Abstract
Deoxynivalenol (DON) is a potent mycotoxin produced by Fusarium molds and affects intestinal nutrient absorption and barrier function in experimental and farm animals. Free DON and the plant metabolite DON-3-β-d-glucoside (D3G) are frequently found in wheat and maize. D3G is stable in the upper human gut, but some human intestinal bacteria release DON from D3G in vitro. Furthermore, some bacteria derived from animal digestive systems degrade DON to a less toxic metabolite, deepoxy-deoxynivalenol (DOM-1). The metabolism of D3G and DON by the human microbiota has not been fully assessed. We therefore conducted in vitro batch culture experiments assessing the activity of the human fecal microbiota to release DON from D3G. We also studied detoxification of DON to DOM-1 by the microbiota and its potential effect on urinary DON excretion in humans. Fecal slurry from five volunteers was spiked with DON or D3G and incubated anaerobically (from 1 h to 7 days), and mycotoxins were extracted into acetonitrile. Mycotoxins were detected in fecal extracts and urine by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The fecal microbiota released DON from D3G very efficiently, with hydrolysis peaking after 4 to 6 h. The fecal microbiota from one volunteer transformed DON to DOM-1. Urine from the same volunteer also contained DOM-1 (4.7% of DON), whereas DOM-1 was not detectable in urine from other volunteers. Our results confirm that the fecal microbiota releases DON from its glycosylated form, hence increasing the toxic burden in exposed individuals. Furthermore, this is first evidence that the human fecal microbiota of one volunteer detoxifies DON, resulting in the appearance of DOM-1 in urine.
INTRODUCTION
Mycotoxins are common food contaminants in most regions of the world. Deoxynivalenol (DON) is produced by Fusarium molds that frequently contaminate wheat, corn, and barley grown in temperate climates in Europe, South and North America, and some parts of Asia (1, 2). DON is a potent mycotoxin and induces severe intestinal symptoms and feed refusal in farm animals when fed at high concentrations (3). On a molecular level, DON is ribotoxic and hampers protein synthesis in cells. In the intestinal epithelium, DON interferes with the differentiation of enterocytes and disrupts intestinal barrier function (4–7). Advances in mycotoxin analysis have revealed that in addition to the free mycotoxin, cereal samples also contain DON-3-β-d-glucoside (D3G), which derives from plant phase II metabolism of DON (8). A recent study has found that all cereal samples that were found to be contaminated with DON also contained D3G at levels ranging between 7 and 29% of detected DON in wheat and 5 and 46% of detected DON in maize (9). This suggests that the mycotoxin burden in cereals could be between 14 and 17% higher if these “masked” mycotoxins are taken into account. A recent in vitro study assessed the release of DON from D3G under simulated human intestinal conditions (10). Authors found D3G to be stable under acidic pH and resistant to digestive pancreatic enzymes and human cytosolic β-glucosidase. Incubations of D3G with selected bacterial strains derived from the human microbiota showed that some bacteria were able to hydrolyze D3G and release free DON. Among the strains tested, Lactobacillus plantarum, Enterococcus faecium, Enterococcus mundtii, Enterococcus durans, and Enterobacter cloacae were highly active and released between 6 and 66% of D3G as DON after 8 h of incubation. However, the hydrolytic activity of a mixed human microbiota has not been assessed.
The gut microbiota of animals has been shown to be capable of another conversion of DON, namely, the degradation of DON to the less toxic deepoxide metabolite deepoxy-deoxynivalenol (DOM-1) (11, 12). Bacterial strains isolated from chicken digesta have so far been found to possess this activity (13, 14). A study by Eriksen and colleagues (15) found that the fecal microbiota from pigs reared in a farm environment converted 100% of 3-acetyl-DON to DOM-1 within 48 h of incubation in McDougall buffer. The same group has investigated the ability of the human fecal microbiota to metabolize 3-acetyl-DON (16), and this study found efficient deacetylation but no deepoxidation activity in human fecal samples (n = 10). The culture conditions used in this study (lack of nutrients) might have not supported optimal bacterial growth over the 48 h of incubation time. We therefore conducted in vitro fecal batch culture experiments assessing the ability of the human fecal microbiota to release DON from D3G and to detoxify DON into the less toxic DOM-1. Furthermore, we collected urine samples from the same volunteers and analyzed them for the presence of DON and DOM-1.
