Abstract
Lactoferrin is an iron-binding glycoprotein found in the milk of most mammals for which various biological functions have been reported, such as antimicrobial activity and bifidogenic activity. In this study, we compared the bifidogenic activity of bovine lactoferrin (bLF) and pepsin hydrolysate of bLF (bLFH), isolated bifidogenic peptide from bLFH, and investigated the bifidogenic spectra of bLF, bLFH, and its active peptide against 42 bifidobacterial strains comprising nine species. Against Bifidobacterium breve ATCC 15700T, minimal effective concentrations of bLF and bLFH were 300 and 10 μg/ml. Against Bifidobacterium longum subsp. infantis ATCC 15697T, the minimal effective concentration of bLFH was 30 μg/ml, and bLF did not show bifidogenic activity within 300 μg/ml. As an active peptide, a heterodimer of A1-W16 and L43-A48 linked by a disulfide bond was isolated. Previously, this peptide was identified as having antibacterial activity. An amino acid mixture with the same composition as this peptide showed no bifidogenic activity. The strains of each species whose growth was highly promoted (>150%) by this peptide at 3.75 μM were as follows: B. breve (7 out of 7 strains [7/7]), B. longum subsp. infantis (5/5), Bifidobacterium bifidum (2/5), B. longum subsp. longum (1/3), Bifidobacterium adolescentis (3/6), Bifidobacterium catenulatum (1/4), Bifidobacterium pseudocatenulatum (0/4), Bifidobacterium dentium (0/5), and Bifidobacterium angulatum (0/3). Growth of none of the strains was highly promoted by bLF at 3.75 μM. We demonstrated that bLFH showed stronger bifidogenic activity than natural bLF, especially against infant-representative species, B. breve and B. longum subsp. infantis; furthermore, we isolated its active peptide. This is the first report about a bifidogenic peptide derived from bLF.
INTRODUCTION
Bifidobacteria are Gram-positive anaerobic bacteria that naturally colonize the human intestinal tract and are considered to be beneficial to human health (1, 2). In the intestinal tract of breast-fed infants, bifidobacteria are predominant. However, after infants are weaned, the bifidobacteria population decreases to 1/10, and other microorganisms increase (3, 4). The bifidobacterial pattern in the human intestinal tract changes with age. In infants, Bifidobacterium breve and Bifidobacterium longum subsp. infantis typify the bifidobacterial flora. However, in adults, B. longum subsp. longum and Bifidobacterium adolescentis typify it (1). Therefore, it is considered that milk components are associated with the formation of infant-specific intestinal flora (5). In fact, some oligosaccharides and other factors in human milk are selectively utilized by infant-representative bifidobacteria (6–8).
Lactoferrin (LF) is a multifunctional iron-binding glycoprotein of the transferrin family (9). It was first discovered in cow's milk and is present in the milk of most mammals (9). Various biological functions of this protein have been reported, such as antimicrobial activity, bifidogenic activity, and regulation of immune responses (9–11). Therefore, LF is considered one of the milk components that modulate the intestinal flora of infants (12). However, the antibacterial activity and bifidogenic activity of natural LF are moderate (13). Previously, it was observed that pepsin hydrolysate of LF (LFH) showed much stronger antibacterial activity than natural LF, and its active peptide, lactoferricin (LFcin), was isolated (14). LFcin showed strong antibacterial activity against a wide range of Gram-positive and Gram-negative microorganisms. However, against bifidobacteria, it showed weak antibacterial activity (15). As for the bifidogenic activity of LF, two bifidogenic peptides from pepsin hydrolysate of human LF (hLFH) were reported (16), and pepsin hydrolysate of bovine lactoferrin (bLFH) was also reported to show bifidogenic activity (17). Therefore, it is expected that the activity of LF to modulate intestinal flora may be exerted by gastric digestion. In this study, we compared bifidogenic activities of bovine LF (bLF), bLFH, and isolated bifidogenic peptide from bLFH and investigated the bifidogenic spectra of bLF, bLFH, and its active peptide against 42 bifidobacterial strains of B. breve, B. longum subsp. infantis, Bifidobacterium bifidum, B. longum subsp. longum, B. adolescentis, Bifidobacterium catenulatum, Bifidobacterium pseudocatenulatum, Bifidobacterium angulatum, and Bifidobacterium dentium.
MATERIALS AND METHODS
Bovine lactoferrin.
The bLF used in this study was produced by Morinaga Milk Industry Co., Ltd. (Zama, Japan), which was prepared from fresh skimmed milk by the method of Law and Reiter (18). The iron content of the bLF was 12.1 mg/100 g as determined by inductively coupled plasma mass spectrometry (ICP-MS).
Pepsin hydrolysate of bLF and whey protein isolate.
bLFH was prepared as described previously with some modifications (14). bLF was dissolved in distilled water at 5% (wt/vol). HCl was added to adjust the pH to 3.0. Porcine pepsin (EC 3.4.23.1; 10 units/mg) (Difco Laboratories, Detroit, MI) was added to a final concentration of 3% (wt/wt of substrate). The hydrolysis reaction was performed at 37°C for 4 h and terminated by heating at 80°C for 10 min. NaOH was added to readjust the pH to 6.0. The hydrolysate was lyophilized and fractured to particles, and homogenized powder was used in the experiments. For comparison with bLFH, pepsin hydrolysate of whey protein isolate, WPI 895 (Fonterra Co., Ltd., Auckland, NZ), was also prepared in the same manner (WPIH).
Microorganisms.
Bifidobacterial strains were obtained from stock cultures maintained in the Morinaga Culture Collection (MCC; Morinaga Milk Industry Co., Ltd., Zama, Japan), American Type Culture Collection (ATCC; Manassas, VA), and Japan Collection of Microorganisms (JCM; Wako, Japan). A total of 42 strains comprising nine species originating from humans or sewage were studied. These microorganisms were cultured at 37°C for 16 h in deMan-Rogosa-Sharpe (MRS) broth (Merck, Darmstadt, Germany), in accordance with a modified protocol, under anaerobic conditions. The medium used was prepared as follows: MRS broth at 5.5% (wt/vol) and l-cysteine-HCl at 0.05% (wt/vol) were dissolved in distilled water with the pH adjusted to 6.5 by HCl and autoclaved at 115°C for 15 min (MRSC broth) (19).
