Abstract
We cloned a Paenibacillus sp. strain E18 5.3-kb xylanolytic gene cluster that contains three open reading frames encoding two family 43 α-l-arabinofuranosidases (Abf43A and Abf43B) and one family 10 xylanase (XynBE18). The deduced amino acid sequences of Abf43A and Abf43B were at most 68% and 63% identical to those of two putative family 43 proteins from Clostridium sp. strain DL-VIII (EHI98634.1 and EHI98635.1), respectively, but were only 11% identical to each other. Recombinant Abf43A and Abf43B had similar activities at 45°C and pH 6.0 but varied in thermostabilities and substrate specificities. Abf43B was active against only 4-nitrophenyl α-l-arabinofuranoside, whereas Abf43A acted on 4-nitrophenyl α-l-arabinofuranoside, wheat arabinoxylan, 4-nitrophenyl α-d-xylopyranoside, and sugar beet arabinan. The sequential and combined effects on xylan degradation by XynBE18, Abf43A, and Abf43B were characterized. For beechwood, birchwood, and oat spelt xylans as the substrates, synergistic effects were found when XynBE18 and Abf43A or Abf43B were incubated together and when the substrates were first incubated with Abf43A or Abf43B and then with XynBE18. Further high-performance liquid chromatography (HPLC) analysis showed that the amounts of xylobiose and xylose increased sharply in the aforementioned reactions. For water-soluble wheat arabinoxylan as the substrate, Abf43A not only released arabinose but also had a synergistic effect with XynBE18. Synergy may arise as the result of removal of arabinose residues from xylans by α-l-arabinofuranosidases, which eliminates steric hindrance caused by the arabinose side chains and which allows xylanases to then degrade the xylan backbone, producing short xylooligosaccharides.
INTRODUCTION
Hemicelluloses are polysaccharides found abundantly in nature (1–3). Xylan, the major component of plant hemicelluloses, is the second most abundant naturally occurring polysaccharide after cellulose (4, 5). It is composed of a β-1,4-linked d-xylose backbone, with l-arabinose, d-galactose, glucuronic, acetic, ferulic, and p-coumaric acid side chains (2). Therefore, complete degradation of xylan requires the actions of several enzymes (6, 7). Xylanases and β-xylosidases act on the xylan backbone, and α-l-arabinofuranosidase, α-glucuronidase, and several esterases act at the xylan branch points (2).
α-l-Arabinofuranosidases (EC 3.2.1.55) cleave α-l-arabinofuranosyl residues in hemicellulosic polysaccharides. On the basis of their amino acid sequence similarities (http://www.cazy.org/), α-l-arabinofuranosidases are classified as family 3, 43, 51, 54, or 62 glycoside hydrolases (GH-3, GH43, etc.). Some GH-43 α-l-arabinofuranosidases have both α-l-arabinofuranosidase and β-xylosidase activities (8, 9). In combination with xylanase, α-l-arabinofuranosidase, as well as other xylanolytic enzymes, such as β-xylosidase, α-glucuronidase, acetyl xylan esterase, and feruloyl esterase, can act synergistically and facilitate the complete hydrolysis of various substrates (10–14).
For the work reported herein, we identified two novel α-l-arabinofuranosidase genes in the Paenibacillus sp. strain E18 xylanolytic gene cluster. We investigated the enzymatic activities of their recombinant gene products against various xylans, sequentially and in combination with the Paenibacillus sp. strain E18 bifunctional xylanase-glucanase XynBE18 (15).
MATERIALS AND METHODS
Strains and culture medium.
Paenibacillus sp. strain E18 CGMCC 3327 (15), maintained in our laboratory, was cultured in Luria-Bertani broth at 37°C for isolation of genomic DNA. Escherichia coli strains trans1-T1 and BL21(DE3) (TransGen) were employed for gene cloning and protein expression, respectively.
Cloning and sequencing of the xylanolytic gene cluster.
Using the known sequence of the Paenibacillus gene xynBE18 (FJ899683) (15), two sets of specific primers and 11 degenerate primers (data not shown) were synthesized to obtain the flanking upstream and downstream fragments in conjunction with thermal asymmetric interlaced PCR (TAIL-PCR) (16). After purification with agarose gel DNA purification kit version 2.0 reagents (TaKaRa), the PCR products were individually ligated into a pGEM-T Easy vector (Promega) for sequencing. The nucleotide sequences were assembled and analyzed using Vector NTI Advance 10.0 software (Invitrogen). BLASTN and BLASTP (http://www.ncbi.nlm.nih.gov/BLAST/) and AlignX from Vector NTI were used to analyze the nucleotide and deduced amino acid sequences, respectively. The SignalP 4.0 server and pSORTb (http://www.psort.org/psortb/) were used to predict the subcellular locations of proteins.