MATERIALS AND METHODS
Mycotoxin reference standards in acetonitrile (DON, D3G, and 13C15-DON from Romer Labs, Runcorn, United Kingdom, and DOM-1 from Sigma-Aldrich, Ltd., Poole, United Kingdom) were used to develop a liquid chromatography-tandem mass spectrometry (LC-MS/MS) methodology. Additionally, working stocks of DON in acetonitrile prepared from powdered DON (Molekula, Gillingham, United Kingdom) were used in fecal incubation experiments following quantification and purity verification by LC-MS/MS.
Volunteers.
Spot fecal and urine samples were collected from 5 volunteers (2 males and 3 females). This study was ethically approved by the Rowett Institute's Ethics Review Panel following favorable consideration by the North of Scotland Research Ethics Committee. All volunteers received a participant information sheet and granted written consent before participating in the study.
Fecal batch culture experiments.
Freshly collected fecal samples were placed in a stomacher bag under CO2 and mixed for 5 min to ensure a homogenous composition. Fecal samples were diluted 1/10 under CO2 with anaerobic M2 medium (17) with the omission of agar and the inclusion at 0.1% of l-cysteine HCl-H2O as reducing agent. The dilutions were vortexed for 1 min, and 1-ml aliquots of slurry were transferred to sterile screw-cap Hungate tubes fitted with butyl septum stoppers (Bellco Glass, Inc., Vineland, NJ) while maintaining anaerobic conditions. In duplicate, 1 ml of fecal slurry was spiked with 1 μg/ml DON or 1 μg/ml D3G in acetonitrile (resulting in a maximum solvent concentration of 2% in all incubations) and incubated anaerobically at 37°C for various periods (from 1 h to 7 days). Samples were then transferred into 15-ml centrifuge tubes, extracted with 3 ml of acetonitrile (vortexed for 1 min), and centrifuged at 2,000 × g for 5 min. The supernatants were transferred to glass Pyrex tubes, evaporated at 50°C under nitrogen, and reconstituted (vortexed for 30 s) in 1 ml of water. The samples were then passed through C18 columns (Agilent, Wokingham, United Kingdom) after preparation of the columns with two 2-ml volumes of methanol and two 2-ml volumes of water. The columns were washed with two 2-ml volumes of water and eluted with 3 ml methanol into glass Pyrex tubes. The methanol eluent was evaporated at 50°C under nitrogen and reconstituted (vortexed for 30 s) in 1 ml of 50% aqueous methanol for LC-MS/MS analysis. Blank fecal incubations were included in all experiments, and no mycotoxins were detectable. Method development was performed by spiking M2 culture medium with DON, D3G, and DOM-1 at 2 concentrations (1 and 0.1 μg/ml) either prior to extraction (spiked M2) or after extraction (spiked M2 extract). Extraction recovery (RE) was calculated using a simplified method from De Baere et al. (19): RE = RA × 100/SSE, where RA (apparent recovery) = concentration of spiked M2 × 100/concentration of standard and SSE (signal suppression/enhancement) = concentration of spiked M2 extract × 100/concentration of standard. Extraction recoveries were 101.1% for DON, 106.3% for D3G, and 100.3% for DOM-1. This was confirmed in fecal slurries from 2 volunteers resulting in extraction recoveries for DON, D3G, and DOM-1 of 91.4% ± 5.8%, 80.1% ± 9.7%, and 116.2% ± 0.7%, respectively. However, signal suppression was substantial, leading to low apparent recoveries (6.6 to 19.3%). Hence, spiked fecal slurries (containing DON, D3G, or DOM-1) without incubation (time zero) were included in all experiments to act as reference points. All results were calculated by comparing mycotoxin concentrations in fecal slurry after incubation to mycotoxin concentrations in fecal slurry at time zero and are expressed as percentages.
Repeatability of extraction was established in 3 independent experiments spiking M2 culture medium with DON, D3G, and DOM-1 at 0.1 and 1 μg/ml. The average RAs of three experiments were 17.9% ± 0.5% and 19.1% ± 0.9% for DON, 7.8% ± 0.8% and 7.6% ± 0.2% for D3G, and 10.3% ± 0.4% and 11.5% ± 0.7% for DOM-1 at 0.1 and 1 μg/ml, respectively. The repeatability was excellent, with relative standard deviations (SD) of between 2.7 and 9.9% for all mycotoxin metabolites at both spiking concentrations. Furthermore, the stability of D3G was assessed in pure M2 culture medium and in autoclaved fecal slurry over 48 h. D3G was recovered at 100.5% ± 3.7% and 100.2% ± 4.7% from M2 and 97.0% ± 1.4% and 89.0% ± 0.7% from fecal slurry after 24 and 48 h of incubation.
