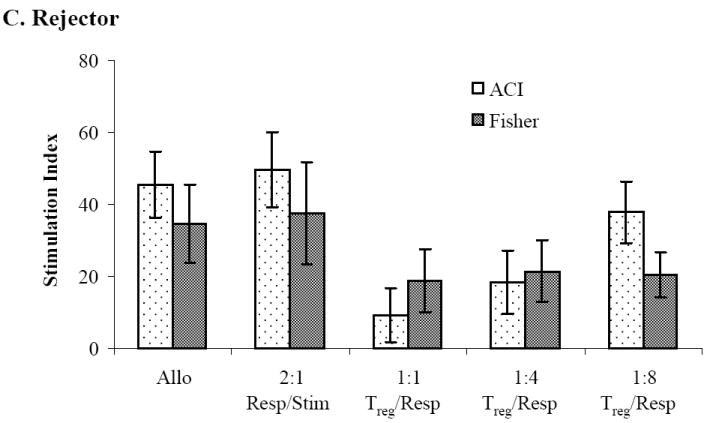
MLR showing WF responder lymph nodes in the presence of sorted CD8-/CD4+/CD25bright Treg upon stimulation with irradiated donor and third-party splenocytes. Effect of sorted CD8-/CD4+/CD25bright Treg cells from (A) CTA naïve, (B) tolerant and (C) rejector animals on suppression of WF responder cells stimulated with ACI donor and Fisher third-party splenocytes, respectively. Data are shown as the mean ± SE (vertical bar) of triplicate wells in a 1:1 Treg/responder ratio from naïve, tolerant and rejector CTA animals from 3-5 independent determinations. The stimulation index (SI) reflecting the ratio of the autoresponse to the alloresponse for all groups is shown on the y-axis. *,**Significant difference from control (P < 0.05; P < 0.01).
Absolute numbers of FoxP3+ Treg in spleen of naïve, acceptor, and rejector animals
We next determined the level of FoxP3 expression in these groups and whether this difference may have been attributed to early graft failure. CD8-/CD4+/CD25+ Treg cells were sorted from naïve, long-term CTA acceptor (6 months post CTA), and rejector animals (1-2 months post CTA), restained for FoxP3 and analyzed by flow cytometry. Over 90% of the 0.4 × 106 sorted CD8-CD4+/CD25+ Treg cells from the spleen of a naïve animal were FoxP3+ (Table 1). Although the percentage of FoxP3+ cells was higher in the naïve untreated animal, the absolute number of CD8-/CD4+/CD25+ FoxP3+ cells was higher in rejector animals compared to either the acceptors or naïve animal.
Table 1.
Absolute numbers of FoxP3+ Treg and CD8-/CD4+/CD25+
| Animal | CD8-/CD4+/CD25+ Total Cell Yield | CD8-/CD4+/CD25+ FoxP3+ cells | Absolute FoxP3+ Treg Cells/ml |
|---|---|---|---|
|
| |||
| Naïve WF | 0.4 × 106 | 90.8% | 0.3 × 106 |
| Acceptor | 1.2 × 106 | 51.2% | 0.9 × 106 |
| Acceptor | 1.9 × 106 | 52.8% | 1.0 × 106 |
| Acceptor | 2.0 × 106 | 58.4% | 1.2 × 106 |
| Rejector | 3.3 × 106 | 58.4% | 1.9 × 106 |
| Rejector | 4.3 × 106 | 47.3% | 2.0 × 106 |
Kinetics of FoxP3+ Treg expression in peripheral blood and detection in CTA acceptor skin
To follow the kinetic of Treg over time post-CTA, PB from acceptor animals was harvested and stained for CD8-/CD4+/CD25+ FoxP3+ Treg. The base line total PB FoxP3+ Treg expression was 39.9% ± 4.2% prior to CTA (Figure 5A). Only 5.9% ± 2.2% of total FoxP3+ Treg were of donor origin prior to CTA but following BMT (Figure 5A). This is not surprising as these animals received donor bone marrow one month prior to CTA. In addition, we observed a 11.9 fold increase in absolute numbers of total FoxP3+ Treg post-CTA in the PB (Figure 5B). Although similar numbers of FoxP3+ Treg were previously discovered in the spleen from acceptor, rejector, and naïve animals after 6 months, there appears to be sinusoidal distribution of Treg in the PB over time. These results suggest that FoxP3+ Treg in the PB might be important for the maintenance of peripheral tolerance as the total number of FoxP3+ Treg increases over time.