Protein expression and purification.
Abf43A and Abf43B had no Sec-type putative signal peptides at the N terminus. The DNA sequences of abf43A and abf43B were PCR amplified from Paenibacillus sp. strain E18 genomic DNA with the expression primer sets abf43A-F (GGGCATATGATGTATCCAAATCCGATTATATGGG) plus abf43A-R (GGGAAGCTTTTAAGAGAGCACCTGATATTGAAAATC) and abf43B-F (CCGGAATTCATGATACCAAAAAAGAAAAGCGAGTTTATCGAGG) plus abf43B-R (ATTTGCGGCCGCCTAATCGCGTTTTACAATGACTTCGATCG), respectively. (In the primer sets, restriction sites are underlined.) The PCR products were purified and cloned downstream of the His-Tag coding sequence of a pET-28a(+) vector (Novagen), and the recombinant plasmids (pET-28a-abf43A and pET-28a-abf43B) were then individually transformed into E. coli BL21(DE3) competent cells. Protein expression was induced at 30°C by 0.6 mM isopropyl-β-d-1-thiogalactopyranoside (IPTG), and the cells were then cultured for an additional 6 h. Cells were centrifuged at 12,000 × g at 4°C for 5 min. The pellet (5 g), resuspended in 25 ml of lysis buffer (20 mM Tris-HCl [pH 7.0]), was sonicated with an Ultrasonic cell disruptor (Scientz) on ice with 50 short bursts at 200 W of 10 s followed by intervals of 20 s for cooling. Cell debris was removed by centrifugation. The supernatant containing Abf43A was subjected to Ni2+-nitrilotriacetic acid (NTA) chromatography with a linear 2 to 300 mM imidazole gradient in 50 mM Tris-HCl–0.5 M NaCl (pH 7.6). Following the same purification procedure of Abf43A, Abf43B didn't achieve apparent homogeneity and was further purified by ammonium sulfate precipitation (30 to 90%), desalting, and ion-exchange chromatography. The supernatant (1 ml) was loaded onto a 5 ml Hitrap Q Sepharose XL fast protein liquid chromatography (FPLC) column (GE Healthcare) equilibrated with 20 mM Tris-HCl (pH 7.0). The elution was performed with a linear gradient of NaCl from 0 to 1.0 M in the same buffer at a flow rate of 3 ml min−1. The fractions exhibiting enzyme activities were pooled, concentrated, and assayed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (17). The protein concentration was determined by the Bradford assay (18) with bovine serine albumin as the standard.
Enzyme assays.
α-l-Arabinofuranosidase activity was assayed as described previously (19, 20), the dinitrosalicylic acid (DNS) method (15, 21) was used to assay xylanase activity, and β-xylosidase activity was determined as reported in reference22. Each reaction solution contained an appropriately diluted enzyme sample and 1 mM 4-nitrophenyl α-l-arabinofuranoside or p-nitrophenyl-β-d-xylopyranoside or 1.0% (wt/vol) xylan and was incubated at 40°C (pH 6.0) for 10 min in 100 mM Na2HPO4-citric acid. One unit of α-l-arabinofuranosidase or β-xylosidase activity was defined as the amount of enzyme that released 1 μmol of 4-nitrophenol or p-nitrophenol per min under the assay conditions. One unit of xylanase activity was defined as the amount of enzyme that released 1 μmol of xylose per min under standard conditions. Each reaction was performed in triplicate, as was each experiment.
Effects of pH and temperature on enzyme activity.
The optimal pH values for Abf43A and Abf43B activities were determined at 50°C using the following solutions: 100 mM Na2HPO4-citric acid (pH 2.0 to 8.0), 100 mM Tris-HCl (pH 8.0 to 9.0), and 100 mM glycine-NaOH (pH 9.0 to 11.0). The stabilities of the enzymes under different pH conditions were assessed by measuring residual enzyme activities under standard conditions after incubation at 37°C for 1 h at the different pH values. The optimal temperatures were determined by measuring activities between 20 and 60°C at the experimentally determined optimal pH.
