Abstract
Neocortical neuronal migration anomalies such as microgyria and heterotopia have been associated with developmental language learning impairments in humans, and rapid auditory processing deficits in rodent models. Similar processing impairments have been suggested to play a causal role in human language impairment. Recent data from our group has shown spatial working memory deficits associated with neocortical microgyria in rats. Similar deficits have also been identified in humans with language learning impairments. To further explore the extent of learning deficits associated with cortical neuronal migration anomalies, we evaluated the effects of neocortical microgyria and test order experience using spatial (Morris water maze) and non-spatial water maze learning paradigms. Two independent groups were employed (G1 or G2) incorporating both microgyria and sham conditions. G1 received spatial testing for five days followed by non-spatial testing, while the reverse order was followed for G2. Initial analysis, including both test groups and both maze conditions, revealed a main effect of Treatment, with microgyric rats performing significantly worse than shams. Overall analysis also revealed a task by order interaction, indicating that each group performed better on the second task as compared to the first, regardless of which task was presented first. Independent analyses of each task revealed a significant effect of Treatment (microgyria worse than sham) only for the spatial water maze condition. Results indicate that prior maze experience (regardless of task type) leads to better subsequent performance. Results suggest that behavioral abnormalities associated with microgyria extend beyond auditory and working memory deficits seen in previous studies, to include spatial but not non-spatial learning impairments and that non-specific test experience may improve behavioral performance.
Keywords: Cortical developmental malformations, learning impairment, spatial learning, non-spatial learning, test experience
1. Introduction
Malformations of neocortical development associated with neuronal migration have been linked to a wide spectrum of neurodevelopmental disorders including cognitive and language deficits [1, 2, 3, 4]. For example, Galaburda and colleagues [4] identified clusters of ectopic molecular layer neurons and abnormality folded microgyric cortices in the brains of dyslexics examined post mortem. More recently, imaging studies have associated microgyria and periventricular nodular heterotopia with specific learning impairments in humans [2, 3, 5, 6]. In concert with these associations, rodent models have revealed a link between a diverse spectrum of injury, genetic and teratogenic mediated neocortical malformations and specific learning and processing impairments [7, 8, 9, 10, 11, 12, 13], which parallel clinical phenotypes.
As with human clinical cases, deficits in learning and auditory processing seen in rodent models of disrupted neuronal migration are often domain specific. For example, rats with cortical malformations resulting from RNA interference of neuronal migration/candidate dyslexia susceptibility gene Kiaa0319 show impairments in complex auditory processing but not working memory or spatial learning [13]. However, deficits in spatial learning are observed in subgroups of animals with cortical and hippocampal malformations induced via genetic disruption or teratogen exposure during early neuronal migration [13, 14]. In addition, rats with neocortical microgyria and cortical heterotopia show auditory processing impairments that are dependent on the rate of stimulus presentation (rapid) [7, 8, 9, 10, 11, 15, 16]. More recently, Fitch and colleagues showed persistent spatial working memory deficits in rats with bilateral cortical microgyria [8]. Importantly, both working memory deficits and impairments in rapid auditory processing are seen in some sub-groups of language learning impaired humans [6, 17, 18, 19, 20, 21, 22]. Although several studies in humans and rodent models of neuronal migration disorders suggest domain specific deficits in learning and processing, the full range of behavioral impairments stemming from early neocortical disruption is still unknown.
In humans, developmental language learning impairments represent a heterogeneous amalgam of disorders largely defined by discrepancy between typical function in non-linguistic domains and poor performance in language learning and processing [23]. While rate specific processing deficits have been proposed to account for some cases of language learning impairments, evidence also suggests impairments in short-term and/or working memory [24, 19], which parallel deficits observed in rodent models of neocortical neuronal migration anomalies [8, 12]. Recently Gabel and associates [25] showed that mice with a Dcdc2 gene mutation, known to regulate neuronal migration, had impairments in visual spatial processing (novel object recognition) despite the absence of gross histological malformations. These findings highlight the heterogeneous nature of developmental learning impairments and illustrate the challenges in identifying specific neurobehavioral substrates of developmental learning impairments in humans. Although a diverse number of behavioral deficits are associated with disorders of neocortical neuronal migration in animal models and humans, clear patterns of abnormal function have begun to emerge, with rapid auditory processing and spatial-working memory deficits commonly identified [7, 8, 10, 11, 12, 13, 25].
Selective deficits in working memory and complex/rapid auditory processing have further been consistently observed in a surprisingly diverse range of models of disrupted neocortical neuronal migration (microgyria, ectopia and heterotopia). However, the extent to which other behavioral domains are specifically affected by various forms of cortical disruption has not been fully explored. In order to further evaluate the extent of behavioral impairments associated with neocortical neuronal migration anomalies we sought to assess spatial learning (Morris water maze) and non-spatial learning in microgyric (MG) and sham rats, previously tested for auditory processing [11]. Given evidence that prior spatial water maze exposure can influence performance of mice in subsequent novel water maze paradigms [26], we also sought to assess possible interactions between the order of task presentation (spatial first followed by non-spatial testing or the reverse order) and subsequent learning performance differences between treated and sham subjects. Based on previous studies showing spatial working memory deficits in inbreed BXSB mice with neocortical molecular layer ectopia, [27, 28], we predicted that microgyric rats would show similar selective deficits. In fact, results showed that MG rats were significantly impaired on the spatial task and unimpaired at the non-spatial task regardless of task order presentation. These findings suggest that working memory and auditory processing deficits observed in microgyric rats extend to generalized spatial reference learning but not non-spatial learning.