Figure 5. Kinetics of percentage and absolute numbers of total and donor-specific FoxP3+ expression in CTA acceptor animals and immunofluorescence staining of FoxP3+ Treg in skin.
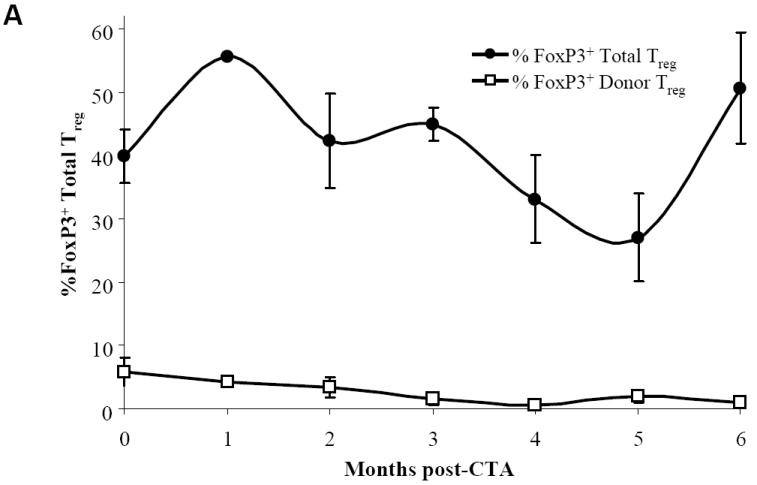
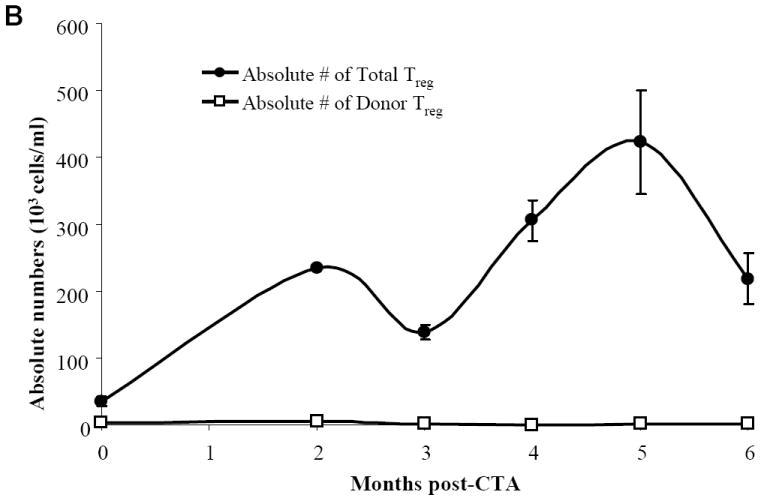
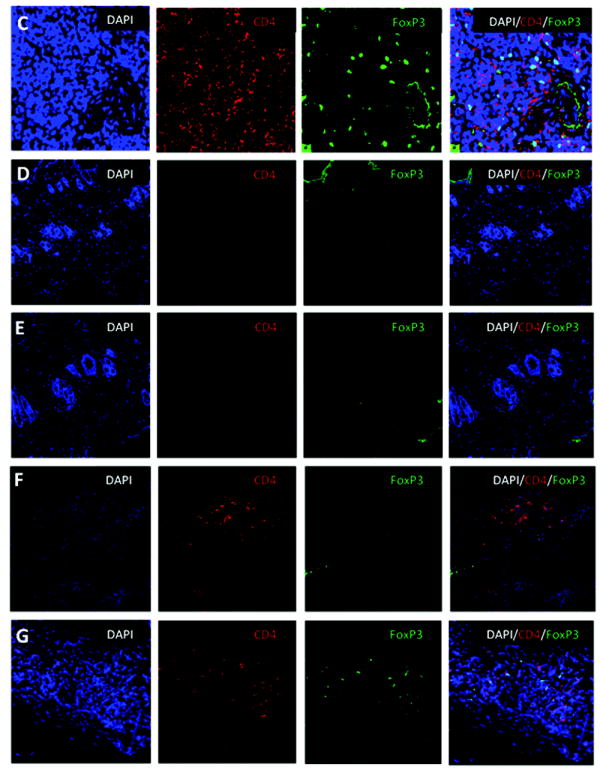
WF recipients were conditioned as previously described. Four CTA acceptor animals were assessed monthly before and after CTA for (A) percentage of total and donor-specific FoxP3 expression and (B) absolute numbers of total and donor-specific FoxP3 expression in the lymphoid gate by flow cytometric analysis. Data represents the means ± SE (vertical bar) of combined data. (C) Naïve ACI spleen sections (+ctrl) were stained for DAPI (nuclear), CD4, and FoxP3 and visualized under a laser confocal microscope. Co-localization of DAPI with FoxP3 was seen as a light blue color in the merged image. (D) Skin sections from naïve ACI non-transplanted animals were isolated and stained for DAPI, CD4, and FoxP3. The merged images show all 3 staining patterns. (E) Skin samples from long-term syngeneic (WF-WF) transplanted animals were isolated and stained for DAPI, CD4, and FoxP3. The merged images show all 3 staining patterns. (F) Skin samples from rejected allogeneic (ACI-WF) transplanted animals were isolated and stained for DAPI, CD4, and FoxP3. Recipient animals did not receive TCD ACI BMC. The merged images show all 3 staining patterns. (G) Skin samples from long-term allogeneic (ACI-WF) CTA acceptor animals were harvested and stained for DAPI, CD4, and FoxP3. The merged images show all 3 staining patterns. An area of staining was further enhanced to visualize the CD4+/FoxP3+ cells. Scale bars are equal to 50 μM.
