Abstract
The effects of temperature and pH on the water treatment performance of a point-of-use (POU) coagulant/disinfectant product were evaluated. Cold temperatures (∼5°C) reduced the bactericidal efficiency of the product with regard to Escherichia coli and total coliform log10 reductions.
TEXT
There is evidence that point-of-use (POU) water treatment and safe storage techniques can be effective interventions to prevent diarrheal diseases in humanitarian emergency contexts (1). Water quality objectives for humanitarian relief (2) consist of no Escherichia coli per 100 ml, a free chlorine residual (FCR) of 0.5 mg/liter (30 min contact time and pH < 8), and turbidity of less than 5 nephelometric turbidity units (NTU).
Coagulant/disinfection products (CDPs) are a POU water treatment option that provide microbial quality improvements, turbidity reductions, and a posttreatment FCR. Most commercially available CDPs are sachets containing a coagulant (e.g., ferric sulfate) and a disinfectant (e.g., calcium hypochlorite) along with other chemical components (e.g., oxidizing agents, flocculant aids, etc.) for a predetermined treatment volume. Cold temperatures (<5°C) can affect coagulant-assisted treatment processes (3), and alkaline pH levels (>8) can render free residual chlorination less effective (4). Thus, the effects of pH and temperature (under extreme conditions) should be an important part of the evaluation of the treatment efficiency of CDPs and were the objective of this study.
Treatment efficiencies (i.e., bacterial removal, turbidity reductions, and FCR levels) were evaluated against current humanitarian water treatment objectives (2) and recent quantitative microbial risk assessment (QMRA)-based criteria for the evaluation of POU treatment options (5). This study focused on bacterial water quality improvements (i.e., E. coli removal), the microbial quality criterion in relief situations.
Treatment steps of a CDP (PUR; Procter & Gamble Co., Pakistan) were adapted to a laboratory setup, namely, mixing (for 5 min), settling (for 5 min), cloth filtration, and continued disinfection (for 20 min). A Kemwater Flocculator 2000 (Kemira, Sweden) stirring paddle was set at 250 rpm to provide uniform mixing. A commercially available cloth (J-Cloth; Associated Brands, Canada) was used as the filtration material to simulate a worst-case scenario for this step. This was in line with the objective of evaluating the CDP's performance under simulated extreme conditions.
The test water was a 1:5 dilution of primary settled wastewater (Station d'epuration Est, Québec) in dechlorinated tap water (5). Treatment efficiencies were tested under different conditions, namely, “reference” (pH 7, 20°C), “extreme pH” (pH 9, 20°C), and “cold temperature” (pH 7, 5°C). When needed, pH was adjusted with NaOH and H2SO4. A crushed ice jacket around the mixing vessel kept test water at 5 ± 1°C. Turbidity was adjusted to approximately 100 NTU using a kaolin clay slurry. In order to further examine the temperature and pH effect on the different underpinning treatment processes (e.g., coagulation and disinfection), experiments under all three conditions were repeated with the addition of sodium thiosulfate before CDP treatment to neutralize its disinfectant, thereby allowing for an estimation of the bacterial removal attributable to the coagulant-assisted (i.e., sedimentation) and filtration steps.
Turbidity, pH, and FCRs were measured using a 2100 P turbidimeter, HQ40d pH meter, and pocket colorimeter, respectively, as specified by the manufacturer (HACH, USA). With the exception of FCRs (sampled only after treatment), all other measurements were made with samples collected before (t = 0 min) and after (t = 30 min) treatment. Triplicate bacteriological sampling was conducted with sterile bottles containing sodium thiosulfate. Enumeration of E. coli and total coliforms was performed with the Colilert Quanti-tray/2000 system (IDEXX Laboratories). Cold temperature and extreme pH effects on microbial indicator reductions in comparison to reference conditions were assessed by Student's t test analyses (α = 0.05). CDP testing under each set of conditions was repeated 3 times.
Performance data of the CDP are summarized in Table 1. Under reference conditions, its performance was comparable to that reported previously (6–8). Any differences could be attributed to the choice of a thinner filtration material used in this study. The method detection limit of 1 most probable number (MPN)/100 ml for bacterial analysis was used in the geometric mean calculations when microbial concentrations were less than 1. Cold temperatures (5°C) affected bacterial and turbidity reductions the most, with the least compliance of recommended quality criteria with regard to E. coli and turbidity (2). FCRs of at least 0.5 mg/liter (2) were not observed under any condition, possibly due to the greater chlorine demand of the primary settled wastewater dilution used. Tests in naturally occurring surface waters should yield FCRs in the range of 0.5 to 1.5 mg/liter (9).