Substrate specificities and kinetic parameters.
Substrate specificities of Abf43A and Abf43B were examined under standard assay conditions with 1 mM 4-nitrophenyl α-l-arabinofuranoside, 4-nitrophenyl β-d-xylopyranoside, 4-nitrophenyl-α-d-galactopyranoside, 2-nitrophenyl β-d-galactopyranoside, 4-nitrophenyl α-d-glucopyranoside, 4-nitrophenyl α-l-arabinopyranoside, p-nitrophenyl acetate, α-l-arabinooligosaccharides, 4-nitrophenyl α-d-glucuronide, and aldouronic acid mixture and 1% (wt/vol) oat spelt xylan, birchwood xylan, beechwood xylan, water-soluble wheat arabinoxylan, sugar beet arabinan, and debranched sugar beet arabinan. All of the substrates were purchased from Sigma, Megazyme, or TCI.
The Km and Vmax values for Abf43A and Abf43B with 4-nitrophenyl α-l-arabinofuranoside (1 to 10 mM) as the substrate were determined for 100 mM Na2HPO4-citric acid (pH 6.0) at 40°C (assay time, 5 min). The data were plotted according to the Lineweaver-Burk method (23).
Xylan degradation.
All reactions were carried out at 37°C in 100 mM Na2HPO4-citric acid (pH 6.0) solutions composed of 900 μl of 0.5% (wt/vol) substrate and 100 μl of enzyme(s) (1.0 U of XynBE18 for beechwood xylan and 0.5 U of XynBE18 for birchwood xylan, oat spelt xylan, and water-soluble wheat arabinoxylan, and 0. 5 U of Abf43A or Abf43B when present). After 12-h incubations, the reactions were stopped by immersing the tubes containing the reaction mixtures in boiling water for 10 min. The reaction system containing each substrate without any enzyme was treated as the blank control. When the synergetic effects of sequential addition of the enzymes on degradation were assessed, heat denaturation was performed prior to addition of the second enzyme solution. The xylose equivalents released (mM) were determined by the DNS method with xylose as the standard. The degree of synergy was defined as the ratio of xylose equivalents released when enzymes were incubated simultaneously or sequentially to the sum of the xylose equivalents released by each enzyme alone.
Sugar compositions determined by HPLC.
Hydrolysis products were centrifuged (10,000 × g, 4°C, 10 min) through a 3-kDa Amicon ultracentrifugal filter (Millipore) to remove the enzymes, and the filtrates were each chromatographed through a column (10 μm by 6.5 mm by 300 mm) coupled to an Alliance high-performance liquid chromatography (HPLC) system (Waters) (24). The mobile phase was 50 mM EDTA-CaNa2, and a flow rate of 0.5 ml min−1 was used. Xylotriose, xylobiose, xylose, and arabinose served as standards.
Nucleotide sequence accession numbers.
The nucleotide sequences of Abf43A and Abf43B have been deposited in GenBank under accession no. FJ899684 and JN673276, respectively.
RESULTS
Gene cloning and sequencing.
A gene fragment of ∼5.3 kb was obtained with the use of TAIL-PCR. The fragment contained a 5′-noncoding 941-bp sequence and three open reading frames (ORFs), two of which (1,821 and 1,536 bp) encode α-l-arabinofuranosidases, denoted Abf43A and Abf43B, respectively. The third ORF (981 bp) encodes the bifunctional xylanase-glucanase XynBE18 (15). No Sec-type signal peptide sequences were predicted for the ORFs by using SignalP. Further pSORTb analysis indicated that Abf43B and XynBE18 are extracellular, and Abf43A is predicted to be cytoplasmic. Of them, Abf43B is similar to the α-l-arabinofuranosidase (α-l-AFase II; P82594.1) from Streptomyces chartreusis GS901, which has a TAT-type signal sequence that involves the twin-arginine secretion pathway (25).
Homology comparison of the deduced Abf43A amino acid sequence with those of known GHs showed that it is most similar to a putative family 43 protein of Clostridium sp. strain DL-VIII (EHI98634.1) (68% identity) and has 48% identity to Bifidobacterium adolescentis DSM20083 arabinofuranohydrolase D3 (AAO67499.1) (9). The deduced Abf43B sequence is most similar (63% identify) to that of Clostridium sp. strain DL-VIII α-l-arabinofuranosidase (EHI98635.1) and has 32% identity to an exo-1,5-α-l-arabinofuranosidase from Streptomyces avermitilis MA-4680 (BAC68753.1; PDB_id 1, 3AKF). However, the sequences of Abf43A and Abf43B are only 11% identical based on the analysis of AlignX from Vector NTI.
