Abstract
Lactobacilli convert linoleic acid to hydroxy fatty acids; however, this conversion has not been demonstrated in food fermentations and it remains unknown whether hydroxy fatty acids produced by lactobacilli have antifungal activity. This study aimed to determine whether lactobacilli convert linoleic acid to metabolites with antifungal activity and to assess whether this conversion can be employed to delay fungal growth on bread. Aqueous and organic extracts from seven strains of lactobacilli grown in modified De Man Rogosa Sharpe medium or sourdough were assayed for antifungal activity. Lactobacillus hammesii exhibited increased antifungal activity upon the addition of linoleic acid as a substrate. Bioassay-guided fractionation attributed the antifungal activity of L. hammesii to a monohydroxy C18:1 fatty acid. Comparison of its antifungal activity to those of other hydroxy fatty acids revealed that the monohydroxy fraction from L. hammesii and coriolic (13-hydroxy-9,11-octadecadienoic) acid were the most active, with MICs of 0.1 to 0.7 g liter−1. Ricinoleic (12-hydroxy-9-octadecenoic) acid was active at a MIC of 2.4 g liter−1. L. hammesii accumulated the monohydroxy C18:1 fatty acid in sourdough to a concentration of 0.73 ± 0.03 g liter−1 (mean ± standard deviation). Generation of hydroxy fatty acids in sourdough also occurred through enzymatic oxidation of linoleic acid to coriolic acid. The use of 20% sourdough fermented with L. hammesii or the use of 0.15% coriolic acid in bread making increased the mold-free shelf life by 2 to 3 days or from 2 to more than 6 days, respectively. In conclusion, L. hammesii converts linoleic acid in sourdough and the resulting monohydroxy octadecenoic acid exerts antifungal activity in bread.
INTRODUCTION
Sourdough bread has an extended mold-free storage life compared to that of conventionally leavened products (1, 2), and metabolites from specific strains of lactobacilli contribute to the prolonged storage life of sourdough bread (3, 4, 5). While the fermentation microbiota of traditional sourdough is controlled by the fermentation conditions and the choice of raw materials, the industrial production of sourdough often relies on single strains of lactobacilli with defined metabolic properties (6, 7). To date, cyclic dipeptides, phenyllactic acid, acetic and propionic acids, and short-chain hydroxy fatty acids have been identified as antifungal metabolites of sourdough lactobacilli (8, 9, 10). However, these compounds are either not produced in effective quantities in sourdough fermentations or adversely affect the quality of the product when produced in active concentrations. Cyclic dipeptides, such as 2,5-diketopiperazines, are produced in quantities 1,000-fold below the MIC against molds and are accompanied by bitter or metallic flavors if present in higher quantities (11). Similarly, the amount of phenyllactic acid produced in sourdough is 1,000 times less than the required amount for activity (8, 12, 13). Cooperative metabolism of Lactobacillus buchneri and Lactobacillus diolivorans in sourdough produced acetic and propionic acids in concentrations of 4 and 3 g liter−1, respectively (10). Acetic and propionic acid formation during sourdough fermentation contributed to mold inhibition in bread (10); however, their concentrations remained below the MIC for mold inhibition, at 7.2 g liter−1 and 4.4 g liter−1, respectively, and increased concentrations adversely affect sensory properties of bread.
Pseudomonas aeruginosa transformed linoleic acid to a mixture of mono-, di-, and trihydroxy fatty acids with antifungal activity against a wide range of crop fungal pathogens (14, 15). However, P. aeruginosa is not suitable for use in food fermentations. Lactic acid bacteria also convert linoleic acid to hydroxy fatty acids (16, 17); however, this conversion was not demonstrated in food fermentations and it remains unknown whether hydroxy fatty acids produced by lactobacilli have antifungal activity. Hence, the aim of this study was to determine whether lactobacilli convert linoleic acid to metabolites with antifungal activity, to assess whether this conversion can be achieved in sourdough fermentation, and to determine whether conversion of linoleic acid in sourdough delays fungal spoilage of bread. The screening of lactobacilli focused on sourdough isolates that were previously shown to convert linoleic and oleic acids to hydroxylated metabolites (18).
MATERIALS AND METHODS
Chemicals and standards.
9-cis-12-cis-Octadecadienoic (linoleic) acid, 9-cis-12-hydroxy-octadecenoic (ricinoleic) acid, 12-hydroxystearic acid, octadecanoic (stearic) acid, 9-cis-octadecenoic (oleic) acid, and distearin with >99% purity were purchased from Nu-Chek Prep, Inc. (Elysian, MN). 9,10-Dihydroxystearic acid (>90%) was supplied by Pfaltz and Bauer (Waterbury, CT). Cysteine-HCl (≥98%), Trizma hydrochloride (>99%), and lipoxidase from Glycine max (soybean) type I-B (≥50,000 units mg−1) were purchased from Sigma-Aldrich, (St. Louis, MO). Fisher Scientific (Ottawa, Canada) supplied microbiological medium, high-performance liquid chromatography (HPLC)-grade chloroform, methanol, and acetic acid. Solvents were of analytical grade unless specified otherwise.
Strains and growth conditions.
Lactobacillus sanfranciscensis ATCC 27651, Lactobacillus reuteri LTH2584, Lactobacillus pontis LTH2587, Lactobacillus hammesii DSM16381, and Lactobacillus plantarum TMW1460 and TMW1701 were cultivated on modified De Man Rogosa Sharpe (mMRS) medium (10). Lactobacilli were incubated under microaerophilic conditions (1% O2, balance N2) for 24 h at 37°C (L. reuteri) or 30°C (all other strains). Mucor plumbeus FUA5003, Aspergillus niger FUA5001, or Penicillium roqueforti FUA5005 (10) was used as a target organism for antifungal assay. Fungal cultures were grown on malt extract agar medium at 25°C for 72 h, and spores were harvested as described previously (10). Spore counts were standardized to 104 or 102 spores ml−1 with a hemocytometer (Fein-Optik, Jena, Germany).
Screening of antifungal activity.
Strains were inoculated into 15 ml of mMRS medium and incubated for 24 h. The cells were washed twice with 0.85% NaCl and resuspended in 10 ml of 0.85% NaCl. For each strain, 5% (vol/vol) washed cells was inoculated into 20 ml mMRS broth. Additionally, linoleic acid at 0, 2, or 4 g liter−1 was added. The inoculated broth was incubated with shaking at 120 rpm for 8 days. After 1, 4, and 8 days of incubation, the culture supernatant was collected by centrifugation and sterilized by filtration. Organic extracts of culture supernatants were obtained by extraction with 2:1 (vol/vol) chloroform. The organic phase was collected by centrifugation, and the upper layer was again extracted in the same manner. Organic extracts were dried under nitrogen gas and tested for antifungal activity.
Sourdough was prepared by mixing 10 g white wheat flour and 10 ml tap water and inoculating the mixture with 5% (vol/vol) lactobacilli suspended in saline. Linoleic acid (0, 2, or 4 g kg−1) was added as a substrate, and the dough was incubated at 30°C or 37°C for 8 days. At days 1, 4, and 8, 2-g samples of sourdough were removed for extraction and analysis. All sourdough fermentations were routinely characterized with regard to cell counts and pH to verify growth of lactobacilli and to ensure the identity of fermentation microbiota with the inoculum. Aqueous extracts were obtained by centrifugation (4,000 × g for 10 min). To obtain organic extracts, 2 ml water and 1.5 ml isopropanol were added to 2 g sourdough. The pH of the mixture was adjusted to 2.5 ± 0.05 (mean ± standard deviation) using 5 M HCl, followed by the addition of NaCl to saturation to obtain phase separation. Solids were removed by centrifugation, and the organic phase was collected.