2. Material and methods
2.1 Subjects
Subjects were 43 male Wistar rats born to time-mated dams (Charles River Laboratory, Wilmington, MA) at the University of Connecticut. Male subjects were assessed, given prior evidence of auditory temporal processing deficits in male but not female rodents with neuronal migration anomalies [29, 30] - findings that parallel higher diagnostic rates of neurodevelopmental disorders (including dyslexia, epilepsy, autism and mental retardation) in human males as compared to females [31, 32]. Subsets of pups received either five-second cortical plate focal freezing lesions (producing microgyria; MG) or sham procedure (see below) on postnatal day 1 (P1) and were culled into litters of 10 (8 males and 2 females, for details see [9, 11, 33]). Male subjects (n = 43) were right or left ear marked and housed into pairs at P21 using a 12:12 light/dark cycle with food and water available ad libitum. Prior to behavioral testing, subjects were assigned to two different groups (G1 or G2), each including balanced numbers of sham and MG subjects. We sought to control for litter effects by assigning pups from each litter to each study condition. Thus, at culling (Postnatal day (P) 1), male subjects within each litter were randomly selected to receive either bilateral freezing lesion, or sham surgery. Group one (G1; sham n = 10, MG n = 11) received spatial (Morris Water Maze, MWM) followed by non-spatial testing (NSWM). Group two (G2; sham n = 12, MG n = 10) received the non-spatial testing first followed by MWM testing. This was done to counterbalance the two tasks and assess for non-specific learning effects related to test experience on two different learning tasks. Prior to maze learning (beginning postnatal day 123) all subjects received testing on a modified acoustic startle paradigm to assess complex auditory processing, the results of which were previously published [11]. All procedures were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, including adequate measures to minimize pain and discomfort. The Institutional Animal Care and Use committee (IACUC) at the University of Connecticut approved all procedures.
2.2 Induction of postnatal day one freezing lesion (microgyria)
On the day of surgery (P1), litters were culled to 10 pups (eight male, two female), with male pups randomly assigned to receive double-pair freezing lesion or sham surgery. Females were retained to equalize litter size and avoid all-male litters. Before surgery, subjects were cryogenically anesthetized using crushed ice. Surgeries involved a 1-mm incision, followed by the placement of a 2-mm stainless steel probe cooled to −70 C in dry ice on the skullcap [34, 35]. Focal lesions were induced as a double-pair (two to each hemisphere) as previously described [11]. This procedure has been shown to lead to the formation of cortical microgyria similar to those identified in the brains of human dyslexics [4]. Sham subjects received similar treatment with a room temperature probe. After surgery, the pups were individually marked with footpad ink injections, warmed under a lamp, and placed back with the mother.
2.3 Histology
At the end of maze testing, subjects were weighed, anesthetized with ketamine/xylazine (100/15 mg/kg), and transcardially perfused with saline followed by 10% phosphate buffered formalin. The brains were removed, lesions were visually confirmed (appearing as indentations (microgyria) on the surface of the neocortex), and the location verified. All the subjects identified as microgyric showed evidence of focal cortical malformations, and no such malformations were seen in sham brains (see fig. 1 for prototypical histological samples).
Figure 1.
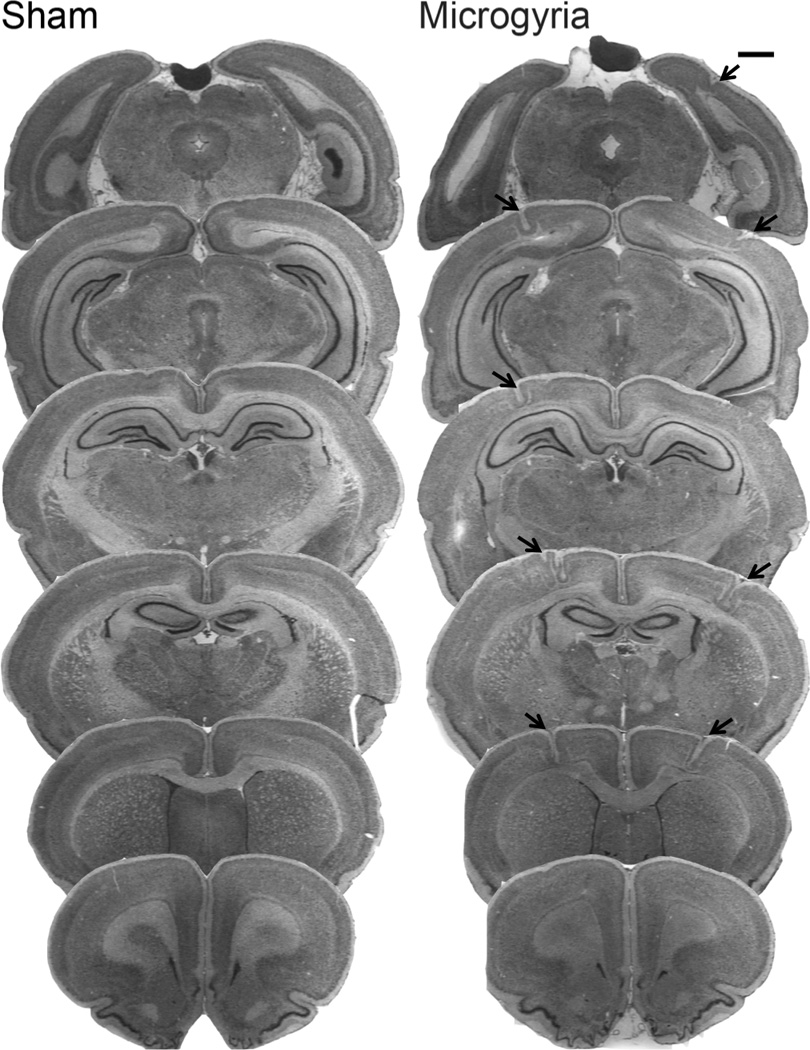
Photomicrographs (1.3×) showing serial coronal sections of a protypical sham and microgyric brain. Arrows indicate the site of bilateral microgyria. Scale bar is 1mm.
