Abstract
Specific and complex interactions between soil bacteria, known as rhizobia, and their leguminous host plants result in the development of root nodules. This process implies a complex dialogue between the partners. Rhizobia synthesize different classes of polysaccharides: exopolysaccharides (EPS), Kdo-rich capsular polysaccharides, lipopolysaccharides, and cyclic β-(1,2)-glucans. These polymers are actors of a successful symbiosis with legumes. We focus here on studying the EPS produced by Rhizobium sullae bacteria that nodulate Hedysarum coronarium L., largely distributed in Algeria. We describe the influence of the carbon source on the production and on the composition of EPS produced by R. sullae A6 and RHF strains. High-molecular-weight EPS preserve the bacteria from desiccation. The structural characterization of the EPS produced by R. sullae strains has been performed through sugar analysis by gas chromatography-mass spectrometry. The low-molecular-weight EPS of one strain (RHF) has been totally elucidated using nuclear magnetic resonance and quantitative time-of-flight tandem mass spectrometry analyses. An unusual fucose-rich EPS has been characterized. The presence of this deoxy sugar seems to be related to nodulation capacity.
INTRODUCTION
The Hedysarum genus is composed of a great number of forage leguminous plant species, distributed throughout Europe, Africa, Asia, and North America (1). Hedysarum coronarium L. (sulla, French honey-suckle, Spanish sainfoin, and Spanish esparcet) is a member of the Leguminosae family originating and growing in the Mediterranean basin. It has been established as a forage crop in several countries (2). These plants play a significant role in maintaining the productivity in agriculture thanks to its ability to fix nitrogen and to grow satisfactorily over a wide range of soil conditions. Moreover, it tolerates drought and coastal conditions (3). Leguminous plants are able to enter in symbiosis with bacteria belonging to the rhizobia family. The latter reduce atmospheric nitrogen into ammonium, which is directly usable by the plants (4, 5). Rhizobium sullae is the specific bacterial partner of H. coronarium L (1, 2). In the symbiotic association of H. coronarium L. with R. sullae there is a clear specificity between the plant ecotype and the corresponding Rhizobium strain (6). The establishing of a symbiotic nitrogen fixation is important for the plant especially when the plant soil is starved in nutrients. This process occurs in the roots of the leguminous plants within specialized organs called nodules. The formation of nodules is engaged in the earliest steps of the process (7).
The Rhizobium-legume symbiosis requires specific chemical signaling between the symbiotic partners (4, 8). In addition to the flavonoids and Nod factors which initiate the symbiotic program, exopolysaccharides (EPS), lipopolysaccharides (LPS), Kdo-rich capsular polysaccharides (KPS), and cyclic β-(1,2)-glucans play essential roles in the formation of the infection thread and in nodule development (9). Nevertheless, the precise functions of these complex carbohydrates are still being investigated.
Rhizobial EPS are species-specific heteropolysaccharides composed of common sugars that can be substituted with noncarbohydrate residues, such as pyruvyl, succinyl, or acetyl groups (10, 11). The relationship between nodulation ability and rhizobial EPS production, in culture, has been reported (12, 13). Production of EPS is influenced by several growth factors, such as carbon and nitrogen sources. These factors also influence the structure and rheological properties of EPS (14, 15).
The rheological properties and peripheral localization of EPS suggest that they protect bacteria against environmental stress and provide a first contact between bacteria and the plant surface (9, 16), contributing to the intrinsic roles of bacterial EPS (17).
However, the production and structure of EPS are essential in a successful nodulation of host plants for the formation of indeterminate nodules (18, 19). H. coronarium L. is a host plant that forms indeterminate nodules. This type of nodule is mostly cylindrical or exhibits an elongated shape with a persistent apical meristem producing a gradient of developmental zones (20). For example, the Sinorhizobium meliloti EPS have to be succinylated to be active on Medicago truncatula.
Orgambide et al. (21) began with studying the glycoconjugates and lipids of R. hedysari IS123. Navarini et al. (22) highlighted the influence of carbon source in EPS production in R. hedysari HCNT isolated from H. coronarium L. The objective of the present study was to determine the composition and size of the EPS produced by R. sullae grown in the presence of different carbon source, to study their symbiotic activities on the plant H. coronarium L.
Finally, we attempted to establish a relationship between the EPS and the ability of strains to nodulate the host plant and to resist drought conditions. Considering the singular composition of these EPS, one structure has been totally elucidated (R. sullae strain RHF grown on mannitol).
Structural determination occurred through nuclear magnetic resonance (NMR), electrospray ionization-mass spectrometry (ESI-MS), and gas chromatography-mass spectrometry (GC-MS) analyses. It revealed the production of fucose-rich (ca. 30%) EPS that have not been previously reported for the Rhizobiaceae family. The relationship between the high fucose content of low-molecular-weight (LMW) EPS and the nodulation efficiency of corresponding strains is discussed here. The production level of high-molecular-weight (HMW) EPS is the key component of the bacterial resistance to drought conditions.
MATERIALS AND METHODS
Media and bacterial growth.
The bacterial strains used in the present study belong to two varieties of R. sullae: R. sullae A6 and R. sullae RHF. The strains were collected in Constantine (Algeria) and in Pisa (Italy), respectively. Rhizobia from sulla are related to Rhizobium etli, Rhizobium leguminosarum, and Sinorhizobium meliloti (23). Strains were grown on the yeast extract mannitol agar (YMA) solid medium (mannitol, 10 g/liter; KH2PO4, 0.5 g/liter; MgSO4·7H2O, 0.2 g/liter; NaCl, 0.1 g/liter; yeast extract, 0.5 g/liter; agar, 15 g/liter) at 28°C for 24 h. The other carbon sources are introduced at 10 g/liter instead of mannitol.
Production, extraction, and purification of EPS.
The production of EPS by strain was tested on solid medium YMA in which mannitol was replaced by other sugars (sucrose, glucose, and sorbitol) to estimate the influence of carbon source on EPS production. Petri dishes were incubated at 28°C for 5 days. Mucoid colonies were scraped with a sterilized spatula and then resuspended in sterile KCl (0.85%) (24).
Bacterial cells were separated from their EPS by centrifugation (12,800 × g for 30 min at 4°C). The supernatant containing the EPS was vacuum filtered through 45-μm-pore-size membranes to eliminate cells and large cellular fragments. EPS secreted in the supernatant are not retained by the 0.45-μm-pore-size filter. The HMW fraction of bacterial EPS was precipitated with 3 volumes of 95% cold ethanol. The supernatant was used to precipitate, by the addition of 7 volumes of ethanol, the LMW EPS fraction. The fraction was collected by centrifugation. The fractions of EPS were then lyophilized.
Colorimetric assay.
EPS were quantified by the Dreywood anthrone-sulfuric acid method (25) and the Blumenkrantz et al. phenol-sulfuric acid method (26) to estimate the amount of neutral and acid sugar contained in each sample.
Relative viscosity of EPS.
Solutions of EPS were prepared in distilled water, at a final concentration 0.1 g/liter, and they were left under stirring (20 rpm) overnight at 25°C. Solutions of EPS were analyzed with a viscometer (Scott Geräte AVS 310) at 25°C with a 5520/II column (diameter, 2 mm; length, 4 cm). The results are based on the determination of the relative viscosity calculated as follows:
where ηrel is the relative viscosity, η is the viscosity of the EPS solution, η0 is the viscosity of solvent (water), t and t0 represent the time for the ball to cross the capillary for the EPS solution and the solvent, respectively, and ρ and ρ0 are their densities.
Recovery of strains after desiccation.
After growth on an agar medium supplemented at 1% with different sugars (mannitol, sucrose, glucose, or sorbitol), mucoid colonies were scraped. Then, 0.1-g portions of the colonies were left to dry on a sterile cellulose filter membrane (65 μm, 47 mm; Millipore, France) at 35°C in a laminar flow hood until the bacteria were completely dried. The time required for drying varied from a strain to another. The filter was cut, and the part that contained all of the dried cells was inoculated into 150 ml of yeast extract mannitol base (YMB; YMA without agar). The flasks were strongly shaken and then incubated at 28°C. The growth rate of bacteria was estimated by measuring the optical density at 600 nm (OD600) for different incubation periods.
Glycosyl composition analyses.
The EPS of each strain were hydrolyzed in 2 M trifluoroacetic acid (TFA) at 110°C for 2 h. The TFA was removed by repeated evaporation with isopropanol. The acidic monosaccharides were methylated by diazomethane in ether. The derivation was performed by silylation, with 20 μl of pyridine, 100 μl of TMCS-HMDS mixture (trimethylchlorosilane and 1,1,3,3,3-hexamethyldisilazane) at 70°C (27). Trimethylsilyl glycoside derivatives of various monosaccharide standards were also prepared and analyzed by GC-MS for comparison to the sample peaks on chromatograms. The response factors of the different sugar types were determined after derivatization of the standards. These specific response coefficients were then applied to quantify each monosaccharide family.
GC-MS analyses of the EPS were performed with 6890N GC interfaced with 5973 MSD by using a HP5MS capillary column (25-m length, 0.25-mm external diameter and 0.25-μm internal diameter). The oven program was 70 to 300°C over 57 min. The scanning mass range was from m/z 50 atomic mass units (amu) to m/z 650 amu. The on-column injected volume was 0.1 μl.
Size exclusion chromatography (SEC).