Urinary DON and DOM-1.
Urinary DON and DOM-1 were analyzed using the method of Turner et al. (18). In brief, urine was centrifuged (2,000 × g, 15 min, 4°C) and 4 ml of urine was spiked with 13C15-DON as the internal standard (5 ng/ml). Following overnight incubation with β-glucuronidase (23,000 U in 1 ml 75 mM KH2PO4 buffer at pH 6.8) to release glucuronidated DON and DOM-1, samples were centrifuged (2,000 × g, 15 min, 4°C), diluted to 16 ml with phosphate-buffered saline (PBS) (pH 7.2), and passed through immunoaffinity columns (DONtest WB; Vicam). Following a washing step, bound DON and DOM-1 were eluted with 4 ml methanol into glass Pyrex tubes, dried under a nitrogen stream, and reconstituted in 250 μl 10% aqueous ethanol for LC-MS/MS analysis.
LC-MS/MS analysis.
The liquid chromatography separation of the mycotoxin metabolites was performed on an Agilent 1200 high-performance liquid chromatography (HPLC) system (Agilent Technologies, Wokingham, United Kingdom) with the metabolites separated on an Agilent Zorbax 5-μm, 150-mm by 4.6-mm C18 column. The mobile phase solvents were 0.1% acetic acid (A) and methanol (B) for fecal incubation experiments (19) and water (A) and methanol (B) for urine analysis. The starting gradient was 25% B, which rose linearly to 95% B over 10 min, was held at 95% B for 2 min, and then returned to 25% B and was reequilibrated for 5 min. The flow rate was 400 μl/min, and the injection volume was 35 μl. The LC eluent was directed into, without splitting, a Q-Trap 4000 triple-quadrupole mass spectrometer (AB Sciex, Warrington, United Kingdom) fitted with a TurboV ion spray (TIS) source. The mass spectrometer was run in negative-ion mode with the following source settings: ion spray voltage, −4,000; temperature, 200°C; gases 1 and 2 set at 24 and 40, respectively; and the curtain gas set at 10. Mycotoxins were quantified using the multiple-reaction monitoring (MRM) technique. Standard solutions of 500 ng/ml were pumped directly into the TIS via a syringe, and their transition values were optimized. Calibration curves were prepared for each metabolite by preparation of a series of standards ranging from 0.1 to 500 ng/ml. For fecal analysis, no internal standard was used and the mobile phase contained acetic acid (19). Therefore, the precursor ions were used as [M+Ac]− and the transitions for DON, DOM-1, and D3G were 355.1 → 265.1, 339.1 → 249.1, and 517.3 → 427.3, respectively.
For urine analysis, 13C15-DON was used as the internal standard and standard curves contained the internal standard at 80 ng/ml. The transitions for DON, DOM-1, and 13C15-DON from urine under neutral conditions were 295.1 → 265.1, 279.1 → 249.1, and 310.1 → 279.1, respectively. Limits of detection (LOD) for DON and DOM-1 were established by spiking experiments in PBS. The LOD were 0.10 ng/ml (0.104 ± 0.001 ng/ml) for DON and 0.05 ng/ml (0.048 ± 0.001 ng/ml) for DOM-1. Two quality controls of spiked PBS (10 ng/ml DON and 1 ng/ml DOM-1) were included in the analysis (DON and DOM-1 at 10.501 ± 0.478 and 0.986 ± 0.142 ng/ml, respectively). Urinary DON and DOM-1 concentrations are expressed as ng/ml urine and ng/mg creatinine. Urinary creatinine was analyzed using alkaline picrate solution on an automated clinical analyzer (Konelab 30; Labmedics, Stockport, United Kingdom).
