Abstract
The bean bug Riptortus pedestris is specifically associated with the Burkholderia gut symbiont and acquires the symbiont from the environment every generation. Here, we investigated the infective dose of the symbiont by experimental administration. The 50% infective dose was remarkably low, only 80 cells, indicating efficient colonization of the symbiont.
TEXT
Endosymbiotic association with microorganisms is omnipresent in nature, which has strikingly affected organismal evolution (1–3). Insects that feed exclusively on nutritionally limited or persistent food sources such as plant phloem sap, vertebrate blood, or wood materials commonly carry symbiotic microorganisms in their bodies (4–6). Symbiotic microorganisms can be essential for host survival and reproduction and play pivotal roles in host metabolism by providing essential nutrients, digesting food materials, and/or influencing host plant use, resistance against parasitoids, and body color change (4, 7–10). To ensure that offspring acquire these irreplaceable partners, insects have evolved sophisticated vertical mechanisms for symbiont transmission, such as ovarial transmission, egg smearing, and coprophagy (5, 6, 11, 12).
The bean bug Riptortus pedestris (Heteroptera: Alydidae), formerly known as Riptortus clavatus, develops a number of sac-like tissues, called crops or crypts, in the posterior region of the midgut, and these crypts are colonized by dense populations of a Burkholderia symbiont (Betaproteobacteria) (13). A comparison between symbiotic and aposymbiotic insects demonstrated that the Burkholderia symbiont strikingly improves the growth of the stinkbug (14). Furthermore, a recent study demonstrated that insecticide-degrading strains of the Burkholderia symbiont confer insecticide resistance to the bean bug (15). Unlike the typical insect-microbe symbiosis, the bean bug does not vertically transmit the Burkholderia symbiont from mother to offspring; instead, the symbiont is acquired from the surrounding environment at the early nymphal stage (14).
Our previous study revealed that Burkholderia symbiont colonization occurs mainly at the second-instar stage, which is strictly correlated with the development of the symbiotic organ (16). Considering the limited period of symbiont colonization and that millions of bacterial species are living in environmental soils (17), colonization of the Burkholderia symbiont is expected to be highly specific and efficient. The Burkholderia symbiont is culturable and can be administered orally to the host insect (16), which provides us with a unique opportunity to experimentally investigate colonization efficiency more fully.
Here, we identified an important ecological aspect of in vivo selection in the stinkbug-Burkholderia symbiosis, that is, colonization efficiency, by administering a given number of Burkholderia symbionts into bean bug nymphs.
R. pedestris collected from a soybean (Glycine max) field in Tsukuba, Ibaraki, Japan, and maintained in a laboratory was used in this study. Bean bugs were reared in petri dishes (diameter, 90 mm; height, 20 mm) at 25°C under a long-day regimen (16 h light, 8 h dark) and fed on dried soybean seeds and distilled water containing 0.05% ascorbic acid (DWA). A rifampin-resistant spontaneous mutant of the Burkholderia symbiont, RPE75 (16), was used for the experiments. The symbiont strain was conserved as frozen stock at −80°C and cultured at 25°C using YG medium (0.5% yeast extract, 0.4% glucose, 0.1% NaCl), as described previously (16).
To determine the 50% infective dose (ID50), defined as the amount of Burkholderia symbiont required for colonization of 50% of tested insects, the symbiont was administered orally as follows (experimental time course is shown in Fig. 1A). Immediately after the first-instar nymphs molted to the second-instar nymphs, the DWA was removed from the rearing case, and the nymphs were denied access to water overnight to induce thirst. The second-instar nymphs were kept separately in small petri dishes (diameter, 40 mm; height, 15 mm) and supplied with 1 μl of symbiont-containing water (Fig. 1B). The inoculation was performed in a humidity chamber to prevent water evaporation, and the nymphs that could not drink the entire 1 μl of water were excluded from the analysis. To prepare the symbiont-containing water, symbiont strain RPE75 was grown to early log phase in YG-RIF (YG medium containing 10 μg/ml of rifampin) on a gyratory shaker (150 rpm, 25°C) and diluted with distilled water so that the water contained a given CFU (from 5 to 125,000 CFU) of Burkholderia symbiont per 1 μl. In total, 21 different doses of the symbiont were tested. The administered CFU was confirmed by plating the symbiont-containing water on YG-RIF agar plates. After administration, the nymphs were reared as described above. Two days after the nymphs molted to the third instar (approximately 5 days after administration), the symbiotic organs were dissected, and colonization of the Burkholderia symbiont was confirmed by diagnostic PCR with a specific primer set of the symbiont, as described previously (13, 14). For each administration group, 20 to 30 nymphs were investigated. To estimate the ID50, the results were evaluated by probit analysis using the statistical program R 2.12.1 (18).