Antifungal activity assay.
The assay used to determine MIC values was performed as serial 2-fold dilutions using a microtiter plate well method described by Magnusson and Schnürer (19). Microtiter plates were inoculated with mMRS broth containing 104 spores ml−1 of A. niger, M. plumbeus, or P. roqueforti and incubated at 25°C. The MIC was determined as the lowest concentration of sample inhibiting growth. Organic solvents in the samples were removed by evaporation under a laminar flow hood prior to the addition of fungal spores. Experiments were performed in triplicate.
Combined liquid chromatography-atmospheric pressure photoionization mass spectrometry (LC-APPI-MS).
Underivatized organic extracts were analyzed by LC-APPI-MS. Separations were conducted on an Agilent 1200 series LC system (Agilent Technologies, Palo Alto, CA) at 25°C using a YMC polyvinyl alcohol-polymerized silica (PVA-Sil) column (150 mm by 2.0 mm inner diameter, 5 μm; Waters Ltd., Mississauga, ON, Canada). Lipid samples dissolved in chloroform were eluted using an injection volume of 5 μl and a gradient of hexane with 0.2% acetic acid (A) and isopropanol with 0.2% acetic acid (B) at a flow rate of 0.2 ml min−1. The gradient was as follows: 0 min, 99% A; 20 min, 70% A; 20.1 min, 99% A. Negative ion APPI-MS was performed on a QStar Elite hybrid orthogonal quadrupole time-of-flight (Q-TOF) mass spectrometer coupled to a PhotoSpray source with Analyst QS 2.0 software (Applied Biosystems/MDS Sciex, Concord, ON, Canada). The source and mass spectrometer conditions were as follows (in arbitrary units unless specified): nebulizer gas, 70; auxiliary gas, 20; curtain gas, 25; ion spray voltage, −1,300 V; source temperature, 400°C; declustering potential (DP), −35 V; focusing potential, −130 V; DP2, −13 V; and scanning mass range, m/z 50 to 700.
Identification and quantitation of antifungal compounds.
For isolation of antifungal compounds, mMRS medium was fermented with L. hammesii or L. sanfranciscensis and 4 g liter−1 linoleic acid for 4 days. Cultures were extracted twice with two volumes of 85:15 (vol/vol) chloroform-methanol. The organic phase was then dried under vacuum at 30°C and was stored at −20°C under nitrogen gas. Up to 25 mg of extracted sample was loaded onto a conditioned 500-mg Sep-Pak silica cartridge (Waters Ltd., Mississauga, ON, Canada) and washed with 20 ml of chloroform, and hydroxy fatty acids were eluted with 10 ml 50% isopropyl alcohol in chloroform (vol/vol). The hydroxy fatty acid fraction was dried under nitrogen and dissolved in chloroform at 30 mg ml−1. For further fractionation according to hydroxyl group number, semipreparative high-performance liquid chromatography was performed on an Agilent 1200 series LC system (Agilent Technologies, Palo Alto, CA). An injection volume of 100 μl was loaded onto a Zorbax Rx-SIL semiprep column (9.4 mm by 250 mm inner diameter, 5 μm; Agilent Technologies). Separations were performed at 23°C at a flow of 3 ml min−1 on a gradient analogous to the analytical column described above. Separations were monitored by a diode array detector at 210 nm and confirmed by splitting the post-column flow to an evaporative light-scattering detector (ELSD) at 60°C with 3.5 standard liters min−1 nitrogen gas. Fractions were collected in 0.1-min time slices, analyzed by mass spectrometry for purity, and assessed for their antifungal activities by MIC assays in triplicate.
For the further fractionation of C18:1 monohydroxy fatty acids, a Supelcosil LC-18-DB column (10 mm by 250 mm inner diameter, 5 μm; Sigma-Aldrich, Oakville, ON, Canada) was employed, using a 3 ml min−1 flow rate and a gradient of 50% acetonitrile and 50% water at 0 min, increasing to 100% acetonitrile at 35 min. A total of 20 μl of a 1 mg ml−1 fatty acid extract in chloroform was injected. Fractions were collected from 13 to 15.5 min, and LC-APPI-MS confirmed the absence of other compounds prior to assessment for antifungal activity.
For relative quantification, peak area percentages of hydroxy fatty acids were compared to the peak area of the same hydroxy fatty acid in extracts from cultures of L. hammesii supplemented with linoleic acid. mMRS medium and sourdough were fermented for 4 days with L. hammesii or L. sanfranciscensis or were chemically acidified with 4:1 (vol/vol) lactic acid-acetic acid to pH 3.5. Linoleic acid was added at either 0 or 4 g liter−1. mMRS medium was extracted directly with methanol-chloroform as described above. Wheat doughs were extracted using the Bligh and Dyer method (20). Briefly, the wheat dough was lyophilized, and 1:2:0.8 (vol/vol/vol) chloroform-methanol-water was added to the dried sample. The mixture was homogenized and left at ambient temperature for 1 h. One part each chloroform and water were added for phase separation, the solution was mixed again, and the lower phase was collected. Each broth and dough lipid extract was dried under nitrogen and reconstituted in 85:15 (vol/vol) chloroform-methanol to 1 mg ml−1 with 5 μg ml−1 distearin added as an internal standard. All samples were then analyzed by LC-APPI-MS, and Analyst software was used to determine peak areas. Each peak was normalized using the response of the distearin internal standard. For optimization of lactobacillus metabolites over time, the same fermentations were prepared and sampled at 12-h intervals over 8 days. All relative quantifications were performed in triplicate independent experiments with a minimum of three technical repeats.
For absolute quantification of the antifungal fatty acid in sourdough starter, dough, and bread, 200-mg lyophilized samples were extracted by the Bligh and Dyer method (20). The lyophilized samples were first spiked with 150 μg of ricinoleic acid standard to measure extraction recovery. Each extraction was adjusted to a volume of 5 ml with chloroform after 25 μg of distearin internal standard was added. An external standard of ricinoleic acid was used to construct a calibration curve, with the assumption that the ionization efficiency was similar to that of the unknown monohydroxy C18:1 product. All samples from triplicate independent experiments were analyzed in duplicate by LC-APPI-MS.
Enzymatic production of coriolic acid.
To test the activity of different components of C18 fatty acids, 9-cis-11-trans-13-hydroxy-octadecadienoic (coriolic) acid was produced in a one-step method (18). Linoleic acid was added at a concentration of 3 mM to a 0.1 M Trizma hydrochloride buffer (pH 9.0) containing cysteine in a 4:1 molar ratio to linoleic acid. After the addition of 0.16 g liter−1 lipoxygenase, the reaction was carried out under a gentle stream of oxygen at room temperature for 5 min, and then the mixture was transferred to an incubator at 25°C with shaking speed of 150 rpm for 25 min. At the end of the incubation period, the buffer was adjusted to pH 2 with 1 N HCl and extracted three times with chloroform containing 15% (vol/vol) methanol. Coriolic acid was purified from unreduced peroxide fatty acids by semipreparative silica chromatography as outlined above. LC-APPI-MS confirmed the identity, the preparation, and the absence of contaminants.