Expression and purification of recombinant Abf43A and Abf43B.
The whole-cell lysates of positive transformants containing pET-28a-abf43A and pET-28a-abf43B had α-l-arabinofuranosidase activities of 77.4 ± 2.6 and 69.5 ± 0.4 U ml−1, respectively, after expression of the enzymes for 6 h at 30°C. The recombinant enzymes were purified to apparent homogeneity according to SDS-PAGE; the apparent molecular masses of Abf43A and Abf43B were 57 and 67 kDa, respectively, similar to their calculated masses (57.5 and 67.6 kDa, respectively).
Enzyme properties.
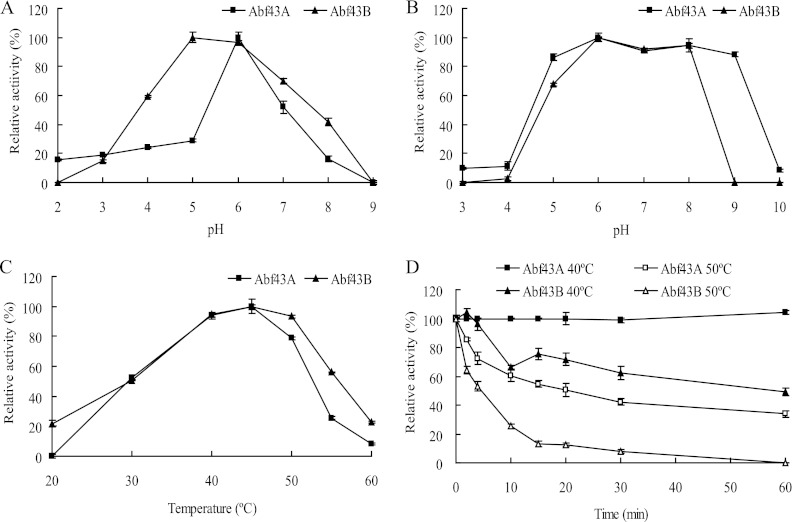
With 4-nitrophenyl α-l-arabinofuranoside as the substrate and a temperature of 40°C, Abf43A and Abf43B were most active at pH 5.0 to 6.0 (Fig. 1A), and they retained their activities between pH 6.0 and 8.0 for 1 h at 37°C (Fig. 1B). When assayed at pH 6.0, both enzymes had temperature optima of 45°C (Fig. 1C). Abf43A was more thermostable than was Abf43B, as Abf43A retained almost all of its initial activity at 40°C for 1 h and half of its activity at 50°C for 1 h (Fig. 1D).
Fig 1.
Characterization of purified Abf43A and Abf43B. (A) Effect of pH on Abf43A and Abf43B activities at 40°C. (B) pH stability at 37°C for 1 h. (C) Effect of temperature on Abf43A and Abf43B activities (pH 6.0). (D) Thermostability at 40 and 50°C.
Abf43A acted on many different substrates (Table 1) and was most active against water-soluble wheat arabinoxylan, moderately active against sugar beet arabinan, slightly active against 4-nitrophenyl α-l-arabinofuranoside and 4-nitrophenyl α-d-xylopyranoside, and inactive against debranched sugar beet arabinan, α-l-arabinooligosaccharides, 4-nitrophenyl α-d-glucuronide, and the aldouronic acid mixture. Abf43B acted solely on 4-nitrophenyl α-l-arabinofuranoside.
Table 1.