Fig 1.
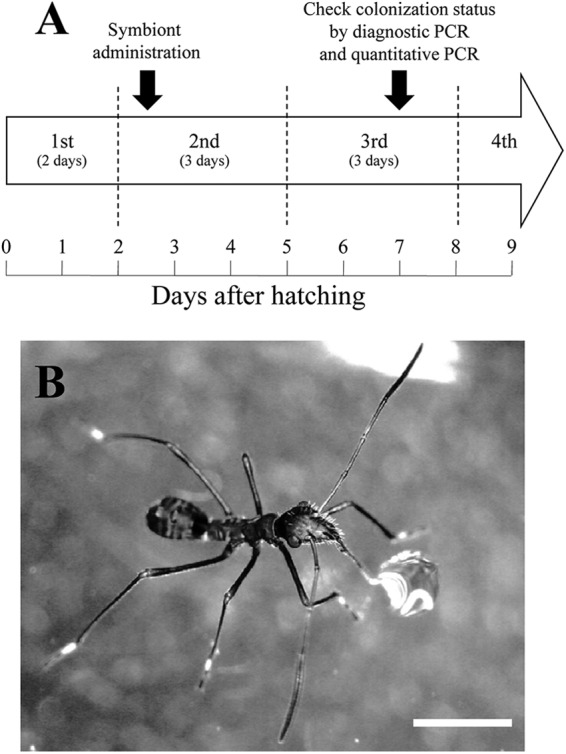
Oral administration of the Burkholderia symbiont. (A) Developmental time course of the bean bug and experimental schedule. (B) An unfed second-instar nymph of Riptortus pedestris sucking 1 μl of distilled water containing cultured Burkholderia symbiont. Bar, 1 mm.
Figure 2 shows the relationship between cell numbers of the administered symbiont and the percent rate of the infected nymphs. When about 80 cells of the Burkholderia symbiont were administered, an average of 50% of R. pedestris nymphs became colonized. Colonization efficiency improved rapidly as inoculum size increased, reaching 100% at levels above 3,500 symbiont cells.
Fig 2.

ID50 of the Burkholderia symbiont for symbiotic colonization. In the oral administration experiments, second-instar nymphs were fed with different numbers of Burkholderia symbiont (strain RPE75) cells, and the percentage of host individuals subsequently colonized was determined by diagnostic PCR. The dose at which 50% of the animals become infected by the third instar, indicated by the dotted lines, was determined using the equation probit (y) = 0.37 ln (x) + 3.38.
To confirm whether the initial cell numbers of inoculated Burkholderia symbiont affect colonization level, the number of symbionts per insect in the infected third-instar nymphs was investigated by quantitative PCR. Among the 21 administration groups, infected individuals of 12 groups were investigated. Real-time PCR quantification of dnaA gene copy numbers of the Burkholderia symbiont was performed using SYBR green (Molecular Probes), an Mx3000P QPCR system (Stratagene), and primer sets BSdnaA-F and BSdnaA-R, as described previously (16).
In infected individuals, quantitative PCR revealed that the number of Burkholderia symbiont was not significantly different between experimental groups (Kruskal-Wallis test; P = 0.4531) (Fig. 3), wherein symbiont density was 6.48 × 107 ± 5.10 × 106 (mean ± standard error) per individual in the third-instar nymphs, based on dnaA gene copy numbers. Although the rate of successful colonization is correlated with the size of the dose of administered symbiont, these results demonstrated that a wide range of symbiont doses is sufficient to initiate colonization, indicating that the Burkholderia symbiont proliferates and reaches a consistent density inside the symbiotic organ by the third-instar stage.