The LMW EPS were dissolved in Milli-Q water at a final concentration of 5 mg/ml, and they were filtered through a 0.22-μm-pore-size membrane. The EPS fractions were separated on a size exclusion column (TSK-Gel G2000SWxl; 7.8-mm diameter, 30-cm height), which is able to separate globular proteins from 5 to 150 kDa. Detection was carried out by an evaporative detector by diffusion of light (ELS; Waters 2420). The 20 μl of the 5-mg/ml sample injected was eluted by ammonium acetate (20 mM) with a 0.8-ml/min fixed flow, and the time of analysis was 30 min. Detector was set at 20 lb/in2 and 100°C.
DOC-PAGE.
Desoxycholic acid-polyacrylamide gel electrophoresis (DOC-PAGE) analyses were performed on a 15% acrylamide-bisacrylamide resolving gel, with a 4% acrylamide-bisacrylamide stacking gel. The 30% acrylamide-bisacrylamide (19:1) solution was obtained from Sigma-Aldrich (Steinheim, Germany). For the migration, a generator from Pharmacia Biotech (Freiburg, Germany) was used at 20 mA for 1.5 h. A prestained protein ladder from Fermentas (Villebon-sur-Yvette, France) was used to estimate the size range of the EPS.
Structural analysis of EPS using NMR.
Nuclear magnetic resonance (1H NMR) spectroscopy was performed on a Brücker AMX500 spectrometer (Wissenbourg, France). The samples were dissolved in D2O (100%). NMR spectra were recorded at 303 K at 500 MHz (1H) and 125.75 MHz (13C) using a cryoprobe. Chemical shifts are indicated in ppm, and the number of accumulated scans was 512. Correlation-observed spectroscopy (COSY) allowed to assign chemical shifts of proton of each residue. The two-dimensional (2D) heteronuclear one-bond proton-carbon correlation experiment was registered in the 1H-detection mode via single-quantum coherence (HSQC), and 32 scans were accumulated. Analysis in heteronuclear multiple bond correlation mode (HMBC) was used to determine the sequence of residues of the repeating unit.
ESI/time-of-flight MS.
Fractions of R. sullae LMW EPS were analyzed in the negative-ion mode on a QqTof system (Ultima; Waters) (the EPS used were from R. sullae RHF cultivated in the presence of mannitol). The settings were as follows: probe, 3 kV; cone, 50 V; Rf lens, 25 V; and collision, 10 V (up to 25 V for MS/MS). Samples were dissolved at a concentration of 2 mg ml−1 in water-methanol (1:1) with 0.1% ammonia. The injection was carried out with a flow of 7 μl min−1.
Plant assays.
Seeds of H. coronarium L. were surface sterilized and germinated as described by Vincent (28). Germinated seeds were aseptically planted in modified Leonard's bottle-jar containing the sand-vermiculite mixture watered with a Fahraeus nutrition solution (29). Ten plants were then inoculated with 2 ml of exponentially grown rhizobia (OD600 of ∼1), cultivated in the presence of different carbon sources (mannitol, glucose, sucrose, or sorbitol) at 28°C. The pots were placed in the culture room at room temperature (the temperature varied from 16 at 21°C during the day and from 8 to 17°C during the night) for 10 weeks. The chamber was equipped with a system that ensured a discontinuous light illumination (16-h day and 8-h night).
RESULTS
Production of EPS.
We studied the EPS produced by two strains of R. sullae—A6 and RHF—that are specific endosymbionts of H. coronarium L. These strains are pure, as well as phenotypically and genotypically characterized, and their taxonomic position was determined. ARDRA, LMM RNA profiles, and DNA-DNA hybridization showed genotypic similarity of 100% between the two strains (1). The study of phenotypic characters carried out by Struffi et al. (6) reveals minor differences between the two RHF and A6 strains. The degree of NaCl tolerance is of 1% for strain A6 and 0.5% for strain RHF, and the nitrite reductase activity is low for strain RHF and absent in strain A6. Strain RHF is more resistant to phages than strain A6, in particular for f123c, FHC, F100C, f19a, f44a, and f44c1. There was also a difference in the plasmid profiles of the two strains.
The production of EPS was affected by the carbon source (Table 1), even if the strain growth remained similar. However, the growth on glucose was quite variable. The A6 strain recorded its maximum EPS production in the presence of sorbitol (11.1 mg/g). In contrast, the RHF strain produced a maximum EPS in the presence of glucose (20.5 mg/g). The proportions of HMW and LMW EPS produced also varied, depending on the carbon source. For example, the LMW/HMW ratio for the same strain (A6) varied from 7/3 (sorbitol) to 3/7 (mannitol). EPS production has been evaluated by weighing the alcoholic precipitate, after lyophilization. The measured weights have been confirmed through colorimetric assays (29).
Table 1.
Effect of carbon source on the production of R. sullae EPS
| Strain and carbon source | Mean production of EPS (mg/g of bacteria) ± SD |
||
|---|---|---|---|
| LMW EPS | HMW EPS | Total EPS | |
| R. sullae A6 | |||
| Mannitol | 2.7 ± 0.9 | 4.8 ± 1.0 | 7.5 ± 2.0 |
| Sucrose | 3.9 ± 1.6 | 6.7 ± 2.8 | 10.6 ± 0.6 |
| Glucose | 6.7 ± 5.0 | 4.0 ± 2.9 | 10.7 ± 1.1 |
| Sorbitol | 7.5 | 3.5 | 11.0 |
| R. sullae RHF | |||
| Mannitol | 6.6 ± 2.0 | 4.8 ± 2.0 | 11.6 ± 1.3 |
| Sucrose | 7.0 ± 0.9 | 6.7 ± 2.4 | 13.7 ± 1.0 |
| Glucose | 15.0 ± 6.0 | 5.6 ± 0.8 | 20.6 ± 2.6 |
| Sorbitol | 1.8 ± 0.6 | 1.6 ± 1.4 | 3.4 ± 1.0 |
Relative viscosity of EPS.
The HMW EPS, due to their ability to make intra- and intermolecular hydrogen bonds and their low solubilities, tend to be more viscous than the LMW ones. The viscosities measured here vary in accordance with the EPS size, which is dependent on the carbon source (Table 2). The viscosity measured at 0.1 g/liter for the two classes of EPS ranged for both strains from 0.96 to 1.11 for the HMW EPS and from 0.89 to 0.98 for the LMW EPS.
Table 2.
Viscosities of R. sullae EPS depending on the carbon source
| Strain and carbon source | Relative viscosity |
|
|---|---|---|
| HMW EPS | LMW EPS | |
| R. sullae A6 | ||
| Mannitol | 1.110 | 0.910 |
| Sucrose | 1.030 | 0.935 |
| Glucose | 1.070 | 0.952 |
| Sorbitol | 1.100 | 0.890 |
| R. sullae RHF | ||
| Mannitol | 1.090 | 0.937 |
| Sucrose | 1.030 | 0.984 |
| Glucose | 1.110 | 0.942 |
| Sorbitol | 0.961 | 0.915 |
Recovery of strains after desiccation.
The growth kinetics measured after the reculture of desiccated strains (Fig. 1) indicated that the RHF strain is on average more resistant to drought. Actually, the latency time was reduced, and the stationary phase reached in <20 h. For the two strains, the totality of the desiccated spot has been inoculated into the medium. The presented curves result from three replicates. After drying, sucrose enabled the two strains to resume growth with a higher speed, among the four sugars tested. High concentrations of EPS in the culture medium resulted in increased turbidity; however, this was observable only after 40 h of growth, corresponding to 18 h of stationary phase. Therefore, the growth kinetics measured at 600 nm over the first 24 h were not affected by this artifact.
Fig 1.
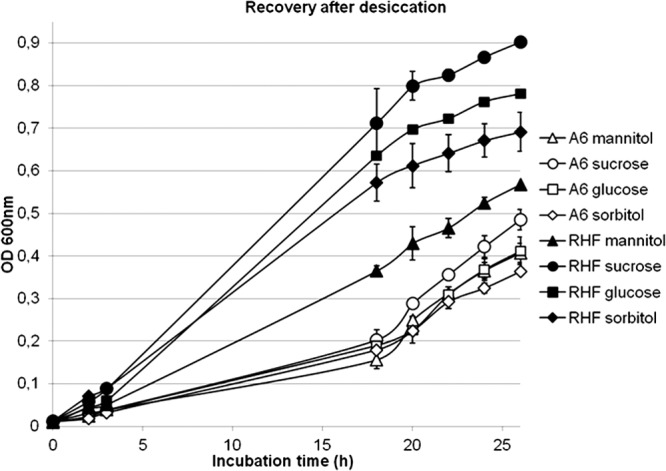
Growth kinetics of recovery after desiccation of the two R. sullae strains A6 (white) and RHF (black) and their four sources of carbon (mannitol, triangles; sucrose, circles; glucose, squares; sorbitol, diamonds).
Glycosyl composition analyses.