2.4 Behavioral testing: water escape and Morris water maze
Subjects were tested on a Morris water maze (spatial learning), and a non-spatial water maze task (non-spatial learning). The order in which each group was tested on the two tasks was counter-balanced. Specifically, each test group (G1 or G2) was assigned to receive either spatial or non-spatial water maze testing first (for one week), before testing on the alternate maze following a one-week break. Thus, G1 received spatial testing first followed by non-spatial, while the reverse order was followed for G2. As a motor, visual and motivation control, all subjects were first tested on a water escape task. The water escape task involved the use of a visible platform (10.2 cm diameter) placed at one end of an oval tub (103 cm × 54.5 cm) filled with water (20 cm) at room temperature (22°C). Subjects were released in the opposite end of the tub from the platform, and the time taken to swim to the platform was recorded.
After completing water escape (on the following day), G1 began Morris water maze (MWM) testing, which was administered over a period of five days. Testing was conducted in a round 122 cm diameter tub filled with water (temp 22° Celsius) with a 20.3 cm diameter submerged (invisible) platform, consistently placed in the southeast (SE) quadrant, 2 cm below the water surface (see fig. 3a for model). Fixed, extra-maze cues were abundant (computer, sink, door, table), while precaution was taken to eliminate intra-maze cues (tub and platform were painted black so the submerged platform blended into a consistent background [36]). On each of five testing days, subjects underwent four trials, with each trial starting from a different randomly selected compass point (N, S, E, W). On day one, trial one, each subject was placed on the platform for 10 sec, removed from the platform and then released from one of the starting locations. Each trial had a maximum time of 45 sec. Subjects unable to reach the platform within this time window were guided to the target and allowed to remain for 5 sec. The latency to reach the platform for each trial was recorded.
Figure 3.
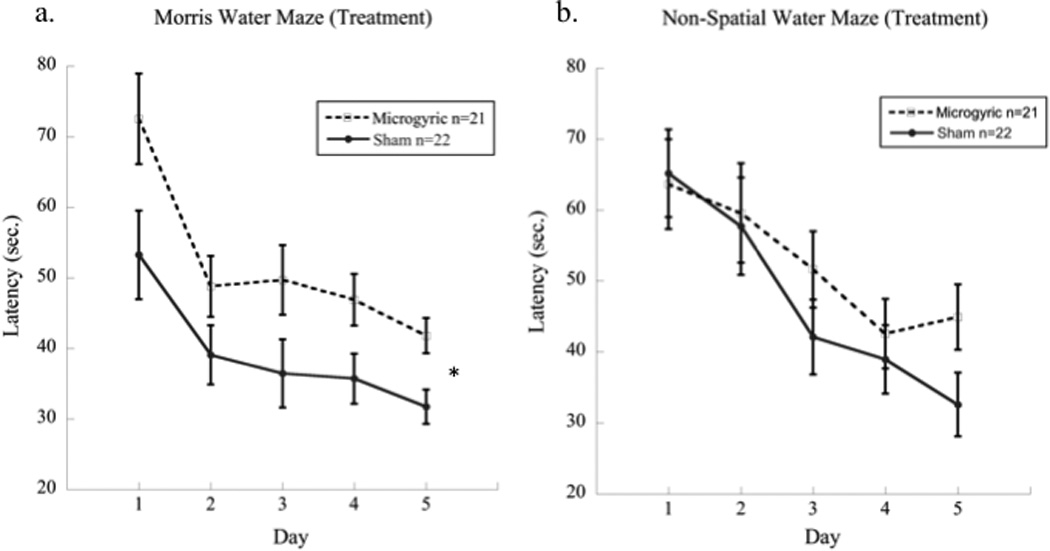
Graphs showing (a) a significant effect of Treatment (microgyric worse then sham) on the spatial water maze, regardless of task presentation order, in contrast to (b) the nonspatial task, which did not elicit a deficit from microgyric animals regardless of task presentation order.
2.5 Non-spatial water maze
The non-spatial water maze has been used to test non-spatial reference learning, i.e., the ability to consistently locate a hidden platform, using intra-maze visual cues that are independent of extra-maze space. Testing took place in the same 122 cm diameter tub as the spatial MWM, with the submerged 10.2 cm diameter platform located 2 cm below the water's surface, but also included an insert characterized by 4 black/white complex visual stimuli (which acted as intra-maze cues for each quadrant of the outer maze wall). Further, non-spatial testing was conducted in an alternate room from the spatial testing to eliminate interference from familiar spatial cues. The intra-maze patterns consisted of black/white vertical stripes; black/white horizontal stripes; white panel background and a grey panel background. These cues were presented on a black background (for more details, see [26, 27, 37]). The platform location was always paired with the vertical lines for each subject, such that learning required an association between the target intra-maze stimulus (i.e., vertical lines) and the platform, irrespective of extra-maze space. While the platform remained in a constant within-maze position relative to the 4 quadrants, the maze itself was randomly rotated across trials with respect to the room. Subjects were released from the same compass point (N) on all trials, and latency to reach the platform was recorded for each trial. All other testing parameters were similar to the spatial version of the MWM (number of trials, testing days, and length of time subject was left on platform).