Fig 3.

Quantification of the Burkholderia symbiont in midgut crypts of third-instar nymphs. The nymphs were experimentally inoculated with different numbers of symbiont cells at the second-instar stage. Symbiont number in terms of symbiont dnaA gene copies per insect.
Probably because of their medical importance, the ID50 or the analogous 50% lethal dose (LD50), which is the bacterial dose that causes death in 50% of infected hosts, has been reported in several pathogens. Although some pathogenic bacteria like Yersinia pestis show low infection doses, the doses of pathogenic bacteria are relatively high. For instance, LD50 is more than 107 cells in Escherichia coli O157 (19), 105 cells in pathogenic Vibrio species (20, 21), and more than 105 cells in Burkholderia pseudomallei, known as an agent of melioidosis (22). In contrast, the number of bacterial cells needed for bacterial colonization could be lower in mutualistic associations involving environmental symbiont transmission. The ID50 is almost 250 symbiont cells in squid-Vibrio luminescent symbiosis (23) and around 104 symbionts in legume-Rhizobium symbiosis (24). The infective dose in the stinkbug-Burkholderia symbiosis, only about 80 symbiont cells (Fig. 2), is remarkably lower than those in the pathogenic and mutualistic associations, strongly suggesting an unusually specific and efficient colonization mechanism in the stinkbug symbiosis.
Several studies have estimated densities of Burkholderia spp. in the soil at around 105 cells/g (25–27). Our recent study, based on quantitative PCR with specific primers, suggested that the density of the Burkholderia symbiont in crop fields is diverse, from 70 cells to 7,500 cells per gram of soil (K. Tago, Y. Kikuchi, A. Nagayama, T. Hori, and M. Hayatsu, unpublished data). In such fluctuating environments, the efficient colonization identified here could ensure that the stinkbug nymphs acquire their beneficial partner, and such low environmental density of the symbiont has probably accelerated the evolution of the highly efficient colonization in the Burkholderia symbiont.
Since most insect-microbe symbioses are maintained by strict vertical transmission and most of the symbionts are unculturable (4, 6), determination of infective dose is generally difficult. In aphids and beewolves, the number of vertically transmitted symbionts was estimated by quantitative PCR and direct cell counting (28, 29). In both insects, almost 900 cells of the symbionts were transmitted from the mother to offspring, and especially in beewolves, researchers estimated that only 100 symbiont cells were enough for colonization inside the symbiotic organ (28). In stinkbugs that vertically transmit their symbionts, the infective dose was experimentally examined in the plataspid stinkbug Megacopta punctatissima, wherein the symbiotic bacterium Ishikawaella capsulata is localized in the midgut crypts and is transmitted by a “symbiont capsule” (30, 31). Experimental manipulation of egg/capsule ratios has demonstrated that the minimum threshold for successful colonization is 1.9 × 106 symbionts in the plataspid symbiosis (32). Such higher colonization doses, compared with those in the bean bug, seem to be affected by symbiont transmission manner. In the plataspid stinkbug, symbiont transmission could be considered a maternal investment (32), in which more symbionts would be better for offspring. In the bean bug, in contrast, a lower number of ingested bacteria might be preferred by nymphs because environmental transmission faces the constant risk of infection by simultaneously ingested pathogens and cheaters. Although this is speculation, it will be of great interest to investigate and compare ecological and molecular factors affecting the infective doses between the two gut symbiotic systems.
The mechanisms ensuring the highly efficient colonization of the Burkholderia symbiont in the bean bug remain unclear. Physiological conditions inside the midgut crypts, such as nutrient availability, pH, osmotic pressure, and possibly antimicrobial agents, would affect the specificity and efficiency of the symbiont infection. In mutualistic associations like coral-Symbiodinium, squid-Vibrio, and legume-Rhizobium symbioses, initial contact between the host and symbiont is mediated by a specific lectin-sugar interaction (33–36). In addition to physiological selectivity, such molecular-level interactions probably play a pivotal role in the stinkbug-Burkholderia mutualistic association. The Burkholderia symbiont is easily culturable (14, 16) and genetically manipulatable (Y. Kikuchi, unpublished data), and RNA interference works well in the bean bug (37), providing us with a unique opportunity to identify the molecular bases involved in the sophisticated insect-microbe gut symbiosis.
