Abstract
Four Lactobacillus strains were isolated from marketed probiotic products, including L. rhamnosus strains from Vifit (Friesland Campina) and Idoform (Ferrosan) and L. casei strains from Actimel (Danone) and Yakult (Yakult Honsa Co.). Their genomes and phenotypes were characterized and compared in detail with L. casei strain BL23 and L. rhamnosus strain GG. Phenotypic analysis of the new isolates indicated differences in carbohydrate utilization between L. casei and L. rhamnosus strains, which could be linked to their genotypes. The two isolated L. rhamnosus strains had genomes that were virtually identical to that of L. rhamnosus GG, testifying to their genomic stability and integrity in food products. The L. casei strains showed much greater genomic heterogeneity. Remarkably, all strains contained an intact spaCBA pilus gene cluster. However, only the L. rhamnosus strains produced mucus-binding SpaCBA pili under the conditions tested. Transcription initiation mapping demonstrated that the insertion of an iso-IS30 element upstream of the pilus gene cluster in L. rhamnosus strains but absent in L. casei strains had constituted a functional promoter driving pilus gene expression. All L. rhamnosus strains triggered an NF-κB response via Toll-like receptor 2 (TLR2) in a reporter cell line, whereas the L. casei strains did not or did so to a much lesser extent. This study demonstrates that the two L. rhamnosus strains isolated from probiotic products are virtually identical to L. rhamnosus GG and further highlights the differences between these and L. casei strains widely marketed as probiotics, in terms of genome content, mucus-binding and metabolic capacities, and host signaling capabilities.
INTRODUCTION
Lactic acid bacteria (LAB) are a phylogenetically related group of Gram-positive bacteria sharing as a common metabolic property the production of lactic acid as the main end product of carbohydrate utilization (1). Many LAB are traditionally used as culture starters in industrial dairy fermentations of raw materials, such as milk, vegetables, and meat. However, in recent years, specific LAB strains have been associated with health benefits and are marketed as probiotics in a highly successful way, reaching market volumes of over $100 billion (2, 3). Most of these marketed strains belong to the genus Lactobacillus, which represents the largest group of LAB, encompassing more than 100 cultivable bacterial species (4). They are found in a large variety of food-related habitats and are naturally associated with mucosal surfaces such as the oral cavity, vagina, and gastrointestinal (GI) tract.
Currently, strains belonging to the species L. acidophilus, L. plantarum, L. johnsonii, L. reuteri, L. paracasei, L. casei, and L. rhamnosus play a predominant role in the probiotics market, where they are known under proprietary brand names (5). Many of these LAB strains marketed as probiotics were selected according to their in vitro abilities to endure to the harsh physical-chemical environment of the human GI tract, i.e., low pH and high concentration of bile salts, and also for their remarkable adhesive properties on human mucus and antipathogenic activity (6). To demonstrate their health-promoting abilities, a number of Lactobacillus strains, including L. rhamnosus GG, have been successfully used in human interventions with subjects suffering from GI disorders and atopic dermatitis (7, 8). Comparative studies have shown that probiotic features and their associated health properties are strain specific and cannot be generalized, indicating that it is essential to characterize the Lactobacillus strains at the genome level, as has been done for a limited number of paradigm probiotics (2). This has promoted rapid insights into the diversity, evolution, and molecular basis underlying the health benefits of these strains, resulting in a research area that has been termed probiogenomics (9). One of the most studied and widely marketed probiotic strain is the human isolate L. rhamnosus GG (commercialized under the name LGG). We have recently characterized this and another L. rhamnosus strain, LC705, at the genomic and phenotypic levels (10). This analysis identified candidate genes contributing to its adaptability in the intestinal tract, and the construction of dedicated knockout mutants contributed to establishing detailed gene-function relationships. Thus, specific surface macromolecules and their role in gastrointestinal fitness of Lactobacillus GG have been characterized. For instance, the long galactose-rich exopolysaccharide (EPS) molecules form a protective shield against antimicrobial peptides secreted by intestinal epithelial cells, promoting the survival of L. rhamnosus GG in the intestinal tract (11). In addition, L. rhamnosus produces two secreted proteins, p75 and p40, reported to signal to the mitogen-activated protein kinase (MAPK) pathway in intestinal cells (12), which were found recently to be the glycosylated d-glutamyl-l-lysyl endopeptidase MspI and an essential cell wall hydrolase, Msp2, respectively (13, 14). Moreover, several surface proteins have been investigated because they mediate the interaction with human host, including the mucus-binding factor (MBF) (15) and the highly repeated protein MabA, which appears to contribute to biofilm formation (16). However, a major driver of adhesion to intestinal mucosa and biofilm formation is the mucus-binding pili of L. rhamnosus GG encoded by the spaCBA-strC gene cluster (10, 17, 18). These pili are protruding protein fibers consisting of multimers of SpaA, decorated by the mucus-binding protein SpaC and covalently linked to the peptidoglycan by the product of spaB (17). Comparative genome analysis has shown that L. rhamnosus and L. casei genomes are highly related (4). This is illustrated by the observation that not only L. rhamnosus but also L. casei strains produce highly identical MspI (p75) and Msp2 (p40) proteins that have similar functions in both species (19, 20). However, in spite of the fact that strains of L. casei are widely marketed as probiotics (5), the genomes of many commercial L. casei strains have yet to be reported. Currently, the complete genome sequences of L. casei ATCC 334 (21), L. casei BL23 (22), L. casei Zhang (23), L. casei LCW2 (24), L. casei W56 (25), and L. casei BD-II (26) are available, and some have been subjected to detailed comparative genome analysis (27, 28). However, only a very limited number of functional studies have been reported. The best-characterized strain is L. casei BL23, which was used in studies that indicated anti-inflammatory properties in an animal model of intestinal inflammation (29) and the capacity to bind extracellular matrix proteins (fibronectin and collagen), ascribed to the FbpA surface and other proteins that are also partly conserved in L. rhamnosus GG (30). Other documented properties of L. casei relate to its resistance to the stresses encountered during gastrointestinal passage mainly due to acid and bile tolerance (31–34).
The aim of the present study is to provide a comparative analysis of widely marketed probiotic Lactobacillus strains belonging to the species L. casei and L. rhamnosus. Hence, L. rhamnosus strains were isolated from the commercial products Vifit and Idoform, while L. casei strains were isolated from products branded as Yakult and Actimel. These strains were characterized at the genotypic and phenotypic levels for their carbohydrate metabolism and adhesive and immunomodulatory properties. The validity of this approach was confirmed by the high level of identity to the reported L. rhamnosus GG genome of the L. rhamnosus isolates from commercial products, testifying to the product stability of this widely used probiotic strain.
MATERIALS AND METHODS
Isolation of strains, growth conditions, and DNA extraction.
The bacterial strains used in this study are listed in Table 1. L. rhamnosus GG (ATCC 53103) was obtained from the Valio culture collection (Valio Ltd., Helsinki, Finland), and L. casei BL23 (ATCC 393) that was cured of its lactose plasmid pLZ15 was kindly provided by the Institute of Agrochemistry and Food Technology (Valencia, Spain). L. rhamnosus strains were isolated as the dominant population from food and pharmaceutical products commercialized as carrying L. rhamnosus GG under the brand names Vifit (Friesland Campina, Netherlands) and Idoform (Ferrosan, Denmark), resulting in L. rhamnosus strains LrV and LrI, respectively. L. casei strains were derived from the food drinks branded as Yakult (Yakult Honsha Co., Japan) and Actimel (Danone, France) and were termed L. casei strains LcY and LcA, respectively. The isolation of strains LrV, LcY, and LcA was carried out by homogenizing 1 ml of product in 9 ml of sterile phosphate-buffered saline (PBS), while one Idoform tablet was dissolved in 10 ml of sterile PBS to isolate LrI. Isolation was carried out by generating single colonies via serial dilution and plating onto MRS broth (Difco BD, NJ) solidified with 1% (wt/vol) agar incubated anaerobically at 37°C for 48 h. Colonies of each product were selected, inoculated into MRS broth, and propagated overnight anaerobically at 37°C. An aliquot from each bacterial culture was used for chromosomal DNA extraction using a Wizard Genomic DNA purification kit (Promega, WI) according to the manufacturer's instructions.
Table 1.