Growth promotion assay.
Bacterial growth was measured by the optical density at 630 nm (OD630) using a microplate reader (MTP-32; Corona Electric, Ibaraki, Japan). Microorganisms were collected by centrifugation, washed once with sterile saline, resuspended with MRSC broth to an OD630 of 0.8/200 μl in a 96-well microplate and used as seed cultures for growth promotion assays. Seed cultures were diluted to 1% (vol/vol) with MRSC broth. Samples were dissolved with distilled water to concentrations of 2.5, 7.5, 25, 75, 250, and 750 μg/ml, with the pH adjusted to 6.5, and filter sterilized (pore size, 0.22 μm). Twofold concentrate of MRSC broth was prepared. In a 96-well microplate, 20 μl of 1% seed culture dilutions (final concentration of 0.1%), 80 μl of sample solutions (final concentrations of 1, 3, 10, 30, 100, and 300 μg/ml), and 100 μl of 2-fold concentrate of MRSC broth were mixed (200 μl), incubated at 37°C for 16 h under anaerobic conditions, and measured for the OD630 value. Mean value and standard deviation were calculated from the data of triplicate assays in parallel. Reproducibility was confirmed in preliminary experiments. These data were compared with the control by a Student's t test, and a P value of <0.05 was considered to be statistically significant. The bifidogenic activity was calculated by the following formula, as described elsewhere (13): bifidogenic activity (%) = OD630(sample added)/OD630(control) × 100.
On the basis of these values, the activity was evaluated as follows: high (H; >150%), medium (M; 120 to 150%), low (L; 80 to 120%), and growth inhibition (I; <80%) (19).
Peptide purification.
Active peptides were purified by reverse-phase high-performance liquid chromatography (RP-HPLC) using a C18 column (Pegasil-300 ODS-II; column size, 10-mm diameter by 250 mm [SSC, Tokyo, Japan]). bLFH (40 mg) was dissolved in the solvent (2 ml) composed of 95% (vol/vol) H2O and 5% (vol/vol) acetonitrile (ACN), containing 0.1% trifluoroacetic acid (TFA), and loaded onto the column. In the first purification step, the elution gradient program was as follows: 0 to 10 min, 95% H2O and 5% ACN; 10 to 60 min, 95 to 50% H2O and 5 to 50% ACN; and 60 to 70 min, 50 to 20% H2O and 50 to 80% ACN. TFA (0.1%) was used as a mobile phase additive. The flow rate was 3 ml/min. The elution of each peak was collected, evaporated with a centrifugal vacuum evaporator, dissolved with distilled water (5 ml), with the pH adjusted to 6.5, filter sterilized (pore size, 0.22 μm), and used in growth promotion assays (the same method as above). B. breve ATCC 15700T and B. longum subsp. infantis ATCC 15697T were used to identify active peaks. For the second purification step, sample solutions of active peaks were evaporated with a centrifugal vacuum evaporator and dissolved in a solvent (2 ml) composed of 95% H2O and 5% ACN containing 0.2% formic acid (FA) and loaded onto the same column. The elution gradient program was the same as above, but the mobile phase additive was changed from TFA (0.1%) to FA (0.2%). The bifidogenic activity of each peak was evaluated by the same method as in the first purification step, and active peaks were identified.
Peptide identification.
Active peaks selected above were analyzed by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Autoflex2; Bruker Daltonics, Billerica, MA). The elution (1 μl) of each active peak in the second purification step was loaded onto the matrix spot of the MALDI target Prespotted AnchorChip (Bruker Daltonics, Billerica, MA) equipped with an α-cyano-4-hydroxycinnamic acid matrix and calibrants (1 to 4 kDa), dried at room temperature, and analyzed. The spectra were recorded in the reflector in positive mode. Calibration was performed using a combination of nine standard peptides: bradykinin 1–7 (757.39 Da), angiotensin II (1,046.54 Da), angiotensin I (1,296.68 Da), neurotensin (1,672.91 Da), renin substrate (1,758.93 Da), adrenocorticotropin hormone (ACTH) clip 1 to 17 (2,093.08 Da), ACTH clip 18 to 39 (2,465.19 Da), ACTH clip 1 to 24 (2,932.58 Da), and ACTH clip 7 to 38 (3,657.92 Da). The N-terminal amino acid sequence of the active peptide was analyzed using protein sequencer PPSQ-23A (Shimadzu, Kyoto, Japan).
Peptide synthesis.
Active peptide was synthesized by the 9-fluorenylmethoxy carbonyl (Fmoc) method at the Toray Research Center Co., Ltd. (Tokyo, Japan). The purity of the peptide (>95%) was guaranteed by RP-HPLC. The molecular weight of bLF is about 80,000 and that of synthesized peptide is 2,673.1. Therefore, the bifidogenic activity of synthesized peptide was compared with bLF or bLFH in terms not of weight concentration but of molecular concentration. The concentrations of bLF or bLFH in the growth promotion assays above were 1, 3, 10, 30, 100, and 300 μg/ml, which correspond approximately to 0.0125, 0.0375, 0.125, 0.375, 1.25, and 3.75 μM, respectively. Therefore, the bifidogenic activity of synthesized peptide was evaluated at 0.0125, 0.0375, 0.125, 0.375, 1.25, and 3.75 μM, as described above.
AAM.
Amino acid mixture (AAM) with the same composition as the active peptide was prepared using Amino Acid Standard Kit 22 (Pierce Chemical Company, IL). Aqueous amino acid solutions of valine, leucine, serine, threonine, asparagine, glutamine, lysine (1 mM), alanine, isoleucine, proline, tryptophan, cysteine, glutamic acid (2 mM), and arginine (3 mM) were prepared. Each solution (100 μl) was mixed, evaporated with a centrifugal vacuum evaporator, and dissolved in distilled water (1 ml). We defined this mixture as 100 μM AAM, corresponding to the constituent amino acids of 100 μM synthesized peptide. This AAM was diluted with the pH adjusted to 6.5, filter sterilized (pore size, 0.22 μm), and used in growth promotion assays. The bifidogenic activity of AAM was evaluated at 0.0125, 0.0375, 0.125, 0.375, 1.25, and 3.75 μM and compared with synthesized peptide as described above.