RESULTS
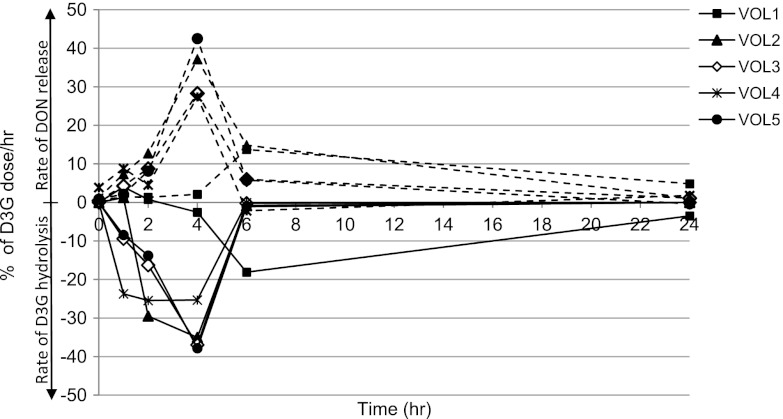
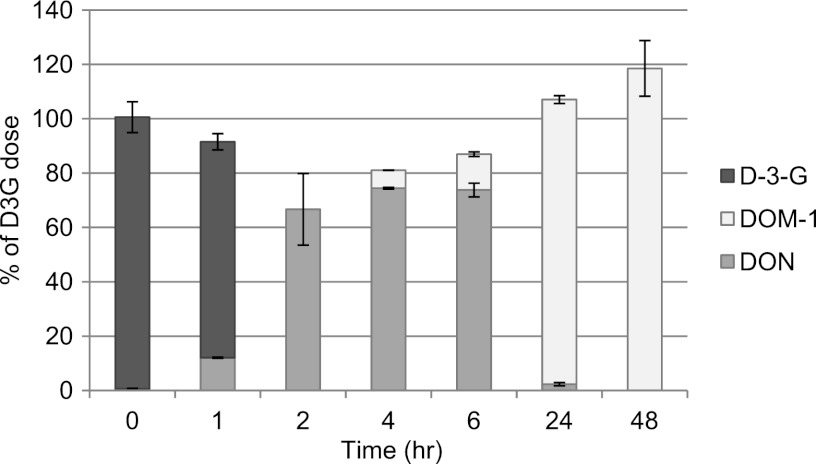
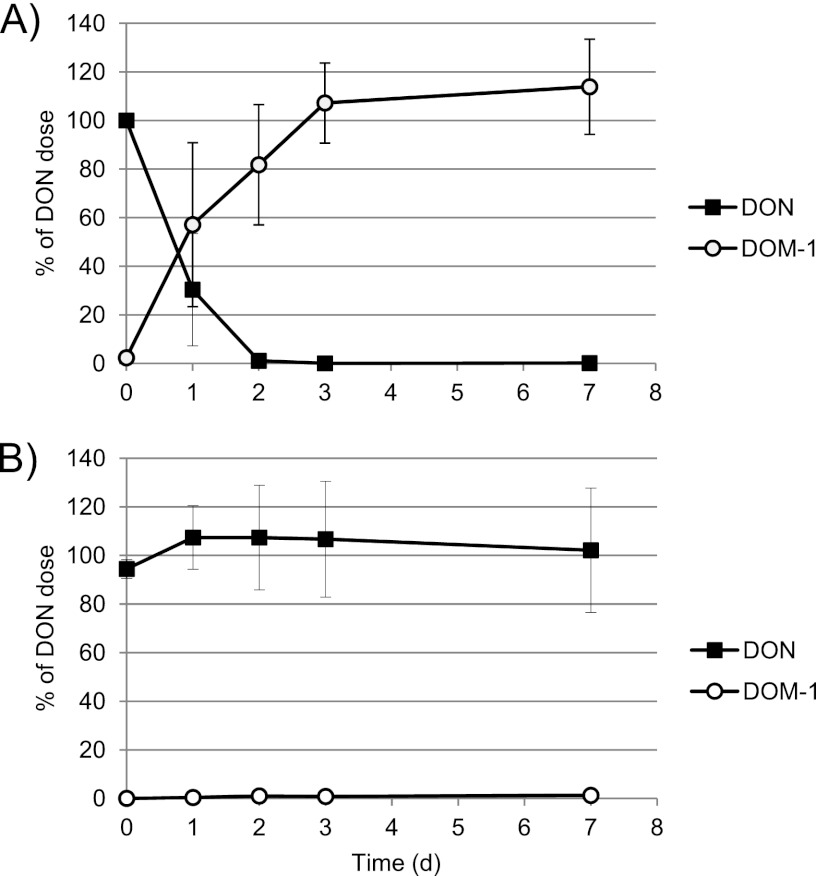
Our results clearly show that the fecal microbiota of all five subjects were able to cleave DON-3-glucoside very rapidly and efficiently. The peak hydrolysis rate varied between 25 and 38% per hour after 4 h of incubation, and 100% of the D3G dose was degraded after 6 h in samples from 4 out of 5 volunteers. However, the microbiota from one volunteer showed a slightly lower rate of hydrolysis of D3G (Fig. 1). Hydrolysis of D3G was mirrored by the release of free DON into the culture medium, with the highest rate of release occurring after 4 h of incubation. The fecal microbiota of one of the 5 volunteers showed rapid release of DON from D3G initially (up to 2 h of incubation), followed by metabolism of DON to DOM-1 starting after 6 h of incubation (Fig. 2). Subsequently, metabolism of DON to DOM-1 was assessed in more detail in fecal incubations from all 5 volunteers for up to 7 days. The metabolism of DON to DOM-1 was observed in all fecal samples collected from volunteer 5 but not in any fecal samples from other volunteers (Fig. 3). The rate of conversion of DON to DOM-1 varied in 4 fecal samples collected from volunteer 5 on different days, with 36 to 107% of the DON dose converted to DOM-1 after 24 h of incubation (average, 57%) (Fig. 3A). Fecal incubations from other volunteers showed that DON was stable for up to 7 days in cultures, and no DOM-1 was produced (Fig. 3B). Analysis of independent spot urine samples collected from each volunteer showed that all volunteers excreted DON in urine (mean 3.62 ± 2.98 ng/ml urine; range, 0.38 to 10.05 ng/ml urine) (Table 1). DOM-1 was only detected in the three urine samples collected from volunteer 5 (0.17, 0.05, and 0.10 ng/ml urine) and represented 4.7, 1.3, and 3.1% of urinary DON, respectively. DOM-1 was below the limit of detection in urine samples from volunteers 1 to 4. Our study is the first to report that the human fecal microbiota of one volunteer detoxifies DON to DOM-1 in vitro. Our work also proves that this detoxification activity of the microbiota results in urinary DOM-1 excretion in the same volunteer.