Strains used in this study
| Strain | Functional producta | Product category | Source or reference |
|---|---|---|---|
| L. rhamnosus GG (ATCC 53103) | Gefilus product family | Buttermilks, yogurts, milk, fruit drinks, dairy drinks, and fermented whey-based drinks | Valio Ltd. culture collection |
| L. rhamnosus LrV | Vifit product family | Yogurts and drinkable yogurts | Friesland Campina |
| L. rhamnosus LrI | Idoform | Tablets | Ferrosan |
| L. casei BL23 (ATCC 393) | NA | Dairy product | 35 |
| L. casei LcY | Yakult | Fermented milk drink | Yakult Honsa Co. |
| L. casei LcA | Actimel | Fermented milk | Danone |
NA, not available.
Molecular typing.
The identification of bacterial isolates to the species level was performed by amplification of the tuf gene, as described previously (36). Briefly, the tuf gene was amplified by PCR using a solution containing 10 mM Tris-HCl; 50 mM KCl; 1.5 mM MgCl2; 200 μM each deoxynucleoside triphosphates (dNTPs) (Finnzymes, Finland); 10 pmol of primers PAR (5′-GACGGTTAAGATTGGTGAC-3′), CAS (5′-ACTGAAGGCGACAAGGA-3′), and RHA (5′-GCGTCAGGTTGGTGTTG-3′); 50 pmol of primer CPR (5′-CAANTGGATNGAACCTGGCTTT-3′); 25 ng of genomic DNA; and 2.5 U of Dynazyme DNA polymerase (Finnzymes, Finland) in a final volume of 50 μl. Multiplex PCR assays were run with a DNA Engine Peltier thermal cycler (Bio-Rad, CA). Amplification products were resolved by DNA gel electrophoresis (Sigma, MO), and gel was stained with ethidium bromide.
Fermentative profiling.
The sugar degradation and other catabolic properties of the Lactobacillus strains were characterized by using an API CH 50 kit (Bio-Merieux, Marcy l'Etoile, France). All strains were grown until the logarithmic phase and then inoculated into API galleries according to the manufacturer's instructions. API galleries were incubated at 37°C for 48 h prior to colorimetric analysis.
Human mucus-binding assay.
Adhesion assays of the Lactobacillus strains radiolabeled by [3H]thymidine were performed as described previously (37). In brief, Maxisorp microtiter plates (Nunc, Denmark) were coated with 100 μl of human intestinal mucus solution in PBS at final concentration of 0.5 mg/ml and incubated overnight at 4°C. The wells were washed with PBS to remove unbound mucus, and 100 μl of [3H]thymidine-radiolabeled bacterial suspensions at an optical density at 600 nm (OD600) of 0.25 ± 0.01 was added. The microtiter plate was incubated at 37°C for 1 h. Next, wells were washed with PBS to remove unbound bacteria and incubated at 60°C for 1 h with a 1% (wt/vol) SDS–0.1 M NaOH solution. The radioactivity of lysed bacterial suspensions was measured by liquid scintillation counting (Wallac 1480 Wizard 3 automatic gamma counter). The percent ratio between radioactivity values of bound bacterial suspension and the total bacterial suspension initially added to the well was used to measure adhesion to human intestinal mucus. For each strain, the binding assay was performed at least in triplicate. An antiserum-mediated mucus-binding assay was also performed for GG, LrV, and LrI in the presence of polyclonal SpaC antiserum exactly as described previously (10). A procedure similar to the one mentioned above was subsequently performed, and radiolabeled bacteria were added to intestinal immobilized mucus upon incubation with a 1:100 dilution of SpaC immune serum.
Bile salt sensitivity.
All strains were propagated in MRS broth at 37°C anaerobically. Next, the bacterial suspensions were adjusted to an OD600 of 1.5 and further diluted in sterile PBS. Three microliters of samples was spotted onto MRS agar plates supplemented with 0.5% (wt/vol) Ox gall bile salts (Sigma, MO). Plates were then incubated for 48 h at 37°C under anaerobic conditions prior to visual examination.
Western blot analysis of cell wall proteins.
Bacterial suspensions (OD600 = 1) were used to extract cell wall-associated proteins from the Lactobacillus strains. Cell pellets were washed once with PBS and disrupted mechanically by bead beating with sterile quartz beads (200- to 800-μm diameter; Merck, Germany). The cell wall fraction was resuspended in 500 μl of PBS, pelleted at 13,000 × g for 30 min at 4°C, and subsequently digested for 3 h at 37°C in 50 μl of lysis buffer containing 50 mM Tris-HCl, 5 mM MgCl2, 5 mM CaCl2, 10 mg/ml lysozyme, and 150 U/ml mutanolysin. Samples were mixed with 12.5 μl of 4× Laemmli buffer (Bio-Rad, CA) and denatured at 99°C for 10 min. Cell wall-associated proteins were separated by SDS-PAGE using a 10% (vol/vol) polyacrylamide gel and then electroblotted onto a 0.2-μm nitrocellulose membrane (Bio-Rad, CA). Polyclonal rabbit SpaA antiserum (1:10,000 dilution) (17) and peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch, United Kingdom) (1:10,000 dilution) were used as primary and secondary antibodies, respectively, in 5% nonfat milk–PBS. Membranes were blocked with 5% nonfat milk–PBS and washed with 0.05% Tween 20–PBS between incubations. Bands were visualized by using chemiluminescence according to the specifications of the supplier (Western Lightning Chemiluminescence Reagent Plus; PerkinElmer, United Kingdom).
TLR response assay.
The HEK-blue hTLR2, HEK-blue hTLR4, and HEK-blue hTLR5 cell lines (Invivogen, CA), which constitutively express the Toll-like receptor (TLR) and an alkaline phosphatase gene fused to the NF-κB gene, were used in TLR response assays. All cell lines were grown and subcultured at 70 to 80% confluence in Dulbecco's modified Eagle medium (DMEM) supplemented with 4.5 g/liter d-glucose, 50 U/ml penicillin, 50 μg/ml streptomycin, 100 μg/ml μg/ml Normocin, 2 mM l-glutamine, and 10% (vol/vol) heat-inactivated fetal bovine serum (Integro BV, Netherlands). For each cell line, an immune response experiment was carried out by splitting HEK-blue cells in flat-bottom 96-well plates and stimulating them by the addition of a bacterial suspension adjusted to an OD600 of 0.1 or TLR-specific ligands. The 96-well plates were incubated for 24 h at 37°C in a 5% CO2 incubator. The receptor ligands PAM(3)CSK(4) (synthetic triacylated lipopeptide; 1 ng/ml for human TLR2 [hTLR2]), LPS-EB (lipopolysaccharide preparation isolated from Escherichia coli O111:Br; 1 ng/ml for hTLR4), and recFLA-ST (recombinant flagellin from Salmonella enterica serovar Typhimurium; 10 ng/ml for hTLR5) were used as positive controls, while maintenance medium without any selective antibiotics was used as the negative control. The activity of the secreted alkaline phosphatase, the product of the reporter gene fused to the NF-κB gene, was then determined by incubating 20-μl samples of the reporter cell line supernatant with 180 μl of QUANTI-Blue (Invivogen, CA) at 37°C, followed by measurement of the OD620 after 1 h, 2 h, and 3 h. All assays were performed in triplicate for each sample.
Immunoelectron microscopy.
Lactobacillus strains were grown to stationary phase and then used for transmission electron microscopy analyses. Sample preparation was done according to an immunogold-labeling protocol described previously (17). Briefly, drops of MRS-grown cultures were incubated on Formvar carbon-coated copper grids for 30 min at room temperature. Grids were washed three times with 0.02 M glycine in PBS and then incubated for 15 min with a blocking solution of 1% (wt/vol) bovine serum albumin (BSA). Next, polyclonal SpaA antibody was diluted 1:100 in 1% BSA, in which the grids were incubated for 1 h, and then washed with 0.1% BSA and incubated for 20 min with protein A gold conjugates (10-nm diameter). Grids were then washed several times in PBS, fixed for 5 min using 1% glutaraldehyde, washed again with MilliQ distilled water, and stained with a 1.8% methylcellulose–0.4% uranyl acetate solution. Grid visualization was carried out by using a Jeol 1200 EX II transmission electron microscope (Jeol Ltd., Japan).
Genome sequencing and bioinformatic analysis of L. rhamnosus and L. casei strains.