RESULTS
Evaluation of bifidogenic activity of bLFH.
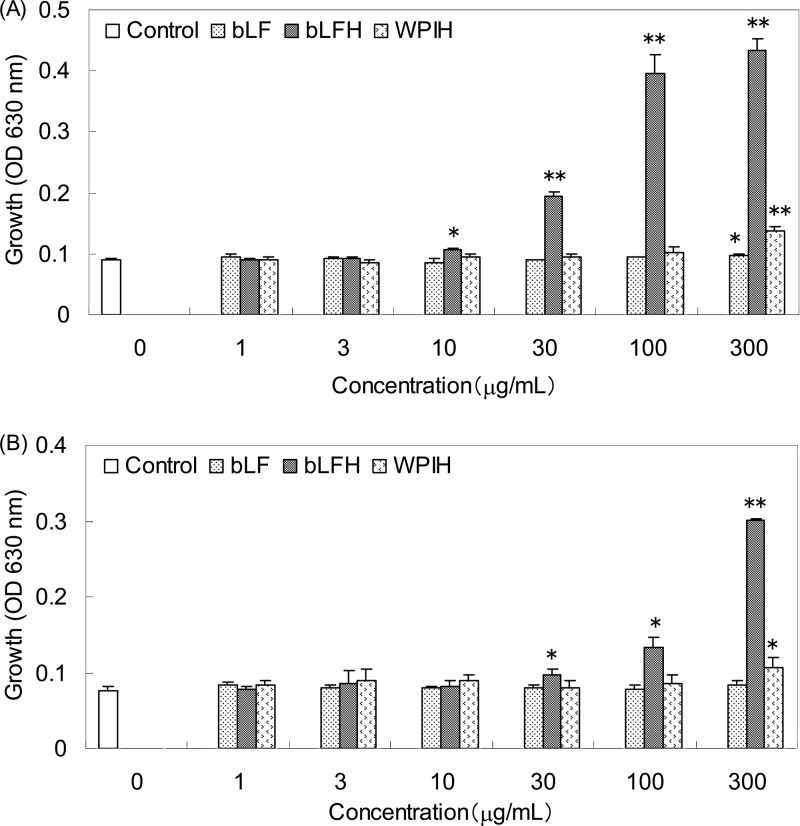
Bifidogenic activities of bLF, bLFH, and WPIH against B. breve ATCC 15700T were compared, as shown in Fig. 1A. Minimal effective concentrations of bLF, bLFH, and WPIH were 300, 10, and 300 μg/ml, respectively. Bifidogenic activities of bLF, bLFH, and WPIH against B. longum subsp. infantis ATCC 15697T were also compared, as shown in Fig. 1B. Minimal effective concentrations of bLFH and WPIH were 30 and 300 μg/ml, respectively, and bLF did not show bifidogenic activity in this concentration range. Against both bifidobacterial strains, bLFH showed higher bifidogenic activity than bLF or WPIH.
Fig 1.
Comparison of bifidogenic activity of bovine lactoferrin (bLF), pepsin hydrolysate of bovine lactoferrin (bLFH), and pepsin hydrolysate of whey protein isolate (WPIH). (A) B. breve ATCC 15700T. (B) B. longum subsp. infantis ATCC 15697T. Data are presented as the mean values, and the error bars represent the standard deviations of three experiments. ⁎, P < 0.05; ⁎⁎, P < 0.001.
Identification of bifidogenic peptide from bLFH.
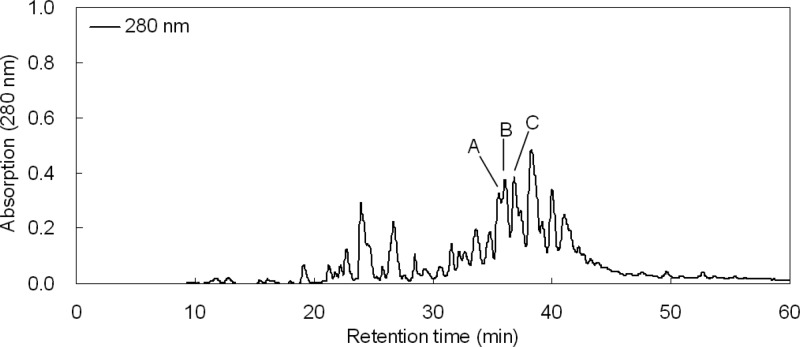
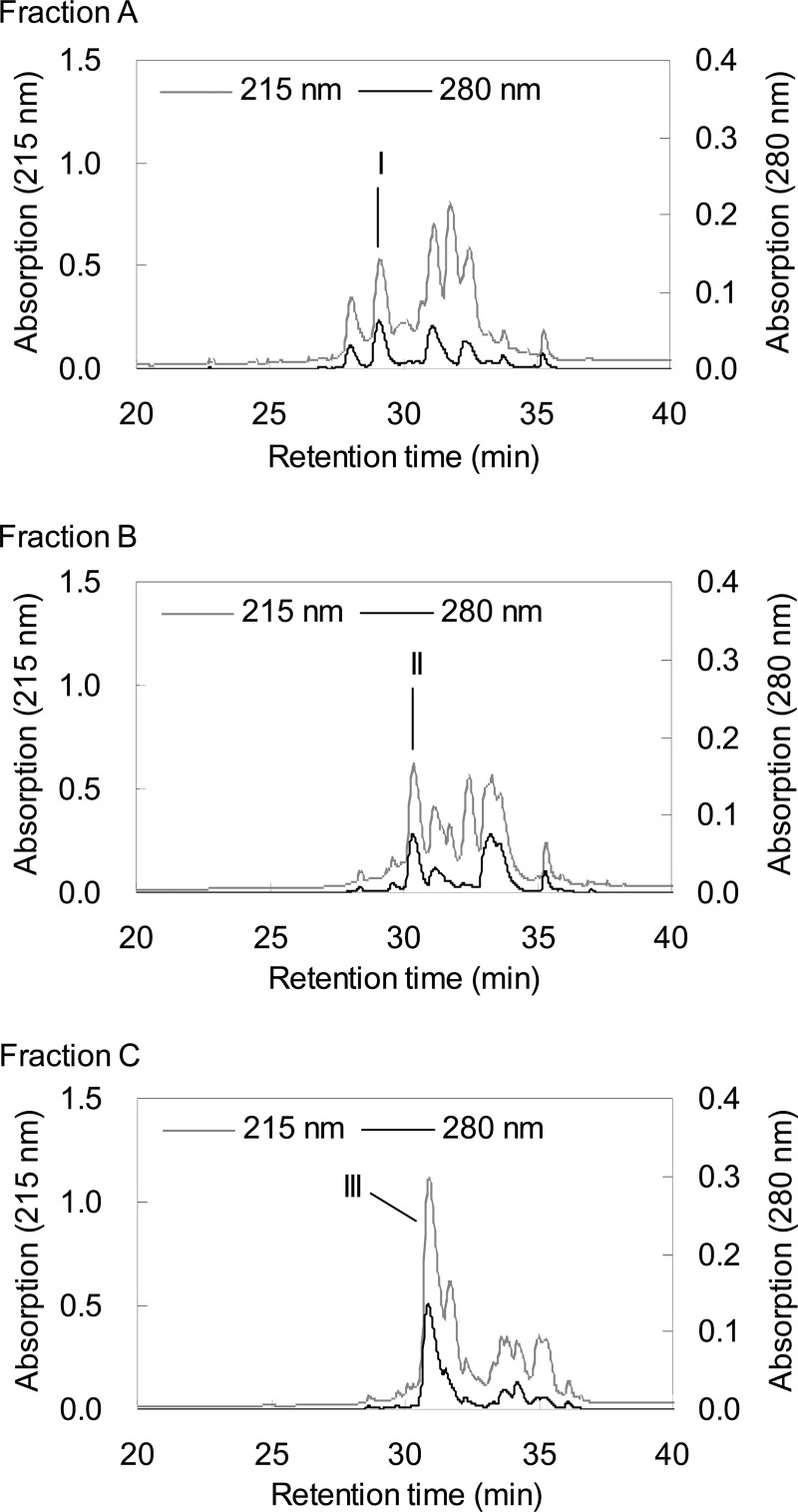
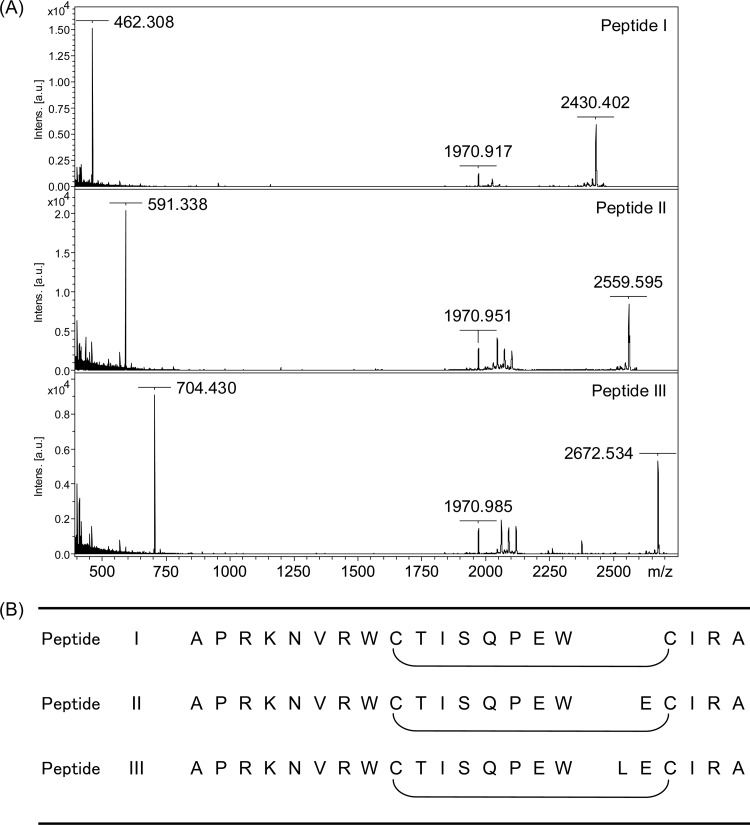
In the first purification step, three peaks (A, B, and C) with retention times of 35.5, 36.0, and 37.0 min mainly showed bifidogenic activity against B. breve ATCC 15700T and B. longum subsp. infantis ATCC 15697T (Fig. 2). In the second purification step of each peak (A, B, and C), three peaks (I, II, and III) with retention times of 29.0, 30.5, and 31.0 min, respectively, showed bifidogenic activity against B. breve ATCC 15700T and B. longum subsp. infantis ATCC 15697T (Fig. 