Substrate specificities of Abf43A and Abf43B
| Substrate | Sp act (U mg−1)a |
|
|---|---|---|
| Abf43A | Abf43B | |
| 4-Nitrophenyl α-l-arabinofuranoside | 0.49 ± 0.01 | 1.13 ± 0.07 |
| 4-Nitrophenyl β-d-xylopyranoside | 0.30 ± 0.01 | − |
| Wheat arabinoxylan | 23.84 ± 0.56 | − |
| Arabinan (Ara-Gal-Rha-GalUA [88:3:2:7]) | 8.54 ± 0.31 | − |
| Debranched arabinan (Ara-Gal-Rha [71:26:3]) | − | − |
| 4-Nitrophenyl α-l-arabinopyranoside | − | − |
| Oat spelt xylan | − | − |
| Birchwood xylan | − | − |
| Beechwood xylan | − | − |
| α-l-Arabinooligosaccharides | − | − |
| 4-Nitrophenyl α-d-glucuronide | − | − |
| Aldouronic acid mixture | − | − |
| 4-Nitrophenyl α-d-galactopyranoside | − | − |
| 2-Nitrophenyl β-d-galactopyranoside | − | − |
| 4-Nitrophenyl α-d-glucopyranoside | − | − |
| p-Nitrophenyl acetate | − | − |
Values are means ± SD (n = 3). −, no activity detected.
With 4-nitrophenyl α-l-arabinofuranoside as the substrate, the Km and Vmax values for Abf43A and Abf43B were 6.6 ± 0.4 mM and 2.9 ± 0.3 μmol min−1 mg−1 and 13.3 ± 0.8 mM and 7.2 ± 0.5 μmol min−1 mg−1, respectively.
Xylan degradation.
The xylose equivalents released by XynBE18 from the birchwood xylan, beechwood xylan, and oat spelt xylan varied between 2.20 and 9.96 mM (Table 2). The effects of different enzyme combinations on xylose equivalents released also varied and depended on the substrate. Similar synergistic patterns were found for birchwood xylan and beechwood xylan as the substrates, with the greatest synergy found when birchwood xylan was first incubated with Abf43A alone and then with XynBE18 (degree of synergy, 1.77-fold). For beechwood xylan, the degree of synergy in the same sequential reaction was 1.25-fold. Incubation of XynBE18 and Abf43A or Abf43B together was also synergistic. Conversely, when XynBE18 was added first and inactivated before the addition of Abf43A or Abf43B, no synergistic effect was observed.
Table 2.
Simultaneous and sequential reactions by Abf43A, Abf43B, and XynBE18 against various xylansf
| Enzyme(s) added in 1st reaction | Enzyme added in 2nd reaction | Birchwood xylana |
Beechwood xylanb |
Oat spelt xylanc |
Water-soluble wheat arabinoxyland |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concn of xylose equivalents (mM) | Degree of synergye | Concn of xylooligosaccharides from HPLC (mg ml−1) | Concn of xylose equivalents (mM) | Degree of synergy | Concn of xylooligosaccharides from HPLC (mg ml−1) | Concn of xylose equivalents (mM) | Degree of synergy | Concn of xylooligosaccharides from HPLC (mg ml−1) | Concn of xylose and arabinose equivalents (mM) | Degree of synergy | Concn of xylooligosaccharides from HPLC (mg ml−1) | ||
| XynBE18 | None | 4.66 ± 0.10 | − | 0.61 ± 0.02 | 9.96 ± 0.17 | − | 1.68 ± 0.06 | 2.20 ± 0.09 | − | 0.39 ± 0.01 | 4.03 ± 0.04 | − | 1.08 ± 0.09 |
| Abf43A | None | 0 | − | 0 | 0 | − | 0 | 0 | − | 0 | 4.33 ± 0.24 | − | 0 |
| Abf43B | None | 0 | − | 0 | 0 | − | 0 | 0 | − | 0 | − | − | 0 |
| XynBE18+ Abf43A | None | 6.68 ± 0.19 | 1.43* | 1.05 ± 0.06 | 11.9 ± 0.49 | 1.19* | 1.76 ± 0.08 | 4.66 ± 0.03 | 2.12* | 0.79 ± 0.04 | 11.4 ± 0.45 | 1.36* | 1.69 ± 0.13 |
| XynBE18 + Abf43B | None | 5.42 ± 0.19 | 1.16* | 0.80 ± 0.06 | 10.7 ± 0.24 | 1.07* | 1.77 ± 0.02 | 3.90 ± 0.03 | 1.77* | 0.59 ± 0.02 | 4.19 ± 0.08 | 1.04 | 1.12 ± 0.09 |
| XynBE18 | Abf43A | 4.66 ± 0.27 | 1.00 | 0.62 ± 0.03 | 10.2 ± 0.29 | 1.02 | 1.64 ± 0.04 | 3.87 ± 0.13 | 1.76* | 0.69 ± 0.03 | 9.08 ± 0.12 | 1.09* | 1.15 ± 0.12 |
| XynBE18 | Abf43B | 4.26 ± 0.15 | 0.91 | 0.56 ± 0.02 | 9.99 ± 0.19 | 1.00 | 1.59 ± 0.06 | 3.55 ± 0.13 | 1.61* | 0.63 ± 0.02 | 4.04 ± 0.08 | 1.00 | 1.09 ± 0.05 |
| Abf43A | XynBE18 | 8.26 ± 0.23 | 1.77* | 1.38 ± 0.09 | 12.5 ± 0.11 | 1.25* | 1.95 ± 0.03 | 6.06 ± 0.11 | 2.76* | 1.09 ± 0.06 | 10.0 ± 0.15 | 1.20* | 1.46 ± 0.07 |
| Abf43B | XynBE18 | 6.99 ± 0.13 | 1.50* | 1.20 ± 0.06 | 10.8 ± 0.05 | 1.08* | 1.83 ± 0.05 | 4.98 ± 0.08 | 2.26* | 0.96 ± 0.05 | 4.70 ± 0.12 | 1.17* | 1.21 ± 0.04 |
Birchwood xylan, arabinose/xylose ratio of ∼1:90.