Since FoxP3+ Treg were detected in the PB of CTA acceptor animals, and the total number of FoxP3+ Treg increased over time post-CTA, experiments were carried out to determine if FoxP3+ Treg were present at the CTA donor graft site. Membranal CD4+ and nuclear FoxP3+ were visible in positive control naïve ACI spleen sections (Figure 5C). No detectable levels of CD4 infiltration or FoxP3 staining were observed in skin samples from non CTA naïve animals (Figure 5D). Non-specific FoxP3 staining was observed on the leading tissue edge as determined by negative CD4 staining (Figure 5D). No detectable levels of FoxP3+ CD4+ infiltrates were observed in skin samples from CTA rejected animals (Figure 5F). FoxP3+ staining was non-specific in rejector tissue as co-localization with DAPI was not observed. In contrast, graft skin from long-term CTA acceptor animals had co-localization of nuclear DAPI and FoxP3 staining as well as membranal CD4 staining in the dermis layer as seen in the merged image (Figure 5G). To confirm that the accumulation of CD4+/FoxP3+ Treg in the skin was not due to the CTA transplantation itself, some WF rats were conditioned in the same manner as previously described, received WF bone marrow and a WF CTA. No detectable levels of membranal CD4+ or nuclear FoxP3+ graft-infiltrating cells were detected (Figure 5E). These results suggest that CTA tolerance may be associated with infiltration of FoxP3+ Treg at the graft site. These Treg may be important in the maintenance of tolerance as they remained at the graft site for several months post-CTA.