Sourdough fermentation and bread preparation.
L. hammesii and L. sanfranciscensis were used to prepare sourdough bread. Cells from an overnight culture in mMRS medium were washed twice and suspended in sterile tap water to a concentration of 109 CFU ml−1. Sourdough was prepared by mixing white wheat flour, sterile tap water, and culture in a ratio of 2:1:1 (wt/wt/wt) and 4 g kg−1 linoleic acid to homogeneity. The dough was fermented at 30°C for 2 days. Samples were taken after 0, 1, and 2 days for analysis of cell counts, pH values, and the concentration of organic acids and ethanol (10). The identity of the fermentation microbiota and the inoculum was verified by observation of uniform and matching colony morphology. During sourdough fermentation, cell counts for L. hammesii and L. sanfranciscensis reached 9 log CFU ml−1 after 24 h and remained constant after 48 h. The pH values of sourdough after 24 h of fermentation were 3.3 ± 0.1 (L. sanfranciscensis) and 3.4 ± 0.1 (L. hammesii). The pH values remained the same after 24 h of fermentation; L. hammesii and L. sanfranciscensis produced 64.9 ± 6.1 and 70.6 ± 2.4 mmol lactate (kg sourdough)−1, respectively, and 11.1 ± 1.6 and 12.5 ± 2.3 mmol acetate (kg sourdough)−1, respectively.
Dough was prepared with the bread formulations shown in Table 1. Bread was prepared with 20% addition of sourdough; nonacidified dough, chemically acidified dough, and dough supplemented with 0.4% (wt/wt) calcium propionate or 0.15% (wt/wt) coriolic acid were prepared as references. Dough was mixed for 8 min (model number K45SS mixer; Kitchen Aid, Hobart, Troy, OH) and proofed for 25 min at 30°C and 85% relative humidity in a proofer (Cres Cor, Mentor, OH). After the first proof, the dough was molded, placed into tins, and proofed under the same conditions for an additional 105 min. The dough was baked in a convection oven (model number X-300L; Bakers Pride Canada, Lachine, Canada) at 180°C for 25 min. The loaves were cooled to room temperature on racks for 120 min, when samples were taken for antifungal testing, pH determination, and quantification of antifungal compounds by LC-APPI-MS.
Table 1.
Bread formulations
| Ingredient | Amt (g) in indicated formulation |
||||
|---|---|---|---|---|---|
| Nonacidified | Chemically acidified | Fermented | Propionate | Coriolic acid | |
| Flour | 200 | 200 | 180 | 200 | 200 |
| Water | 130 | 130 | 110 | 130 | 130 |
| Salt | 4 | 4 | 4 | 4 | 4 |
| Yeast | 4 | 4 | 4 | 4 | 4 |
| Acid mixa | 0 | 1.3 | 0 | 0 | 0 |
| Sourdoughb | 0 | 0 | 40 | 0 | 0 |
| Calcium propionate | 0 | 0 | 0 | 0.8 | 0 |
| Coriolic acid | 0 | 0 | 0 | 0 | 0.3 |
Mixture of lactic and acetic acid (4:1 [vol/vol]) to yield a dough pH of 3.9 ± 0.5.
Fermented by either L. hammesii or L. sanfranciscensis supplemented with 4 g kg−1 linoleic acid.
Bread pH was measured by homogenizing a 10-fold dilution of bread crumb in deionized water. Growth of mold on bread was measured by slicing the bread in 25-mm-thick uniform slices under sterile conditions and placing into sealed sterile plastic bags with filter tips inserted to allow the exchange of oxygen. Bread slices were inoculated with a spore suspension containing 102 spores ml−1 in a 0.9% (wt/vol) NaCl, 0.1% (wt/vol) Tween 80 solution. The spore suspension was sprayed five times in each corner of the bread slice and once in the middle, delivering 89.1 ± 3.1 μl of spore suspension for P. roqueforti and 90.3 ± 3.3 μl of spore suspension for A. niger with each spray. Additional samples were sliced in an open baking area to allow environmental contamination without inoculation. Slices were incubated for 15 days at 20°C and monitored every 12 h. The time to visible mycelial growth is reported as mold-free shelf life.
The effect of sourdough fermentation was determined in triplicate independent experiments (triplicate sourdough fermentation and baking). Statistical analysis was done with Tukey's pairwise multiple comparison test using SAS 9.3. Significant differences were reported at a confidence level of P values of ≤0.05.
RESULTS
Selection of sourdough lactobacilli with antifungal activity.
Seven strains of lactobacilli that are known to convert linoleic acid (18) were screened for antifungal activity to identify lactobacilli that specifically convert linoleic acid to antifungal metabolites. The inhibitory activities of culture supernatant or organic extracts from cultures in mMRS medium or sourdough were investigated against A. niger and M. plumbeus. The antifungal activity increased from 1 to 4 days of incubation and was maintained until 8 days (data not shown). Table 2 shows the activity of culture supernatants after 4 days of fermentation with A. niger as the indicator strain. Comparable results were obtained with M. plumbeus (see Table S1 in the supplemental material). L. plantarum TMW1460, L. reuteri LTH2584, and L. hammesii DSM16381 exhibited the strongest activities in both mMRS broth and sourdough medium. However, L. hammesii was the only strain for which higher concentrations of linoleic acid led to a stronger antifungal effect. The organic extracts exhibited higher activities than the culture supernatants (Table 3). The strongest activities of the organic extracts from mMRS broth were from L. reuteri, L. pontis, and L. hammesii; the strongest activities in sourdough extracts were observed with L. sanfranciscensis, L. plantarum, and L. hammesii. Again, L. hammesii exhibited a strong effect in either medium, and the addition of linoleic acid increased the antifungal effect against A. niger (Table 3) and M. plumbeus (see Table S2 in the supplemental material). Overall, the results indicate that L. hammesii converts linoleic acid to a hydrophobic compound with antifungal activity.
Table 2.
MICs of aqueous extracts from cultures in mMRS medium and sourdough with differing levels of linoleic acida
| Starter culture | Mean MIC (ml · liter−1) ± SD in medium with indicated concn (g · liter−1) of linoleic acid |
|||||
|---|---|---|---|---|---|---|
| mMRS broth |
Sourdough |
|||||
| 0 | 2 | 4 | 0 | 2 | 4 | |
| L. sanfranciscensis ATCC 27051 | 83 ± 0 | 70 ± 17 | 83 ± 0 | 35 ± 10 | 17 ± 5 | 42 ± 0 |
| L. plantarum TMW1460 | 70 ± 20 | 9 ± 2 | 42 ± 0 | 28 ± 10 | 42 ± 0 | 17 ± 6 |
| L. plantarum TMW1701 | 56 ± 20 | 10 ± 0 | 70 ± 24 | 83 ± 0 | 83 ± 0 | 35 ± 12 |
| L. reuteri LTH2584 | 42 ± 0 | 9 ± 2 | 56 ± 24 | 17 ± 5 | 21 ± 0 | 14 ± 6 |
| L. pontis LTH2587 | 70 ± 20 | 83 ± 0 | 70 ± 24 | 42 ± 0 | 42 ± 0 | 21 ± 0 |
| L. hammesii DSM16381 | 14 ± 5 | 17 ± 5 | 3 ± 2 | 28 ± 10 | 21 ± 0 | 10 ± 0 |
Samples were extracted at 4 days of fermentation and tested for activity using Aspergillus niger as an indicator. MIC analysis was performed after 2 days of indicator growth; data are from triplicate independent experiments.