Table 1.
CDP performance summary
| Parameter | Mean (range) CDP performance summarya |
|||||
|---|---|---|---|---|---|---|
| Room temp, no pH adjustment (reference) |
5°C, no pH adjustment |
Room temp, pH 9 |
||||
| Before treatment | 30 min after treatment | Before treatment | 30 min after treatment | Before treatment | 30 min after treatment | |
| E. coli (MPN/100 ml)b | 7.3 × 105 (5.9 × 105 to 1.0 × 106) | 1.0 × 100 (<1 × 100 to 2.0 × 100) [78] | 7.3 × 105 (5.9 × 105 to 1.0 × 106) | 3.3 × 100 (<1 × 100 to 1.3 × 101) [11] | 4.6 × 105 (2.7 × 105 to 7.0 × 105) | 1.2 × 100 (<1 × 100 to 3 × 101) [56] |
| Total coliforms (MPN/100 ml)b | 2.1 × 106 (1.4 × 106 to 2.8 × 106) | 4.0 × 100 (<1 × 100 to 4.3 × 101) [11] | 2.1 × 106 (1.7 × 106 to 2.8 × 106) | 1.5 × 102 (3.7 × 100 to 5.5 × 102) [0] | 1.8 × 106 (1.5 × 106 to 2.8 × 106) | 2.6 × 101 (1 × 100 to 1.7 × 102) [0] |
| Turbidity (NTU) | 97 (91 to 113) | 1.7 (1.5 to 1.9) [100] | 97 (92 to 104) | 9.2 (6.0 to 14) [0] | 101 (93 to 111) | 2.5 (1.8 to 3.7) [100] |
| Free Cl2 (mg/liter) | ND | 0.09 (0.08 to 0.11) [0] | ND | 0.07 (0.07 to 0.08) [0] | ND | 0.09 (0.07 to 0.11) [0] |
| pH | 7.2 (7.2 to 7.2) | 6.4 (6.3 to 6.5) [100] | 7.2 (7.2 to 7.2) | 6.4 (6.3 to 6.5) [100] | 9.0 (8.9 to 9.2) | 6.8 (6.7 to 6.9) [100] |
| Temp (°C) | 21.0 (20.6 to 21.3) | 21.4 (20.9 to 21.7) | 6.0 (5.3 to 6.4) | 6.6 (6.2 to 6.8) | 20.8 (20.7 to 20.9) | 21.1 (21.1 to 21.2) |
Unless otherwise indicated, results are shown as mean (range) [% of samples within recommended guidelines (2) or below detection limit (for total coliforms)]. ND, not determined.
Geometric means used for microbial indicator data.
Cold temperature had a statistically significant (P = 0.005) effect on log reductions of E. coli and total coliforms in comparison to results under reference conditions (Fig. 1). E. coli log reduction calculations for reference and extreme pH conditions were bound by the method's limit of detection, as many samples were below 1 MPN/100 ml after treatment. This also explains why observed differences were greater when considering total coliform log reductions (Table 1), since the coliforms had a higher initial concentration. Despite the observed temperature effect, the CDP still achieved the minimum default QMRA-based 4-log reduction (5) of bacterial indicator organisms. Extreme pH trials also showed a statistically significant (P < 0.001) difference relative to reference condition bacterial indicator log reductions. However, this difference may not be attributed to the effect of pH on free chlorination efficiency, as the final measured pH was near neutral (Table 1). pH changed to nearly the final pH values within a short time after the product addition (data not shown). The composition of the product (9) contains components known to affect solution pH (e.g., ferric sulfate and sodium carbonate) and is thought to bring the final pH to similar near-neutral values.
Fig 1.