3. Results
3.1. Histology results
Analysis of post mortem brains revealed consistent location and relative size of microgyric malformations for all of the subjects that received P1 freezing lesion treatment. Malformations were primarily observed in sensorimotor cortex (SM-1), with some extension into frontal, temporal, and occipital cortices. None of the sham subjects showed any cortical malformations (see fig. 1).
3.2 Water escape, spatial maze learning & non-spatial water maze
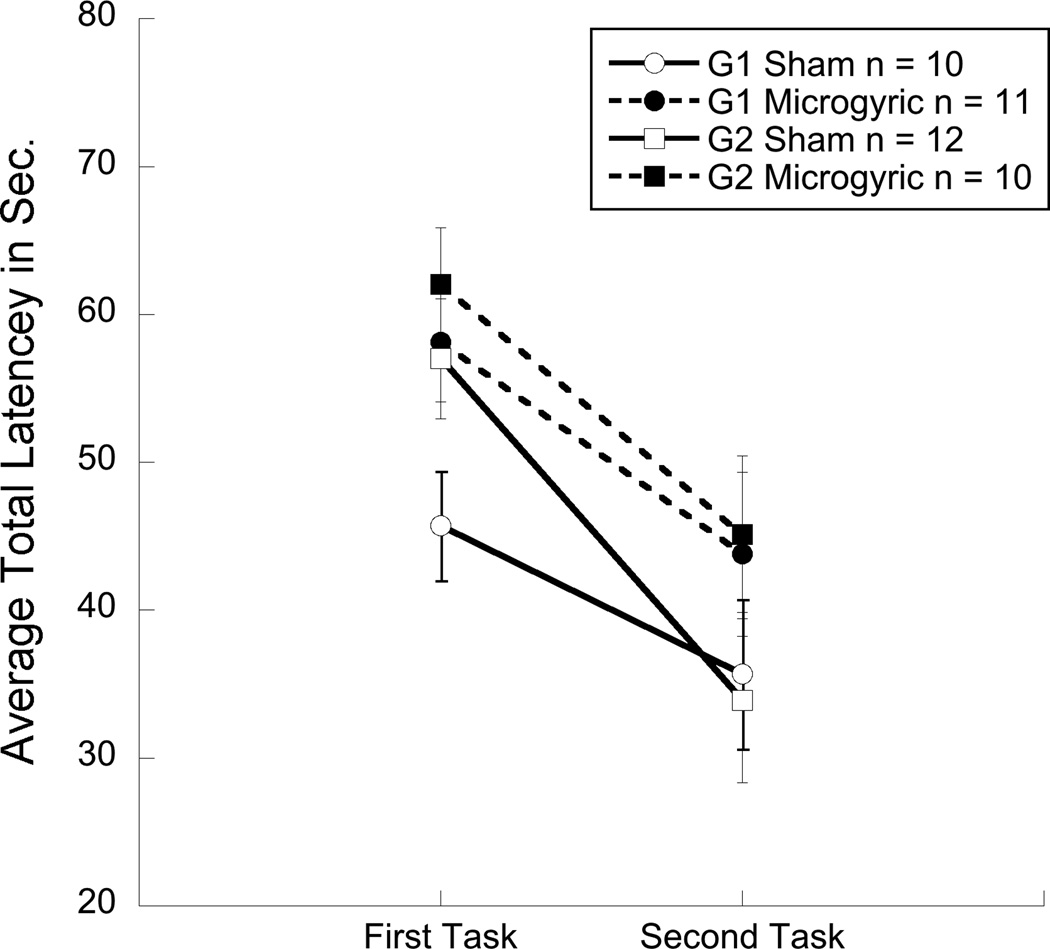
No group differences were observed for the water escape task, indicating comparable performance in swimming skill and finding a visible platform (ns). An overall 2 (Order, G1 & G2) × 2 (Treatment, MG & sham) × 2 (Task, MWM & NSWM) × 5 (Day) repeated measures ANOVA revealed a main effect of Treatment [F(1,39) = 4.8, p < 0.05], with microgyric subjects taking longer to complete each task as compared to shams. In addition, this analysis revealed a Task×Order interaction [F(1,39) = 57.6, p < 0.001], indicating that each group performed better on the second task regardless of the order of task presentation or the presence of microgyria (see fig. 2). However, we did not observe an overall effect of Task (ns), suggesting that both tasks were of comparable difficulty. Further, individual 2 (Group) × 2 (Treatment) × 5 (Day) repeated measures analyses were conducted for each task. For the spatial task (MWM), results revealed a significant effect of Treatment [F (1,39) = 9.2, p < 0.01] (see figure 3a), and a significant effect of presentation Order [F (1,39) = 10.1, p < 0.01], reflecting a Task×Order effect (second task benefits from prior experience). Results for the spatial maze also revealed an effect of Day [F (1, 39) = 43.8, p < 0.001] indicating that the time to reach the platform decreased from day one to day five. For the non-spatial task (NSWM), results also showed a significant effect of Order [F (1, 39) = 13.6, p < 0.01], again indicating improved performance on the non-spatial task when presented on the second week. A day effect was also observed in the non-spatial task [F (1, 39) = 46.4, p <0.001]. However, unlike the spatial maze, analysis on the NSWM showed no significant effect of Treatment (ns; see figure 3b), suggesting that the overall treatment effect observed when both tasks were analyzed together was primarily a result of poor MG performance, as compared to shams, on the spatial task.