ACKNOWLEDGMENTS
This study was supported by Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI grant number 24117525 and by the Programme for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry.
Footnotes
Published ahead of print 4 January 2013
REFERENCES
- 1. Margulis L, Fester R. (ed). 1991. Symbiosis as a source of evolutionary innovation. MIT Press, Cambridge, MA: [PubMed] [Google Scholar]
- 2. Moran NA. 2007. Symbiosis as an adaptive process and source of phenotypic complexity. Proc. Natl. Acad. Sci. U. S. A. 104:8627–8633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruby E, Henderson B, McFall-Ngai M. 2004. We get by with a little help from our (little) friends. Science 303:1305–1307 [DOI] [PubMed] [Google Scholar]
- 4. Bourtzis K, Miller TA. 2003. Insect symbiosis. CRC Press, Boca Raton, FL [Google Scholar]
- 5. Buchner P. 1965. Endosymbiosis of animals with plant microorganisms. Interscience, New York, NY [Google Scholar]
- 6. Kikuchi Y. 2009. Endosymbiotic bacteria in insects: their diversity and culturability. Microbes Environ. 24:195–204 [DOI] [PubMed] [Google Scholar]
- 7. Douglas AE. 1998. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 43:17–37 [DOI] [PubMed] [Google Scholar]
- 8. Oliver KM, Russell JA, Moran NA, Hunter MS. 2003. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. U. S. A. 100:1803–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsuchida T, Koga R, Fukatsu T. 2004. Host plant specialization governed by facultative symbiont. Science 303:1989. [DOI] [PubMed] [Google Scholar]
- 10. Tsuchida T, Koga R, Horikawa M, Tsunoda T, Maoka T, Matsumoto S, Simon JC, Fukatsu T. 2010. Symbiotic bacterium modifies aphid body color. Science 330:1102–1104 [DOI] [PubMed] [Google Scholar]
- 11. Inoue T, Kitade O, Yoshimura T, Yamaoka I. 2000. Symbiotic association with protists. In Abe T, Bignell DE, Higashi M. (ed), Termites: evolution, sociality, symbioses, ecology. Springer, Dordrecht, The Netherlands [Google Scholar]
- 12. Miura T, Braendle C, Shingleton A, Sisk G, Kambhampati S, Stern DL. 2003. A comparison of parthenogenetic and sexual embryogenesis of the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidoidea). J. Exp. Zool. B. 295:59–81 [DOI] [PubMed] [Google Scholar]
- 13. Kikuchi Y, Meng XY, Fukatsu T. 2005. Gut symbiotic bacteria of the genus Burkholderia in the broad-headed bugs Riptortus clavatus and Leptocorisa chinensis (Heteroptera: Alydidae). Appl. Environ. Microbiol. 71:4035–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kikuchi Y, Hosokawa T, Fukatsu T. 2007. Insect-microbe mutualism without vertical transmission: a stinkbug acquires beneficial gut symbiont from environment every generation. Appl. Environ. Microbiol. 73:4308–4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kikuchi Y, Hayatsu M, Hosokawa T, Nagayama A, Tago K, Fukatsu T. 2012. Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. U. S. A. 109:8618–8622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kikuchi Y, Hosokawa T, Fukatsu T. 2011. Specific developmental window for establishment of an insect-microbe gut symbiosis. Appl. Environ. Microbiol. 77:4075–4081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roesch LF, Fulthorpe RR, Riva A, Casella G, Hadwin AK, Kent AD, Daroub SH, Camargo FA, Farmerie WG, Triplett EW. 2007. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 1:283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. R Developmental Core Team 2010. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org [Google Scholar]
- 19. Pai CH, Kelly JK, Meyers GL. 1986. Experimental infection of infant rabbits with verotoxin-producing Escherichia coli. Infect. Immun. 51:16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu PC, Chen YC, Huang CY, Lee KK. 2000. Virulence of Vibrio parahaemolyticus isolated from cultured small abalone, Haliotis diversicolor supertexta, with withering syndrome. Lett. Appl. Microbiol. 31:433–437 [DOI] [PubMed] [Google Scholar]