Beechwood xylan, arabinose/xylose ratio of ∼1:90.
Oat spelt xylan, arabinose/xylose/glucose ratio of ∼10:75:15.
Water soluble what arabinoxylan, arabinose/xylose/other sugars ratio of ∼37:61:2.
The degree of synergy is defined as the ratio of xylose equivalents from simultaneous or sequential enzyme reactions to the sum of the xylose equivalents released by the individual enzymes. −, not detected; *, the enzymatic reaction is significantly synergistic at P < 0.05 (Tukey's test by OriginPro 8).
−, not detected
All combinations of sequential or simultaneous enzyme additions had very significant synergistic effects on oat spelt xylan degradation (1.54- to 2.78-fold). The same as toward birchwood xylan and beechwood xylan, prehydrolysis by Abf43A alone followed by XynBE18 gave the highest synergism.
Wheat arabinoxylan digestion by the various enzyme combinations was significantly distinct from those for the other three xylans. Addition of Abf43A alone could release oligosaccharides from water-soluble wheat arabinoxylan. Moreover, high synergies were detected when Abf43A and XynBE18 were incubated with water-soluble wheat arabinoxylan in combination or sequentially.
Hydrolysis product compositions.
The product composition for each xylan substrate after digestion by the enzymes individually or in combination was determined by HPLC (Fig. 2). XynBE18 released xylobiose and xylotriose from oat spelt and birchwood xylan and xylose, xylobiose, and xylotriose from beechwood xylan and water-soluble wheat arabinoxylan. Abf43A liberated arabinose from wheat arabinoxylan. When XynBE18 was combined with Abf43A or Abf43B, the hydrolysis product compositions varied substantially.
Fig 2.
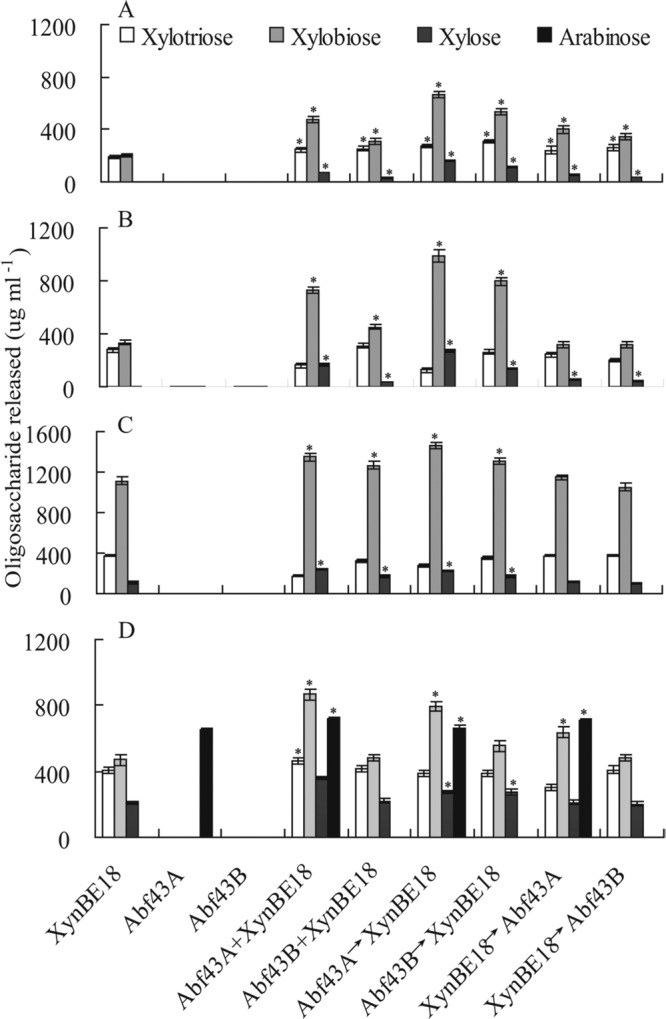
Hydrolysis products of oat spelt xylan (A), birchwood xylan (B), beechwood xylan (C), and wheat arabinoxylan (D). White bars, xylotriose; light gray bars, xylobiose; dark gray bars, xylose; black bars, arabinose. An asterisk indicates that the amount of oligosaccharide released by enzyme combinations is significantly higher than that released by XynBE18 or Abf43A alone at P < 0.05 (Tukey's test by OriginPro 8).