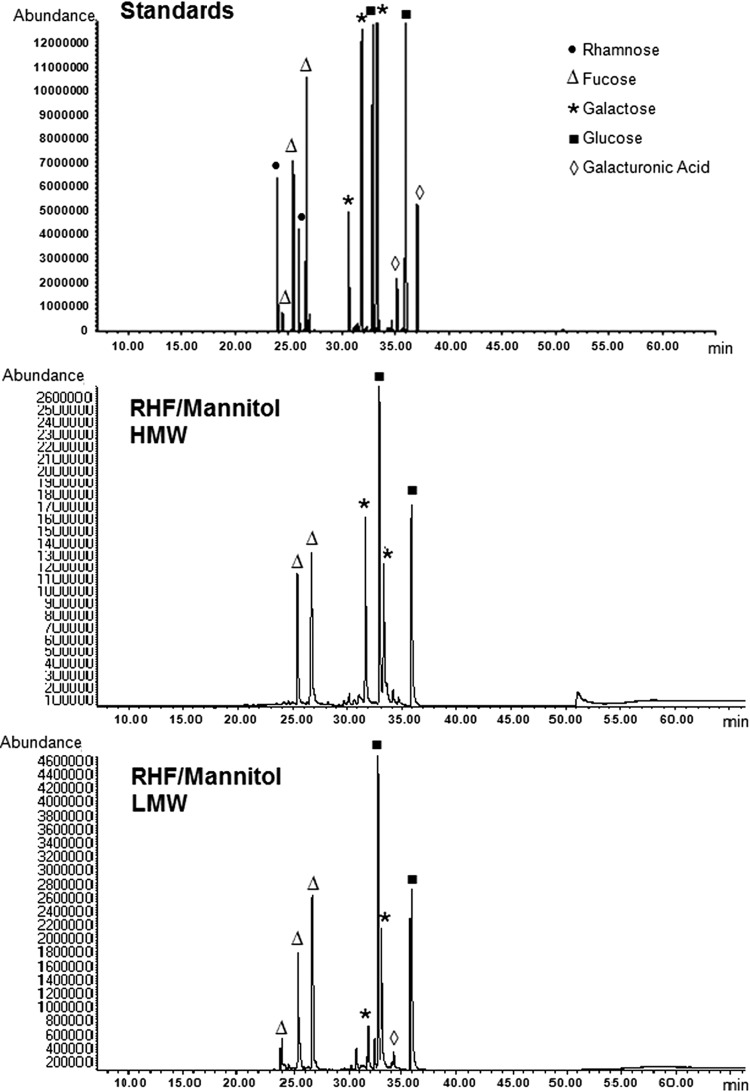
The identification of sugars is carried out using GC-MS by comparing the retention times and EI+ mass spectra to those of standards (our own and those of the NIST database). To ensure the retention time attribution, the chromatograms obtained for the polysaccharide samples are superimposed with those of the standards. The EPS of R. sullae are made up mainly of glucose (Glc), galactose (Gal), and fucose (Fuc). Their proportions depend on the carbon source used for the strain growth (Table 3). We observed that the compositions of HMW and LMW EPS differ, indicating that there are two different populations of polysaccharide and not simply different polymerization degrees for only one. Galacturonic acid (GalA) proportions determined by GC-MS are less precisely presented in Table 3 because the silylation of this uronic acid leads to underestimated and variable results.
Table 3.
Monosaccharide composition of R. sullae EPS as determined by GC-MS
| EPS and strain | Carbon source | Monosaccharide composition (%)a |
||||||
|---|---|---|---|---|---|---|---|---|
| Glc | Gal | Fuc | Man | GalAb | Rha | Rib | ||
| LMW EPS | ||||||||
| R. sullae A6 | Mannitol | 32 ± 1 | 30.5 ± 2.5 | 37 ± 4 | <1 | <1 | <1 | ND |
| Sucrose | 16.5 ± 3.5 | 32.5 ± 7.5 | 51 ± 11 | <1 | <1 | ND | ND | |
| Sorbitol | 60.5 ± 10.5 | 22.5 ± 5.5 | <1 | 8.5 ± 8.5 | <1 | 8.5 ± 0.5 | ND | |
| R. sullae RHF | Mannitol | 38 ± 5 | 30.5 ± 3.5 | 30.5 ± 2.5 | <1 | <1 | <1 | ND |
| Sucrose | 33.5 ± 16.5 | 29 ± 4 | 37.5 ± 12.5 | <1 | <1 | <1 | ND | |
| Sorbitol | 37 ± 12 | 34 ± 3 | 27.5 ± 9.5 | <1 | <1 | ND | ND | |
| HMW EPS | ||||||||
| R. sullae A6 | Mannitol | 45 ± 5 | 32.5 ± 7.5 | 10 ± 10 | ND | >10 | <1 | ND |
| Sucrose | 30 ± 10 | 36.5 ± 3.5 | 17 ± 16 | 3.5 ± 3.5 | >10 | <1 | ND | |
| Sorbitol | 41.5 ± 8.5 | 25 ± 8 | ND | 8.5 ± 8.5 | <10 | 17 ± 0 | ND | |
| R. sullae RHF | Mannitol | 28.5 ± 14.5 | 28 ± 0 | 14 ± 14 | 28.5 ± 28.5 | ND | ND | ND |
| Sucrose | 46 ± 21 | 28.5 ± 3.5 | 12.5 ± 12.5 | <1 | >10 | ND | ND | |
| Sorbitol | 33 ± 0 | 33 ± 0 | 33 ± 0 | ND | <1 | ND | ND | |
| Total EPS | ||||||||
| R. sullae A6 | Mannitol | 42.7 ± 6.5 | 33.4 ± 1.0 | 19.9 ± 13.5 | <1 | <10 | <1 | ND |
| Sucrose | 24.7 ± 6.7 | 34.9 ± 2.0 | 30.4 ± 17.0 | 2.3 ± 1.5 | <10 | <1 | ND | |
| Sorbitol | 54.4 ± 9.5 | 23.3 ± 1.2 | <1 | 8.5 ± 0 | <10 | 11.2 ± 4.2 | ND | |
| R. sullae RHF | Mannitol | 34.1 ± 4.7 | 29.5 ± 1.2 | 23.6 ± 8.2 | 12.3 ± 12.3 | <1 | <1 | ND |
| Sucrose | 39.5 ± 6.3 | 28.8 ± 0.3 | 25.5 ± 12.5 | <1 | <10 | <1 | ND | |
| Sorbitol | 35.5 ± 2.0 | 33.6 ± 0.5 | 29.5 ± 2.7 | <1 | <1 | ND | ND | |
Glc, glucose; Gal, galactose; Fuc, fucose; Man, mannose; GalA, galacturonic acid; Rha, rhamnose; Rib, ribose; ND, not detected. When glucose was used as a carbon source, the composition was quite variable. Values are expressed as means ± standard deviations where applicable.
GalA does not demonstrate a good response factor when derivatized with TMS.
Even if the purification is performed through a two-step precipitation, the produced fractions appear to be clean. Actually, GC-MS analyses of derivatized sugars before and after hydrolysis revealed neither amino acids, nor ribose (Table 3), nor lipids (Fig. 2). Moreover, ESI-MS analyses performed in the two negative and positive ionization modes exhibited only saccharides in the m/z 700 to 2,500 domain. In the higher mass range, the combined problems of bad ionization potential and less efficient solubilization of big carbohydrates could explain the absence in our spectra of the heavier saccharides. To confirm this hypothesis, the efficiency of the precipitation has been controlled through SEC and DOC-PAGE analysis. These studies indicated that in the HMW domain, no EPS exceeded 150 kDa and that in the LMW domain the sizes ranged effectively from 1 to 60 kDa.
Fig 2.
Chromatograms from GC-MS analyses of the monosaccharide composition of standard silylated monosaccharides and of RHF/mannitol hydrolyzed and silylated HMW and LMW EPS.
NMR spectrometric experiments.
NMR analysis allowed us to determine the configuration of carbohydrates and to assess most of the linkages in the polysaccharide. The 1H and 13C chemical shifts for each unit of the polysaccharide are given in Table 4.
Table 4.
Chemical shift data for glycosyl residues of LMW EPS produced by R. sullae RHF grown on mannitol as determined by two-dimensional NMR in D2O at 500 MHz with the use of a cryoprobea
| Designation | Glycosyl residue | Assignments (δ, ppm)b |
|||||
|---|---|---|---|---|---|---|---|
| H-1/C-1 | H-2/C-2 | H-3/C-3 | H-4/C-4 | H-5/C-5 | H-6/C-6 | ||
| A | →4αGal1→ | 5.41/99.1 | 3.84/68.1 | 4.13/66.5 | 3.92/71.8* | 3.72/62.5 | 3.82; 3.77/60.6 |
| →4αGal(6 Succ)1→ | 3.92; 3.79/64.8 | ||||||
| B | →3αFuc1→ | 5.33/96.7 | 4.85/70.8 | 4.12/71.0* | 4.04/68.5* | 4.38/67.2 | 1.22/14.5 |
| C | →3αFuc1→ | 5.30/98.1 | 5.02/69.9 | 4.23/76.5 | 4.20/69.1 | 4.43/66.7 | 1.07/14.7 |
| D | α(4.6 Pyr)Glc1→ | 5.09/92.1 | 3.54/72.1 | 3.71/79.8 | 3.39/75.9 | 3.64/69.5 | 3.91; 3.82/67.7 |
| E | →4βGalA1→ | 4.88/100.8 | 3.86/80.0 | 3.90/78.4 | 3.73/76.5* | 3.68/76.8 | 175.0 |
| F | →3αFuc(4 Ac)1→ | 4.57/103.7 | 3.31/72.9 | 3.69/76.2 | 3.78/71.5 | 4.06/71.0 | 1.27/19.7 |
| G | →3βGlc | 4.53/96.0 | 3.25/74.1 | 3.51/82.6 | 3.42/67.7 | 3.67/61.5* | 3.70; 3.64/60.6 |
| Succ | 2.08/20.0 | 173.0 | 183.0 | ||||
| Pyr | 1.37/24.8 | 100.1 | 176.0 | ||||
| Ac | 1.86/22.4 | 181.1 | |||||
Succ, succinate; Pyr, pyruvate; Ac, acetate. All other abbreviations are as defined in Table 3, footnote a.