3). The peptide masses of these fractions were analyzed by MALDI-TOF MS (Fig. 4A). Peptide I in fraction I had a mass of 2,430, and its fragments (1,971 and 462) were observed. Peptide II in fraction II had a mass of 2,560, and its fragments (1,971 and 591) were observed. Peptide III in fraction III had a mass of 2,673, and its fragments (1,971 and 704) were observed. In another study, a bLF peptide with the same molecular weight as peptide III was reported (20). Then, peptide III was analyzed using a protein sequencer, and the N-terminal amino acid sequence was read in the following order: (A, L) (i.e., A and L were detected simultaneously), (P, E), (R, −) (i.e., R was detected but C was not), (K, I), (N, R), (V, A), (R), (W), (−), (T). Two cysteines were not detected, probably because of the disulfide bond. These results agreed with the known peptide (20). Therefore, we identified peptide III as the known peptide. From the information on peptide III, the sequences of the other two peptides were assigned (Fig. 4B). Each peptide differs in only one amino acid, so the peptides are virtually identical. We named peptide III BLP (bifidogenic lactoferrin peptide) as a representative of these peptides. It was reported that the pepsin hydrolysis of bLF was almost complete within 30 min under conditions similar to ours (21). Therefore, BLP is considered to be not a reaction intermediate but an end product.
Fig 2.
Separation of bifidogenic peptides from pepsin hydrolysate of bovine lactoferrin (bLFH) by HPLC in the first purification step. Fractions A, B, and C (retention times of 35.5, 36.0, and 37.0 min, respectively) showed bifidogenic activity. The largest peak (retention time of 38.3 min) contained LFcin B.