Fig 1.
Rate of hydrolysis of D3G and release of DON by the fecal microbiota of 5 volunteers (VOL). Data are presented as D3G hydrolyzed per hour (% of D3G dose [solid lines]) and DON released per hour (% of D3G dose [dashed lines]).
Fig 2.
Metabolism of D3G by the human microbiota of volunteer 5. Results are presented as means of duplicate incubations for each time point.
Fig 3.
Kinetics of metabolism of DON to DOM-1 by the fecal microbiota of volunteer 5 (A) and volunteers 1 to 4 (B). Results in panel A are means of 4 independent experiments ± SD. Results in panel B are means of fecal incubations from 4 volunteers ± SD. All fecal incubation experiments were performed in duplicate for each time point.
Table 1.
DON and DOM-1 concentrations detected in urine samples from 5 volunteers
| Volunteer | Collection | Concn of mycotoxina |
||||
|---|---|---|---|---|---|---|
| DON |
DOM-1 |
|||||
| Urine (ng/ml) | Creatinine (ng/mg) | Urine (ng/ml) | Creatinine (ng/mg) | % of DON | ||
| 1 | A | 1.55 ± 0.17 | 2.99 ± 0.33 | ND | ND | |
| B | 1.65 ± 0.06 | 3.70 ± 0.14 | ND | ND | ||
| 2 | A | 2.88 ± 0.10 | 4.95 ± 0.17 | ND | ND | |
| B | 2.59 ± 0.06 | 2.17 ± 0.05 | ND | ND | ||
| 3 | A | 0.38 ± 0.02 | 0.93 ± 0.04 | ND | ND | |
| B | 1.66 ± 0.08 | 2.91 ± 0.10 | ND | ND | ||
| 4 | A | 10.05 ± 0.72 | 11.33 ± 0.81 | ND | ND | |
| B | 8.41 ± 0.51 | 7.62 ± 0.46 | ND | ND | ||
| 5 | A | 3.72 ± 0.21 | 3.80 ± 0.21 | 0.17 ± 0.05 | 0.18 ± 0.05 | 4.7 |
| B | 3.78 ± 0.11 | 3.29 ± 0.10 | 0.05 ± 0.01 | 0.04 ± 0.01 | 1.3 | |
| C | 3.17 ± 0.11 | 2.82 ± 0.10 | 0.10 ± 0.01 | 0.09 ± 0.01 | 3.1 | |
Data are presented as ng mycotoxin/ml urine and ng mycotoxin/mg creatinine. Values are means of quadruplicate analyses (duplicate extractions in 2 independent experiments) of each urine sample ± SD. Limits of detection: DON, 0.10 ng/ml urine; DOM-1, 0.05 ng/ml urine. ND, not detected.