Genomic DNAs of L. rhamnosus strains LrV and LrI and L. casei strains LcA and LcY were sequenced on a SOLiD sequencer platform (Life Technologies, CA). Sequence alignments were generated by mapping SOLiD color space reads to the L. rhamnosus GG genome (10) or the L. casei BL23 genome (22) as reference genomes, using SOLiD BioScope software (Life Technologies, CA) and SAM tools (38). In order to transfer annotation from a reference genome (GG or BL23) to an unannotated query genome, sequences were compared with “nucmer” to identify regions that share synteny; those regions were extracted out as base range in the query and base range in the reference genome (39). In-house custom-made scripts were used to transfer annotation. The nucleotide sequence identities between synteny blocks were more than or equal to 40%. In the case of the L. rhamnosus strains, initial detection of single-nucleotide polymorphisms (SNPs) and insertions/deletions (InDels) was performed, and chromosomal regions with identified mutations were analyzed further. We considered only unequivocal SNPs with sufficient sequence coverage (>18) and verified them by PCR amplification using High-Fidelity Phusion DNA polymerase (Thermo Scientific, MA) according to the manufacturer's instructions. The PCR amplicons were then sequenced and compared to the reference L. rhamnosus strain GG genome. Orthologous genes between GG and BL23 genomes were calculated by using BLASTP (40) with the standard scoring matrix BLOSUM62 and an initial E value cutoff of 1 × 10E04. The score of every BLAST hit was set in proportion to the best score possible, the score of a hit of the query gene against itself. This resulted in a so-called score ratio value (SRV) between 0 and 100 that reflected the quality of the hit much better than the raw BLAST bit score (41). Two genes were considered orthologous if there existed a reciprocal best BLAST hit between the genes and both hits had an SRV of >35. Genomes were assigned to clusters of orthologous groups (COGs) by using rps-BLAST (reverse position-specific BLAST) and the NCBI Conserved Domain Database (CDD).
Primer extension.
The transcriptional start site (TSS) and the promoter region of the SpaCBA pilus gene cluster were identified by primer extension according to a procedure previously described by Tu et al. (42). Briefly, 5′-6-carboxyfluorescein (FAM)-labeled cDNA was generated from 2 μg total L. rhamnosus GG RNA by using a FAM-labeled primer (5′-GTACCATTAGCATCGGTTTG-3′) (Oligomer Oy, Finland) and a RevertAid Premium reverse transcriptase kit (Thermo Scientific, MA), according to the manufacturer's instructions. The cDNA mixture was then run on an ABI 3730 capillary sequencer in parallel with a Sanger sequencing reaction using the same primer.
RESULTS AND DISCUSSION
Isolation and metabolic characterization of the Lactobacillus strains.
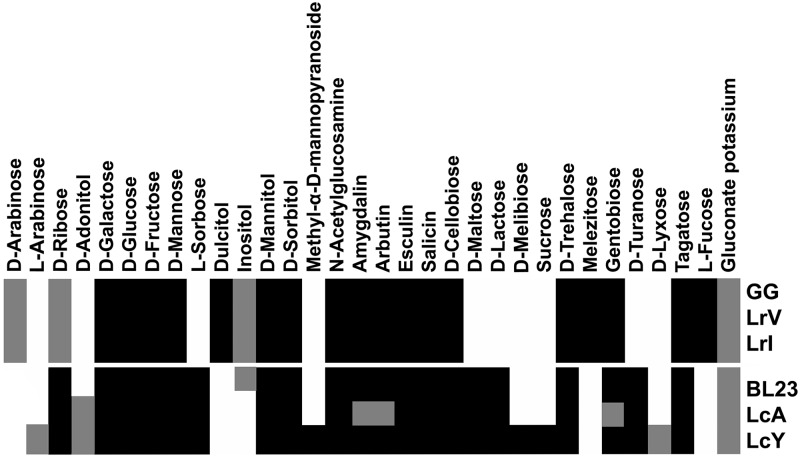
L. rhamnosus strains LrV and LrI were isolated from food and pharmaceutical products commercialized as carrying L. rhamnosus GG under the brand names Vifit (Friesland Campina, Netherlands) and Idoform (Ferrosan, Denmark), respectively. L. casei strains LcY and LcA were isolated from the food drinks branded as Yakult (Yakult Honsha Co., Japan) and Actimel (Danone, France), respectively. Genomic DNA was isolated and used for 16S rRNA and tuf gene analysis (36) to confirm the correctness of their species identification (data not shown), which was corroborated by SOLiD genome mapping. Subsequently, the metabolic properties of the isolated Lactobacillus strains were compared with those of the well-characterized strains L. rhamnosus GG and L. casei BL23 (Fig. 1). Both isolated L. rhamnosus strains showed identical sugar utilization profiles characteristic of L. rhamnosus GG, including the capacity to convert l-fucose but the inability to use d-lactose or l-rhamnose (10). In contrast, the L. casei strains showed considerable variation, indicating that the isolates from probiotic products are not identical. Specifically, they all converted d-lactose, d-maltose, and l-sorbose but not l-fucose, while strain LcY, isolated from the Yakult product, could also utilize d-melibiose and sucrose (Fig. 1).
Fig 1.
Metabolic profiles of the studied Lactobacillus strains. The fermentative capabilities of each strain are color-coded in black and gray for complete and partial carbohydrate utilization, respectively. Carbohydrates that were not fermented are shown in white. The results are those observed after 48 h of incubation. Not used by any of the strains were glycerol, erythritol, d-xylose, l-xylose, methyl-β-d-xylopyranoside, l-rhamnose, methyl-α-d-glucopyranoside, inulin, d-raffinose, amidon, glycogen, xylitol, d-fucose, d-arabitol, l-arabitol, 2-ketogluconate potassium, and 5-ketogluconate potassium.
Bile salt resistance, mucus binding, and intestinal signaling of Lactobacillus strains.
Several desirable traits for a potentially probiotic strain were analyzed in the Lactobacillus strains isolated from probiotic products in comparison with the well-studied strains L. rhamnosus GG and L. casei BL23. Bile salt resistance was tested in a plate agar system or medium containing taurocholic and glycocholic acids derived from the used Ox gall bile. All L. casei strains (BL23, LcY, and LcA) and all L. rhamnosus strains (GG, LrV, and LrI) were found to be resistant to 0.5% (wt/vol) Ox gall bile salts. This is in agreement with previous data on the bile sensitivity of lactobacilli (31, 43) and detailed information on the proteomic bile response of L. casei strains (32), including the two strains used to produce Yakult and Actimel (34) and L. rhamnosus GG (44). This indicates that all L. casei and L. rhamnosus strains possess functional bile resistance mechanisms, an essential trait for persisting in the intestinal tract.
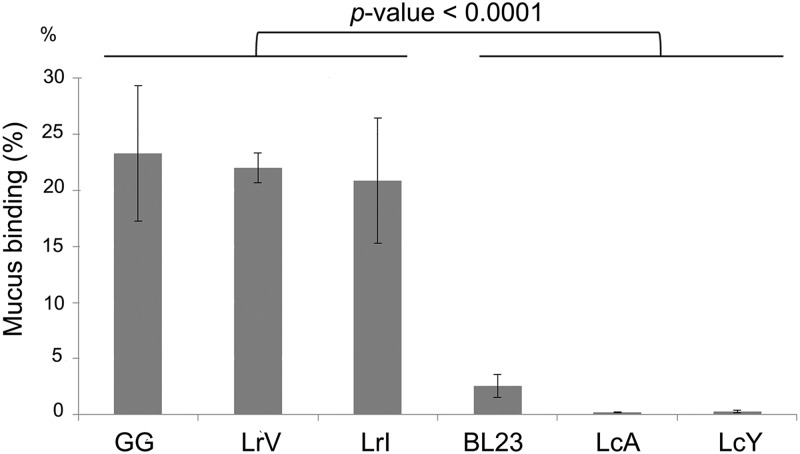
The ability of a variety of Lactobacillus strains marketed as probiotics to adhere to human mucus was previously reported to be highly variable, with L. rhamnosus GG as the highest-binding strain tested (45). Hence, we compared the properties of adhesion of all used Lactobacillus strains to human intestinal mucus (Fig. 2). Indeed, all L. rhamnosus isolates (GG, LrV, and LrY) showed very high-level mucus binding, while the L. casei strains showed only moderate binding (BL23) or virtually no binding (LcY and LcA) under the conditions tested.
Fig 2.
Binding profiles of L. rhamnosus and L. casei strains expressed as percent adhesion to human intestinal mucus. The binding data are expressed as means ± standard deviations. The differences between data sets are considered significant (P ≤ 0.0001).