*, assignments that could be exchanged with one other. Assignments indicated in italics were determined by COSY 1H/1H and HSQC 13C/1H.
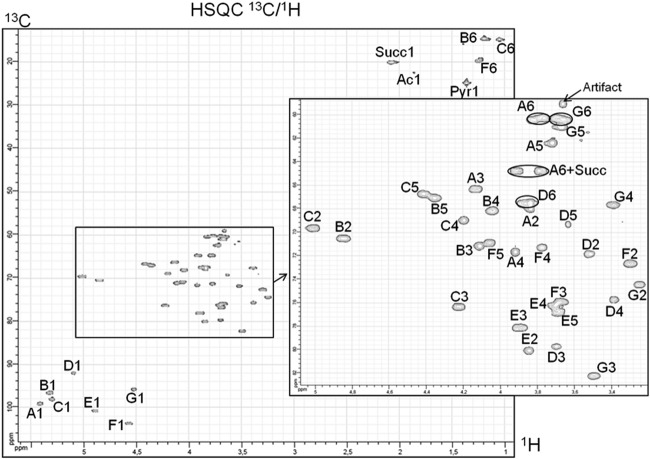
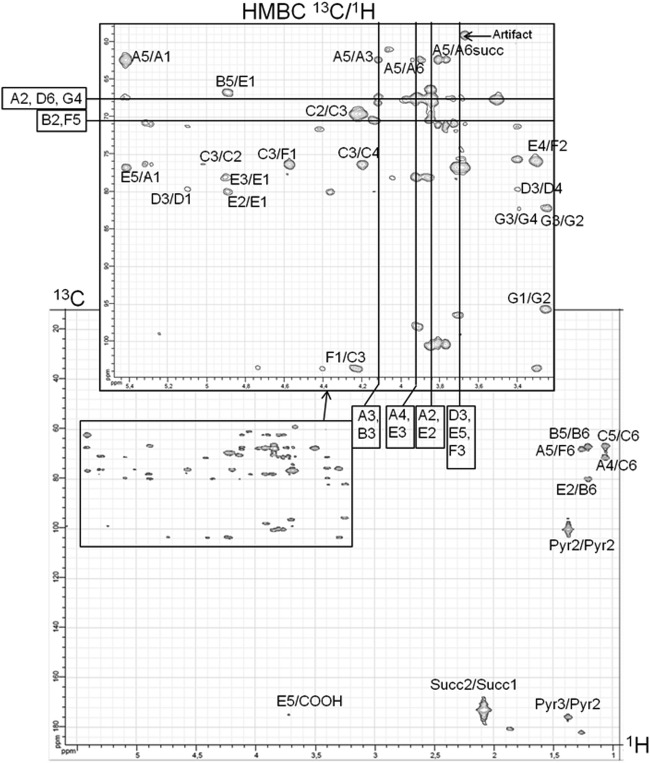
The 500-MHz spectrum of R. sullae LMW EPS (RHF cultivated in the presence of mannitol) showed the presence of a hexasaccharide repeating unit containing Fuc, Glc, Gal, and GalA in a 2:2:1:1 ratio. One can observe a slight difference between this composition and the one presented in Table 3. This is due to the fact that in NMR we observed a single sample (the GC-MS analysis of this sample fit perfectly [Fig. 2]), whereas Table 3 presents the mean composition values over three cultures. It also revealed the presence of the O-acetyl, pyruvyl, and succinyl groups. In order to achieve the best resolution, the NMR study was performed on a 10-volume ethanol-precipitated fraction. This contains oligosaccharides, resulting in fewer molecular interactions and therefore being more soluble. Among the different EPS tested, those of strain RHF cultivated in the presence of mannitol exhibited sharper NMR peaks (Fig. 3). These analyses allowed us to determine the types of linkages between each saccharide residue. This interpretation is complicated by the fact that at least five compounds are present as a mixture in the NMR probe. Systematically, cross peaks could be observed in the COSY experiments between the anomeric positions (H-1) and the neighbor H-2, followed by H-3. These 1H signals could then be correlated with the 13C signals by studying the HSQC map (Fig. 4). For the attribution of the 1H and 13C chemical shifts of positions 4 to 6, interpretation of the HMBC correlations was necessary (Fig. 5). The determined chemical shifts were compared to those in the literature (24, 30–36). To establish the EPS sequence, HMBC correlations were observed between the 13C of acetylated fucose (δ 103.7 ppm) and 1H-3 Fuc (δ 4.23 ppm). α and β configurations were determined through the COSY 1H/1H coupling constants (3JH1,H2). The presence of three substitutions on the sugar backbone could be observed first on the 1H spectrum in the aliphatic CH2/CH3 region (succinate [Succ] CH2, 2.08 ppm; pyruvate [Pyr] CH3, 1.37 ppm; acetate [Ac] CH3, 1.86 ppm), as well as the CH3 of the desoxy sugars (CH3; 1.05, 1.19, and 1.25 ppm). A strong HMBC signal was found for the pyruvyl between 13C (δ = 100.1 ppm) and 1H (δ = 3.82, 3.88, and 3.70 ppm) corresponding respectively to the two H-6 and to H-3 or H-4 of the hexose. Attribution of the chemical shifts corresponding to the quaternary carbons (C and COOH) of the substituents was performed using the HMBC data.
Fig 3.
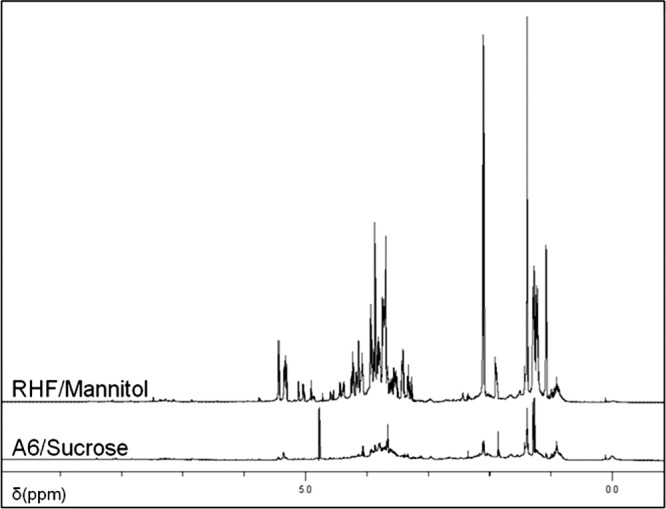
1H NMR spectra of RHF/mannitol LMW EPS (well resolved) and A6/sucrose LMW EPS (less resolved).
Fig 4.
13C/1H HSQC NMR correlation map of RHF/mannitol LMW EPS. The annotated correlation peaks correspond to the attributions found in Table 4.
Fig 5.
13C/1H HMBC NMR correlation map of RHF/mannitol LMW EPS. The annotated correlation peaks correspond to the attributions found in Table 4.
Only one signal can be observed for the pyruvylated hexose, indicating that pyruvylation is complete, as confirmed by the MS data (see Materials and Methods). Since acetylation is partial on the position 4 of the fucose, two types of signals can be clearly observed for the same sugar. Similarly, a partial succinylation could be observed.
ESI-QqTof MS/MS analyses.
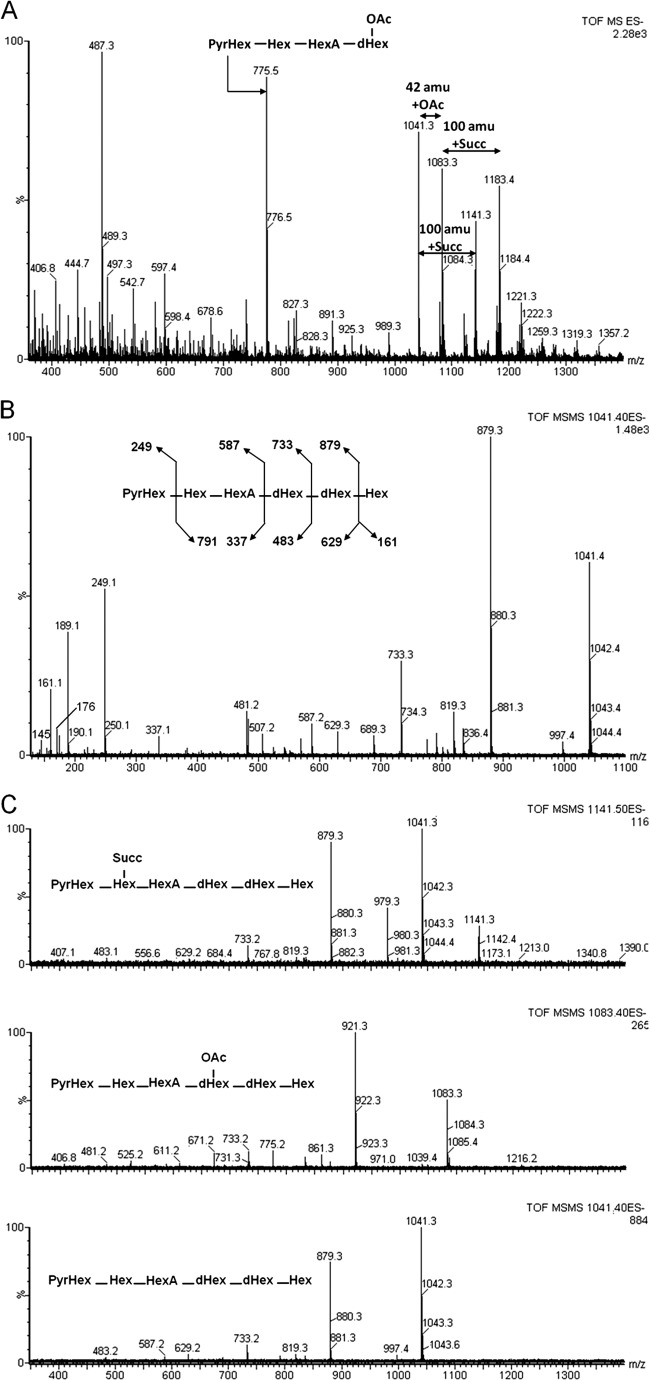
We studied using MS the same samples as for the NMR experiments. The results presented here are for the RHF strain cultivated on mannitol. The m/z 1,041.31 ion corresponds to the molecular ion [M-H]−. This mass corresponds to a hexasaccharide carrying a pyruvyl group. This polymer can be substituted by the O-acetyl (m/z 1,083.4 ion) and/or succinyl (m/z 1141.4 ion) (Fig. 6A). The fragmentation of ion [M-H]− at m/z 1,041.41 was analyzed by MS/MS experiments. The spectra showed B and Y fragmentation patterns.