DISCUSSION
Microbial degradation of mycotoxins is widely studied as a potential means to detoxify contaminated crops or to protect the host from toxicities (20). In the human fecal microbiota, no such degrading activity for DON has been reported to date (16, 21). We have further investigated this topic, conducting carefully controlled anaerobic incubations of human mixed fecal microbiota. We have assessed the potential of the fecal microbiota to cleave DON-3-glucoside as it is found in plant materials and to metabolize free DON to its less toxic metabolite, DOM-1. Our study is the first to demonstrate that the mixed fecal microbiota of humans is capable of hydrolyzing D3G. We have found that cleavage of the glycoside bond occurred very rapidly, with on average 80% (range, 5 to 100%) of the D3G dose hydrolyzed after 4 h of incubation. This is substantially higher than the hydrolysis rates reported for individual strains of colonic bacteria (0.2 to 34% of D3G hydrolysis after 4 h) (10), which is not surprising given the complexity of the microbiota and the vast numbers of bacteria present in mixed fecal incubations. This high rate of hydrolysis observed in all 5 volunteers studied suggests that the human microbiota will completely hydrolyze DON-3-glucoside within the normal residence time in the human colon. β-Glycosidase activity is common among intestinal bacteria, and most human colonic microbiota will possess this activity (22). The release of DON from D3G suggests that the colonic epithelium will be exposed to significant levels of free DON, as the masked mycotoxin D3G is present at approximately 17% of free DON in cereal samples (8). By this mechanism, the overall body burden of DON and the exposure of the colonic epithelium in particular are increased. One recent study in rats suggests that D3G is of less toxicological significance than free DON as D3G dosing resulted in lower urinary levels and higher fecal excretion of DON and metabolites than dosing with free DON (23). However, the relevance of these findings to extrapolate the toxicological consequences of D3G in humans is limited, since the metabolic activity of the rodent microbiota could be expected to differ greatly from that of humans.
Human drug-metabolizing enzymes do not degrade DON to protect the body from DON toxicities (21). The potential of microbial DON detoxification is therefore of considerable interest. Our findings are the first to demonstrate that members of the human fecal microbiota, present in one of our volunteers, are capable of detoxifying DON to DOM-1. Detoxification by fecal microbiota was slow (80% and 100% conversion after 48 and 72 h) but comparable to detoxification rates found in chicken digesta (13) and pig feces (15). The fecal microbiota compositions were not different between pigs with and without DON detoxification activity, suggesting that detoxifying bacteria are a low-abundance group within the pig microbiota (15). A similar situation can also be anticipated in the human microbiota. Nevertheless, this detoxification pathway could be biologically relevant in humans since complete DON detoxification occurred within the normal large intestinal transit time of 24 to 48 h. This hypothesis is further supported by the finding that the detoxification activity of the microbiota resulted in urinary DOM-1 excretion in the same volunteer. This volunteer was able to detoxify DON and excreted up to 4.7% of urinary DON as DOM-1, whereas other volunteers did not excrete DOM-1 in their urine. This finding confirms a published report that urinary DOM-1 detected in French farmers represented on average 4.7% of the urinary DON concentration (24). In the same study, DOM-1 was found frequently in urine from French farmers (34% of DON-positive urine samples), whereas DOM-1 was found more rarely in other populations (0 and 3% of DON-positive urine samples from United Kingdom adults [24, 25] and 3% of DON-positive urine samples from Egyptian women [26]). More work is needed to establish the frequency of the occurrence of DON-degrading bacteria in the human fecal microbiota in different populations and to identify these bacteria and the enzymatic pathway necessary for detoxification.
The lack of microbial detoxification activity in most individuals further stresses the fact that the human body is not able to protect itself against toxicities from DON. The toxicological consequences of chronic low-level DON exposure on the intestinal epithelium are poorly understood (3, 7). Epidemiological studies are presently aiming to link DON exposure to adverse health outcomes in humans. A high incidence of squamous cell carcinomas of the esophagus in some parts of China might be linked to high exposure to Fusarium mycotoxins, but no biomarker-driven epidemiological evidence has been published to date (2).