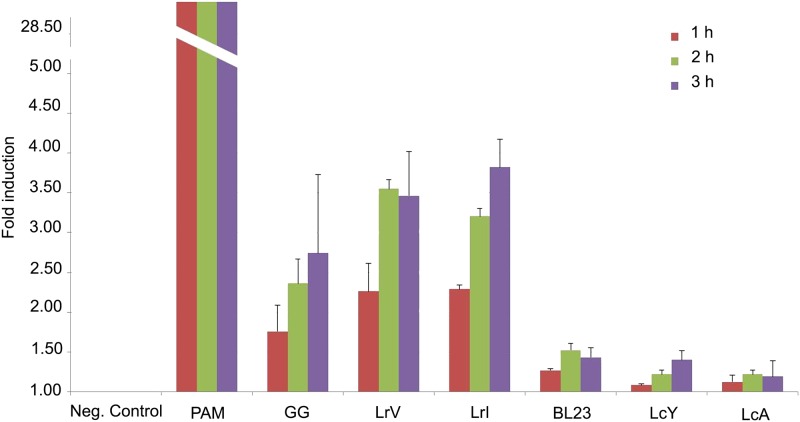
Finally, we compared the capacity of the strains to signal via Toll-like receptors (TLRs) in a mammalian cell line. No significant signaling response was found via TLR4 and TLR5, which is line with the absence of their key ligands (the lipopolysaccharides and the flagellins, respectively) in these lactobacillus strains (data not shown). In contrast, specific and reproducible responses were obtained in a TLR2 reporter cell line, where the NF-κB response was determined via a reporter fusion (Fig. 3). Under the conditions tested, all L. rhamnosus strains (GG, LrV, and LrI) showed significant and similar signaling via TLR2, and L. casei BL23 showed a moderate response, but isolates LcY and LcA showed only background signaling in this in vitro system.
Fig 3.
Response of HEK-blue hTLR2 cells to L. rhamnosus and L. casei strains. HEK-blue hTLR2 cells were stimulated with one of the six LAB strains for 24 h, after which the cell culture supernatant was incubated for 1 h, 2 h, and 3 h for detection of NF-κB activation. NF-κB-induced secreted embryonic alkaline phosphatase (SEAP) activity was measured by a spectrophotometer and converted to fold changes. The data are expressed as means ± standard deviations. PAM, PAM(3)CSK(4).
This different signaling response via TLR2 is remarkable since cell wall components such as peptidoglycan and lipoteichoic acids that signal to TLR2 are generally present in all lactobacilli. One possible explanation for this finding could be that the nonsignaling strains LcY and LcA lack the pili that are known to be present in L. rhamnosus GG (10) and that were recently found to be the primary factors involved in promoting intestinal signaling (46). L. rhamnosus GG pili are decorated by the mucus-binding protein SpaC (10, 17), and L. rhamnosus strains lacking the expression of pili do not display any mucus-binding ability, which may also explain the observed absence of high-level adhesion to mucus by the studied L. casei strains. Hence, we performed a comparative analysis of the genomes of the L. rhamnosus isolates (GG, LrV, and LrI) and the L. casei strains (BL23, LcA, and LcY). However, this showed clearly that the genomes of the L. rhamnosus strains (GG, LrV, and LrI) and all L. casei strains (BL23, LcA, and LcY) contained highly identical sequences for the spaCBA-srtC gene cluster, and therefore, we focused on a detailed analysis of the expression of these pilus genes.
Analysis of pilus gene-encoded cell wall-associated proteins.
The pili of L. rhamnosus GG can be detected by using antibodies against the major pilus protein SpaA or the mucus-binding protein SpaC (17). Western blotting using polyclonal SpaA antibody (Fig. 4) showed that the cell envelope fractions of all L. rhamnosus strains (GG, LrV, and LrI) contained the protein multimers characteristic of pili with different sizes (17). In contrast, when the same experiment was applied to the L. casei strains (BL23, LcA, and LcY), no such SpaA multimers or even monomers were detected (Fig. 4). This was confirmed by overexposing the Western blots or by spotting whole cells, supernatants, or cell extracts of the L. casei strains followed by incubation with anti-SpaA or anti-SpaC antibodies (data not shown). Hence, we conclude that none of the L. casei strains produced the mucus-binding pili characteristic of L. rhamnosus strains GG, LrV, and LrI under the conditions tested.
Fig 4.
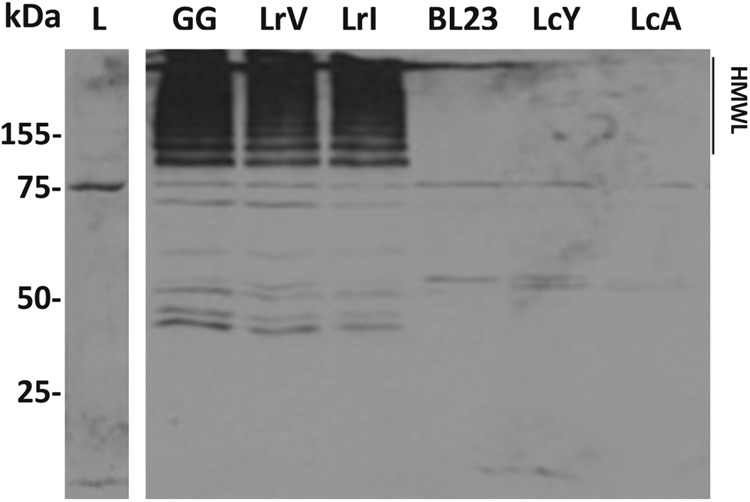
Immunoblotting analysis of cell wall-associated proteins of L. rhamnosus GG (lane 1), LrV (lane 2), and LrI (lane 3) and L. casei BL23 (lane 4), LcY (lane 5), and LcA (lane 6). The membrane was probed with polyclonal serum directed against the SpaA pilin subunit. HMWL, high-molecular-mass ladder; L, ladder.
Subsequently, we used immunogold-labeled anti-SpaA antibodies in an immunoelectron microscopy experiment with the aim to identify the ultrastructure of the pili (see Fig. S1 in the supplemental material). As expected, all L. rhamnosus strains produced similar pilus phenotypes characteristic of L. rhamnosus GG (17), while no such pilus structures could be identified in any of the L. casei strains. Altogether, these experiments indicate that while L. rhamnosus GG and its reisolates from probiotic products produce the mucus-binding pili, these are not present in L. casei BL23 or strains LcA and LcY isolated from the probiotic products Actimel and Yakult. We cannot exclude the possibility that pili in L. casei might be differentially regulated and therefore expressed under in vivo conditions under the control of environmental factors, as seen for Bifidobacterium breve (47). However, exposure of both L. casei and L. rhamnosus strains to conditions mimicking the intestinal tract, i.e., incubation in the presence of bile salts and low pH, did not trigger the expression of pili in L. casei strains, whereas in L. rhamnosus, pili remained expressed (data not shown). Finally, the use of SpaC antiserum in mucus-binding assays abolished the mucus-binding ability of LrV and LrI to background levels, as previously reported for GG (10). This further supports the important role of the SpaCBA pili in the interaction with human intestinal mucosa.
Comparative genome analysis of L. rhamnosus and L. casei strains.