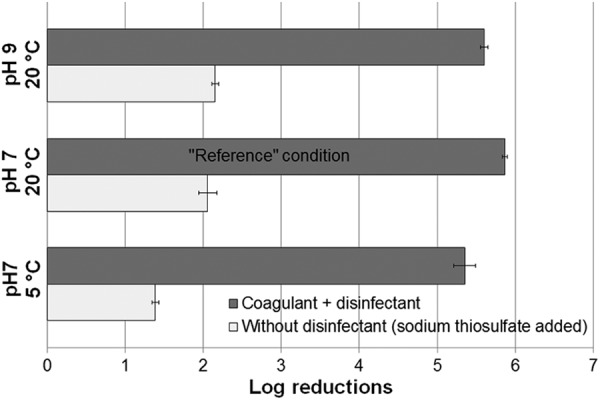
Average E. coli log reductions (error bars represent 1 standard error; n = 9 for each condition).
Under reference conditions, up to approximately 2 log reductions of E. coli were due to the combined action of the coagulant (i.e., coagulation, flocculation, and sedimentation) and filter. This log reduction was decreased by nearly 30% at cold temperatures. The relative disinfectant potential (i.e., calculated E. coli removal solely attributed to disinfectant) remained constant, indicating that differences were likely attributed to coagulation-related processes (i.e., coagulation, flocculation, sedimentation, and filtration). Similar trends were observed for total coliforms (not shown).
Both E. coli removal and turbidity reductions were affected mostly by cold temperatures. While turbidity is not a health-related parameter, perceived water clarity could be an important factor in the acceptability of CDPs (10). Studies (6, 10) have shown that a similar product can have a field performance inferior to that found in laboratory testing (8). Therefore, it is possible that under cold-water conditions, CPD performance can be further compromised in field applications. The buffering effect of the CDP's formulation explains the lack of effect of pH on product treatment performance. These results shed light on water quality and operational limitations that should be taken into account when deploying such products in relief situations.
Footnotes
Published ahead of print 18 January 2013
REFERENCES
- 1. Lantagne D, Clasen T. 2012. Use of household water treatment and safe storage methods in acute emergency response: case study results from Nepal, Indonesia, Kenya, and Haiti. Environ. Sci. Technol. 46:11352–11360 [DOI] [PubMed] [Google Scholar]
- 2. The Sphere Project 2011. The Sphere handbook 2011: humanitarian charter and minimum standards in humanitarian response, 3rd ed Practical Action Publishing, Bourton on Dunsmore, United Kingdom [Google Scholar]
- 3. Bratby J. 2008. Coagulation and flocculation with an emphasis on water and wastewater treatment, 2nd ed International Water Association, London, United Kingdom [Google Scholar]
- 4. Edzwald J. 2011. Water quality & treatment: a handbook on drinking water, 6th ed McGraw-Hill Professional, New York, NY [Google Scholar]
- 5. WHO 2011. Evaluating household water treatment options: health-based targets and microbiological performance specifications. World Health Organization, Geneva, Switzerland [Google Scholar]
- 6. Crump JA, Okoth GO, Slutsker L, Ogaja DO, Keswick BH, Luby SP. 2004. Effect of point-of-use disinfection, flocculation and combined flocculation–disinfection on drinking water quality in western Kenya. J. Appl. Microbiol. 97:225–231 [DOI] [PubMed] [Google Scholar]
- 7. McLennan SD, Peterson LA, Rose RB. 2009. Comparison of point-of-use technologies for emergency disinfection of sewage-contaminated drinking water. Appl. Environ. Microbiol. 75:7283–7286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Souter PF, Cruickshank GD, Tankerville MZ, Keswick BH, Ellis BD, Langworthy DE, Metz KA, Appleby MR, Hamilton N, Jones AL, Perry JD. 2003. Evaluation of a new water treatment for point-of-use household applications to remove microorganisms and arsenic from drinking water. J. Water Health 1:73–84 [PubMed] [Google Scholar]
- 9. Reller ME, Mendoza CE, Lopez MB, Alvarez M, Hoekstra RM, Olson CA, Baier KG, Kewick BH, Luby SP. 2003. A randomized controlled trial of household-based flocculant-disinfectant drinking water treatment for diarrhea prevention in rural Guatemala. Am. J. Trop. Med. Hyg. 69:411–419 [PubMed] [Google Scholar]
- 10. Rangel JM, Lopez B, Mejia MA, Mendoza C, Luby S. 2003. A novel technology to improve drinking water quality: a microbiological evaluation of in-home flocculation and chlorination in rural Guatemala. J. Water Health 1:15–22 [PubMed] [Google Scholar]