Table 3.
MICs of organic extracts from both mMRS growth medium and sourdough starter with differing levels of linoleic acid added for conversiona
| Starter culture | Mean MIC (ml · liter−1) ± SD in medium with indicated concn (g · liter−1) of linoleic acid |
|||||
|---|---|---|---|---|---|---|
| mMRS broth |
Sourdough |
|||||
| 0 | 2 | 4 | 0 | 2 | 4 | |
| L. sanfranciscensis ATCC 27051 | 2 ± 0 | 2 ± 0 | 6 ± 2 | 1 ± 0 | 5 ± 2 | 6 ± 2 |
| L. plantarum TMW 1460 | 2 ± 0 | 8 ± 0 | 8 ± 0 | 2 ± 0 | 2 ± 0 | 3 ± 1 |
| L. plantarum TMW 1701 | 6 ± 2 | 2 ± 0 | 3 ± 1 | 4 ± 0 | 1 ± 0 | 8 ± 0 |
| L. reuteri LTH 2584 | 1 ± 0 | 1 ± 0 | 3 ± 1 | 5 ± 2 | 8 ± 0 | 3 ± 1 |
| L. pontis LTH 2587 | 8 ± 0 | 1 ± 0 | 8 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 |
| L. hammesii DSM 16381 | 4 ± 1 | 2 ± 0 | 1 ± 0 | 6 ± 2 | 5 ± 2 | 1 ± 0 |
Samples were extracted at 4 days of fermentation and tested for activity using Aspergillus niger as an indicator. MIC analysis was performed after 2 days of indicator growth; data are from triplicate independent experiments.
Preliminary characterization of antifungal compounds.
Antifungal compounds were fractionated from the organic extracts of L. hammesii. The corresponding extracts from L. sanfranciscensis were also fractionated for comparison. The organic extracts from both strains in mMRS medium were shown by normal-phase LC-APPI-MS analysis to be a mixture of carbon 18 fatty acid isomers with from 0 to 3 hydroxyl groups and 0 to 3 double bonds (Fig. 1). Detection with ELSD revealed that monohydroxy fatty acids in extracts from cultures of L. hammesii accounted for more than 90% of the peak area in the chromatogram (data not shown). The peak areas for monohydroxy fatty acids in extracts from L. hammesii were 6.5 times larger than those in extracts from L. sanfranciscensis; di- and trihydroxy fatty acids were at or below the limit of detection for LC-ELSD for both strains (data not shown). Fatty acids were fractionated by hydroxyl group number and tested for antifungal activity. Monohydroxy fatty acids from either L. hammesii or L. sanfranciscensis exhibited antifungal activity (Table 4). The MICs of dihydroxy and trihydroxy fatty acids were greater than 20 g liter−1. LC-MS analysis of the monohydroxy fraction from L. hammesii indicated that it consisted almost exclusively of a single compound. The main compound in the monohydroxy fraction of L. hammesii produced an [M-H]− ion with m/z 297.2403, indicating a monohydroxy C18:1 fatty acid with the composition C18H33O3. Contrary to this, the same fraction from L. sanfranciscensis consisted of many monohydroxy fatty acids and their isomers.
Fig 1.
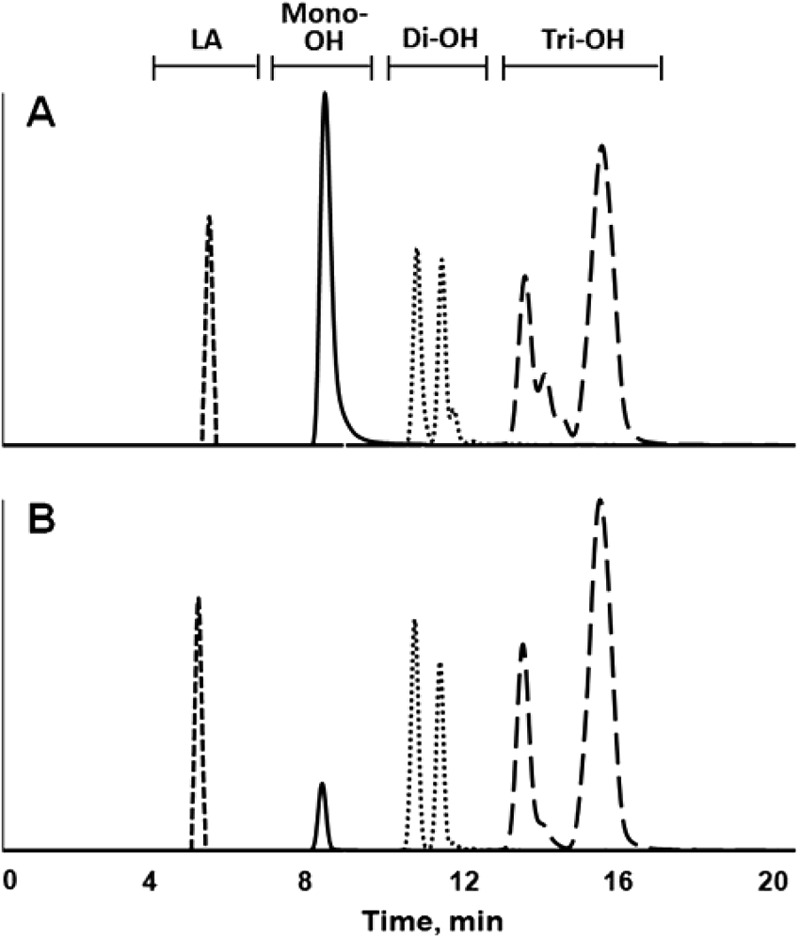
LC-APPI-MS extracted-ion chromatogram overlay of organic extract of sourdough fermented with L. hammesii in the presence of 4 g liter−1 linoleic acid (A) and extract of sourdough fermented with L. sanfranciscensis in the presence of 4 g liter−1 linoleic acid (B). Shown are the [M-H]− ions of m/z 279, corresponding to linoleic acid (LA), m/z 293 to 299, corresponding to saturated, mono-, di-, and triunsaturated monohydroxy C18 fatty acids (solid line), m/z 309 to 315, corresponding to saturated, mono-, di-, and triunsaturated dihydroxy C18 fatty acids (dotted line), and m/z 325 to 331, corresponding to saturated, mono-, di-, and triunsaturated trihydroxy C18 fatty acids (dashed line). Separations were performed on a Waters YMC silica column.
Table 4.