Fig 3.
Separation of bifidogenic peptides from fractions A, B, and C by HPLC in the second purification step. Fractions I, II, and III (retention times of 29.0, 30.5, and 31.0 min, respectively) showed bifidogenic activity.
Fig 4.
Identification of peptides I, II, and III. (A) MALDI-TOF MS analyzed using Autoflex2 (Bruker Daltonics, Billerica, MA). The MALDI target Prespotted AnchorChip (Bruker Daltonics, Billerica, MA) equipped with an α-cyano-4-hydroxycinnamic acid matrix and calibrants (1 to 4 kDa) was used. (B) N-terminal amino acid sequence determined using protein sequencer PPSQ-23A (Shimadzu, Kyoto, Japan). From the information on peptide III, the sequences of the other two peptides were assigned. Intens, intensity; au, arbitrary units.
Evaluation of bifidogenic activity of synthesized BLP.
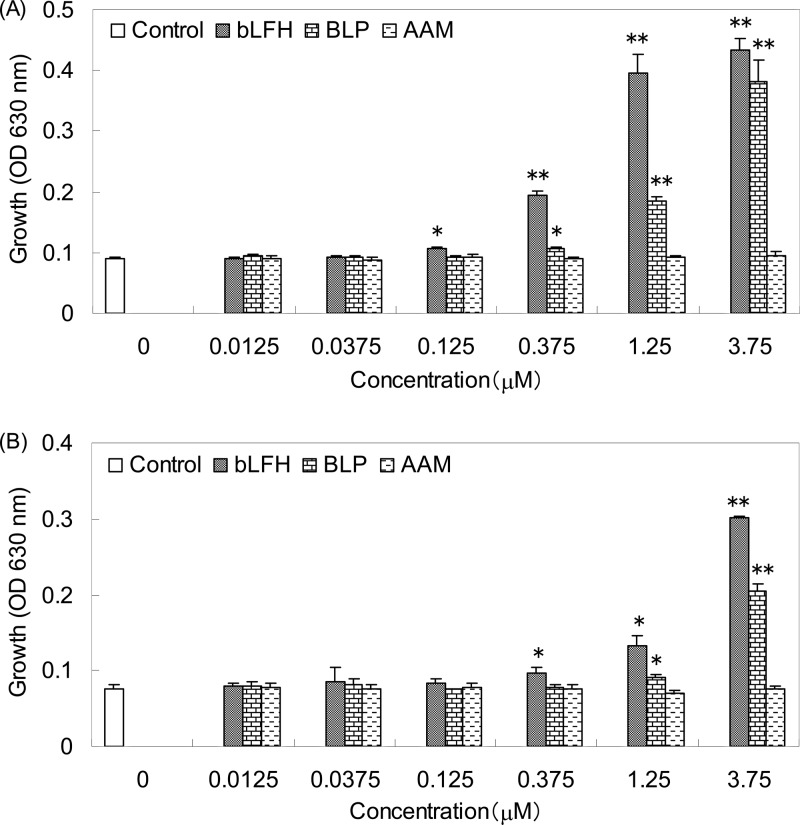
Bifidogenic activities of bLFH, synthesized BLP, and AAM with the same composition as BLP against B. breve ATCC 15700T were compared, as shown in Fig. 5A. Minimal effective concentrations of bLFH and BLP were 0.125 and 0.375 μM, respectively, and AAM did not show bifidogenic activity. The bifidogenic activities of bLFH, BLP, and AAM against B. longum subsp. infantis ATCC 15697T were also compared, as shown in Fig. 5B. Minimal effective concentrations of bLFH and BLP were 0.375 and 1.25 μM, respectively, and AAM did not show bifidogenic activity.
Fig 5.
Comparison of bifidogenic activity of pepsin hydrolysate of bovine lactoferrin (bLFH), synthesized BLP (bifidogenic lactoferrin peptide), and an amino acid mixture with the same composition as BLP (AAM). (A) B. breve ATCC 15700T. (B) B. longum subsp. infantis ATCC 15697T. Data are presented as the mean values, and the error bars represent the standard deviations of three experiments. ⁎, P < 0.05; ⁎⁎, P < 0.001.
Bifidogenic spectra.
Bifidogenic spectra of bLF, bLFH, and synthesized BLP against 42 bifidobacterial strains comprising nine species were summarized (Table 1). Growth of all B. breve (7 out of 7 strains [7/7]) and B. longum subsp. infantis (5/5) strains, of some B. bifidum (2/5), B. longum subsp. longum (1/3), B. adolescentis (2 to 3/6), and B. catenulatum (1/4) strains, and of no B. pseudocatenulatum (0/4), B. dentium (0/5), and B. angulatum (0/3) strains investigated in this study was highly promoted (>150%) by bLFH or BLP at 3.75 μM. Growth of none of the bifidobacterial strains investigated in this study was highly promoted by bLF at 3.75 μM.
Table 1.