DISCUSSION
The fundamental goal in solid organ transplantation and CTA is to achieve long-term donor-specific tolerance and avoid the chronic use of immunosuppressive drugs. Skin is a highly antigenic challenge and remains a critical immunological barrier that must be overcome in CTA. One of the most successful central tolerance strategies to overcome this barrier is through BMT to achieve mixed donor/host chimerism. In 1985, Ildstad et al. (18), demonstrated that mixed chimerism induces tolerance to skin allografts and xenografts. Mixed chimerism has been shown to induce tolerance to cardiac, lung, and composite tissue allografts (19-21) and has allowed for the development of lower-intensity conditioning approaches that reduce the risk associated with conditioning. To our knowledge, we were the first to develop a nonfunctional heterotopic hindlimb flap model to show long-term tolerance to CTA using a nonmyeloablative conditioning approach (22). Previous studies demonstrated that recipient conditioning with anti-lymphocyte serum (ALS) and tacrolimus reduced the minimum TBI dose for engraftment from 950 cGy to 500 cGy (22). Here we show that the addition of anti-αβ-TCR preconditioning on day -3, the minimum TBI dose can further be reduced to 400 cGy. This conditioning approach was associated with 95% engraftment that was multilineage for lymphoid and myeloid cells.
We found that chimerism levels in both CTA acceptor and rejector recipients gradually decreased throughout the follow-up period. These findings are similar to our previous experience (17) and that of others (23). Throughout the 150 day follow-up period, the CTA acceptor animals demonstrated functional donor-specific tolerance, whereas animals with chimerism levels less than 1% rejected their CTA grafts. The reduction of donor-reactive T cells and maintenance of donor chimerism is a prerequisite for inducing tolerance towards MHC-mismatched allografts (24,25). In addition, maintenance of tolerance requires Treg that can act on both any remaining reactive T cells and on new thymic emigrants (26). Under conditions where donor chimerism cannot be sustained, control of immune responsiveness can be achieved through additional mechanisms involving Treg (27). Previous studies with a higher TBI dose demonstrated that long-term CTA acceptance occurred in transiently chimeric animals that subsequently lost PB donor chimerism but retained chimerism in the donor and recipient bone (17). Here, the lack of Treg expression in skin of rejecting tissue may promote early graft rejection. These results suggest a transient immunomodulatory effect of bone marrow cells on rejection.
The induction and maintenance of tolerance to transplanted tissues are believed to be an active process involving multiple pathways. Donor-specific chimerism promotes graft acceptance, may influence homing of FoxP3+ Treg to target organs and may promote the induction of Treg thereby acquiring a Tr1 suppressive phenotype (28,29). The mechanism of Treg induction is unknown, but may involve direct cell-cell contact, contact with antigen presenting cells, or other soluble factors. Recently, Chen et al. demonstrated that TGF-β can promote the conversion of nonregulatory T cells to a suppressive phenotype (30). In addition, culture of naïve human lymphocytes in the presence of TGF-β promotes the generation of CD4+/CD25+ Treg in vitro (31). In thymectomized mice, Karim et al. showed that CD25- precursors can develop into CD4+/CD25+ Treg that functionally suppress skin allograft rejection by inhibiting donor-directed T cell responses (32). These results suggest that naturally occurring Treg in the periphery may not be required to promote allograft survival if inducible Treg are present.
Here, we found that Treg retained their suppressive function when sorted from either acceptor, naïve or graft rejector animals, demonstrating that graft rejection was not directly due to the lack of Treg function in these animals. In addition, we found that although acceptor animals were never exposed to third-party Fisher alloantigen, suppression was dose-dependent and occurred regardless of the source, including third-party antigen. When sorted splenocytes from long-term animals were restained with FoxP3, we found that the absolute numbers of Treg was similar in naïve and acceptor animals and elevated in rejector animals. The lack of high frequencies of Treg in splenocytes from long-term tolerant animals did not suggest that these cells were not involved in tolerance, but perhaps played a role in the induction of tolerance by increasing their numbers and then reaching homeostatic levels once immunologic conditions are normalized. Indeed, when we measured the kinetics of FoxP3 expression we determined that, though similar numbers of FoxP3+ Treg were previously present in the spleen from acceptor, rejector, and naïve animals, there appeared to be sinusoidal increase in total Treg in the PB over time, the majority of which were of recipient and not donor origin. This may represent a subset of newly induced Treg that may home to target tissue in high numbers for extended periods of time like T effector cells, thereby fulfilling the requirements for distribution of Treg to target organs when needed.