MICs of fatty acids isolated from supernatants of cultures using L. hammesii and L. sanfranciscensis, reference compounds, and enzymatically produced coriolic acida
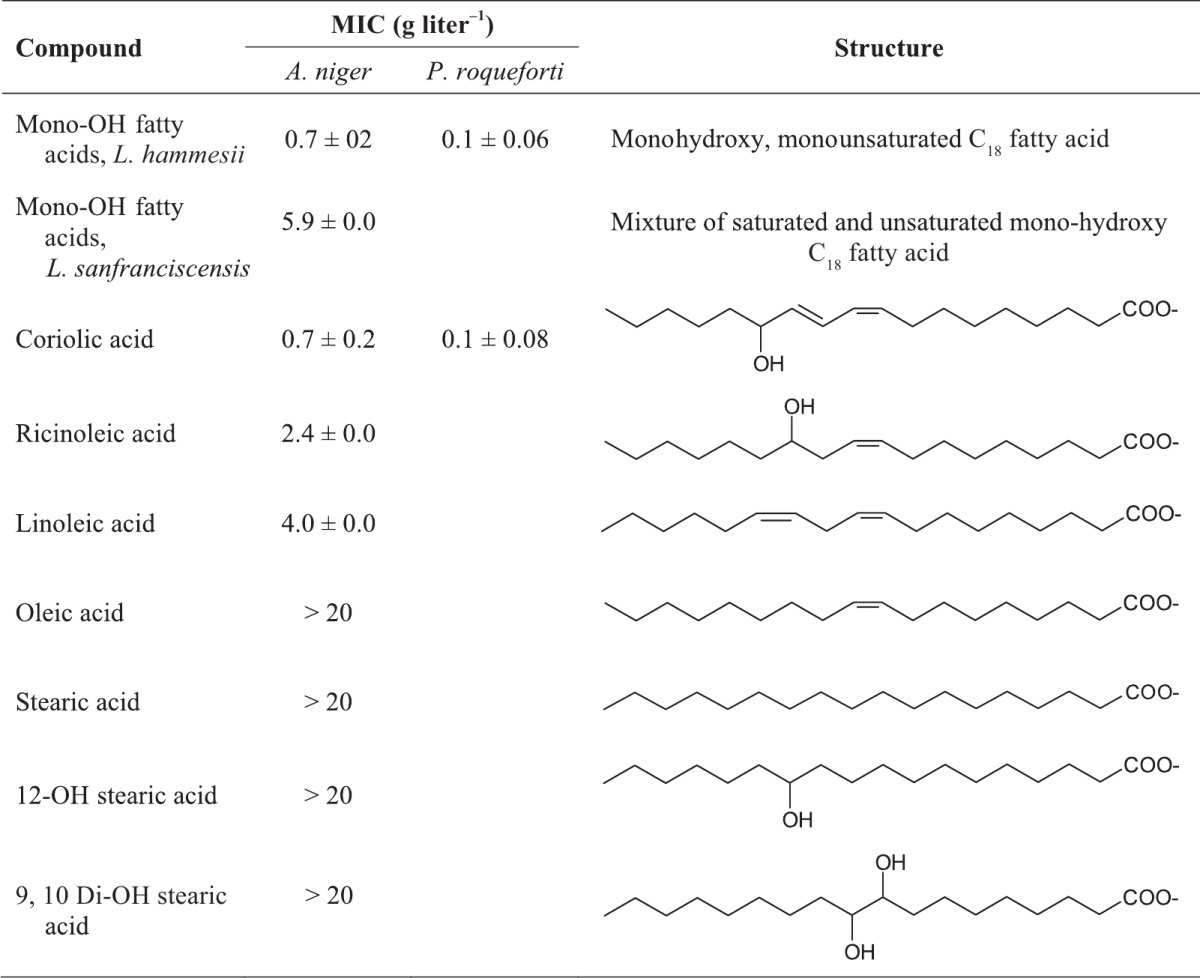
MIC analysis was performed after 3 days of growth with Aspergillus niger or 5 days of growth with Penicillium roqueforti as an indicator strain; data are from triplicate independent experiments. The MICs of di- and trihydroxy fatty acids from L. hammesii and L. sanfranciscensis were higher than 20 g liter−1.
Because monohydroxy fatty acids from L. hammesii or L. sanfranciscensis differed in their antifungal activities, the influence of fatty acid structure on antifungal activity was examined. The monohydroxy fractions from L. hammesii and coriolic acid were the most active, with MICs of 0.7 and 0.1 g liter−1 using A. niger and P. roqueforti, respectively, as indicator strains. The MIC of ricinoleic acid against A. niger was 2.4 g liter−1. Oleic and stearic acids and saturated hydroxy fatty acids exhibited no antifungal activity (Table 4). The C18:1 monohydroxy fatty acid from L. hammesii was purified by reverse-phase chromatography to attribute antifungal activity to a single compound. The purified compound inhibited A. niger with a MIC of 0.7 ± 0.2 g liter−1.
Quantification of conversion products.
In sourdough, hydroxy fatty acids may be produced by enzymatic or chemical oxidation in addition to microbial metabolism. In particular, the oxidation of linoleic acid by lipoxygenase, followed by chemical reduction to coriolic acid, may contribute to the pool of hydroxy fatty acids (18). To distinguish between chemical, enzymatic, and microbial conversions, a quantification of fatty acids in organic extracts from mMRS medium and sourdough was performed. Since authentic standards were not available, hydroxy fatty acids were quantified relative to the concentration of the same compounds in culture supernatants of L. hammesii. Relative quantification was performed in extracts from cultures of L. hammesii or L. sanfranciscensis in mMRS medium and sourdough and from chemically acidified controls (Table 5). The peak area for each fatty acid was expressed as the percentage of the peak area of the same fatty acid in the supernatant of L. hammesii grown in mMRS medium with 4 g liter−1 linoleic acid.
Table 5.
Relative quantitation of C18 hydroxy fatty acids in mMRS medium and sourdough by LC-MSa
| Strain/matrix | Relative concn (mean ± SD) of fatty acids with indicated no. of hydroxyl groups and of double bonds |
|||
|---|---|---|---|---|
| Mono-OH C18 fatty acids |
Di-OH C18 fatty acids |
|||
| 0 | 1 | 0 | 1 | |
| L. hammesii/mMRS + LA | 100 ± 12 | 100 ± 3 | 100 ± 11 | 100 ± 4 |
| L. hammesii/mMRS | 258 ± 43 | 1 ± 0 | 249 ± 24 | 9 ± 1 |
| L. sanfranciscensis/mMRS + LA | 190 ± 31 | 30 ± 3 | 126 ± 28 | 28 ± 2 |
| L. sanfranciscensis/mMRS | 341 ± 51 | 0 ± 0 | 203 ± 28 | 7 ± 3 |
| L. hammesii/dough + LA | 31 ± 6 | 29 ± 1 | 58 ± 11 | 176 ± 8 |
| L. hammesii/dough | 15 ± 2 | 15 ± 1 | 35 ± 13 | 20 ± 7 |
| L. sanfranciscensis/dough + LA | 8 ± 2 | 3 ± 0 | 36 ± 10 | 123 ± 8 |
| L. sanfranciscensis/dough | 7 ± 2 | 4 ± 1 | 10 ± 5 | 9 ± 0 |
| Chemically acidified mMRS + LA | 5 ± 1 | 1 ± 0 | 16 ± 1 | 21 ± 5 |
| Chemically acidified dough + LA | 2 ± 1 | 0 ± 0 | 12 ± 1 | 17 ± 0 |
Hydroxy fatty acid concentrations are expressed relative to the concentration of the same compound extracted from L. hammesii grown in mMRS in the presence of linoleic acid (L. hammesii/mMRS + LA), which was used as a reference (100%). Data are from triplicate independent experiments. LA, addition of 4 g liter−1 linoleic acid.