Compared with the hydrolysis products of oat spelt xylan produced by XynBE18 alone (Fig. 2A), all combinations of XynBE18 and each of the arabinofuranosidases produced substantially more xylotriose, xylobiose, and xylose. For the sequential addition of Abf43B and XynBE18, xylotriose was the most abundant product (2.46-fold increase over that produced by XynBE18 alone) (Table 2). When incubated with Abf43A followed by XynBE18, xylobiose and xylose were the most abundant products. The greatest oligosaccharide yield was produced by the sequential addition of Abf43A and XynBE18 (2.79-fold increase in comparison with that produced by XynBE18 alone) (Table 2).
All enzyme combinations released smaller or similar amounts of xylotriose (P > 0.05) and more xylobiose and xylose from birchwood and beechwood xylans than did XynBE18 alone (Fig. 2B and C). Incubation with Abf43A followed by XynBE18 produced the greatest oligosaccharide yields for birchwood and beechwood xylans (2.26- and 1.16-fold increases in yield compared with that for XynBE18 alone, respectively) (Table 2).
Hydrolysis of water-soluble wheat arabinoxylan produced relatively complex mixtures (Fig. 2D). Abf43A alone released a large amount of arabinose. Combined and sequential addition of Abf43A and XynBE18 had synergistic effects according to the DNS assay: substantially more oligosaccharides were released by the combination of enzymes than by XynBE18 alone (1.96-fold increase in yield) (Table 2). Simultaneous incubation of water-soluble wheat arabinoxylan with Abf43A and XynBE18 produced the greatest oligosaccharide yield (1.36-fold increase in yield) (Table 2).
DISCUSSION
Previously, we identified a bifunctional Paenibacillus sp. strain E18 xylanase-glucanase (15). Because complete xylan degradation requires the action of several different enzymes, we assumed that the gene for XynBE18 was part of a xylanolytic gene cluster. This assumption prompted the present study that identified the neighboring genes and the effects of the gene products on xylan degradation. The xylanolytic gene cluster contains two α-l-arabinofuranosidase genes (gene products Abf43A and Abf43B) in addition to the gene encoding XynBE18. The gene products were expressed in E. coli, purified, and biochemically characterized, and the effects of their action on xylan degradation were investigated. Abf43A, Abf43B, and XynBE18 have no putative Sec-type signal peptides; however, based on pSORTb analysis, Abf43A is cytoplasmic, and Abf43B and XynBE18 are both extracellular. Abf43B is supposed to have a TAT-type signal sequence based on sequence similarity. Further studies on their subcellular locations and hydrolase transcription may shed light on what and how polysaccharides are taken up by Paenibacillus sp. strain E18.