- 21. Liu PC, Chen YC, Lee KK. 2001. Pathogenicity of Vibrio alginolyticus isolated from diseased small abalone Haliotis diversicolor supertexta. Microbios 104:71–77 [PubMed] [Google Scholar]
- 22. Santanirand P, Harley VS, Dance DA, Drasar BS, Bancroft GJ. 1999. Obligatory role of gamma interferon for host survival in a murine model of infection with Burkholderia pseudomallei. Infect. Immun. 67:3593–3600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCann J, Stabb EV, Millikan DS, Ruby EG. 2003. Population dynamics of Vibrio fischeri during infection of Euprymna scolopes. Appl. Environ. Microbiol. 69:5928–5934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhuvaneswari TV, Turgeon BG, Bauer WD. 1980. Early events in the infection of soybean (Glycine max L. Merr) by Rhizobium japonicum. Plant Physiol. 66:1027–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. King EB, Parke JL. 1996. Population density of the biocontrol agent Burkholderia cepacia AMMDR1 on four pea cultivars. Soil Biol. Biochem. 28:307–312 [Google Scholar]
- 26. Ramette A, LiPuma JJ, Tiedje JM. 2005. Species abundance and diversity of Burkholderia cepacia complex in the environment. Appl. Environ. Microbiol. 71:1193–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tabacchioni S, Bevivino A, Dalmastri C, Chiarini L. 2002. Burkholderia cepacia complex in the rhizosphere: a minireview. Ann. Microbiol. 52:103–117 [Google Scholar]
- 28. Kaltenpoth M, Goettler W, Koehler S, Strohm E. 2010. Life cycle and population dynamics of a protective insect symbiont reveal severe bottlenecks during vertical transmission. Evol. Ecol. 24:463–477 [Google Scholar]
- 29. Mira A, Moran NA. 2002. Estimating population size and transmission bottlenecks in maternally transmitted endosymbiotic bacteria. Microb. Ecol. 44:137–143 [DOI] [PubMed] [Google Scholar]
- 30. Fukatsu T, Hosokawa T. 2002. Capsule-transmitted gut symbiotic bacterium of the Japanese common plataspid stinkbug, Megacopta punctatissima. Appl. Environ. Microbiol. 68:389–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hosokawa T, Kikuchi Y, Nikoh N, Shimada M, Fukatsu T. 2006. Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol. 4:e337 doi:10.1371/journal.pbio.0040337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hosokawa T, Kikuchi Y, Fukatsu T. 2007. How many symbionts are provided by mothers, acquired by offspring, and needed for successful vertical transmission in an obligate insect-bacterium mutualism? Mol. Ecol. 16:5316–5325 [DOI] [PubMed] [Google Scholar]
- 33. Downie JA. 2010. The roles of extracellular proteins, polysaccharides and signals in the interactions of rhizobia with legume roots. FEMS Microbiol. Rev. 34:150–170 [DOI] [PubMed] [Google Scholar]
- 34. Kvennefors EC, Leggat W, Hoegh-Guldberg O, Degnan BM, Barnes AC. 2008. An ancient and variable mannose-binding lectin from the coral Acropora millepora binds both pathogens and symbionts. Dev. Comp. Immunol. 32:1582–1592 [DOI] [PubMed] [Google Scholar]
- 35. Nyholm SV, Stabb EV, Ruby EG, McFall-Ngai MJ. 2000. Establishment of an animal-bacterial association: Recruiting symbiotic vibrios from the environment. Proc. Natl. Acad. Sci. U. S. A. 97:10231–10235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wood-Charlson EM, Hollingsworth LL, Krupp DA, Weis VM. 2006. Lectin/glycan interactions play a role in recognition in a coral/dinoflagellate symbiosis. Cell. Microbiol. 8:1985–1993 [DOI] [PubMed] [Google Scholar]
- 37. Futahashi R, Tanaka K, Matsuura Y, Tanahashi M, Kikuchi Y, Fukatsu T. 2011. Laccase2 is required for cuticular pigmentation in stinkbugs. Insect Biochem. Mol. Biol. 41:191–196 [DOI] [PubMed] [Google Scholar]