The monohydroxy octadecenoic acid concentrations in fermentations with L. hammesii including linoleic acid were 20-fold higher than the concentrations in controls containing no linoleic acid or chemically acidified controls without bacterial metabolism (Table 5), demonstrating that it is a microbial metabolite from linoleic acid. The relative concentration of the monohydroxy C18:1 antifungal metabolite was higher in mMRS medium than in sourdough and more abundant in fermentations with L. hammesii than with L. sanfranciscensis. Absolute quantification of the monohydroxy C18:1 from L. hammesii revealed that it is produced in sourdough to a concentration of 0.73 ± 0.03 g kg−1, a level that is equivalent or higher than the MIC.
Culture supernatants from mMRS medium without the addition of linoleic acid show large amounts of mono- and dihydroxy saturated fatty acids (Table 5). These products thus likely result from the metabolism of other fatty acids, i.e., the hydration of oleic acid (17), a component of Tween 80. Sourdough fermentations with added linoleic acid generated a large amount of dihydroxy octadecenoic acid, suggesting that flour-derived enzymes or microbial conversion of fatty acids present in dough play a role in their formation. The relative concentrations of monohydroxy fatty acids with two or three double bonds and dihydroxy fatty acids with two or three double bonds were high in the chemically acidified controls, and their absolute concentrations were low (see Table S3 in the supplemental material). This result indicates that these compounds result from chemical or enzymatic oxidation rather than microbial metabolism.
Concentrations of hydroxy fatty acids in dough and bread.
Initially, the fermentation time to achieve high concentrations of C18 hydroxy fatty acids was optimized. Sourdough was fermented with L. hammesii and L. sanfranciscensis for up to 8 days, and samples were taken every 12 h for analysis with LC-APPI-MS. The peak areas for the antifungal C18:1 hydroxy fatty acid peaked at 2 days of fermentation for L. hammesii and L. sanfranciscensis and remained at constant levels throughout subsequent incubation (data not shown). Therefore, sourdoughs for use in bread making were fermented for 2 days. Bread dough prepared with L. hammesii sourdough contained 0.13 ± 0.02 g kg−1 monohydroxy C18:1 after proofing. After baking, 0.11 ± 0.02 g kg−1 remained, corresponding to a loss of 14%. Coriolic acid was also present in the bread fermented with L. hammesii at a concentration of 0. 13 ± 0.03 g kg−1, confirming its formation from linoleic acid, enzymes, and reducing agents in the wheat flour. Bread supplemented with 1.5 g kg−1 coriolic acid contained 1.2 ± 0.09 g kg−1 coriolic acid after proofing and 1.1 ± 0.02 g kg−1 coriolic acid after heating, corresponding to a baking loss of 12%. The pH value of bread was 5.3 ± 0.1 for nonacidified control and bread supplemented with calcium propionate or coriolic acid. Chemically acidified bread and sourdough bread fermented with L. sanfranciscensis or L. hammesii had pH values of 4.1 ± 0.1, 4.3 ± 0.1, and 4.4 ± 0.1, respectively.
Effect of sourdough fermentation and coriolic acid on fungal spoilage of bread.
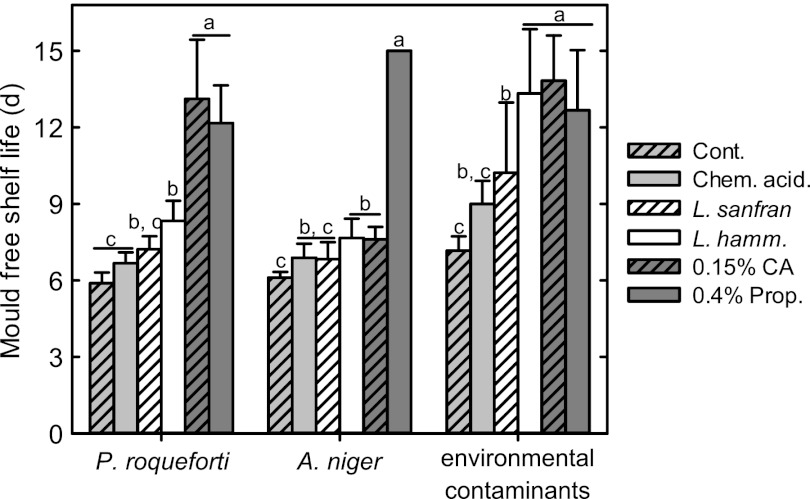
The effect of sourdough fermentation on fungal spoilage was evaluated by challenge with two different fungal strains and after environmental contamination. Sourdough bread was compared to bread prepared in a straight dough process without additives and to bread with 0.4% calcium propionate or 0.15% coriolic acid. Chemical acidification or the addition of sourdough fermented with L. sanfranciscensis had no effect on the growth of A. niger or P. roqueforti compared to their growth in the control. Environmental contaminants, however, were inhibited by the inclusion of sourdough fermented with L. sanfranciscensis (Fig. 2). Bread prepared with L. hammesii sourdough inhibited the growth of all molds relative to their growth in the control and showed delayed growth of environmental contaminants compared to the growth of molds in bread prepared with L. sanfranciscensis sourdough or the chemically acidified control. Coriolic acid-supplemented bread exhibited inhibitory effects against all molds compared to the results for the control, and coriolic acid was as effective as 0.4% calcium propionate for bread inoculated with P. roqueforti and environmental contaminants.
Fig 2.
Mold-free shelf life in days (d) of bread (control [Cont.]), chemically acidified bread (Chem. acid.), sourdough bread fermented with L. sanfranciscensis (L. sanfran) or L. hammesii (L. hamm.), and bread supplemented with 0.15% coriolic acid (0.15% CA) or 0.4% Ca-propionate (0.4% Prop.). Sourdough was supplemented with 4 g kg−1 linoleic acid and fermented with L. hammesii or L. sanfranciscensis. Bread slices were inoculated with A. niger or P. roqueforti or contaminated by environmental fungal spores during handling and stored until visible mold growth or for 15 days. Data are shown as means ± standard deviations of the results of triplicate independent experiments. Values for bread inoculated with the same mold that do not share a letter are significantly different (P < 0.05).
DISCUSSION
This study demonstrated that L. hammesii DSM16381, an isolate from sourdough (21), converts linoleic acid to a monohydroxy octadecenoic acid with antifungal activity. Thus, hydroxy fatty acids produced by food-fermenting lactic acid bacteria (16, 17, 18, 22) exhibit antifungal activity. Moreover, linoleic acid metabolism by lactic acid bacteria was not previously observed in food fermentations (16, 17). L. hammesii produced higher quantities of the monohydroxy C18:1 fatty acid than L. sanfranciscensis and demonstrated higher levels of antifungal activity than other lactobacilli. Moreover, coriolic acid exhibits antifungal activity, and its activity is comparable to that of the hydroxy fatty acid produced by L. hammesii. Sourdough bread prepared with L. hammesii and bread prepared with coriolic acid showed delayed fungal spoilage for up to 15 days.