Fig 6.
Negative-mode ESI-MS results obtained with a QqTof system for LMW EPS fraction from strain A6 grown on sucrose. (A) MS spectrum of R. sullae LMW EPS fraction; (B) MS/MS spectrum of ion [M-H]− m/z 1,041.4; (C) MS/MS spectra of the observed structural variations—nude, acetylated, or succinylated.
The atomic mass unit difference of 162.1 represents one hexose (Glc or Gal). Such a neutral loss corresponds to sugar elimination from one extremity of the saccharide. Since the charge state is conserved, the elimination occurs on the opposite side. The molecular mass of the oligosaccharide corresponds to a hexamer formed by two deoxyhexoses (dHex), two hexoses (Hex), a uronic acid (HexA), and a pyruvyl-hexose (PyrHex), as confirmed by the fragmentation pattern (Fig. 1B). Actually, in the low-mass domain, m/z 145, 161, 176, and 249 ions could be observed corresponding, respectively, to dHex, Hex, HexA, and PyrHex losses. The fragment m/z 733 corresponds to the hexasaccharide having lost a hexose-deoxyhexose disaccharide (Fig. 6B).
The fragment at m/z 587 is consistent with the HexA-Hex-HexPyr entity. Since the fragment m/z 337 is systematically present (as well as the corresponding neutral loss of 338 amu) and since the neutral loss of HexA could not be observed, it is reasonable to hypothesize that the hexasaccharide is under a completely linear form.
In conclusion, this experiment performed with the LMW EPS of R. sullae RHF grown on mannitol showed the presence of a hexasaccharide always carrying a pyruvate and bearing partially an additional succinyl and/or acetyl group. This saccharide contains three Hex, two dHex, and one HexA (Fig. 6C). The sequence deduced from the MS/MS analyses, as previously shown using NMR (see 3.5), indicated that the polar components (Succ and Pyr) are located on one side of the molecule and that more hydrophobic parts (Fuc and Ac) are located on the other side.
Nodulation tests.
H. coronarium L. (sulla) plants which were inoculated with R. sullae A6 and R. sullae RHF were tall and green. The production of EPS (see Materials and Methods) showed that the carbon source used by the bacteria can influence the production rate of EPS. To investigate whether the quantities or quality of EPS produced have an effect on the number and size of nodules formed, the germinated seeds were inoculated with 2 ml of bacteria in the presence of the different carbon sources (mannitol, sucrose, glucose, or sorbitol according to the method of Navarini et al. [22]). The maximal number of nodules was recorded for the plants inoculated by R. sullae A6 cultivated in the presence of sucrose (10 nodules) (Fig. 7), and about 6 nodules were obtained in the presence of glucose. In the presence of sorbitol, the minimal number of nodules was obtained with R. sullae RHF (1 nodule).
Fig 7.
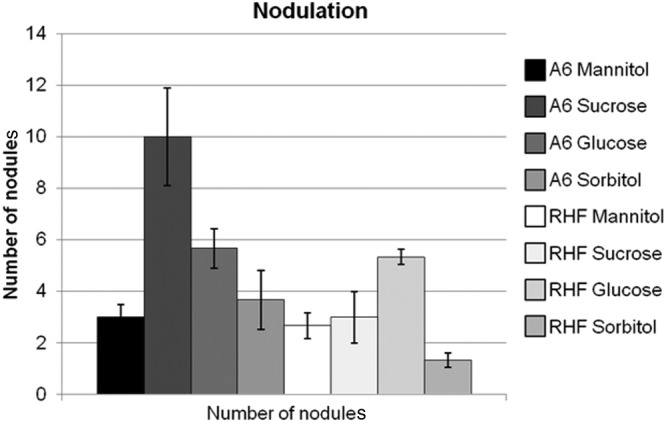
Nodulation abilities of the two R. sullae strains grown on different carbon sources.
DISCUSSION
EPS production depends on the carbon source.
Bacteria belonging to the Rhizobium genus produce important amounts of surface polysaccharides. We have shown that R. sullae specific partner of H. coronarium L., can produce different EPS present in different mass distribution. The HMW and LMW fractions differ in saccharide composition. The amount of EPS is significantly influenced by the carbon source used to cultivate the strain.
Navarini et al. (22) observed high secretion of EPS when R. hedysarum HCNT1 strain are grown on glucose and sucrose. For the Rhizobium strain D110 isolated from Dalbergia lanceolaria, mannitol was the most suitable promoter, followed by glucose and galactose (13).
EPS synthesis is a genetically controlled process. Genes directing and regulating the biosynthesis of EPS (the exo/exs or pss gene) form large clusters located either on the genome or on megaplasmids (11). The biosynthesis of EPS in Rhizobium is influenced by various environmental parameters. The existence of a very complex transcriptional and posttranslational process has been reported (37).
Various factors have been shown to regulate both the quantity and structure of EPS; these factors include osmolarity, nitrogen starvation, phosphate limitation, and growth conditions (38, 39). In S. meliloti, EPS I and EPS II exhibit parallel biosynthesis. A multitude of genes regulate their biosynthesis. The transformation of glucose-6-phosphate uridyl-diphosphate (UDP-Glc) and UDP-galactose (UDP-Gal) is at the base of the biosynthesis of all polysaccharides. Some groups of genes, such as exo and wgg genes, are expressed to build EPS, while others regulate positively or negatively the biosynthesis of EPS I and EPS II, respectively. HMW EPS I are obtained through ExoQ. The succinoglycans of LMW EPS I are obtained either directly or by degradation of the HMW EPS I via ExoK and ExoH proteins. This pathway is repressed in conditions of nitrogen deficiency and high osmolarity. The LMW EPS I can also be exported by the proteins ExoP and ExoT (9, 11, 39, 40). exoR is expressed to inhibit the biosynthesis of succinoglycans (EPS I) (41), whereas exoS stimulates this biosynthesis under high osmolarity (for example, in a highly saline environment). WggR (or MucS) proteins, as well as ExpR, increase the synthesis of galactoglucans (EPS II). The MucR protein is a key regulator that promotes the biosynthesis of the EPS I at the expense of the EPS II biosynthesis. The latter can be produced in another way when media are phosphate starved (40). In S. meliloti, the SyrM protein is also involved in regulating the relative ratio of LMW and HMW proteins from EPS I (42). We observed on R. sullae important effects on EPS production/g of bacteria and on the HMW/LMW proportion, as well as on the carbohydrate composition, according to the carbon source.
EPS composition depends on the carbon source.
R. sullae EPS are mainly constituted by neutral sugars (d-glucose, d-galactose, l-fucose, d-mannose, and sometime l-rhamnose). EPS glycosyl linkages are mainly 1,3- or 1,4- in α or β associations (11). It is important to note that the presence of fucose is rare for bacterial EPS. Pawlicki-Jullian et al. (43) found that fucose presents 40% of the EPS of Enterobacter ludwigii, 35% of the EPS of Klebsiella terrigena, 30% of the EPS in Raoultella terrigena, and between 10% and 35% of the EPS in Raoultella terrigena. The presence of fucose and, more specifically, its important ratio reported here indicate that we are in the presence of a novel type of rhizobial EPS. For the RHF strain, the carbon source influences the composition of EPS and more precisely their fucose content, varying by 30% where the A6 strain fucose content varies by 100%.
Fraysse et al. (9) reported in their review that the EPS monosaccharide composition may depend on the carbon source used for growth. For example, when grown on arabinose, gluconate, or mannitol, Bradyrhizobium japonicum 2143 exhibits the EPS composition described earlier (Man-Glc-Gal-GalA [1:2:1:1]) but, when cultured on malate, the EPS became extremely enriched in Gal (novel EPS) (12). Modification of EPS has been described frequently as a response to different environmental and physiological living conditions of bacteria (44).
In the present study, the presence of EPS enriched in deoxy sugars is highlighted. Its composition and production depend on the carbon sources of the bacteria. The apparition of mannose-rich HMW EPS occurs when the strain is grown on mannitol, and the synthesis of rhamnose instead of fucose happens when the strain is cultivated on sorbitol. This could indicate that direct importation processes exist in the strains studied here and that these sugars are not strictly used as carbon sources for energy.
Viscosity of EPS and their role in resistance to drought conditions.
The influence of the size and composition of EPS on viscosity is already well documented and depends on several factors, such as the composition of the culture medium, the type of strain, and the growth conditions (pH, concentration of oxygen, and amount of agitation) (45). The purpose here was to try to find out whether the composition or the size (both influence the viscosity) is the primary parameter determining the symbiotic activity.