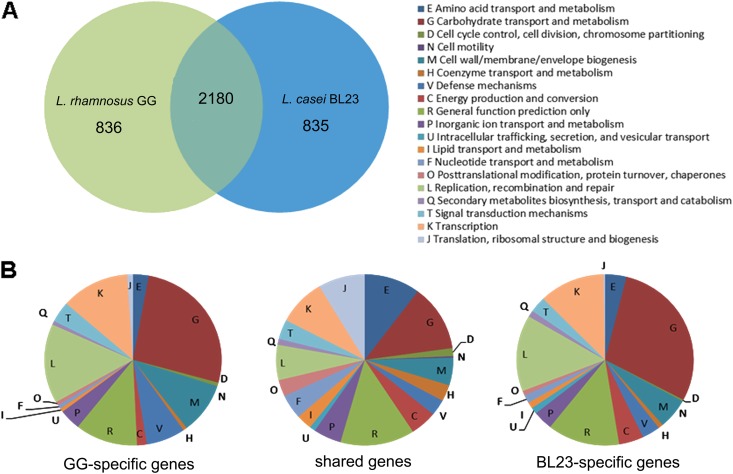
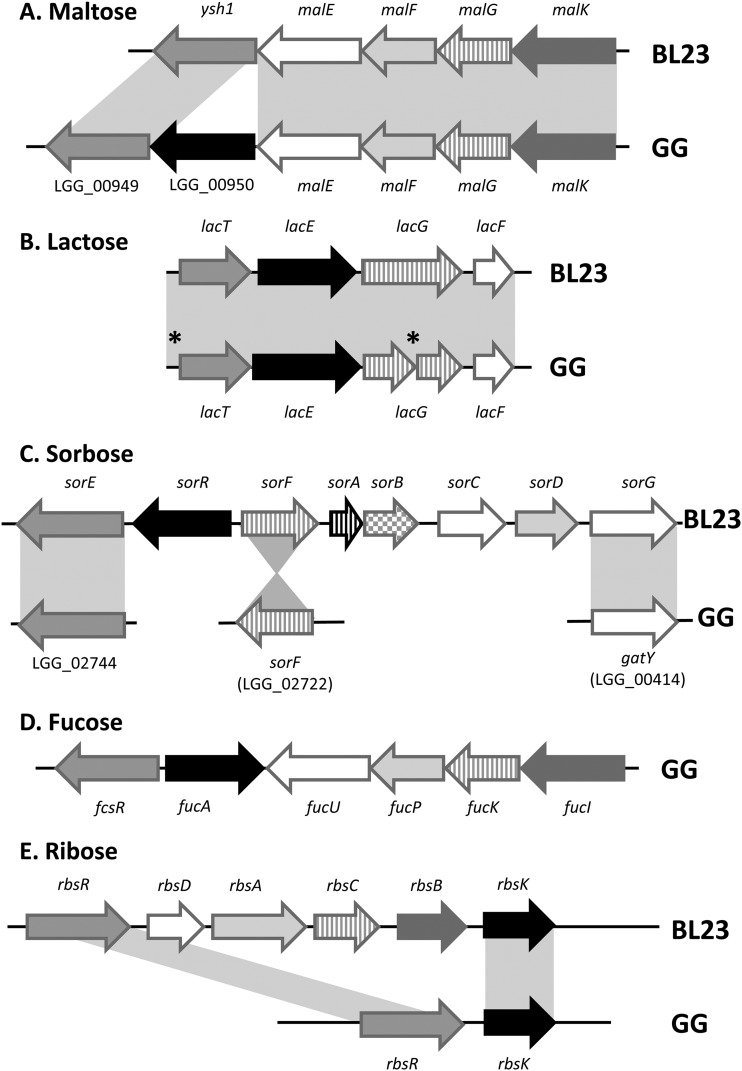
To further characterize the Lactobacillus strains isolated from probiotic products, we determined their genome sequences and analyzed these sequences based on a comparison of the well-established genomes of L. rhamnosus GG and L. casei BL23 (10, 22). The 3-Mb genomes of the latter strains are similarly sized (10, 22), among the largest in the genus Lactobacillus (4), and include no plasmids, unlike L. rhamnosus LC705 (10) and L. casei ATCC 334 (21). Moreover, the genomes of L. rhamnosus GG and L. casei BL23 show a high degree of synteny, with only a few regions disrupted throughout the chromosome (as revealed by ACT [Artemis Comparison Tool] and Gepard dot plot alignments) (Fig. 5). These regions consist mostly of genomic islands encoding prophages, transposases, and sugar transport systems, illustrating the preponderant role of horizontal gene transfer in bacterial evolution, promoting metabolic diversity and life-style adaptability between different lactobacilli. Protein predictions indicated that a total of 2,180 proteins with a high amino acid identity score were shared (including the identical spaCBA-srtC gene cluster), while 836 and 835 proteins were specific for L. rhamnosus GG and L. casei BL23, respectively (Fig. 6). In contrast to L. casei strain BL23, the spaCBA-srtC pilus gene cluster in L. rhamnosus strain GG is located in a chromosomal region rich in transposase genes and poorly conserved among the publicly available sequenced genomes of L. rhamnosus strains, suggesting its acquisition by horizontal gene transfer. Also, the COG distribution revealed that a significant proportion of strain-specific genes were involved in carbohydrate transport and metabolism, supporting the observed metabolic differences (Fig. 5 to 7). We further examined some salient metabolic features of L. rhamnosus GG and L. casei BL23 genomes, i.e., utilization of d-ribose, l-sorbose, d-maltose, d-lactose, and l-fucose. The capacity to metabolize carbohydrates relies on functional transport systems and intact metabolic pathways. The maltose locus was found to be disrupted in L. rhamnosus GG by the insertion of a conserved coding region (LGG_00950) between the genes encoding the maltose-specific transport system (malEFGK) and the hydrolase (LGG_00949) (Fig. 7) (10). In contrast, L. casei BL23 has a continuous and intact maltose locus with no gene insertion (57), which is in agreement with its ability to convert maltose (Fig. 7). It is noteworthy that the insertional inactivation of the maltose locus found in L. rhamnosus strain GG is not present in all L. rhamnosus strains (data not shown), suggesting that only a sublineage of the L. rhamnosus species lost the ability to metabolize maltose. Metabolic profiling also indicated that L. rhamnosus GG cannot metabolize d-lactose, unlike L. casei BL23, which could be verified at the genome level. The L. rhamnosus GG genome possesses a lactose locus, where the genes encoding the antiterminator (lacT) and the phospho-β-galactosidase (lacG) are nonfunctional (10), whereas the homologous lactose locus in BL23 is intact (58) (Fig. 7). L. casei BL23 is also able to convert l-sorbose, which could be associated with the presence of an intact sorbose locus, sorERFABCDG, previously described by Yebra and colleagues (48). No such locus could be identified in L. rhamnosus GG, and only some genes homologous to sorE, sorF, and sorG were found scattered throughout the genome, indicating that L. rhamnosus GG does not possess the complete machinery necessary to utilize l-sorbose. Interestingly, L. rhamnosus GG does not use d-ribose as efficiently as BL23 (Fig. 1), which could be explained by the fact that the ribose-specific ABC transporter rbsABC is missing in GG compared to BL23 (Fig. 7). This also suggests that L. rhamnosus GG uses alternative transporter systems, as it still converts d-ribose. Originally isolated from the human intestinal tract, L. rhamnosus GG is able to convert l-fucose, a sugar compound commonly present in the intestinal tract (49). A close inspection of both genomes revealed that L. rhamnosus has a fucose locus which is not present in L. casei BL23 (Fig. 7). Differences between L. rhamnosus GG and L. casei BL23 genomes are clearly reflected at the phenotypic level and show how different genetic events may contribute to the bacterial diversity within the same genus, i.e., loss, decay, or acquisition of a locus and gene mutation.
Fig 5.
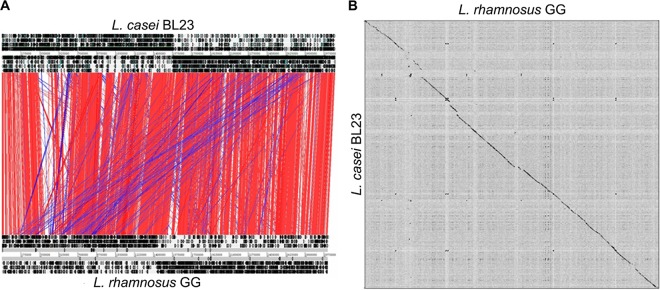
Genomic comparison of L. casei BL23 and L. rhamnosus GG. (A) ACT (Artemis Comparison Tool) comparison of L. rhamnosus GG (bottom chromosome) and L. casei BL23 (top chromosome) (55). Red and blue bars indicate similar regions between GG and BL23 (BLASTN hits), respectively, that have the same orientation or have been inverted. (B) Dot plot alignments of GG and BL23 using Gepard (56).
Fig 6.
Comparative genomic overview of L. rhamnosus GG and L. casei BL23. (A) Number of shared and strain-specific genes. (B) COG distribution of the different subset of genes shown in panel A.
Fig 7.
Metabolic cluster comparison between L. rhamnosus GG and L. casei BL23 genomes. Panels illustrate some of the genes associated with the indicated carbohydrates. Gray shading indicates homologous genes. The black asterisks indicate mutations previously reported (10).