Figure 2.
Graph showing average total latency collapsed across five days of testing, highlighting the significant effects of test experience and an over all effect of treatment. Group 1 (G1) received spatial testing first followed by non-spatial testing and Group 2 (G2) received the opposite presentation sequence.
4. Discussion
The present study assessed the effects of early neocortical injury (MG) and test order experience in two different learning paradigms using spatial (MWM) and non-spatial water mazes (NSWM). The overall analysis revealed a Task by Order interaction, indicating that each group performed better on the second task as compared to the first, regardless of which task was presented first. Separate analyses of each task (including both treatment groups) confirmed the significance of the Task by Order interaction for both sham and microgyric rats. Specifically, G1 (which received MWM testing first) performed significantly better than G2 on the non-spatial task (NSWM), whereas the reverse was true when comparing both groups on the spatial task (MWM). These results indicate that prior maze experience (regardless of task type) leads to better subsequent performance. Similar experience dependent improvements have been observed on tasks assessing auditory discrimination in rodent models [38, 11]. In addition, for both tasks, significant day effects indicate improved performance and learning in both mycrogyric and sham conditions across the five days of testing. Further, spatial testing elicited a significant deficit in MG subjects as compared to the non-spatial task. These results are the first to show spatial reference learning impairments in rats with neocortical microgyria using the Morris Water Maze. These findings complement previous reports showing widespread alterations neural system organization along with deficits in rapid auditory processing and working memory in rats following disruption to the developing neocortex [8, 39, 40].
It is important to note that both tasks used in the present study share a number of transferable variables including the size and shape of the tub, the presence of an escape platform, reward strength and relevance and swimming skill. These similarities where noted by Stavenzer and colleagues [26] who assessed C57BL/6J mice for spatial and non-spatial learning using a counter balanced design similar to that of the current study. However, unlike the present study, Stavenzer and associates [26] found that subjects receiving spatial testing first performed better overall as compared to the group that received non-spatial testing first. They suggested that initial spatial testing facilitated later performance on the non-spatial task in C57BL/6J mice, whereas the non-spatial first group was unable to transfer knowledge to the successive spatial task. In contrast, our results suggest that both spatial and non-spatial water maze-testing (prior to testing in the alternate paradigm) lead to facilitation of information transfer for use in subsequent maze learning, at least in Wistar rats. Interestingly, these effects were seen in both MG and sham subjects. Cross species differences may play a role in the discrepancy between our findings in Wistar rats and those of Stavenzer and associates [26] in C57BL/6J mice.
In addition to the observed experience dependent improvements for each testing group, our findings suggest that the presence of MG has a greater influence on spatial as compared to non-spatial learning. The apparent selectivity of observed impairments in spatial learning, as compared to non-spatial learning in microgyric rats, may parallel subtle memory impairments observed in human learning-disabled populations [41, 42, 43, 44, 45]. Although less frequently reported then working memory or auditory processing deficits, spatial learning deficits have been reported in children with developmental language and learning impairments [45]. However, other studies have reported visuospatial strengths in some dyslexic individuals highlighting the heterogeneous nature of this type of developmental disorder [46]. Of importance, a number of studies have shown widespread changes in cortical and sub-cortical reorganization as a result of early neocortical disruption leading to heterogeneous processing and learning deficits [39, 40]. Although speculative, it is possible that observed reorganization following the formation for microgyria could have altered hippocampal function indirectly. For example, in a model of cortical heterotopia resulting from disrupted neocortical neuronal migration, alterations in hippocampal-cortical circuits were observed, contributing to changes in hippocampal cellular response properties which could be a source of behavioral disruption in the present model [47]. Alternatively, it is possible that the current observed spatial learning deficits in microgyric rats were the direct result of cortical freezing lesions on the dorsal hippocampus, which lies under the freezing probe application site and has been suggested to be partially disrupted by this procedure [8]. This later assertion is consistent with prior evidence that genetically induced disruptions of neocotical neuronal migration (i.e., via RNAi of Dyx1c1 or Kiaa0319; [48, 13]) lead to spatial learning deficits on MWM only when malformations extend to the hippocampus. In the case of injury-induced MG as reported here, a more consistent disruption of the hippocampus may prevail among treated subjects. Ongoing identification of such domain-specific behavioral phenotypes, as well as delineating how such domain-specific deficits are associated with region-specific alterations in circuitry (as seen under different forms of disruption), will be critical to fully understanding the etiology of various developmental disabilities and the subtle differences in behavioral phenotypes within and across these disorders [39, 8, 11, 48]. Future assessments of cortical and hippocampal alterations in microgyric rats (as well as other neural structures) might begin to address the anatomical and physiological origins of the observed spatial learning impairments.