Interestingly, we observed higher absolute numbers of FoxP3+ Treg in the Spleen of rejector animals than either naïve or CTA acceptors. Bunnag et al. demonstrated that in 83 human renal transplant biopsies, the level of FoxP3 mRNA expression was higher in rejected tissues than in non-rejected (33). Louis et al. showed that FoxP3 mRNA transcript levels in PBMC from kidney allograft recipients with chronic rejection were higher than levels in PBMC of either tolerant allograft recipients or healthy individuals (34). It remains to be determined whether FoxP3 expression in the blood necessarily reflects the levels of FoxP3 expression in the graft itself. Here, the increased numbers of FoxP3+ Treg in the spleen of rejector animals correlates with the lack of FoxP3+ Treg expression in the skin of rejector animals. A pool of FoxP3+ Treg that home to sites where their action may be required may exist. Recently, FoxP3 expression has been demonstrated in human hand allograft biopsies from 3 to 6 years after transplantation (11). These results suggest that FoxP3+ Treg in the PB might be important for both the induction and maintenance of peripheral tolerance to CTA.
In summary, these results suggest that hind-limb allotransplant acceptance may be associated with infiltration of FoxP3+ Treg into the transplanted donor skin, and these infiltrating Treg may promote tolerance induction and long-term allograft survival. These data may promote further studies to develop a mechanistically rational approach to characterizing and treating graft rejection.
MATERIALS AND METHODS
Animals
6- to 10-week-old Wistar Furth (WF; RT1Au), August Copenhagen Irish (ACI; RT1Aabl), and Fisher (F344) male rats were purchased from Harlan Sprague Dawley (Indianapolis, IN). Animals were maintained in a barrier facility at the Institute for Cellular Therapeutics, University of Louisville.
Mixed chimera preparation
WF rats were conditioned with anti-αβ-TCR monoclonal antibodies (mAb) on day -3 and 400 cGy TBI on day -1. WF rats were transplanted with 100 × 106 T cell depleted ACI donor BMC on day 0 and received FK-506 (Prograf, Astellas, Deerfield, IL) at 1 mg/kg/day from days 0-10, and a single dose of 5 mg rabbit anti-rat lymphocyte serum (ALS) (Research Diagnostics, Inc., Concord, MA) IP on day 10 after bone marrow transplantation (BMT) (Figure 1).
Chimerism and multilineage typing
Whole blood was isolated at 1 month and stained with either anti-RT1Aabl FITC (anti-donor ACI) (Pharmingen, San Diego, CA) and biotinylated RT1Au-Streptavidin PE-Texas red (anti-recipient WF) mAb or anti-RT1abl in combinations with anti-OX62-PE, anti-CD4-APC, anti-CD8a-PerCP, anti-αβ-TCR-PerCP, anti-NKR-P1A-APC, anti-CD45RA-PE, and anti-CD11b/ c-APC (Pharmingen). Immunostaining was carried out by flow cytometry and analyzed using CellQuest software.
Composite tissue allotransplantation
A heterotopic osteomyocutaneous flap procedure was utilized as previously described (17). Animals were monitored for up to 150 days for clinical signs of rejection or graft-versus-host disease.
Cell sorting
CD8-/CD4+/CD25+ Treg were sorted as previously described (35,36). Briefly, splenocytes were isolated from CTA acceptor, rejectors, or naive WF recipients, and labeled with FITC-labeled anti-CD8α, PE-labeled anti-CD25 and APC-labeled anti-CD4 mAb (BD Biosciences, San Jose, CA). Cells were sorted using a sterile cell sorter (FACS Vantage SE and BD FACS ARIA; Becton Dickinson, Mountainview, CA). The CD8-/CD4+/CD25+bright cell population comprised of the highest percentage of FoxP3+ cells was collected from the live lymphoid gate and determined to be >97% pure.