Subsequently, the genomes of the L. rhamnosus strains (LrV and LrI) and the L. casei strains (LcY and LcA) were analyzed by SOLiD sequencing with paired-end and single reads (50-bp forward reads and 35-bp reverse reads), totaling 8.9 million to 12 million reads and amounting to over 100 Mbp for each genome. The SOLiD sequencing reads were mapped to the L. rhamnosus GG and L. casei BL23 genomes, providing sufficient information to gain insights into gene content, genetic order, and single-nucleotide polymorphisms. In the SOLiD mapping approach, tandem repeats, mononucleotide repeats, and low-complexity sequences present in the genomes may not be correctly mapped in some cases, as previously reported (50, 51). Using the annotation method (39), comparative analysis of the genomes of the L. rhamnosus isolates and that of L. rhamnosus GG (10) revealed strains LrV and LrI to be virtually syntenous at the genome level. We also identified 4 and 2 SNPs to be present in LrV and LrI, respectively (Table 2). Remarkably, the 2 SNPs were identical and were located in intercistronic regions, suggesting that these might be hot spots for mutations. Strain LrV later acquired two additional SNPs, which are not expected to have an impact on the phenotype as observed in the present study. These SNPs were either located in a lipoprotein gene or affected the glvA gene, which is involved in dysfunctional maltose metabolism (Fig. 1). This illustrates the genomic stability of L. rhamnosus strain GG used in various food or other products, including Idoform and Vifit, but this also demonstrates the attention paid by the dairy industry to ensure the stability and integrity of stock culture propagation and use, i.e., inoculation with bacterial cells grown from a frozen and carefully preserved seed culture in large bioreactors and optimal handling in order to prevent any decline in cell viability. As shown by the very low numbers of SNPs found in both isolates, opportunities for evolutionary change in the culture over time are purposely kept to a minimum. It is noteworthy that in the course of this analysis, we identified two sequencing errors present in the original L. rhamnosus GG genome sequence (10), located at bp 615483 (T>C) and bp 1883242 (C>A). The deposited NCBI GenBank sequence (accession number FM179322) was corrected accordingly.
Table 2.
Summary of SNPs identified in L. rhamnosus strains LrV and LrIb
| SNP coordinate in GG | Mutationa |
Nucleotide change | Gene | aa change | Description | |
|---|---|---|---|---|---|---|
| LrI | LrV | |||||
| 1030390 | × | × | T→G | LGG_01017 | H294Q | Lipoprotein |
| 1373568 | × | G→A | NA | NA | Intercistronic region between converging LGG_1372 (conserved protein) and LGG_1371 (conserved protein) | |
| 2649651 | × | G→T | NA | NA | Intercistronic region upstream of LGG_01853, ABC transporter, substrate-binding protein | |
| 2765383 | × | × | G→A | LGG_02701 | H98N | Maltose-6′-phosphate glucosidase GlvA |
× indicates the strain in which the mutation occurred.
NA, not applicable; aa, amino acid.
As no genomic information relating to the L. casei strains used in Actimel and Yakult was available, all SOLiD reads of strains LcA and LcY were mapped to the genome of L. casei strain BL23 (22). This showed that L. casei BL23 and strain LcA isolated from Actimel are highly similar. Using the annotation transfer method (39), all genes of strain BL23 were found to be potentially present in L. casei LcA, including the identical spaCBA-srtC gene cluster. A closer look at the consensus sequence shows that there were only 158 undetermined nucleotides, suggesting potential SNPs or InDels. These were not further addressed in this study, as they need more extensive high-resolution sequence analysis. Using a similar approach, a total of 34 genes from L. casei BL23 were not shared with strain LcY isolated from Yakult, indicating a further phylogenetic distance than strain LcA isolated from the Actimel product, which is reflected at a functional level when comparing the metabolic capacities of the L. casei strains (Fig. 1). The genes lacking in L. casei strain LcY include 34 genes encoding a prophage (see Table S1 in the supplemental material). However, the spaCBA-srtC gene cluster was intact in the genome of L. casei LcY. In addition, the large number of approximately 70 undefined nucleotides suggested more SNPs and InDels that differentiated L. casei strains BL23 and LcY. With the genomic resequencing approaches used here, we could not identify genes not present in BL23, but a more comprehensive high-resolution sequence analysis of all four strains, which is presently ongoing, indicated that we were able to cover 99 and 97% of the genomic information for L. casei strains LcA and LcY isolated from the Actimel and Yakult products, respectively (our unpublished data).
Identification of the transcriptional start site of the spaCBA pilus operon.
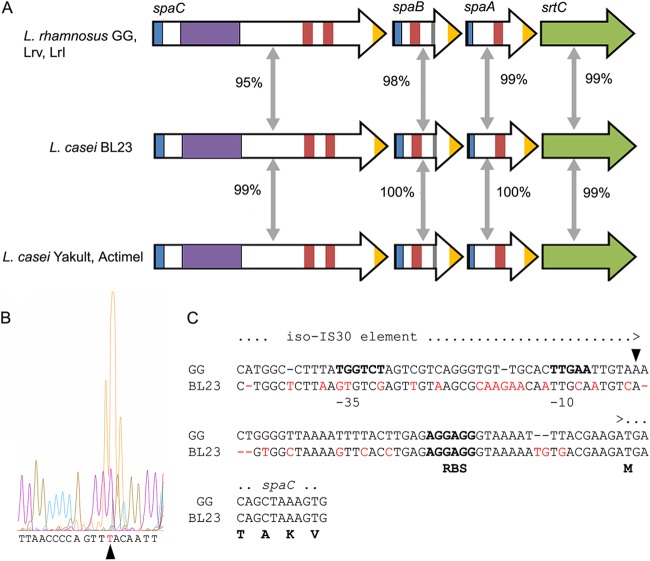
As all of the tested L. rhamnosus strains but none of the L. casei strains produced pili, in spite of the high level of conservation and sequence identity of the spaCBA-srtC pilus gene cluster (Fig. 8), we inspected the sequence upstream of this gene cluster (Fig. 8). A number of differences were evident, which may affect the expression of the pilus genes, notably those in the presumed promoter region. To define the transcription initiation of this cluster, we performed primer extension analysis, which resulted in the identification of the promoter region of the spaCBA pilus gene cluster in L. rhamnosus GG. We observed that the transcriptional start site is located 47 nucleotides upstream of the spaC start codon (ATG). Putative −10 and −35 regions were proposed (Fig. 8). Interestingly, the putative promoter region identified in L. rhamnosus GG differs significantly from the sequences present in the L. casei strains: the differences result in a loss of the consensus −35 and −10 regions and the transcriptional start site (TSS). In spite of the fact that the L. rhamnosus promoter sequence does not resemble the canonical promoter, it shows high levels of expression of the pilus genes. The possibility that alternative sigma factors are used by this promoter is unlikely, as these have not been identified as a major control system in LAB (52). Moreover, the spacing between the predicted Shine-Dalgarno sequence and the initiation codon of the spaC gene is 2 nucleotides longer in the L. casei genomes, suggesting that apart from a transcriptional defect, translation would also be less efficient than that in L. rhamnosus strains. This would explain the absence of any detectable pili in the L. casei strains and correlate with the absence of mucus binding. In L. rhamnosus GG and the virtually identical strains LrV and LrI, an insertion sequence (IS) element is present upstream of the spaC gene, in contrast to the L. casei strains (27), suggesting that the integration of the IS element resulted in the activation of pilus gene expression in these strains but not in the L. casei strains. Such transcriptional activation is reminiscent of various other bacterial systems where gene expression is enhanced or altered by the introduction of IS elements (53, 54).
Fig 8.
Comparison of SpaCBA pilus clusters from L. rhamnosus and L. casei strains. (A) BLAST results and corresponding amino acid conservation percentages for each gene. The presence of different motifs is color/pattern-coded as follows: green arrow for sortase, white arrow for pilus subunit, blue for secretion signal, yellow for the LPxTG motif, purple for the von Willebrand type A domain, and red for the Cna protein B-type domain. (B) Primer extension analysis of the SpaCBA pilus promoter. The peaks (yellow) detected during the analysis are indicated on the sequencing chromatogram. (C) Sequence alignment of the upstream region of the spaC gene in L. casei and L. rhamnosus strains with the position of the transcriptional start site, the putative −10 and −35 regions, and the ribosome-binding site (RBS). Nucleotides highlighted in red in the L. casei BL23 sequence differ from GG.
Conclusions.
We characterized four probiotic-marketed strains at the genomic and phenotypic levels. The two L. rhamnosus strains, LrV and LrI, were virtually similar to GG in terms of genomes and phenotypes, showing the product stability and integrity of the widely used probiotic strain L. rhamnosus GG. Remarkably, the identification of SNPs in the genomes of the L. rhamnosus strains isolated from the Vifit and Idoform probiotic products may indicate the presence of hot spots for mutations. The two L. casei strains isolated showed more heterogeneity than L. casei BL23 regarding genome content and carbohydrate utilization. Interestingly, when looking at the presence of SpaCBA pilus structures in L. rhamnosus and L. casei strains by immunoblotting analysis, electron microscopy, and mucus-binding assays, only L. rhamnosus strains displayed functional pili that correlated with their mucus-binding abilities and possibly are responsible for the TLR2 response. Although characterized under in vitro conditions, our data indicate that L. rhamnosus strains are and remain piliated under different stress conditions, i.e., bile salts and low pH. In contrast, no expression of pili in L. casei could be observed under the conditions tested, even when mimicking intestinal tract conditions. This finding indicates that L. casei strains are not piliated when consumed by humans and may remain so when transiting in the intestinal tract. Although there is a possibility that environmental or stress factors might trigger pilus expression in L. casei under in vivo conditions, our present phenotypic and genomic data indicate that it is unlikely. The identification of the transcriptional start site of the spaCBA operon also suggested that the expression of pili was triggered by the insertion of an IS element in L. rhamnosus strains, in contrast to L. casei strains. The transposition of an IS element upstream of the spaC gene appeared to have a significant impact on the evolution of L. rhamnosus species by conferring a beneficial trait to colonize and persist in mucosa-associated niches, such as the human gastrointestinal tract.