Lactobacilli hydrate linoleic acid to 13-hydroxy-9-octadecenoic acid or 10-hydroxy-12-octadecenoic acid (16, 22). The hydratase of lactic acid bacteria converting linoleic and oleic acids to hydroxy fatty acids was recently characterized (17, 23). Hydratases of lactobacilli produce predominantly 10-hydroxy-octadecenoic acid (23). In Lactobacillus acidophilus, the proportion of hydroxy fatty acids in the cytoplasmic membrane increased at a higher growth temperature (24), suggesting a role of hydroxy fatty acids in membrane homeostasis. Correspondingly, overexpression of the hydratase in lactic acid bacteria increased their heat resistance (25). Cells change the fatty acid composition of the plasma membrane in response to altered environmental conditions to maintain a liquid-crystalline state (26, 27). Hydroxy C18:1 fatty acids decreased the phase transition temperature of the membrane, stabilizing the liquid-crystalline state (28). Under direct comparison, the same unsaturated fatty acid had much less impact on membrane properties (28).
The antifungal activity of hydroxy fatty acids (14) is likely also linked to their interaction with membranes. Partitioning of hydroxy fatty acids into fungal membrane has been proposed to increase membrane permeability (29, 30, 31). Our results demonstrate that antifungal activity is highly dependent on the fatty acid structure. Unsaturated monohydroxy fatty acids exhibited antifungal activity, but saturated hydroxy fatty acids or unsaturated fatty acids were not active. This suggests that at least one double bound and one hydroxyl group along a C18 aliphatic chain are required for antifungal activity. Remarkably, the monohydroxy C18:1 fatty acid produced by L. hammesii had higher activity than the mixture of monohydroxy fatty acids extracted from L. sanfranciscensis. Moreover, the 13-hydroxy-9-cis,11-trans-octadecadienoic (coriolic) acid had higher antifungal activity than 12-hydroxy-9-cis-octadecenoic (ricinoleic) acid. The trans configuration in coriolic acid has no effect on antifungal activity (32, 33), indicating that the exact positioning and configuration of hydroxyl groups and double bonds also affects antifungal activity.
The antifungal activity of metabolites from lactic acid bacteria in bread has, to date, been attributed not to a single compound but, rather, to their synergistic activity with substrate- or yeast-derived compounds (4, 5, 8, 10). This study demonstrated that enzymatic and microbial activities generate antifungal hydroxy fatty acids from linoleic acid. Coriolic acid, the product of enzymatic conversion of linoleic acid, has antifungal activity that is equivalent to the linoleic acid metabolite from L. hammesii. The conversion of linoleic acid to coriolic acid depends on lipoxygenase activity to generate fatty acid peroxides and thiols to reduce fatty acid peroxides to hydroxy fatty acids (18); both lipoxygenase activity and low-molecular-weight thiols are present in wheat (sour)dough (34, 35). Chemical and enzymatic oxidation generates additional hydroxy fatty acids in wheat dough, although identification of all of the isomeric structures has not yet been achieved. Microbial conversion of linoleic acid during growth of L. hammesii and L. sanfranciscensis produced C18:1 monohydroxy fatty acids. Monohydroxy C18:1 fatty acids produced at the dough stage were relative stable, with a baking loss of less than 15% after baking. The concentration of the antifungal hydroxy C18:1 fatty acid from L. hammesii in bread was at or below the MICs; nevertheless, bread prepared with L. hammesii sourdough delayed the growth of A. niger, P. roqueforti, and environmental contaminants. The comparison to bread prepared with L. sanfranciscensis sourdough indicates that microbial conversion of linoleic acid contributes to the antifungal activity of sourdough. The antifungal activity of 0.15% coriolic acid—a concentration exceeding the MICs against A. niger and P. roqueforti by 2- to 10-fold—was comparable to the preservative effect of 0.4% calcium propionate. The bitter taste threshold levels for di- and trihydroxy fatty acids were 4 and 2 g liter−1, respectively (36); monohydroxy fatty acids have a higher taste threshold (37). Monohydroxy fatty acids thus delay or prevent fungal spoilage of bread without adverse impact on the sensory properties of bread.
In conclusion, L. hammesii converts linoleic acid to a monohydroxy octadecenoic acid with antifungal activity. This conversion was observed in sourdough fermentations supplemented with linoleic acid, but the generation of hydroxy fatty acids in sourdough also occurred through enzymatic or chemical oxidation. Monohydroxy octadecenoic acid in combination with substrate-derived coriolic acid inhibited the growth of mold on sourdough bread. The use of coriolic acid and antifungal metabolites from linoleic acid as natural antifungals is not limited to food preservation. Antifungal metabolites from lactobacilli may complement or substitute for these fungicides for use in seed treatment and crop protection (38).
ACKNOWLEDGMENTS
The National Science and Engineering Research Council of Canada and the Ernst Böcker GmbH are acknowledged for funding. Brenna Black acknowledges support from the Canadian Wheat Board, Michael Gänzle acknowledges support from the Canada Research Chairs program, and Jonathan Curtis acknowledges support from the NSERC Discovery program.
Footnotes
Published ahead of print 11 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03784-12.
REFERENCES
- 1. Salovaara H. 2004. Lactic acid bacteria in cereal-based products, 3rd ed, p 431–452 In Salminen S, von Wright A, Ouwehand A. (ed), Lactic acid bacteria. CRC Press, Boca Raton, FL [Google Scholar]
- 2. Smith JP, Daifas DP, El-Khoury W, Koukoutsis J, El-Khoury A. 2004. Shelf life and safety concerns of bakery products. Crit. Rev. Food Sci. Nutr. 44:19–55 [DOI] [PubMed] [Google Scholar]
- 3. Coda R, Rizzello CG, Nigro F, De Angelis M, Arnault P, Gobbetti M. 2008. Long-term fungal inhibitory activity of water-soluble extracts of Phaseolus vulgaris cv. Pinto and sourdough lactic acid bacteria during bread storage. Appl. Environ. Microbiol. 74:7391–7398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coda R, Cassone A, Rizzello CG, Nionelli L, Cardinali G, Gobbetti M. 2011. Antifungal activity of Wickerhamomyces anomalus and Lactobacillus plantarum during sourdough fermentation: identification of novel compounds and long-term effect during storage of wheat bread. Appl. Environ. Microbiol. 77:3484–3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ryan LAM, Zannini E, Dal Bello F, Pawlosksa A, Koehler P, Arendt EK. 2011. Lactobacillus amylovorus DSM19280 as a novel food-grade antifungal agent for bakery products. Int. J. Food Microbiol. 146:276–283 [DOI] [PubMed] [Google Scholar]