Although it is unclear from the present study how cortical injury may influence spatial specific learning, emerging evidence suggests frontal cortical involvement (which is partially disrupted in the present MG model) in the retrieval of spatial memory. For example, Leon and associates [49] utilized a modified one day version of the Morris water maze to investigate early cell signaling in the medial prefrontal cortex (mPFC) and hippocampus during consolidation and memory retrieval phases. They showed elevated levels of extra-cellular regulated kinase (ERK, involved in synaptic plasticity) in the mPFC immediately following retrieval (probe trial). Other researchers have shown that when task demand on a Morris water maze increases, as in the case of multiple test trials or modification of distal cues, the medial prefrontal cortex is engaged [50]. Further, Romanelli and colleagues [51] showed significant NMDA dependent activation of early immediate genes in the cerebral cortex of rats exposed to a novel spatial Lat-maze. In relation to the current study it is possible that frontal cortical circuits mediating the retrieval of early spatial memories were disrupted. However, it is still unclear why non-spatial learning appears intact. Future studies using both spatial and non-spatial learning paradigms should evaluate early protein expression (ERK) and RNA synthesis across brain regions with the goal of identifying unique processing systems for these seemingly divergent learning domains.
However, based on the data presented here it is clear that focal bilateral damage to the developing rat neocortex, on postnatal day one, leads to selective deficits in spatial, but not non-spatial learning. Further, although prior water maze experience does lead to improved overall performance on a second round of testing, these improvements do not appear to be enough to eliminate spatial learning impairments in microgyric rats, since deficits are present regardless of testing order.
Research Highlights.
We assessed test experience, microgyria, spatial and non-spatial learning in rats.
All improved performance on a second week of testing regardless of task order.
Microgyric rats showed spatial (not non-spatial) learning deficits.
These deficits where seen regardless of task order.
Acknowledgements
This research was supported by NIH Grant HD20806, the Rhode Island Idea Network for Biomedical Research Excellence (RI-INBRE) and NIH National Center for Research Resources (5P20RR016457-11).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guerrini R, Parrini E. Neuronal migration disorders. Neurobiol Dis. 2010;38(2):154–166. doi: 10.1016/j.nbd.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Chang B, Ly J, Appignani B, Bodell A, Apse K, Ravenscroft R, Sheen V, Doherty M, Hackney D, O’Connor M, Galaburda A, Walsh C. Reading impairment in the neuronal migration disorder of periventricular nodular heterotopia. Neurology. 2005;64(5):799–803. doi: 10.1212/01.WNL.0000152874.57180.AF. [DOI] [PubMed] [Google Scholar]
- 3.Guerreiro M, Hage S, Guimaraes C, Abramides D, Fernandes W, Pacheco P, Piovesana A, Montenegro M, Cendes F. Developmental language disorder associated with polymicrogyria. Neurology. 2002;59:245–250. doi: 10.1212/wnl.59.2.245. [DOI] [PubMed] [Google Scholar]
- 4.Galaburda A, Sherman G, Rosen G, Aboitiz F, Geschwind N. Developmental Dyslexia: Four Consecutive Patients with Cortical Abnormalities. Ann Neurol. 1985;18(2):222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- 5.Hage S, Cendes F, Montenegro M, Abramides D, Guimaraes C, Guerreiro M. Specific Language Impairment: Linguistic and neurobiological aspects. Arq Neuropsiquiatr. 2006;64(2-A):173–180. doi: 10.1590/s0004-282x2006000200001. [DOI] [PubMed] [Google Scholar]
- 6.Boscariol M, Guimarzaes CA, Hage SR, Garcia VL, Schmutzler KMR, Cendes F, Guerreiro MM. Auditory processing disorder in patients with language-learning impairment and correlation with malformation of cortical development. Brain and Dev. 2011;33:824–831. doi: 10.1016/j.braindev.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Fitch RH, Tallal P, Brown C, Galaburda A, Rosen G. Induced microgyria and auditory temporal processing in rats: a model for language impairment? Cereb Cortex. 1994;4(3):260–270. doi: 10.1093/cercor/4.3.260. [DOI] [PubMed] [Google Scholar]