ACKNOWLEDGMENTS
The present study was financially supported by the ERC grant Microbes Inside (grant 250172), the Center of Excellence in Microbial Food Safety Research (CoE-MiFoSa), the Academy of Finland (grant 141140), and the University of Helsinki. François P. Douillard was sponsored by a postdoctoral research fellowship from the Academy of Finland (grant 252123).
We are also grateful to Pia Rasinkangas, Elina Nummenmaa, Päivi Laamanen, Kirsi Lipponen, and Eeva-Marja Turkki for excellent technical assistance and Edward Alatalo for help in bioinformatics. We thank Vincente Monedero from the Institute of Agrochemistry and Food Technology (Valencia, Spain) for kindly providing L. casei strain BL23. We thank Satu Vesterlund, University of Turku, and Heikki Huhtinen, Turku University Central Hospital, Turku, Finland, for collecting the human intestinal mucus samples.
Footnotes
Published ahead of print 11 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03467-12.
REFERENCES
- 1. Kandler O. 1983. Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 49:209–224 [DOI] [PubMed] [Google Scholar]
- 2. de Vos WM. 2011. Systems solutions by lactic acid bacteria: from paradigms to practice. Microb. Cell Fact. 10(Suppl 1):S2 doi:10.1186/1475-2859-10-S1-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. FAO/WHO 2002. Guidelines for the evaluation of probiotics in food. World Health Organization, Geneva, Switzerland [Google Scholar]
- 4. Kant R, Blom J, Palva A, Siezen RJ, de Vos WM. 2011. Comparative genomics of Lactobacillus. Microb. Biotechnol. 4:323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saxelin M, Tynkkynen S, Mattila-Sandholm T, de Vos WM. 2005. Probiotic and other functional microbes: from markets to mechanisms. Curr. Opin. Biotechnol. 16:204–211 [DOI] [PubMed] [Google Scholar]
- 6. Marco ML, Pavan S, Kleerebezem M. 2006. Towards understanding molecular modes of probiotic action. Curr. Opin. Biotechnol. 17:204–210 [DOI] [PubMed] [Google Scholar]
- 7. Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. 2003. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 361:1869–1871 [DOI] [PubMed] [Google Scholar]
- 8. Szajewska H, Mrukowicz JZ. 2001. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J. Pediatr. Gastroenterol. Nutr. 33(Suppl 2):S17–S25 doi:10.1097/00005176-200110002-00004 [DOI] [PubMed] [Google Scholar]
- 9. Ventura M, O'Flaherty S, Claesson MJ, Turroni F, Klaenhammer TR, van Sinderen D, O'Toole PW. 2009. Genome-scale analyses of health-promoting bacteria: probiogenomics. Nat. Rev. Microbiol. 7:61–71 [DOI] [PubMed] [Google Scholar]
- 10. Kankainen M, Paulin L, Tynkkynen S, von Ossowski I, Reunanen J, Partanen P, Satokari R, Vesterlund S, Hendrickx APA, Lebeer S, De Keersmaecker SCJ, Vanderleyden J, Hamalainen T, Laukkanen S, Salovuori N, Ritari J, Alatalo E, Korpela R, Mattila-Sandholm T, Lassig A, Hatakka K, Kinnunen KT, Karjalainen H, Saxelin M, Laakso K, Surakka A, Palva A, Salusjarvi T, Auvinen P, de Vos WM. 2009. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc. Natl. Acad. Sci. U. S. A. 106:17193–17198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lebeer S, Claes IJJ, Verhoeven TLA, Vanderleyden J, De Keersmaecker SCJ. 2011. Exopolysaccharides of Lactobacillus rhamnosus GG form a protective shield against innate immune factors in the intestine. Microb. Biotechnol. 4:368–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. 2007. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132:562–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Claes IJ, Schoofs G, Regulski K, Courtin P, Chapot-Chartier MP, Rolain T, Hols P, von Ossowski I, Reunanen J, de Vos WM, Palva A, Vanderleyden J, De Keersmaecker SC, Lebeer S. 2012. Genetic and biochemical characterization of the cell wall hydrolase activity of the major secreted protein of Lactobacillus rhamnosus GG. PLoS One 7:e31588 doi:10.1371/journal.pone.0031588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lebeer S, Claes IJ, Balog CI, Schoofs G, Verhoeven TL, Nys K, von Ossowski I, de Vos WM, Tytgat HL, Agostinis P, Palva A, Van Damme EJ, Deelder AM, De Keersmaecker SC, Wuhrer M, Vanderleyden J. 2012. The major secreted protein MspI/p75 is O-glycosylated in Lactobacillus rhamnosus GG. Microb. Cell Fact. 11:15 doi:10.1186/1475-2859-11-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. von Ossowski I, Satokari R, Reunanen J, Lebeer S, De Keersmaecker SCJ, Vanderleyden J, de Vos WM, Palva A. 2011. Functional characterization of a mucus-specific LPXTG surface adhesin from probiotic Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 77:4465–4472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Velez MP, Petrova MI, Lebeer S, Verhoeven TLA, Claes I, Lambrichts I, Tynkkynen S, Vanderleyden J, De Keersmaecker SCJ. 2010. Characterization of MabA, a modulator of Lactobacillus rhamnosus GG adhesion and biofilm formation. FEMS Immunol. Med. Microbiol. 59:386–398 [DOI] [PubMed] [Google Scholar]
- 17. Reunanen J, von Ossowski I, Hendrickx APA, Palva A, de Vos WM. 2012. Characterization of the SpaCBA pilus fibers in the probiotic Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 78:2337–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. von Ossowski I, Reunanen J, Satokari R, Vesterlund S, Kankainen M, Huhtinen H, Tynkkynen S, Salminen S, de Vos WM, Palva A. 2010. Mucosal adhesion properties of the probiotic Lactobacillus rhamnosus GG SpaCBA and SpaFED pilin subunits. Appl. Environ. Microbiol. 76:2049–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bauerl C, Perez-Martinez G, Yan F, Polk DB, Monedero V. 2010. Functional analysis of the p40 and p75 proteins from Lactobacillus casei BL23. J. Mol. Microbiol. Biotechnol. 19:231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Regulski K, Courtin P, Meyrand M, Claes IJ, Lebeer S, Vanderleyden J, Hols P, Guillot A, Chapot-Chartier MP. 2012. Analysis of the peptidoglycan hydrolase complement of Lactobacillus casei and characterization of the major gamma-D-glutamyl-L-lysyl-endopeptidase. PLoS One 7:e32301 doi:10.1371/journal.pone.0032301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Makarova K, Slesarev A, Wolf Y, Sorokin A, Mirkin B, Koonin E, Pavlov A, Pavlova N, Karamychev V, Polouchine N, Shakhova V, Grigoriev I, Lou Y, Rohksar D, Lucas S, Huang K, Goodstein DM, Hawkins T, Plengvidhya V, Welker D, Hughes J, Goh Y, Benson A, Baldwin K, Lee JH, Diaz-Muniz I, Dosti B, Smeianov V, Wechter W, Barabote R, Lorca G, Altermann E, Barrangou R, Ganesan B, Xie Y, Rawsthorne H, Tamir D, Parker C, Breidt F, Broadbent J, Hutkins R, O'Sullivan D, Steele J, Unlu G, Saier M, Klaenhammer T, Richardson P, Kozyavkin S, Weimer B, Mills D. 2006. Comparative genomics of the lactic acid bacteria. Proc. Natl. Acad. Sci. U. S. A. 103:15611–15616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maze A, Boel G, Zuniga M, Bourand A, Loux V, Yebra MJ, Monedero V, Correia K, Jacques N, Beaufils S, Poncet S, Joyet P, Milohanic E, Casaregola S, Auffray Y, Perez-Martinez G, Gibrat JF, Zagorec M, Francke C, Hartke A, Deutscher J. 2010. Complete genome sequence of the probiotic Lactobacillus casei strain BL23. J. Bacteriol. 192:2647–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang WY, Yu DL, Sun ZH, Wu RN, Chen X, Chen W, Meng H, Hu SN, Zhang HP. 2010. Complete genome sequence of Lactobacillus casei Zhang, a new probiotic strain isolated from traditional homemade koumiss in Inner Mongolia, China. J. Bacteriol. 192:5268–5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen C, Ai LZ, Zhou FF, Wang L, Zhang H, Chen W, Guo BH. 2011. Complete genome sequence of the probiotic bacterium Lactobacillus casei LC2W. J. Bacteriol. 