Bifidogenic spectra of bLF, bLFH, and synthesized BLP against 42 bifidobacterial strains of nine species
| Species | Strain | Origin | Growth promotion pattern ona: |
||
|---|---|---|---|---|---|
| bLF | bLFH | BLP | |||
| B. breve | ATCC 15700T | Feces of infant | L | H | H |
| MCC 167 | Feces of infant | M | H | H | |
| MCC 1093 | Feces of infant | L | H | H | |
| MCC 1098 | Feces of infant | L | H | H | |
| MCC 1117 | Feces of infant | L | H | H | |
| MCC 1129 | Feces of infant | L | H | H | |
| MCC 1274 | Feces of infant | L | H | H | |
| B. longum subsp. infantis | ATCC 15697T | Feces of infant | L | H | H |
| MCC 9 | Feces of infant | L | H | H | |
| MCC 171 | Feces of infant | L | H | H | |
| MCC 1124 | Feces of infant | L | H | H | |
| MCC 1297 | Feces of infant | L | H | H | |
| B. bifidum | ATCC 29521T | Feces of infant | L | I | L |
| ATCC 15696 | Intestine of infant | L | H | H | |
| MCC 1092 | Feces of infant | L | H | H | |
| MCC 1113 | Feces of infant | L | L | M | |
| MCC 1122 | Feces of infant | L | M | L | |
| B. longum subsp. longum | ATCC 15707T | Feces of adult | L | L | L |
| ATCC 15708 | Feces of infant | L | H | H | |
| ATCC BAA-999 | Feces of infant | L | L | L | |
| B. adolescentis | ATCC 15703T | Feces of adult | L | L | L |
| ATCC 15704 | Feces of adult | L | L | L | |
| ATCC 15705 | Feces of adult | I | I | H | |
| MCC 1560 | Feces of adult | L | I | L | |
| MCC 1104 | Feces of infant | I | H | H | |
| MCC 1138 | Feces of infant | I | H | H | |
| B. catenulatum | MCC 76 | Feces of adult | L | M | L |
| MCC 78 | Feces of adult | L | M | M | |
| MCC 248 | Feces of adult | L | H | H | |
| MCC 249 | Feces of adult | L | I | M | |
| B. pseudocatenulatum | JCM 7040 | Feces of adult | L | M | L |
| MCC 188 | Feces of adult | L | L | L | |
| MCC 1123 | Feces of infant | L | L | L | |
| MCC 1137 | Feces of infant | L | I | L | |
| B. dentium | ATCC 15424 | Pleural fluid from adult | L | L | L |
| ATCC 27678 | Feces of human | I | L | L | |
| ATCC 27680 | Human dental caries | L | M | L | |
| MCC 311 | Feces of adult | L | M | L | |
| MCC 1102 | Feces of infant | L | L | L | |
| B. angulatum | ATCC 27535T | Feces of human | L | M | M |
| ATCC 27669 | Sewage | L | M | L | |
| ATCC 27671 | Sewage | L | L | M | |
Bifidogenic activity (%) was determined as OD630(sample added)/OD630(control) × 100. H, high (>150%); M, medium (120 to 150%); L, low (80 to 120%), I, growth inhibition (<80%).
DISCUSSION
In this study, we demonstrated that bLFH showed stronger bifidogenic activity than natural bLF. As active peptides in bLFH, we isolated heterodimer of A1-W16 and L43-A48 linked by a disulfide bond (named BLP) and its analogs. While synthesized BLP showed bifidogenic activity, an amino acid mixture with the same composition as BLP showed none. As for the bifidogenic spectra, we demonstrated that bLFH and BLP exerted bifidogenic activity, especially against infant-representative species, B. breve and B. longum subsp. infantis.
In these experiments, bifidobacterial growth was evaluated at 16 h after inoculation so as to measure the turbidity (OD630) within the unsaturated range. In preliminary experiments with B. breve ATCC 15700T, we measured the OD630 at multiple time points. At 4 h or 8 h, there was little difference between the control and bLFH. At 16 h, the value was higher in bLFH than in the control as described here (control, 0.09; 300 μg/ml bLFH, 0.43). Furthermore, the difference was greater at 20 h (control, 0.24; 300 μg/ml bLFH, 0.96). After that, the turbidity of bLFH was saturated, and that of the control varied little. Therefore, bLFH was considered to have effects on both the growth rate and the final population of bifidobacteria. On the other hand, our results in MRSC broth might not always match what would be obtained in milk. The effect of bLFH-fortified milk on the growth of bifidobacteria remains to be evaluated. As for previous studies in vivo, the effect of bLF- or bLFH-fortified milk on the intestinal flora of mice was evaluated (22). Although the growth of enterobacteria was inhibited, the growth of bifidobacteria was not promoted. However, bifidobacterial species of mice are different from those of humans (1). In a study of human infant flora-associated mice, bLF-fortified milk promoted the growth of bifidobacteria (23). Therefore, it would be important to use human infant flora to evaluate the effect of bLFH-fortified milk on the growth of bifidobacteria in vivo.
We isolated BLP (bifidogenic lactoferrin peptide) from bLFH. This is the first report about bifidogenic peptide derived from bLF. Previously, the same peptide as BLP was identified as having moderate antibacterial activity (20). Therefore, BLP may promote the growth of bifidobacteria selectively and inhibit the growth of other microorganisms. Bifidogenic sequences of LF have certain features. BLF consists of a homologous N lobe (residues 1 to 339) and C lobe (residues 340 to 689) (24). The sequence of BLP is located on the N-terminal region of the N lobe of bLF and is adjacent to bovine LFcin (LFcin B). The sequences of two bifidogenic peptides from human LF (hLF) are located on the N-terminal region of the N lobe and the homologous C-lobe region (16, 24). The homology of the N lobe and the C lobe in hLF is 37% (25). Therefore, the N-terminal sequences of these two lobes might have similar functions. In our study, we could not find bifidogenic peptides from the C lobe of bLF. However, in another study, the N-terminal sequence of the C lobe of bLF was defined as an antibacterial peptide under different hydrolysis conditions (26). This antibacterial peptide may show bifidogenic activity. A bifidogenic peptide from the N-terminal region of hLF is human LFcin (LFcin H) corresponding to the region expanding from BLP to LFcin B in bLF (14, 16). In our study, BLP did not cover all the bifidogenic activity of bLFH, and the fraction containing LFcin B (the fraction with a retention time of 38.3 min in Fig. 2) also seemed to have bifidogenic activity against B. breve ATCC 15700T and B. longum subsp. infantis ATCC 15697T (data not shown). Meanwhile, it was reported that LFcin B showed weak antibacterial activity against bifidobacteria (15), and in our study, some bifidobacterial strains of B. bifidum, B. adolescentis, B. catenulatum, and B. pseudocatenulatum were growth inhibited by bLFH, probably because of LFcin B. Further investigations are required to determine whether LFcin B has bifidogenic activity.