In vitro suppression assay
The in vitro suppression assay was carried out as previously described (17). Briefly, irradiated (2,000 cGy) stimulator splenocytes from ACI, WF, or Fisher animals were plated with naïve WF lymphoid responder cells and sorted CD8-/CD4+/CD25+ cells in varying ratios for 4 days. The cells were pulsed with 10 μCi [3H] thymidine (PerkinElmer, Boston, MA), harvested, and counted. [3H] thymidine uptake by stimulator/CD8-/CD4+/CD25+ cells was subtracted from stimulator/responder/CD8-/CD4+/CD25+ cells to account for CD8-/CD4+/CD25+ blasting and to obtain an accurate responder cell response. The data are expressed as a stimulation index, the ratio of counts per million generated by the host cells in response to a given stimulator relative to the auto-response of the host.
FoxP3 cytometric analysis
Splenocytes were either harvested or collected after cell sorting and surface stained with appropriately diluted anti-CD8α, anti-CD4 and anti-CD25 mAbs (BD Biosciences). Labeled splenocytes were fixed, permeabilized, blocked and labeled with anti-rat FoxP3 mAb and then analyzed on the LSR flow cytometer (Becton Dickinson).
Immunohistochemistry
OCT (Tissue-Tek, Hatfield, PA) embedded tissues were fixed, permeabilized, blocked and then simultaneously labeled with rat anti-mouse/rat FoxP3-FITC and mouse anti-rat CD4-PE mAb (BD Biosciences). Sections were then incubated with 0.5% 4’, 6-diamidino-2-phenylindole dilactate (DAPI; Invitrogen, Carlsbad, CA), mounted, and imaged using a Leica TCS SP5 laser confocal microscope and the Leica Application Suite Advanced Fluorescence software. FITC-labeled rat IgG2a (BD Bioscience) was used in isotype controls.
Statistical analysis
All values are expressed as means ± SEM. Statistical comparison of data was performed using two-tailed unpaired student t tests. A value of P < 0.05 was considered to be statistically significant.
Acknowledgments
The authors thank Dr. Haval Shirwan for review of the manuscript and helpful comments; Carolyn DeLautre for manuscript preparation; and the staff of the University of Louisville animal facility for outstanding animal care.
Abbreviations
- APC
antigen presenting cell
- ALS
anti-lymphocyte serum
- ACI
August Copenhagen Irish
- BM
bone marrow
- CTA
composite tissue allotransplant
- F344
Fisher 344
- FoxP3
forkhead box P3
- GVHD
graft-versus-host disease
- MLR
mixed leukocyte reactions
- mAb
monoclonal antibodies
- NK
natural killer cell-mediated
- PB
peripheral blood
- Treg
regulatory T cells
- TCD
T cell-depleted
- TBI
total body irradiation
- WF
Wistar Furth
Footnotes
This work was supported in part by NIH T32HL076138; JDRF 1-2006-146; and The Department of the Navy, Office of Naval Research. This publication was also made possible by Award No. W81XWH-07-1-0185 and W81XWH-09-2-0124 from the U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick, MD,21702-5014 (Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the Office of Army Research); the Commonwealth of Kentucky Research Challenge Trust Fund; the W. M. Keck Foundation; and The Jewish Hospital Foundation. Research was conducted in compliance with the Animal Welfare Act Regulations and other Federal statues relating to animals and experiments involving animals and adheres to the principles set forth in the Guide for Care and Use of Laboratory Animals, National Research Council, 1996.