The intrinsic viscosity is a function of the molecular weight and the hydrodynamic radius of the polymer. Therefore, the viscosity is higher for a higher molecular weight and for a more rigid chain (14), when the composition is constant.
Skorupska et al. (11) showed that EPS I and II produced by S. meliloti are secreted in two major fractions, reflecting different degrees of subunit polymerization: the HMW fraction, consisting of hundreds to thousands of repeating units (polymers of 106 to 107 Da), and the LMW fraction, which represents monomers, dimers, and trimers in the case of EPS I and oligomers (24, 30) in the case of EPS II. The average molecular mass of the LMW EPS was determined here by SEC. The sizes ranged from 1,000 to 60,000 Da. The HMW EPS are >150,000 Da but are mostly <106 Da (based on DOC-PAGE analyses).
The relative viscosity of the R. sullae LMW EPS was never >1 at a concentration of 0.1 g/liter (Table 2) but was near 1 when the distribution in molecular mass increased to 60 kDa. Two exceptions should be noted: R. sullae A6 grown on sorbitol had a viscosity of 0.9 even if its size is close to 60 kDa, and R. sullae RHF grown on glucose exceeded 0.9, with a size of <10 kDa. These findings can be explained by important composition changes.
H. coronarium (sulla) is one legume known for its broad tolerance to various environmental stresses and its ability to thrive without signs of chlorosis in desert soils. Sulla is well exploited as a forage crop due to its pronounced drought resistance and its strong tolerance to alkaline soils (46). This uncommon behavior, associated with the singular EPS composition of its specific host observed here, allowed us to hypothesize that the two could be correlated. Therefore, we studied the resistance of the strains to drought and their ability to resume proliferation after drying. As EPS can protect the bacteria against abiotic stress (17), we were interested in establishing a relationship between their growth resumption after desiccation, viscosity, composition, and production.
The presence of deoxyhexoses in EPS could suggest that the viscosity of the EPS may be more important (47, 48). The LMW EPS fraction follows in general this rule.
In contrast, the viscosity of HMW EPS does not appear to be influenced by the deoxyhexose content (Fig. 2). So, the viscosity appears to depend primarily on the size of the EPS, and then on the deoxyhexose ratio.
The adaptation of bacteria to environmental conditions (desiccation, temperature, pressure, and salinity) has been reported (49). We observed that when a strain produced a great amount of HMW EPS, its resistance to drought was important (Fig. 8). Actually, the better survival explains the better restart of strain RHF in liquid media. Even if less noticeable, the same relationship for the A6 strain could be demonstrated. The HMW EPS are viscous and allow a better restart. When HMW EPS are produced by the bacteria, their envelope presents better gelification triggering and enhanced resistance to drought.
Fig 8.
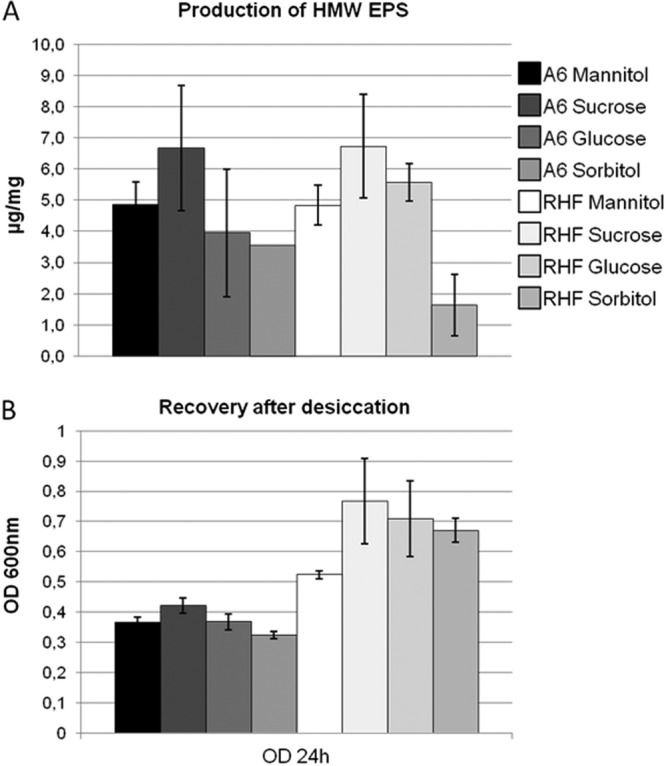
Relationship between the production of HMW EPS of the two strains of R. sullae grown on different carbon sources (A) and the ability of the two strains grown on different carbon sources to resume proliferation in a liquid medium after desiccation (B).
Structure of R. sullae LMW EPS.
A large diversity in EPS chemical structure can be found within the Rhizobium genus, considering the sugar composition and linkage in the single subunit, the repeating unit size, and the degree of polymerization, as well as the noncarbohydrate decoration (11, 50).
EPS are species-specific complex carbohydrate polymers. However, the structures of EPS produced by Rhizobium and by many other Proteobacteria consist of large heteropolymers formed by repeating the subunit structure (40).
In the present study, our attention has focused on fucose-rich EPS and especially on LMW EPS-producing strains exhibiting important biological activity. ESI-MS analysis of the LMW EPS of R. sullae RHF grown in mannitol (similar to R. sullae A6 grown on sucrose) showed that the subunit of this polymer consists of a hexamer (Fig. 6). The results of the QqTof MS/MS showed that the repeating unit of LMW EPS is composed of two deoxy sugars, three hexoses, and an uronic acid. This hexamer is pyruvylated and can carry a supplementary O-succinyl or O-acetyl group.
1H NMR has been performed on the LMW EPS of strain A6 grown in sucrose (with the strongest effect on nodulation), on strain RHF grown with mannitol exhibiting the same HMW/LMW ratio as strain A6 in sucrose, and on strains A6 and RHF grown in sorbitol for their opposite production yields. Their respective 1H NMR spectra indicated a high similarity in carbohydrate signals but a slight variation in the succinylation ratio. Since polysaccharides are difficult to solubilize at the molecular level, we investigated using NMR the most resolved NMR signal corresponding to the LMW EPS from strain RHF grown on mannitol (Fig. 3).
The combination of NMR and QqTof MS data confirms the structure of repeating unit of RHF grown on mannitol. It consists of two fucoses, two glucoses, one galactose, and a galacturonic acid, coupled by α-1,3, α-1,4, and β-1,3 glycosidic bonds. The repeating unit is always substituted by a pyruvyl carried by glucose. The combination of the two analytical techniques makes it possible to determine that ca. 40% of the molecules are succinylated on the galactose and that 40% of all the molecules also bear an additional acetyl group on a fucose residue (Fig. 9). Finally, NMR, especially HMBC, was useful for determining the type of junction but, due to the numerous 1H and 13C overlappings, only ESI-MS determined the sequence, highlighting the importance of combining NMR and MS methods.
Fig 9.
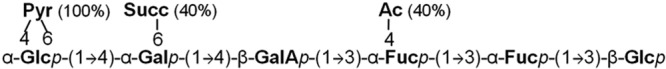
Structure of the repeating unit of the R. sullae LMW EPS fraction from strain RHF grown on mannitol (similar to the LMW EPS fraction from A6 grown on sucrose).
Glucose, galactose, and glucuronic acid are commonly found in the EPS of different rhizobial species such as S. meliloti, R. leguminosarum bv. viciae, R. leguminosarum bv. trifolii, M. loti, and A. radiobacter (9, 40, 45). The presence of mannose and galacturonic acid characterize the EPS of B. japonicum. Rhamnose and galacturonic acid are found in Sinorhizobium fredii HH303 (9), and the rhamnose-glucuronic acid characterizes the repeating unit of the nodular EPS of Bradyrhizobium japonicum and Bradyrhizobium elkanii (9).
However, it is uncommon to find as many deoxy sugars as are described here in rhizobial EPS. Moreover, this is the first time that fucose was found in the structure of EPS of bacteria belonging to the Rhizobium genus. This sugar, rare in the EPS of bacteria, is usually found in Enterobacter EPS (43) or in rhizobial LPS (e.g., B. japonicum, R. leguminosarum bv. trifolii and bv. viciae, and R. etli, respectively), as reported by Carrion et al. (50–53). The total absence of lipid A in the MS analyzes, as well as the precipitation protocol used for EPS, indicated that fucose is not released from LPS hydrolysis.
Symbiotic role of these EPS.
Many studies performed on rhizobial mutants showed that EPS play a major role in the infection of the leguminous plant (39). EPS are involved in early steps of plant infection, such as attachment of bacteria to the roots, structuring of the infection threads, development of bacteroids, suppression of plant defense responses (54–56), and protection against plant antimicrobial compounds (9, 11).
The LMW EPS I, EPS II, and KPS of S. meliloti were described as required for the extension of the infection thread, although the three are not equally efficient in promoting this process (57, 58). Gonzalez et al. (59) reported that the addition of LMW EPS to an exo mutant restores the capacity for nodulation, which suggests that EPS has a signaling function rather than a structural function in the formation of an infection thread (13). However, their role seems to be more important in indeterminate nodule host plants. As H. coronarium is one of these, we studied the infectivity of the strain producing variable EPS content and composition by enumeration of the nodules.
To understand which parameter influences nodulation the most, we studied the structure-activity relationship.