The mechanism of action of BLP is still unknown. With regard to peptides derived from other bovine milk proteins, the pepsin hydrolysate of WPI showed little bifidogenic activity. WPI contains casein glycomacropeptide and major whey proteins such as α-lactalbumin and β-lactoglobulin. This suggests that peptides derived from bovine milk proteins do not always show bifidogenic activity. The amino acid mixture (AAM) with the same composition as BLP showed no bifidogenic activity. This indicates that the bifidogenic activity of BLP is not due to the nutritional supplementation (nitrogen source) it provides in the MRSC broth. In our experiments, we used cysteine as a component of the AAM. Because there is a disulfide bond in BLP, an AAM containing cystine was also tested in a preliminary experiment but showed no bifidogenic activity (data not shown). In addition, it is reported that the addition of cysteine or cystine alone did not promote the growth of bifidobacteria (27). As for the bifidogenic mechanism of LF, it is reported that bifidobacteria have an LF-binding protein on the surface of the cell membrane, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a candidate for this protein (28). In some bacteria, GAPDH may be involved in the oxidative stress response (29, 30). Therefore, LF binding to GAPDH might modulate the defense response to oxidant stress. In B. longum subsp. infantis, it is reported that genes that are necessary for the utilization of human milk oligosaccharide were upregulated by hLF or bLF (31). LF might stimulate the incorporation of nutrients from the environment. It is possible that BLP acts in the same manner as LF. In other studies, it was suggested that the disulfide/sulfhydryl residues in peptides might act as nutrient carriers for bifidobacteria (27, 32). Indeed, BLP or bifidogenic peptides from hLF also contain disulfide residues. Therefore, the disulfide residues in bifidogenic peptides would be important for exerting their activity. We would like to study the bifidogenic mechanism of BLP as our next subject of research.
In this study, BLP showed stronger bifidogenic activity than natural bLF. In addition, the effective concentration of BLP seemed to be similar to that of bifidogenic peptides from hLF (16). Therefore, we consider that pepsin digestion is essential for LF to exert its potential bifidogenic activity. It is often said that the level of gastric pepsin of infants is lower than that of adults (33). Because the effective concentration of LFcin H (16) seems to be much lower (1/10 to 1/100) than the concentration of hLF in milk (>30 μM) (34), only a small proportion of hLF in the milk may need to be hydrolyzed by gastric pepsin to exert its bifidogenic activity, and this might be possible even in infants (35). The concentration of bLF fortified in some commercial infant formulas is about 1 μM. Considering our results, if about half of bLF in infant formula is hydrolyzed by gastric pepsin, bifidogenic activity is expected. When the digestion ability of an infant is insufficient (e.g., preterm infants were found to hydrolyze only 15% of the milk protein in their stomachs [36]), the use of bLFH as a substitute for bLF might be an option. In addition, bLFH might be used in peptide-based formulas with low allergenicity. Most of the bifidus factors that have been intensively investigated are oligosaccharides (37). They are not digested or absorbed by the host but are metabolized by intestinal microorganisms, including bifidobacteria, as a carbon source. We consider that it is possible to use bLFH in combination with oligosaccharides and expect that bLFH assists these oligosaccharides to maintain infant-representative bifidobacteria by promoting their growth or inhibiting the growth of other microorganisms.
The studies of bLF peptides including BLP and LFcin B suggest the possibility that the gastric digestion of LF from a mother's milk contributes to promote the growth of bifidobacteria, inhibit the growth of other microorganisms, and establish the composition of infant-specific healthy intestinal flora. Furthermore, this effect may be expected not only from a mother's milk but also from infant formula containing bLF or bLFH.
ACKNOWLEDGMENTS
We thank Sachiko Takahashi and Daisuke Ochi at Morinaga Milk Industry Co., Ltd., for technical support.
Footnotes
Published ahead of print 11 January 2013
REFERENCES
- 1. Mitsuoka T, Kaneuchi C. 1977. Ecology of the bifidobacteria. Am. J. Clin. Nutr. 30:1799–1810 [DOI] [PubMed] [Google Scholar]
- 2. Tissier H. 1905. Repartition des microbes dans l'intenstin du nourrinson. Ann. Inst. Pasteur 19:109–123 [Google Scholar]
- 3. Mitsuoka T. 1992. Intestinal flora and aging. Nutr. Rev. 50:438–446 [DOI] [PubMed] [Google Scholar]
- 4. Mitsuoka T, Hayakawa K. 1972. Die Faekalflora bei Menschen. I. Mitteilung: die Zusammensetzung der Faekalflora der verschiedenen Altersgruppen. Zentralbl. Bakteriol. Orig. 223:333–342 [PubMed] [Google Scholar]
- 5. Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, Welling GW. 2000. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 30:61–67 [DOI] [PubMed] [Google Scholar]
- 6. Azuma N, Yamauchi K, Mitsuoka T. 1984. Bifidus growth-promoting activity of a glycomacropeptide derived from human κ-casein. Agric. Biol. Chem. 48:2159–2162 [Google Scholar]
- 7. Haarman M, Knol J. 2005. Quantitative real-time PCR assay to identify and quantify fecal Bifidobacterium species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 71:2318–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xiao JZ, Takahashi S, Nishimoto M, Odamaki T, Yaeshima T, Iwatsuki K, Kitaoka M. 2010. Distribution of in vitro fermentation ability of lacto-N-biose I, a major building block of human milk oligosaccharides, in bifidobacterial strains. Appl. Environ. Microbiol. 76:54–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farnaud S, Evans RW. 2003. Lactoferrin—a multifunctional protein with antimicrobial properties. Mol. Immunol. 40:395–405 [DOI] [PubMed] [Google Scholar]