Farm animals are exposed to significantly higher levels of DON than humans, and microbial detoxification of DON has been suggested as a potentially protective mechanism in some species (27). Ruminant animals, for example, are much less susceptible to DON toxicities than monogastric animals (21). This has led to the development of a probiotic feed additive, based on a Eubacterium strain isolated from the bovine rumen, which has been shown to counteract DON toxicities in pig intestines (11). Even though human exposure to DON is expected to be significantly lower, members of the human gut microbiota might still offer modest protection against possible subclinical effects of DON on the intestinal epithelium.
In conclusion, our results clearly confirm that hydrolysis of DON-3-glucoside is a common feature of the human fecal microbiota. We also show that DOM-1, which is occasionally found in urine of individuals exposed to DON, derives from detoxification activity of human microbiota. Our work indicates that a deeper understanding of the role of the human microbiota in the release of masked mycotoxins and their degradation is needed to properly assess dietary mycotoxin exposure and the potential risk posed to humans.
ACKNOWLEDGMENTS
We thank all volunteers for participating in the study.
This work has been funded by the Scottish Government Rural and Environment Science and Analytical Services division (RESAS).
Footnotes
Published ahead of print 11 January 2013
REFERENCES
- 1. Schothorst RC, van Egmond HP. 2004. Report from SCOOP task 3.2.10 “Collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states.” Subtask: trichothecenes. Toxicol. Lett. 153:133–143 [DOI] [PubMed] [Google Scholar]
- 2. Turner PC, Flannery B, Isitt C, Ali M, Pestka J. 2012. The role of biomarkers in evaluating human health concerns from fungal contaminants in food. Nutr. Res. Rev. 25:162–179 [DOI] [PubMed] [Google Scholar]
- 3. Pestka JJ. 2010. Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 84:663–679 [DOI] [PubMed] [Google Scholar]
- 4. Turner PC, Wu QK, Piekkola S, Gratz S, Mykkanen H, El-Nezami H. 2008. Lactobacillus rhamnosus strain GG restores alkaline phosphatase activity in differentiating Caco-2 cells dosed with the potent mycotoxin deoxynivalenol. Food Chem. Toxicol. 46:2118–2123 [DOI] [PubMed] [Google Scholar]
- 5. Kasuga F, Hara-Kudo Y, Saito N, Kumagai S, Sugita-Konishi Y. 1998. In vitro effect of deoxynivalenol on the differentiation of human colonic cell lines Caco-2 and T84. Mycopathologia 142:161–167 [DOI] [PubMed] [Google Scholar]
- 6. Pinton P, Nougayrede JP, Del Rio JC, Moreno C, Marin DE, Ferrier L, Bracarense AP, Kolf-Clauw M, Oswald IP. 2009. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 237:41–48 [DOI] [PubMed] [Google Scholar]
- 7. Maresca M, Fantini J. 2010. Some food-associated mycotoxins as potential risk factors in humans predisposed to chronic intestinal inflammatory diseases. Toxicon 56:282–294 [DOI] [PubMed] [Google Scholar]
- 8. Berthiller F, Dall'Asta C, Schuhmacher R, Lemmens M, Adam G, Krska R. 2005. Masked mycotoxins: determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 53:3421–3425 [DOI] [PubMed] [Google Scholar]
- 9. Berthiller F, Dall'asta C, Corradini R, Marchelli R, Sulyok M, Krska R, Adam G, Schuhmacher R. 2009. Occurrence of deoxynivalenol and its 3-beta-d-glucoside in wheat and maize. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 26:507–511 [DOI] [PubMed] [Google Scholar]
- 10. Berthiller F, Krska R, Domig KJ, Kneifel W, Juge N, Schuhmacher R, Adam G. 2011. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol. Lett. 206:264–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schatzmayr G, Zehner F, Taubel M, Schatzmayr D, Klimitsch A, Loibner AP, Binder EM. 2006. Microbiologicals for deactivating mycotoxins. Mol. Nutr. Food Res. 50:543–551 [DOI] [PubMed] [Google Scholar]