- 8.Fitch RH, Breslawski H, Rosen GD, Chrobak JJ. Persistent spatial working memory deficits in rats with bilateral cortical microgyria. Behav Brain Funct. 2008;4(1):45. doi: 10.1186/1744-9081-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Threlkeld, et al. Developmental timeframes for induction of microgyria and rapid auditory processing deficits in the rat. Brain Res. 2006;1109(1):22–31. doi: 10.1016/j.brainres.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Threlkeld SW, Rosen GD, Fitch RH. Age at developmental cortical injury differentially alters corpus callosum volume in the rat. BMC Neurosci. 2007;8:94. doi: 10.1186/1471-2202-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Threlkeld SW, Hill CA, Rosen GD, Fitch RH. Early acoustic discrimination experience ameliorates auditory processing deficits in male rats with cortical developmental disruption. Int. J. Devl Neuroscience. 2009;27(4):321–328. doi: 10.1016/j.ijdevneu.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szalkowski CE, Hinman JR, Threlkeld SW, Wang Y, LePack A, Rosen GD, Chrobak JJ, LoTurco JJ, Fitch RH. Persistent spatial working memory deficits in rats following in utero RNAi of Dyx1c1. Genes Brain Behav. 2011;10(2):244–252. doi: 10.1111/j.1601-183X.2010.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szalkowski CE, Fiondella CG, Galaburda AM, Rosen GD, Loturco JJ, Fitch RH. Neocortical disruption and behavioral impairments in rats following in utero RNAi of candidate dyslexia risk gene Kiaao319. Int J Dev Neurosci. 2012;30(4):293–302. doi: 10.1016/j.ijdevneu.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Threlkeld SW, Hill CA, Cleary CE, Truong DT, Rosen GD, Fitch RH. Developmental learning impairments in a rodent model of nodular heterotopia. J Neurodev Disord. 2009;1(3):237–250. doi: 10.1007/s11689-009-9026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark MG, Rosen GD, Tallal P, Fitch RH. Impaired two-tone processing at rapid rates in male rats with induced microgyria. Brain Res. 2000;871(1):94–97. doi: 10.1016/s0006-8993(00)02447-1. [DOI] [PubMed] [Google Scholar]
- 16.Peiffer AM, McClure MM, Threlkeld SW, Rosen GD, Fitch RH. Severity of focal microgyria and associated rapid auditory processing deficits. Neuroreport. 2004;15(12):1923–1926. doi: 10.1097/00001756-200408260-00018. [DOI] [PubMed] [Google Scholar]
- 17.Beneventi H, Tønnessen FE, Ersland L, Hugdahl K. Executive working memory processes in dyslexia: behavioral and fMRI evidence. Scand J Psychol. 2010;51(3):192–202. doi: 10.1111/j.1467-9450.2010.00808.x. [DOI] [PubMed] [Google Scholar]
- 18.Bishop DVM. Genes, cognition, and communication: insights from neurodedevelopmental disorders. Ann. N.Y. Acad. Sci. 2009;1156:1–18. doi: 10.1111/j.1749-6632.2009.04419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gathercole SE, Alloway TP. Practitioner review: short-term working memory impairments in neurodevelopmental disorders: diagnosis and remedial support. J Child Psychol Psychiatry. 2006 Jan;47(1):4–15. doi: 10.1111/j.1469-7610.2005.01446.x. [DOI] [PubMed] [Google Scholar]
- 20.Benasich AA, Choudhury N, Friedman JT, Realpe-Bonilla T, Chojnowska C, Gou Z. The infant as a prelinguistic model for language learning impairments: predicting from event-related potentials to behavior. Neuropsychologia. 2006;44(3):396–411. doi: 10.1016/j.neuropsychologia.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boets B, Vandermosten M, Poelmans H, Luts H, Wouters J, Ghesquière P. Preschool impairments in auditory processing and speech perception uniquely predicts future reading problems. Res Dev Disabil. 2011;32(2):560–570. doi: 10.1016/j.ridd.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Gaab N, Gabrieli J, Deutsch G, Tallal P, Temple E. Neuronal correlates of rapid auditory processing are disrupted in children with developmental dyslexia and ameliorated with training: An fMRI study. Bestor Neurol Neurosci. 2007;25(3–4):295–310. [PubMed] [Google Scholar]
- 23.Snowling M, Bishop DV, Stothard SE. Is preschool language impairment a risk factor for dyslexia in adolescence? J Child Psychol Psychiatry. 2000;41(5):587–600. doi: 10.1111/1469-7610.00651. [DOI] [PubMed] [Google Scholar]
- 24.Smith-Spark JH, Fisk JE. Working memory functioning in developmental dyslexia. Memory. 2007;15(1):34–56. doi: 10.1080/09658210601043384. [DOI] [PubMed] [Google Scholar]
- 25.Gabel LA, Marin I, LoTurco JJ, Che A, Murphy C, Manglani M, Kass S. Mutation of the dyslexia-associated gene Dcdc2 impairs LTM and visuo-spatial performance in mice. Genes Brain Behav. 2011 Nov;10(8):868–875. doi: 10.1111/j.1601-183X.2011.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stavenezer A, Hyde L, Bimonte H, Armstrong C, Denenberg V. Differential learning strategies in spatial and nonspatial versions of the Morris water maze in the C57BL/6J inbred mouse strain. Behav Brain Res. 2002;133(2):261–270. doi: 10.1016/s0166-4328(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 27.Hyde L, Stavenzer A, Bimonte H, Sherman G, Denenberg V. Spatial and nonspatial morris maze learning: impaired behavioral flexibility in mice with ectopias located in the prefrontal cortex. Behav. Brain Res. 2002;133:247–259. doi: 10.1016/s0166-4328(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 28.Balogh SA, Sherman GF, Hyde LA, Denenberg VH. Effects of neocortical ectopias upon the acquisition and retention of a non-spatial reference memory task in BXSB mice. Brain Res Dev Brain Res. 1998;111(2):291–293. doi: 10.1016/s0165-3806(98)00138-2. [DOI] [PubMed] [Google Scholar]
- 29.Peiffer AM, Rosen GD, Fitch RH. Sex differences in rapid auditory processing deficits in ectopic BXSB/MpJ mice. Neuroreport. 2002;13(17):2277–2280. doi: 10.1097/00001756-200212030-00021. [DOI] [PubMed] [Google Scholar]