- 10. Actor JK, Hwang SA, Kruzel ML. 2009. Lactoferrin as a natural immune modulator. Curr. Pharm. Des. 15:1956–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petschow BW, Talbott RD. 1991. Response of Bifidobacterium species to growth promoters in human and cow milk. Pediatr. Res. 29:208–213 [DOI] [PubMed] [Google Scholar]
- 12. Roberts AK, Chierici R, Sawatzki G, Hill MJ, Volpato S, Vigi V. 1992. Supplementation of an adapted formula with bovine lactoferrin: 1. Effect on the infant faecal flora. Acta Paediatr. 81:119–124 [DOI] [PubMed] [Google Scholar]
- 13. Saito H, Miyakawa H, Ishibashi N, Tamura Y, Hayasawa H, Shimamura S. 1996. Effect of iron-free and metal-bound forms of lactoferrin on the growth of bifidobacteria, E. coli and S. aureus. Biosci. Microflora 15:1–7 [Google Scholar]
- 14. Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K, Tomita M. 1992. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1121:130–136 [DOI] [PubMed] [Google Scholar]
- 15. Bellamy W, Takase M, Wakabayashi H, Kawase K, Tomita M. 1992. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J. Appl. Bacteriol. 73:472–479 [DOI] [PubMed] [Google Scholar]
- 16. Liepke C, Adermann K, Raida M, Magert HJ, Forssmann WG, Zucht HD. 2002. Human milk provides peptides highly stimulating the growth of bifidobacteria. Eur. J. Biochem. 269:712–718 [DOI] [PubMed] [Google Scholar]
- 17. Kim WS, Rahman MM, Kumura H, Shimazaki K. 2005. Comparison of growth promoting effects on Bifidobacterium spp. by bovine lactoferrin hydrolysates. Biosci. Microflora 24:119–123 [Google Scholar]
- 18. Law BA, Reiter B. 1977. The isolation of bacteriostatic properties of lactoferrin from bovine milk whey. J. Dairy Res. 44:595–599 [DOI] [PubMed] [Google Scholar]
- 19. Rahman MM, Kim WS, Kumura H, Shimazaki K. 2010. Screening of Bifidobacterium spp. based on in vitro growth responses to bovine lactoferrin. Int. J. Food Sci. Technol. 45:453–458 [Google Scholar]
- 20. Dionysius DA, Milne JM. 1997. Antibacterial peptides of bovine lactoferrin: purification and characterization. J. Dairy Sci. 80:667–674 [DOI] [PubMed] [Google Scholar]
- 21. Tomita M, Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K. 1991. Potent antibacterial peptides generated by pepsin digestion of bovine lactoferrin. J. Dairy. Sci. 74:4137–4142 [DOI] [PubMed] [Google Scholar]
- 22. Teraguchi S, Shin K, Ogata T, Kingaku M, Kaino A, Miyauchi H, Fukuwatari Y, Shimamura S. 1995. Orally administered bovine lactoferrin inhibits bacterial translocation in mice fed bovine milk. Appl. Environ. Microbiol. 61:4131–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hentges DJ, Marsh WW, Petschow BW, Thal WR, Carter MK. 1992. Influence of infant diets on the ecology of the intestinal tract of human flora-associated mice. J. Pediatr. Gastroenterol. Nutr. 14:146–152 [DOI] [PubMed] [Google Scholar]
- 24. Pierce A, Colavizza D, Benaissa M, Maes P, Tartar A, Montreuil J, Spik G. 1991. Molecular cloning and sequence analysis of bovine lactotransferrin. Eur. J. Biochem. 196:177–184 [DOI] [PubMed] [Google Scholar]
- 25. Metz-Boutigue MH, Jolles J, Mazurier J, Schoentgen F, Legrand D, Spik G, Montreuil J, Jolles P. 1984. Human lactotransferrin: amino acid sequence and structural comparisons. Eur. J. Biochem. 145:659–676 [DOI] [PubMed] [Google Scholar]
- 26. Elbarbary HA, Abdou AM, Park EY, Nakamura Y, Mohamed HA, Sato K. 2010. Novel antibacterial lactoferrin peptides generated by rennet digestion and autofocusing technique. Int. Dairy J. 20:646–651 [Google Scholar]
- 27. Poch M, Bezkorovainy A. 1991. Bovine milk κ-casein trypsin digest is a growth enhancer for the genus Bifidobacterium. J. Agric. Food Chem. 39:73–77 [Google Scholar]
- 28. Shimazaki K, Kushida T, Takase M. 2011. Lactoferrin-binding protein detected in bifidobacteria is GAPDH: a hypothesis of growth promotion of bifidobacteria by lactoferrin using the text mining approach, abstr O-III-3, p 38 Abstr. 10th Int. Conf. Lactoferrin, Mazatlan Sinaloa, Mexico [Google Scholar]
- 29. Carreté R, Reguant C, Bordons A, Constantí M. 2005. Relationship between a stress membrane protein of Oenococcus oeni and glyceraldehyde-3-phosphate dehydrogenases. Appl. Biochem. Biotechnol. 127:43–51 [DOI] [PubMed] [Google Scholar]
- 30. Morigasaki S, Shimada K, Ikner A, Yanagida M, Shiozaki K. 2008. Glycolytic enzyme GAPDH promotes peroxide stress signaling through multistep phosphorelay to a MAPK cascade. Mol. Cell 30:108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garrido D, Nwosu C, Ruiz-Moyano S, Aldredge D, German JB, Lebrilla CB, Mills DA. 2012. Endo-β-N-acetylglucosaminidases from infant gut-associated bifidobacteria release complex N-glycans from human milk glycoproteins. Mol. Cell. Proteomics 11:775–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ibrahim SA, Bezkorovainy A. 1994. Growth-promoting factors for Bifidobacterium longum. J. Food Sci. 59:189–191 [Google Scholar]
- 33. Agunod M, Yamaguchi N, Lopez R, Luhby L, Glass GBJ. 1969. Correlative study of hydrochloric acid, pepsin, and intrinsic factor secretion in newborns and infants. Am. J. Dig. Dis. 14:400–414 [DOI] [PubMed] [Google Scholar]
- 34. Ronayne de Ferrer PA, Baroni A, Sambucetti ME, Lopez NE, Ceriani Cernadas JM. 2000. Lactoferrin levels in term and preterm milk. J. Am. Coll. Nutr. 19:370–373 [DOI] [PubMed] [Google Scholar]
- 35. Prentice A, MacCarthy A, Stirling DM, Vasquez-Velasquez L, Ceesay SM. 1989. Breast-milk IgA and lactoferrin survival in the gastrointestinal tract—a study in rural Gambian children. Acta Paediatr. Scand. 78:505–512 [DOI] [PubMed] [Google Scholar]
- 36. Henderson TR, Hamosh M, Armand M, Mehta NR, Hamosh P. 2001. Gastric proteolysis in preterm infants fed mother's milk or formula. Adv. Exp. Med. Biol. 501:403–408 [DOI] [PubMed] [Google Scholar]
- 37. Mitsuoka T. 2002. Prebiotics and intestinal flora. Biosci. Microflora 21:3–12 [Google Scholar]