All work should be attributed to: Institute for Cellular Therapeutics, University of Louisville, 570 S. Preston Street, Suite 404, Louisville, KY 40202-1760
Reference List
- 1.Ravindra KV, Wu S-L, Bozulic L, Xu H, Breidenbach WC, Ildstad ST. Composite Tissue Transplantation: A Rapidly Advancing Field. Transplant Proc. 2008;40:1237–1248. doi: 10.1016/j.transproceed.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ildstad ST, Wren SM, Bluestone JA, Barbieri SA, Sachs DH. Characterization of mixed allogeneic chimeras: Immunocompetence, in vitro reactivity and genetic specificity of tolerance. J Exp Med. 1985;162:231–244. doi: 10.1084/jem.162.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colson YL, Zadach K, Nalesnik M, Ildstad ST. Mixed allogeneic chimerism in the rat. Donor-specific transplantation tolerance without chronic rejection for primarily vascularized cardiac allografts. Transplantation. 1995;60:971–980. [PubMed] [Google Scholar]
- 4.Luo B, Nanji SA, Schur CD, Pawlick RL, Anderson CC, Shapiro AM. Robust tolerance to fully allogeneic islet transplants achieved by chimerism with minimal conditioning. Transplantation. 2005;80:370–377. doi: 10.1097/01.tp.0000167724.38038.ae. [DOI] [PubMed] [Google Scholar]
- 5.Wekerle T, Sayegh MH, Ito H, et al. Anti-CD154 or CTLA4Ig obviates the need for thymic irradiation in a non-myeloablative conditioning regimen for the induction of mixed hematopoietic chimerism and tolerance. Transplantation. 1999;68:1348–1355. doi: 10.1097/00007890-199911150-00022. [DOI] [PubMed] [Google Scholar]
- 6.Tomita Y, Sachs DH, Khan A, Sykes M. Additional monoclonal antibody (mAB) injections can replace thymic irradiation to allow induction of mixed chimerism and tolerance in mice receiving bone marrow transplantation after conditioning with anti-T cell mABs and 3-GY whole body irradiation. Transplantation. 1996;61:469–477. doi: 10.1097/00007890-199602150-00027. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 10.Bashuda H, Kimikawa M, Seino K, et al. Renal allograft rejection is prevented by adoptive transfer of anergic T cells in nonhuman primates. J Clin Invest. 2005;115:1896–1902. doi: 10.1172/JCI23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eljaafari A, Badet L, Kanitakis J, et al. Isolation of regulatory T cells in the skin of a human hand-allograft, up to six years posttransplantation. Transplantation. 2006;82:1764–1768. doi: 10.1097/01.tp.0000250937.46187.ca. [DOI] [PubMed] [Google Scholar]
- 12.Barao I, Hanash AM, Hallett W, et al. Suppression of natural killer cell-mediated bone marrow cell rejection by CD4+CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:5460–5465. doi: 10.1073/pnas.0509249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanash AM, Levy RB. Donor CD4+CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation. Blood. 2005;105:1828–1836. doi: 10.1182/blood-2004-08-3213. [DOI] [PubMed] [Google Scholar]
- 14.Weng L, Dyson J, Dazzi F. Low-intensity transplant regimens facilitate recruitment of donor-specific regulatory T cells that promote hematopoietic engraftment. Proc Natl Acad Sci U S A. 2007;104:8415–8420. doi: 10.1073/pnas.0701031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehr T, Takeuchi Y, Kurtz J, Wekerle T, Sykes M. Early regulation of CD8 T cell alloreactivity by CD4+ Eur J Immunol. 2005;35:2679–2690. doi: 10.1002/eji.200526190. [DOI] [PubMed] [Google Scholar]
- 16.Cendales LC, Kirk AD, Moresi JM, Ruiz P, Kleiner DE. Composite tissue allotransplantation: classification of clinical acute skin rejection. Transplantation. 2006;81:418–422. doi: 10.1097/01.tp.0000185304.49987.d8. [DOI] [PubMed] [Google Scholar]