193:3419–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hochwind K, Weinmaier T, Schmid M, van Hemert S, Hartmann A, Rattei T, Rothballer M. 2012. Draft genome sequence of Lactobacillus casei W56. J. Bacteriol. 194:6638 doi:10.1128/JB.01386-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ai LZ, Chen C, Zhou FF, Wang L, Zhang H, Chen W, Guo BH. 2011. Complete genome sequence of the probiotic strain Lactobacillus casei BD-II. J. Bacteriol. 193:3160–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Broadbent J, Neeno-Eckwall E, Stahl B, Tandee K, Cai H, Morovic W, Horvath P, Heidenreich J, Perna N, Barrangou R, Steele J. 2012. Analysis of the Lactobacillus casei supragenome and its influence in species evolution and lifestyle adaptation. BMC Genomics 13:533 doi:10.1186/1471-2164-13-533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cai H, Thompson R, Budinich M, Broadbent JR, Steele JL. 2009. Genome sequence and comparative genome analysis of Lactobacillus casei: insights into their niche-associated evolution. Genome Biol. Evol. 1:239–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, Dewulf J, Brassart D, Mercenier A, Pot B. 2007. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J. Gastroenterol. 13:236–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Munoz-Provencio D, Perez-Martinez G, Monedero V. 2010. Characterization of a fibronectin-binding protein from Lactobacillus casei BL23. J. Appl. Microbiol. 108:1050–1059 [DOI] [PubMed] [Google Scholar]
- 31. Alcántara C, Revilla-Guarinos A, Zúñiga M. 2011. Influence of two-component signal transduction systems of Lactobacillus casei BL23 on tolerance to stress conditions. Appl. Environ. Microbiol. 77:1516–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alcántara C, Zúñiga M. 2012. Proteomic and transcriptomic analysis of the response to bile stress of Lactobacillus casei BL23. Microbiology 158:1206–1218 [DOI] [PubMed] [Google Scholar]
- 33. Broadbent JR, Larsen RL, Deibel V, Steele JL. 2010. Physiological and transcriptional response of Lactobacillus casei ATCC 334 to acid stress. J. Bacteriol. 192:2445–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hamon E, Horvatovich P, Bisch M, Bringel F, Marchioni E, Aoude-Werner D, Ennahar S. 2012. Investigation of biomarkers of bile tolerance in Lactobacillus casei using comparative proteomics. J. Proteome Res. 11:109–118 [DOI] [PubMed] [Google Scholar]
- 35. Hansen PA, Lessel EF. 1971. Lactobacillus casei (Orla-Jensen) comb. nov. Int. J. Syst. Bacteriol. 21:69–71 [Google Scholar]
- 36. Ventura M, Canchaya C, Meylan V, Klaenhammer TR, Zink R. 2003. Analysis, characterization, and loci of the tuf genes in Lactobacillus and Bifidobacterium species and their direct application for species identification. Appl. Environ. Microbiol. 69:6908–6922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vesterlund S, Paltta J, Karp M, Ouwehand AC. 2005. Measurement of bacterial adhesion—in vitro evaluation of different methods. J. Microbiol. Methods 60:225–233 [DOI] [PubMed] [Google Scholar]
- 38. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kurtz S, Phillippy A, Delcher A, Smoot M, Shumway M, Antonescu C, Salzberg S. 2004. Versatile and open software for comparing large genomes. Genome Biol. 5:R12 doi:10.1186/gb-2004-5-2-r12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lerat E, Daubin V, Moran NA. 2003. From gene trees to organismal phylogeny in prokaryotes: the case of the γ-proteobacteria. PLoS Biol. 1:E19 doi:10.1371/journal.pbio.0000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tu WY, Pohl S, Gizynski K, Harwood CR. 2012. The iron-binding protein Dps2 confers peroxide stress resistance on Bacillus anthracis. J. Bacteriol. 194:925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jacobsen CN, Rosenfeldt Nielsen V, Hayford AE, Moller PL, Michaelsen KF, Paerregaard A, Sandstrom B, Tvede M, Jakobsen M. 1999. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 65:4949–4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koskenniemi K, Laakso K, Koponen J, Kankainen M, Greco D, Auvinen P, Savijoki K, Nyman TA, Surakka A, Salusjarvi T, de Vos WM, Tynkkynen S, Kalkkinen N, Varmanen P. 2011. Proteomics and transcriptomics characterization of bile stress response in probiotic Lactobacillus rhamnosus GG. Mol. Cell. Proteomics 10:M110.002741. doi:10.1074/mcp.M110.002741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ouwehand AC, Kirjavainen PV, Grönlund M-M, Isolauri E, Salminen SJ. 1999. Adhesion of probiotic micro-organisms to intestinal mucus. Int. Dairy J. 9:623–630 [Google Scholar]
- 46. Lebeer S, Claes I, Tytgat HL, Verhoeven TL, Marien E, von Ossowski I, Reunanen J, Palva A, Vos WM, Keersmaecker SC, Vanderleyden J. 2012. Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl. Environ. Microbiol. 78:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. O'Connell Motherway M, Zomer A, Leahy SC, Reunanen J, Bottacini F, Claesson MJ, O'Brien F, Flynn K, Casey PG, Moreno Munoz JA, Kearney B, Houston AM, O'Mahony C, Higgins DG, Shanahan F, Palva A, de Vos WM, Fitzgerald GF, Ventura M, O'Toole PW, van Sinderen D. 2011. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc. Natl. Acad. Sci. U. S. A. 108:11217–11222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yebra MJ, Veyrat A, Santos MA, Pérez-Martínez G. 2000. Genetics of l-sorbose transport and metabolism in Lactobacillus casei. J. Bacteriol. 182:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lebeer S, Vanderleyden J, De Keersmaecker SC. 2010. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat. Rev. Microbiol. 8:171–184 [DOI] [PubMed] [Google Scholar]
- 50. Bormann Chung CA, Boyd VL, McKernan KJ, Fu Y, Monighetti C, Peckham HE, Barker M. 2010. Whole methylome analysis by ultra-deep sequencing using two-base encoding. PLoS One 5:e9320 doi:10.1371/journal.pone.0009320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang W, Messing J. 2011. High-throughput sequencing of three Lemnoideae (duckweeds) chloroplast genomes from total DNA. PLoS One 6:e24670 doi:10.1371/journal.pone.0024670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stevens MJA, Molenaar D, de Jong A, De Vos WM, Kleerebezem M. 2010. σ54-mediated control of the mannose phosphotransferase system in Lactobacillus plantarum impacts on carbohydrate metabolism. Microbiology 156:695–707 [DOI] [PubMed] [Google Scholar]
- 53. Poirel L, Decousser J-W, Nordmann P. 2003. Insertion sequence ISEcp1B is involved in expression and mobilization of a blaCTX-M β-lactamase gene. Antimicrob. Agents Chemother. 47:2938–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reynolds AE, Mahadevan S, LeGrice SF, Wright A. 1986. Enhancement of bacterial gene expression by insertion elements or by mutation in a CAP-cAMP binding site. J. Mol. Biol. 191:85–95 [DOI] [PubMed] [Google Scholar]
- 55. Carver TJ, Rutherford KM, Berriman M, Rajandream M-A, Barrell BG, Parkhill J. 2005. ACT: the Artemis comparison tool. Bioinformatics 21:3422–3423 [DOI] [PubMed] [Google Scholar]
- 56. Krumsiek J, Arnold R, Rattei T. 2007. Gepard: a rapid and sensitive tool for creating dotplots on genome scale. Bioinformatics 23:1026–1028 [DOI] [PubMed] [Google Scholar]
- 57. Monedero V, Yebra MJ, Poncet S, Deutscher J. 2008. Maltose transport in Lactobacillus casei and its regulation by inducer exclusion. Res. Microbiol. 159:94–102 [DOI] [PubMed] [Google Scholar]
- 58. Gosalbes MJ, Monedero V, Péresz-Martínez G. 1999. Elements involved in catabolite repression and substrate induction of the lactose operon in Lactobaccillus casei. J. Bacteriol. 181:3928–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]