We found that the deoxyhexose ratio in the LMW EPS (Table 3), influences the nodulation (Fig. 7), depending on the amount of LMW EPS (Table 1). Considering strain RHF, a relationship between the three parameters is evident. For example, the highest fucose ratio (37,5%) was reached when the carbon source was sucrose, allowing the highest nodulation (5.3 nodules on average). When the carbon source was sorbitol, the fucose content was lower (27%), and the nodulation was less efficient (1.6 nodules on average). This fucose content difference is reinforced by the fact that the production of LMW was high (7 mg/g) when grown on sucrose but low (1.8 mg/g) when grown on sorbitol. This supports the hypothesis that the fucose content in the LMW influences nodulation.
For the A6 strain, there was significant biological variability (large standard deviations for the production and composition), especially when the carbon source was glucose. The relationship between production, composition, and nodulation are therefore more difficult to interpret. However, when sucrose was the carbon source, the fucose was ca. 50%, and the total number of nodule was the largest (10 average). When the A6 strain had sorbitol as the carbon source, no fucose was produced, but another deoxy sugar—rhamnose—was detected (Table 3). Nodulation appeared not to be influenced by the fact that the deoxyhexose is different. The great production of LMW protein (7.5 mg/g) seemed to compensate for the relatively low deoxyhexose ratio (8.5%). This increased production makes A6/sorbitol comparable to A6/mannitol on nodulation. The latter combination exhibited quite high fucose content 33% but a reduced production of 2.6 mg/g. For both, nodulation was similar (3.7 and 3.0 nodules). More generally, the fucose ratio in the LMW fraction corrected by the production level determines nodulation. For the RHF strain, nodulation was less important than for A6, despite a deoxyhexose ratio and production levels that are in the same range, indicating that A6 might be for other reasons a better symbiont of H. coronarium.
Here we demonstrate that the LMW EPS and the HMW EPS do not systematically exhibit the same structures and are dependent on the growth conditions, giving new insights into studying the symbiotic role of the EPS.
Conclusion.
The proportions of the LMW and HMW EPS produced by R. sullae, as well as their compositions, varied based on the carbon source used. We report here, for the first time, that EPS produced by a Rhizobium sp. can contain an important amount of fucose. One trend regarding the biological activity seems to be that an increased production of fucose-rich LMW EPS is more efficient for nodulation. A high production of HMW EPS can modulate the resistance of strains to drought conditions, indicating a probable adaptation to the dryness characteristic of the southern Italian and Algerian regions.
For one strain, R. sullae RHF grown in mannitol, a complete structural analysis was performed. Combined analysis of the NMR and MS data demonstrated the presence of fucose, glucose, and galactose, as well as galacturonic acid coupled mainly by α-1,3, α-1,4, and β-1,3 glycosidic bonds. As described previously for S. meliloti, we observed a highly substituted repeating unit (the presence of acetyl, pyruvyl, and succinyl groups).
Some interesting trends concerning the activity of fucose-rich EPS could be highlighted. However, it appears necessary to deepen the hypotheses presented here by studying, for example, the restoration of nodulation by coinoculating these EPS with mutant strains deficient in EPS production.
ACKNOWLEDGMENTS
We thank Jean-Christophe Garrigues and Christian Labau for help with the SEC and MS analyses and Yacine Benhizia for useful discussions.
This study was completed at the Laboratory of Microbial Ecology (UMC, Algeria) and the Laboratoire IMRCP (Toulouse, France).
Footnotes
Published ahead of print 26 November 2012
REFERENCES
- 1. Squartini A, Struff P, Doring H, Selenska-Pobell S, Tola E, Giacomini A, Vendramin E, Velazquez E, Mateos PF, Martinez-Molina E, Dazzo FB, Casella S, Nuti MP. 2002. Rhizobium sullae sp. nov. (formerly “Rhizobium hedysari”), the root-nodule microsymbiont of Hedysarum coronarium L. Int. J. Syst. Evol. Microbiol. 52:1267–1276 [DOI] [PubMed] [Google Scholar]
- 2. Benguedouar A, Corich V, Giacomini A, Squartini A, Nuti MP. 1997. Characterization of symbiotic bacteria from the Mediterranean legume crop Hedysarum coronarium (sulla) by multilocus enzyme electrophoresis. Agric. Mediterr. 127:173–177 [Google Scholar]
- 3. Douglas GB. 1984. Seed production of sulla: a plant for soil conservation. Proc. N. Z. Grassland Assoc. 45:239–242 [Google Scholar]
- 4. Dénarié J, Debellé F, Rosenberg C. 1992. Signaling and host range variation in nodulation. Annu. Rev. Microbiol. 46:497–531 [DOI] [PubMed] [Google Scholar]
- 5. Lindström K, Terefework Z, Suominen L, Lortet G. 2002. Signaling and development of Rhizobium-legume symbioses. Biol. Environ. 102:61–64 [Google Scholar]
- 6. Struffi P, Corich V, Giacomini A, Benguedouar A, Squartini A, Casella S, Nuti MP. 1998. Metabolic properties, stress tolerance, and macromolecular profiles of rhizobia nodulating Hedysarum coronarium. J. Appl. Microbiol. 84:81–89 [DOI] [PubMed] [Google Scholar]
- 7. Murray JD. 2011. Invasion by invitation: rhizobial infection in legumes. Mol. Plant Microbe Interact. 24:631–639 [DOI] [PubMed] [Google Scholar]
- 8. Cooper JE. 2007. Early interactions between legumes and rhizobia: disclosing complexity in a molecular dialogue. J. Appl. Microbiol. 103:1355–1365 [DOI] [PubMed] [Google Scholar]
- 9. Fraysse N, Couderc F, Poinsot V. 2003. Surface polysaccharide involvement in establishing the Rhizobium-legume symbiosis. Eur. J. Biol. Chem. 270:1365–1380 [DOI] [PubMed] [Google Scholar]
- 10. González JE, York GM, Walker GC. 1996. Rhizobium meliloti exopolysaccharides: synthesis and symbiotic function. Gene 179:141–146 [DOI] [PubMed] [Google Scholar]
- 11. Skorupska A, Janczarek M, Marczak M, Mazur A, Krol J. 2006. Rhizobial exopolysaccharides: genetic control and symbiotic functions. Microb. Cell Fact. 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karr DB, Liang RT, Reuhs BL, Emerich DW. 2000. Altered exopolysaccharides of Bradyrhizobium japonicum mutants correlate with impaired soybean lectin binding, but not with effective nodule formation. Planta 211:218–226 [DOI] [PubMed] [Google Scholar]
- 13. Ghosh AC, Ghosh S, Basu PS. 2005. Production of extracellular polysaccharides by a Rhizobium species from root nodules of the leguminous tree Dalbergia lanceolaria. Eng. Life Sci. 5:378–382 [Google Scholar]
- 14. Ibarburu I, Soria-Díaz ME, Rodríguez-Carvajal MA, Velasco SE, Tejero-Mateo P, Gil-Serrano AM, Irastorza A, Dueñas MT. 2007. Growth and exopolysaccharide (EPS) production by Oenococcus oeni I4 and structural characterization of their EPSs. J. Appl. Microbiol. 103:477–486 [DOI] [PubMed] [Google Scholar]
- 15. Staudt AK, Wolfe LG, Shrout JD. 2012. Variations in exopolysaccharide production by Rhizobium tropici. Arch. Microbiol. 194:197–206 [DOI] [PubMed] [Google Scholar]
- 16. D'Haeze W, Glushka J, De Rycke R, Holsters M, Carlson RW. 2004. Structural characterization of extracellular polysaccharides of Azorhizobium caulinodans and importance for nodule initiation on Sesbania rostrata. Mol. Microbiol. 52:485–500 [DOI] [PubMed] [Google Scholar]
- 17. Potts M. 1994. Desiccation tolerance of prokaryotes. Commun. Microbiol. Rev. 58:755–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laus MC, Logman TJ, Van Brussel AA, Carlson RW, Azadi P, Gao MY, Kijne JW. 2004. Involvement of exo5 in production of surface polysaccharides in Rhizobium leguminosarum and its role in nodulation of Vicia sativa subsp. nigra. J. Bacteriol. 186:6617–6625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Janczarek M, Skorupska A. 2003. Exopolysaccharide synthesis in Rhizobium leguminosarum bv. trifolii is related to various metabolic pathways. Res. Microbiol. 154:433–442 [DOI] [PubMed] [Google Scholar]
- 20. Patriarca EJ, Tate R, Iaccarino M. 2002. Key role of bacterial NH4+ metabolism in rhizobium-plant symbiosis. Microbiol. Mol. Biol. Rev. 66:203–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Orgambide GG, Philip-Hollingsworth S, Tola E, Cedergren RA, Squartini A, Dazzo FB, Hollingsworth RI, Nuti MP. 1996. Glycoconjugate and lipid components of Rhizobium “hedysari” IS123, a root nodule symbiont of the stress-tolerant legume, Hedysarium coronarium. Can. J. Microbiol. 42:340–345 [Google Scholar]