- 30.Peiffer AM, Rosen GD, Fitch RH. Sex differences in rapid auditory processing deficits in microgyric rats. Brain Res Dev Brain Res. 2004;148(1):53–57. doi: 10.1016/j.devbrainres.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. J Child Psychol Psychiatry. 2003;44(8):1092–1115. doi: 10.1111/1469-7610.00194. [DOI] [PubMed] [Google Scholar]
- 32.Liederman J, Kantrowitz L, Flannery K. Male vulnerability to reading disability is not likely to be a myth: a call for new data. J Learn Disabil. 2005;38(2):109–129. doi: 10.1177/00222194050380020201. [DOI] [PubMed] [Google Scholar]
- 33.Rosen GD, Galaburda AM. Single cause, polymorphic neuronal migration disorders: an animal model. Dev Med Child Neurol. 2000;42(10):652–662. doi: 10.1017/s0012162200001213. [DOI] [PubMed] [Google Scholar]
- 34.Dvorak K, Feit J, Jurankova Z. Experimentally Induced Focal Microgyria and Status Verrucosus Deformis in Rats - Pathogenesis and Interrelation Histological and Autoradiographical Study. Acta Neuropathol. 1978;44:121–129. doi: 10.1007/BF00691477. [DOI] [PubMed] [Google Scholar]
- 35.Humphreys P, Rosen G, Press D, Sherman G, Galaburda A. Freezing Lesions of the Developing Rat Brain: A Model For Cerebrocortical Microgyria. J Neuropathol Exp Neurol. 1991;50:145–160. doi: 10.1097/00005072-199103000-00006. [DOI] [PubMed] [Google Scholar]
- 36.D’Hooge R, De Deyn PP. Application of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 37.McClure MM, Threlkeld SW, Fitch RH. The effects of erythropoietin on auditory processing following neonatal hypoxic-ischemic injury. Brain Res. 2007;1132(1):203–209. doi: 10.1016/j.brainres.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Friedman JT, Peiffer AM, Clark MG, Benasich AA, Fitch RH. Age and experiencerelated improvements in gap detection in the rat. Brain Res Dev Brain Res. 2004;152(2):83–91. doi: 10.1016/j.devbrainres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Rosen G, Burstein D, Galaburda A. Changes in Efferent and Afferent Connectivity in Rats With Induced Cerebrocortical Microgyria. J Comp Neurol. 2000;418:423–440. [PubMed] [Google Scholar]
- 40.Jacobs KM, Graber KD, Kharazia VN, Parada I, Prince DA. Postlesional epilepsy: The ultimate brain plasticity. Epilepsia. 2000;41(6):S153–S161. doi: 10.1111/j.1528-1157.2000.tb01574.x. 152(2):83–91. [DOI] [PubMed] [Google Scholar]
- 41.Capellini SA, Padula NA, Santos LC, Lourenceti MD, Carrenho EH, Ribeiro LA. Phonological awareness, working memory, reading and writing performances in familial dyslexia. Pro Fono. 2007;19(4):374–380. doi: 10.1590/s0104-56872007000400009. [DOI] [PubMed] [Google Scholar]
- 42.Ramus F, Szenkovits G. What phonological deficit? Q J Exp Psychol (Hove) 2008;61(1):129–141. doi: 10.1080/17470210701508822. [DOI] [PubMed] [Google Scholar]
- 43.Temple E. Brain mechanisms in normal and dyslexic readers. Curr Opin Neurobiol. 2002;12(2):178–183. doi: 10.1016/s0959-4388(02)00303-3. [DOI] [PubMed] [Google Scholar]
- 44.Howard D, Hickin J, Redmond T, Clark P, Best W. Re-visiting “semantic facilitation” of word retrieval for people with aphasia: facilitation yes but semantic no. Cortex. 2006;42(6):946–962. doi: 10.1016/s0010-9452(08)70439-8. [DOI] [PubMed] [Google Scholar]
- 45.Roach NW, Hogben JH. Spatial cueing deficits in dyslexia reflect generalized difficulties with attentional selection. Vision Res. 2008;48(2):193–207. doi: 10.1016/j.visres.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Attree EA, Turner MJ, Cowell N. A virtual reality test identifies the visuospatial strengths of adolescents with dyslexia. Cyberpsychol Behav. 2009;12(2):163–168. doi: 10.1089/cpb.2008.0204. [DOI] [PubMed] [Google Scholar]
- 47.Colacitti C, Sancini G, Franceschetti S, Cattabeni F, Avanzini G, Spreafico R, Di Luca M, Battaglia G. Altered connections between neocortical and heterotopic areas in methylazoxymethanol-treated rat. Epilepsy Res. 1998;32(1–2):49–62. doi: 10.1016/s0920-1211(98)00039-4. [DOI] [PubMed] [Google Scholar]
- 48.Threlkeld SW, McClure MM, Bai J, Wang Y, LoTurco JJ, Rosen GD, Fitch RH. Developental disruptions and behavioral impairments in rats following in utero RNAi of Dyx1c1. Brain Res Bull. 2007;71(5):508–514. doi: 10.1016/j.brainresbull.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leon WC, Bruno MA, Allard S, Nader K, Cuello CA. Engagement of the PFC in consolidation and recall of recent spatial memory. Learn. Mem. 2010;17:297–305. doi: 10.1101/lm.1804410. [DOI] [PubMed] [Google Scholar]
- 50.Compton DM, Griffith HR, McDaniel WF, Foster RA, Davis BK. The flexible use of multiple cue relationships in spatial navigation: A comparison of water maze performance following hippocampal, medial septal, prefrontal cortex, or posterior parietal cortex lesions. Neurobiol Learn Mem. 1997;68:117–132. doi: 10.1006/nlme.1997.3793. 1997. [DOI] [PubMed] [Google Scholar]
- 51.Romanelli P, Di Matteo L, Cobellis G, Varriale B, Menegazzi M, Gironi Carnevale UA, Ruocco LA, Sadile AG. Transcription factor expression, RNA synthesis and HADPH-diaphorase across the rat brain and exposure to spatial novelty. Behav. Brain. Res. 2007;184:91–100. doi: 10.1016/j.bbr.2007.06.021. [DOI] [PubMed] [Google Scholar]