- 12. Danicke S, Hegewald AK, Kahlert S, Kluess J, Rothkotter HJ, Breves G, Doll S. 2010. Studies on the toxicity of deoxynivalenol (DON), sodium metabisulfite, DON-sulfonate (DONS) and de-epoxy-DON for porcine peripheral blood mononuclear cells and the intestinal porcine epithelial cell lines IPEC-1 and IPEC-J2, and on effects of DON and DONS on piglets. Food Chem. Toxicol. 48:2154–2162 [DOI] [PubMed] [Google Scholar]
- 13. Young JC, Zhou T, Yu H, Zhu H, Gong J. 2007. Degradation of trichothecene mycotoxins by chicken intestinal microbes. Food Chem. Toxicol. 45:136–143 [DOI] [PubMed] [Google Scholar]
- 14. Yu H, Zhou T, Gong J, Young C, Su X, Li XZ, Zhu H, Tsao R, Yang R. 2010. Isolation of deoxynivalenol-transforming bacteria from the chicken intestines using the approach of PCR-DGGE guided microbial selection. BMC Microbiol. 10:182 doi:10.1186/1471-2180-10-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eriksen GS, Pettersson H, Johnsen K, Lindberg JE. 2002. Transformation of trichothecenes in ileal digesta and faeces from pigs. Arch. Tierernaehr. 56:263–274 [DOI] [PubMed] [Google Scholar]
- 16. Sundstol Eriksen G, Pettersson H. 2003. Lack of de-epoxidation of type B trichothecenes in incubates with human faeces. Food Addit. Contam. 20:579–582 [DOI] [PubMed] [Google Scholar]
- 17. Hobson PN. 1969. Rumen bacteria. Methods Microbiol. 3:133–149 [Google Scholar]
- 18. Turner PC, Burley VJ, Rothwell JA, White KL, Cade JE, Wild CP. 2008. Dietary wheat reduction decreases the level of urinary deoxynivalenol in UK adults. J. Expo. Sci. Environ. Epidemiol. 18:392–399 [DOI] [PubMed] [Google Scholar]
- 19. De Baere S, Goossens J, Osselaere A, Devreese M, Vandenbroucke V, De Backer P, Croubels S. 2011. Quantitative determination of T-2 toxin, HT-2 toxin, deoxynivalenol and deepoxy-deoxynivalenol in animal body fluids using LC-MS/MS detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 879:2403–2415 [DOI] [PubMed] [Google Scholar]
- 20. Kabak B, Dobson AD, Var I. 2006. Strategies to prevent mycotoxin contamination of food and animal feed: a review. Crit. Rev. Food Sci. Nutr. 46:593–619 [DOI] [PubMed] [Google Scholar]
- 21. Pestka JJ, Smolinski AT. 2005. Deoxynivalenol: toxicology and potential effects on humans. J. Toxicol. Environ. Health B Crit. Rev. 8:39–69 [DOI] [PubMed] [Google Scholar]
- 22. McIntosh FM, Maison N, Holtrop G, Young P, Stevens VJ, Ince J, Johnstone AM, Lobley GE, Flint HJ, Louis P. 2012. Phylogenetic distribution of genes encoding beta-glucuronidase activity in human colonic bacteria and the impact of diet on faecal glycosidase activities. Environ. Microbiol. 14:1876–1887 [DOI] [PubMed] [Google Scholar]
- 23. Nagl V, Schwartz H, Krska R, Moll WD, Knasmuller S, Ritzmann M, Adam G, Berthiller F. 2012. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in rats. Toxicol. Lett. 213:367–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Turner PC, Hopton RP, Lecluse Y, White KL, Fisher J, Lebailly P. 2010. Determinants of urinary deoxynivalenol and de-epoxy deoxynivalenol in male farmers from Normandy, France. J. Agric. Food Chem. 58:5206–5212 [DOI] [PubMed] [Google Scholar]
- 25. Turner PC, Hopton RP, White KL, Fisher J, Cade JE, Wild CP. 2011. Assessment of deoxynivalenol metabolite profiles in UK adults. Food Chem. Toxicol. 49:132–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Piekkola S, Turner PC, Abdel-Hamid M, Ezzat S, El-Daly M, El-Kafrawy S, Savchenko E, Poussa T, Woo JC, Mykkanen H, El-Nezami H. 2012. Characterisation of aflatoxin and deoxynivalenol exposure among pregnant Egyptian women. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 29:962–971 [DOI] [PubMed] [Google Scholar]
- 27. Awad WA, Ghareeb K, Bohm J, Zentek J. 2010. Decontamination and detoxification strategies for the Fusarium mycotoxin deoxynivalenol in animal feed and the effectiveness of microbial biodegradation. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 27:510–520 [DOI] [PubMed] [Google Scholar]