- 22. Navarini L, Stredansky M, Matulova M, Bertocchi C. 1997. Production and characterization of an exopolysaccharide from Rhizobium hedysari HCNT 1. Biotechnol. Lett. 19:1231–1234 [Google Scholar]
- 23. Evguenieva-Hackenberg E, Selenska-Pobell S. 1995. Variability of the 5′-end of the large subunit rDNA and presence of a new short class of rRNA in Rhizobiaceae. Lett. Appl. Microbiol. 21:402–405 [DOI] [PubMed] [Google Scholar]
- 24. Kaci Y, Heyraud A, Barakat M, Heulin T. 2005. Isolation and identification of an EPS-producing Rhizobium strain from arid soil (Algeria): characterization of its EPS and the effect of inoculation on wheat rhizosphere soil structure. Res. Microbiol. 152:522–531 [DOI] [PubMed] [Google Scholar]
- 25. Dreywood R. 1946. Qualitative test for carbohydrates. Ind. Eng. Chem. Res. 18:499 [Google Scholar]
- 26. Blumenkrantz N, Asboe-Hansen G. 1973. New method for quantitative determination of uronic acids. Anal. Biochem. 54:484–489 [DOI] [PubMed] [Google Scholar]
- 27. Rojas-Escudero E, Alarcón-Jiménez AL, Elizalde-Galván P, Rojo-Callejas F. 2004. Optimization of carbohydrate silylation for gas chromatography. J. Chromatogr. A 1027:117–120 [DOI] [PubMed] [Google Scholar]
- 28. Vincent JM. 1970. A manual for the practical study of the root-nodule bacteria. IBP handbook 15 Blackwell Scientific Publishers, Oxford, England [Google Scholar]
- 29. Fahraeus G. 1957. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J. Gen. Microbiol. 16:374–381 [DOI] [PubMed] [Google Scholar]
- 30. Ruas-Madiedo P, De Los Reyes-Gavilan CG. 2005. Methods for the screening, isolation, and characterization of exopolysaccharides produced by lactic acid bacteria. J. Dairy Sci. 88:843–856 [DOI] [PubMed] [Google Scholar]
- 31. Evans LR, Linker A, Impallomeni G. 2000. Structure of succinoglycan from an infectious strain of Agrobacterium radiobacter. Int. J. Biol. Macromol. 27:319–326 [DOI] [PubMed] [Google Scholar]
- 32. Plock A, Beyer G, Hiller K, Gründemann E, Krause E, Nimtz M, Wray V. 2001. Application of MS and NMR to the structure elucidation of complex sugar moieties of natural products: exemplified by the steroidal saponin from Yucca filamentosa L. Phytochemistry 57:489–496 [DOI] [PubMed] [Google Scholar]
- 33. Cescutti P, Kallioinen A, Impallomeni G, Toffanin R, Pollesello P, Laisola M, Eerikaïinen T. 2005. Structure of the exopolysaccharide produced by Enterobacter amnigenus. Carbohydr. Res. 340:439–447 [DOI] [PubMed] [Google Scholar]
- 34. Perry MB, MacLean LL, Patrauchan MA, Vinogradov E. 2007. The structure of the exocellular polysaccharide produced by Rhodococcus sp. RHA1. Carbohydr. Res. 342:2223–2229 [DOI] [PubMed] [Google Scholar]
- 35. Ihara H, Hanashima S, Okada T, Ito R, Yamaguchi Y, Taniguchi N, Ikeda Y. 2010. Fucosylation of chitooligosaccharides by human α1,6-fucosyltransferase requires a nonreducing terminal chitotriose unit as a minimal structure. Glycobiology 20:1021–1033 [DOI] [PubMed] [Google Scholar]
- 36. MacLean LL, Vinogradov E, Pagotto F, Farber JM, Perry MB. 2010. The structure of the O-antigen of Cronobacter sakazakii HPB 2855 isolate involved in a neonatal infection. Carbohydr. Res. 345:1932–1937 [DOI] [PubMed] [Google Scholar]
- 37. Zhao L, Chen Y, Ren S, Han Y, Cheng H. 2010. Studies of chemical structure and antitumor activity of an exopolysaccharide from Rhizobium sp. N613. Carbohydr. Res. 345:637–643 [DOI] [PubMed] [Google Scholar]
- 38. Becker A, Pühler A. 1998. Production of exopolysaccharides in Rhizobiaceae, p 97–118 In Spaink HP, Kondorosi A, Hooykaas PJJ. (ed), The Rhizobiaceae. Kluwer Academic Publishers, Dordrecht, Netherlands [Google Scholar]
- 39. Colpin DL, Cook D. 1990. Molecular genetics of extracellular polysaccharide biosynthesis in vascular phytopathogenic bacteria. Mol. Plant Microbe Interact. 3:271–279 [DOI] [PubMed] [Google Scholar]
- 40. Spaink HP. 2000. Root nodulation and infection factors produced by rhizobial bacteria. Annu. Rev. Microbiol. 54:257–288 [DOI] [PubMed] [Google Scholar]
- 41. Janczarek M. 2011. Environmental signals and regulatory pathways that influence exopolysaccharide production in rhizobia. Int. J. Mol. Sci. 12:7898–7933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Glenn SA, Gurich N, Feeney MA, Gonzalez JE. 2007. The ExpR/Sin quorum-sensing system controls succinoglycan production in Sinorhizobium meliloti. J. Bacteriol. 189:7077–7088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pawlicki-Jullian N, Courtois B, Pillon M, Lesur D. 2010. Exopolysaccharide production by nitrogen-fixing bacteria within nodules of Medicago plants exposed to chronic radiation in the Chernobyl exclusion zone. Res. Microbiol. 161:101–108 [DOI] [PubMed] [Google Scholar]
- 44. Lloret J, Wullf BH, Rubio JM, Dowine JA, Bonilla I, Rivila R. 1998. Exopolysaccharide II production is regulated by salt in the halotolerant strain Rhizobium meliloti EFB1. Appl. Environ. Microbiol. 64:1024–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Duta FP, De França FP, De Almeida Lopes LM. 2006. Optimization of culture conditions for exopolysaccharides production in Rhizobium sp. using the response surface method. Electron. J. Biotechnol. 9:391–399 [Google Scholar]
- 46. Tola E, Henriquez-Sabà JL, Polone E, Dazzo FB, Concheri G, Casella S, Squartini A. 2009. Shovel roots: a unique stress-avoiding developmental strategy of the legume plant Hedysarum coronarium L. Plant Soil 322:25–37 [Google Scholar]
- 47. Yemmas L, Soetaert W, Vandamme EJ. 2003. Enzymatic conversion of the clavan exopolysaccharide by Streptomyces sp. YSDL-20. Commun. Agric. Appl. Biol. Sci. 68:327–330 [PubMed] [Google Scholar]
- 48. Meyer W, Luz S, Schnapper A. 2009. Lectin histochemical aspects of mucus function in the oesophagus of the reticulated python (Python reticulatus). Anat. Histol. Embryol. 38:316–318 [DOI] [PubMed] [Google Scholar]
- 49. Poli A, Kazak H, Gurleyendag B, Tommonaro G, Pieretti G, Toksoy Oner E, Nicolaus B. 2009. High level synthesis of levan by a novel Halomonas species growing on defined media. Carbohydr. Polymers 78:651–657 [Google Scholar]
- 50. Carrion M, Bhat UR, Reuhs B, Carlson RW. 1990. Isolation and characterization of the lipopolysaccharides from Bradyrhizobium japonicum. J. Bacteriol. 172:1725–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dazzo FB, Truchet GL, Hollingsworth RI, Hrabak EM, Pankratz HS, Philip-Hollingsworth S, Salzwedel JL, Chapman K, Appenzeller L, Squartini A. 1991. Rhizobium lipopolysaccharide modulates infection thread development in white clover root hairs. J. Bacteriol. 173:5371–5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang Y, Hollingsworth RI, Priefer UB. 1992. Characterization of structural defects in the lipopolysaccharides of symbiotically impaired Rhizobium leguminosarum biovar viciae VF-39 mutants. Carbohydr. Res. 231:261–271 [DOI] [PubMed] [Google Scholar]
- 53. Noel KD, Box JM, Bonne VJ. 2004. 2-O-methylation of fucosyl residues of a rhizobial lipopolysaccharide is increased in response to host exudate and is eliminated in a symbiotically defective mutant. Appl. Environ. Microbiol. 70:1537–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Niehaus K, Kapp D, Pühler A. 1993. Plant defense and delayed infection of alfalfa pseudonodules induced by an exopolysaccharide (EPSI)-deficient Rhizobium meliloti mutant. Planta 190:415–425 [Google Scholar]
- 55. Niehaus K, Baier R, Kohring B, Flashl E, Pühler A. 1997. Symbiotic suppression of the Medicago sativa plant defence system by Rhizobium meliloti oligosaccharides, p 110–114 In Legoki A, Bothe H, Pühler A. (ed), Biological fixation of nitrogen for ecology and sustainable agriculture. Springer-Verlag, Heidelberg, Germany [Google Scholar]
- 56. van Workum WAT, van Slageren S, van Brussel AAN, Kijne JW. 1998. Role of exopolysaccharides of Rhizobium leguminosarum bv. viciae as host plant-specific molecules required for infection thread formation during nodulation of Vicia sativa. Mol. Plant Microbe Interact. 11:1233–1241 [Google Scholar]
- 57. Doherty D, Leigh JA, Glazebrook J, Walker GC. 1988. Rhizobium meliloti mutants that overproduce the R. meliloti acidic calcofluor-binding exopolysaccharide. J. Bacteriol. 170:4249–4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brewin NJ. 1998. Tissue and cell invasion by Rhizobium: the structure and development of infection threads and symbiosomes, p 417–429 In Spaink HP, Kondorosi A, Hooykaas JJ. (ed), The Rhizobiaceae. Kluwer Academic Publishers, Dordrecht, Netherlands [Google Scholar]
- 59. Gonzalez JE, Reuhs B, Walker GC. 1996. Low molecular weight EPS II of Rhizobium meliloti promotes nodule invasion of alfalfa. Proc. Natl. Acad. Sci. U. S. A. 93:8636–8641 [DOI] [PMC free article] [PubMed] [Google Scholar]