- 17.Adamson LA, Huang W, Breidenbach WC, et al. A modified model of hindlimb osteomyocutaneous flap for the study of tolerance to composite tissue allotransplantation. Microsurgery. 2007;27:630–636. doi: 10.1002/micr.20414. [DOI] [PubMed] [Google Scholar]
- 18.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 19.Colson YL, Xu H, Huang Y, Ildstad ST. Mixed xenogeneic chimerism induces donor-specific humoral and cellular immune tolerance for cardiac xenografts. J Immunol. 2004;173:5827–5834. doi: 10.4049/jimmunol.173.9.5827. [DOI] [PubMed] [Google Scholar]
- 20.Foster RD, Ascher NL, McCalmont TH, Neipp M, Anthony JP, Mathes SJ. Mixed allogeneic chimerism as a reliable model for composite tissue allograft tolerance induction across major and minor histocompatibility barriers. Transplantation. 2001;72:791–797. doi: 10.1097/00007890-200109150-00009. [DOI] [PubMed] [Google Scholar]
- 21.Pham SM, Mitruka SN, Youm W, et al. Mixed hematopoietic chimerism induces donor-specific tolerance for lung allografts in rodents. Am J Respir Crit Care Med. 1999;159:199–205. doi: 10.1164/ajrccm.159.1.9712041. [DOI] [PubMed] [Google Scholar]
- 22.Foster RD, Fan L, Neipp M, et al. Donor-specific tolerance induction in composite tissue allografts [corrected; erratum to be published] Am J Surg. 1998;176:418–421. doi: 10.1016/s0002-9610(98)00248-7. [DOI] [PubMed] [Google Scholar]
- 23.Nasir S, Bozkurt M, Krokowicz L, Klimczak A, Siemionow M. Correlation of chimerism with graft size and revascularization in vascularized and nonvascularized skin allografts. Ann Plast Surg. 2009;62:430–438. doi: 10.1097/SAP.0b013e3181877ad7. [DOI] [PubMed] [Google Scholar]
- 24.Wells AD, Li XC, Li Y, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 25.Lechler RI, Garden OA, Turka LA. The complementary roles of deletion and regulation in transplantation tolerance. Nat Rev Immunol. 2003;3:147–158. doi: 10.1038/nri1002. [DOI] [PubMed] [Google Scholar]
- 26.Walsh PT, Taylor DK, Turka LA. Tregs and transplantation tolerance. J Clin Invest. 2004;114:1398–1403. doi: 10.1172/JCI23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurtz J, Wekerle T, Sykes M. Tolerance in mixed chimerism - a role for regulatory cells? Trends Immunol. 2004;25:518–523. doi: 10.1016/j.it.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med. 2002;196:255–260. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected] J Exp Med. 2002;196:247–253. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282–7289. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 32.Josien R, Douillard P, Guillot C, et al. A critical role for transforming growth factor-beta in donor transfusion-induced allograft tolerance. J Clin Invest. 1998;102:1920–1926. doi: 10.1172/JCI4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bunnag S, Allanach K, Jhangri GS, et al. FOXP3 expression in human kidney transplant biopsies is associated with rejection and time post transplant but not with favorable outcomes. Am J Transplant. 2008;8:1423–1433. doi: 10.1111/j.1600-6143.2008.02268.x. [DOI] [PubMed] [Google Scholar]
- 34.Louis S, Braudeau C, Giral M, et al. Contrasting CD25hiCD4+T cells/FOXP3 patterns in chronic rejection and operational drug-free tolerance. Transplantation. 2006;81:398–407. doi: 10.1097/01.tp.0000203166.44968.86. [DOI] [PubMed] [Google Scholar]
- 35.Kaufman CL, Colson YL, Wren SM, Watkins SL, Simmons RL, Ildstad ST. Phenotypic characterization of a novel bone-marrow derived cell that facilitates engraftment of allogeneic bone marrow stem cells. Blood. 1994;84:2436–2446. [PubMed] [Google Scholar]
- 36.Huang Y, Rezzoug F, Chilton PM, Grimes HL, Cramer DE, Ildstad ST. Matching at the MHC Class I K locus is essential for long-term engraftment of purified hematopoietic stem cells: a role for host NK cells in regulating HSC engraftment. Blood. 2004;104:873–880. doi: 10.1182/blood-2003-11-3910. [DOI] [PubMed] [Google Scholar]