- 6. Brandt MJ. 2007. Sourdough products for convenient use in baking. Food Microbiol. 24:161–164 [DOI] [PubMed] [Google Scholar]
- 7. Corsetti A. 2013. Technology of sourdough fermentation and sourdough applications, p 183–217 In Gobbetti M, G̈anzle MG. (ed), Handbook of sourdough biotechnology. Springer, Heidelberg, Germany [Google Scholar]
- 8. Lavermicocca P, Valerio F, Evidente A, Lazzaroni S, Corsetti A, Gobbetti M. 2000. Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl. Environ. Microbiol. 66:4084–4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schnürer J, Magnusson J. 2005. Antifungal lactic acid bacteria as preservatives. Trends Food Sci. Technol. 16:70–78 [Google Scholar]
- 10. Zhang C, Brandt MJ, Schwab C, Gänzle MG. 2010. Propionic acid production by cofermentation of Lactobacillus buchneri and Lactobacillus diolivorans in sourdough. Food Microbiol. 27:390–395 [DOI] [PubMed] [Google Scholar]
- 11. Ryan LAM, Dal Bello F, Arendt EK, Koehler P. 2009. Detection and quantitation of 2,5-diketopiperazines in wheat sourdough and bread. J. Agric. Food Chem. 57:9563–9568 [DOI] [PubMed] [Google Scholar]
- 12. Ryan LAM, Dal Bello F, Czerny M, Koehler P, Arendt EK. 2009. Quantification of phenyllactic acid in wheat sourdough using high resolution gas chromatography–mass spectrometry. J. Agric. Food Chem. 57:1060–1064 [DOI] [PubMed] [Google Scholar]
- 13. Valerio F, Lavermicocca P, Pascale M, Visconti A. 2004. Production of phenyllactic acid by lactic acid bacteria: an approach to the selection of strains contributing to food quality and preservation. FEMS Microbiol. Lett. 233:289–295 [DOI] [PubMed] [Google Scholar]
- 14. Hou CT. 2008. New bioactive fatty acids. Asia Pac. J. Clin. Nutr. 17(Suppl 1):192–195 [PubMed] [Google Scholar]
- 15. Martin-Arjol I, Bassas-Galia M, Bermudo E, Garcia F, Manresa A. 2010. Identification of oxylipins with antifungal activity by LC-MS/MS from the supernatant of Pseudomonas 42A2. Chem. Phys. Lipids 163:341–346 [DOI] [PubMed] [Google Scholar]
- 16. Kishimoto N, Yamamoto I, Toraishi K, Yoshioka S, Saito K, Masuda H, Fujita T. 2003. Two distinct pathways for the formation of hydroxy FA from linoleic acid by lactic acid bacteria. Lipids 38:1269–1274 [DOI] [PubMed] [Google Scholar]
- 17. Volkov A, Liavonchanka A, Kamneva O, Fiedler T, Goebel C, Kreikemeyer B, Feussner I. 2010. Myosin cross-reactive antigen of Streptococcus pyogenes M49 encodes a fatty acid double bond hydratase that plays a role in oleic acid detoxification and bacterial virulence. J. Biol. Chem. 285:10353–10361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shahzadi A. 2011. Bio-transformation of fatty acids. Ph.D. thesis. University of Alberta, Edmonton, Alberta, Canada [Google Scholar]
- 19. Magnusson J, Schnürer J. 2001. Lactobacillus coryniformis subsp. Coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl. Environ. Microbiol. 67:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911–917 [DOI] [PubMed] [Google Scholar]
- 21. Valcheva R, Korakli M, Onno B, Prévost H, Ivanona I, Ehrmann MA, Dousset X, Gänzle MG, Vogel RF. 2005. Lactobacillus hammesii sp. nov., isolated from French sourdough. Int. J. Syst. Evol. Microbiol. 55:763–767 [DOI] [PubMed] [Google Scholar]
- 22. Ogawa J, Matsumura K, Kishino S, Omura Y, Shimizu S. 2001. Conjugated linoleic acid accumulation via 10-hydroxy-12-octadecaenoic acid during microaerobic transformation of linoleic acid by Lactobacillus acidophilus. Appl. Environ. Microbiol. 67:1246–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang B, Chen H, Song Y, Chen YQ, Zhang H, Chen W. 2013. Myosin-cross-reactive antigens from four different lactic acid bacteria are fatty acid hydratases. Biotechnol. Lett. 35:75–81 [DOI] [PubMed] [Google Scholar]
- 24. Fernández Murga ML, Bernik D, Font de Valdez G, Disalvo AE. 1999. Permeability and stability properties of membrane formed by lipids extracted from Lactobacillus acidophilus grown at different temperatures. Arch. Biochem. Biophys. 364:115–121 [DOI] [PubMed] [Google Scholar]
- 25. Rosberg-Cody E, Liavonchanka A, Göbel C, Ross RP, O'Sullivan O, Fitzgerald GF. 2011. Myosin-cross-reactive antigen (MCRA) protein from Bifidobacterium breve is a FAD-dependent fatty acid hydratase which has a function in stress protection. BMC Biochem. 12:9–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Annous BA, Becker LA, Bayles DO, Labeda DP, Wilkinson BJ. 1997. Critical role of anteiso-C15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 63:3887–3894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Quinn PJ. 1981. The fluidity of cell membranes and its regulation. Prog. Biophys. Mol. Biol. 38:1–104 [DOI] [PubMed] [Google Scholar]
- 28. Jenske R, Lindström F, Gröbner G, Vetter W. 2008. Impact of free hydroxylated and methyl-branched fatty acids on the organization of lipid membranes. Chem. Phys. Lipids 154:26–32 [DOI] [PubMed] [Google Scholar]
- 29. Pohl CH, Kock JLF, Thibane VS. 2011. Antifungal free fatty acids: a review, p 61–71 In Méndez-Vilas A. (ed), Science against microbial pathogens: communicating current research and technological advances. Formatex Research Center, Badajoz, Spain [Google Scholar]
- 30. Pohl EE, Voltchenko AM, Rupprecht A. 2008. Flip-flop of hydroxy fatty acids across the membrane as monitored by proton-sensitive microelectrodes. Biochim. Biophys. Acta 1778:1292–1297 [DOI] [PubMed] [Google Scholar]
- 31. Sjögren J, Magnusson J, Broberg A, Schnürer J, Kenne L. 2003. Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl. Environ. Microbiol. 69:7554–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Avis TJ. 2007. Antifungal compounds that target fungal membranes: applications in plant disease control. Can. J. Plant Pathol. 29:323–329 [Google Scholar]
- 33. Kobayashi Y, Okamoto S, Shimazaki T, Ochiai Y, Sato F. 1987. Synthesis and physiological activities of both enantiomers of coriolic acid and their geometric isomers. Tetrahedron Lett. 28:3959–3962 [Google Scholar]
- 34. Belitz HD, Grosch W, Schieberle P. (ed). 2009. Food chemistry, 4th ed, p 670–742 Springer, Berlin, Germany [Google Scholar]
- 35. Vermeulen N, Kretzer J, Machalitza H, Vogel RF, Gänzle MG. 2006. Influence of redox-reactions catalysed by homo- and heterofermentative lactobacilli on gluten in wheat sourdoughs. J. Cereal Sci. 43:137–143 [Google Scholar]
- 36. Baur C, Grosch W, Wieser H, Jugel H. 1977. Enzymatic oxidation of linoleic acid: formation of bittertasting fatty acids. Z. Lebensm. Unters. Forsch. 164:171–176 [DOI] [PubMed] [Google Scholar]
- 37. Biermann VU, Wittmann A, Grosch W. 1980. Occurrence of bitter hydroxy fatty acids in oat and wheat. Fette Seifen Anstrichm. 82:236–240 [Google Scholar]
- 38. Hernández AF, Parrón T, Tsatsakis AM, Requena M, Alarcón R. 20 June 2012. Toxic effects of pesticide mixtures at a molecular level: their relevance to human health. Toxicology [Epub ahead of print.] doi:10.1016/j.tox.2012.06.009 [DOI] [PubMed] [Google Scholar]