Abf43A and Abf43B are members of family 43, but these two enzymes have low sequence identity (11%). They have similar pH and temperature optima but different thermostabilities and substrate specificities. As with most bacterial α-l-arabinofuranosidases that are optimally active under acidic and mesothermal conditions (26, 27), Abf43A and Abf43B are most active between pH 5.0 and 6.0 at 45°C and are stable between pH 6.0 and 8.0 at 37°C for 1 h. Abf43A is more thermostable than is Abf43B. At 40°C after 1 h, Abf43A retained all of its activity, but Abf43B retained only 50% of its activity. Moreover, the two enzymes have different substrate specificities. Abf43A acts as a bifunctional α-l-arabinofuranosidase/β-xylosidase with greater α-l-arabinofuranoside activity than β-xylosidase activity (0.49 versus 0.30 U mg−1). Many bifunctional α-l-arabinofuranosidase/β-xylosidases belong to the GH-43 family: e.g., Paenibacillus woosongensis XylC (27), Penicillium purpurogenum ABF3 (8), and Butyrivibrio fibrisolvens XylB (28). Abf43A hydrolyzed wheat arabinoxylan and beet sugar arabinan but not debranched beet sugar arabinan. Therefore, Abf43A hydrolyzes α-(1→2)- or α-(1→3)-linked arabinosyl residues from xylans but not α-(1→5)-linked backbone arabinoses. Given its mode of action and substrate specificity, Abf43A can be classified as an arabinofuranosidase of type B (29). Conversely, Abf43B is active against only 4-nitrophenyl α-l-arabinofuranoside and therefore is an arabinofuranosidase of type A. Compared with other arabinofuranosidase counterparts, Abf43A and Abf43B showed slightly higher specific activities (0.49 and 1.13 U mg−1, respectively) toward 4-nitrophenyl α-l-arabinofuranoside than AbfA (0.25 U mg−1) and AXH-d3 (0.10 U mg−1) from Bifidobacterium adolescentis (9, 30), but lower specific activities than SaAraf43A (2.92 U mg−1) from S. avermitilis NBRC14893 (31), α-l-AFase II (3.16 U mg−1) from S. chartreusis GS901 (25), and RuXyn1 (14.2 U mg−1) from the rumen metagenome (32). Characterization of the structures of these GH-43 arabinofuranosidases would be of great interest to increase our understanding of their modes of action and how their substrate specificities are determined.
Abf43A or Abf43B and XynBE18 acted synergistically on birchwood, beechwood, and oat spelt xylans. Initial action by Abf43A alone on the xylans improved the ability of XynBE18 to degrade the substrates. Similar observations have been reported for arabinofuranosidases from Penicillium sp. strain AHT-1 and Streptomyces sp. strain PC22 (13, 33). Moreover, except for wheat arabinoxylan, the other three xylans were degraded synergistically by the enzymes in all simultaneous combinations. However, no reducing sugar or oligosaccharide was identified by both DNS and HPLC methods when the three xylan substrates were incubated with Abf43A or Abf43B alone. The low contents of arabinose in birchwood, beechwood (<1%) (34), and oat spelt xylans (≤10%) (35) might be the reason why no arabinose released by Abf43A or Abf43B was detected. On the other hand, significant arabinose (0.65 mg ml−1) was detected in water-soluble wheat arabinoxylan, which contains 37% arabinose (35).
We used HPLC to identify the hydrolysis products of each xylan and found that sequential addition of Abf43A alone followed by XynBE18 produced more xylooligosaccharides from birchwood, beechwood, and oat spelt xylans, and simultaneous combinations of Abf43A and XynBE18 produced the most xylooligosaccharides from water-soluble wheat arabinoxylan. Combined with the data for total reducing sugar released by the enzymes individually and in combination, some conclusions can be drawn. (i) Compared with Abf43B, Abf43A has broad substrate specificity and different modes of action and shows a higher degree of synergy when birchwood, beechwood, and oat spelt xylans are the substrates (Table 2). Thus, Abf43A shows greater degradation efficiency and represents a useful accessory enzyme for the bioconversion of economically important agricultural resources. (ii) For Abf43B, the synergies on birchwood and beechwood xylan are different from that on oat spelt xylan. This might be ascribed to the different structures of xylan of various sources. In fact, oat spelt xylan contains more α-(1→3)-linked arabinosyl residues than birchwood and beechwood xylans. (iii) Based on the xylooligosaccharide composition analysis from HPLC (Fig. 2), the amounts of xylobiose and xylose released by XynBE18 were increased when all xylans were first incubated with Abf43A or Abf43B. The reason might be that removal of arabinose residues from xylans by α-l-arabinofuranosidases eliminates the steric hindrance caused by the arabinose side chains (14), which allows xylanases to degrade the xylan backbone to produce short xylooligosaccharides. Thus, to achieve the highest level of degradation, xylan hydrolysis strategies should incorporate different enzymes introduced in different orders according to the substrate to be degraded.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (grant 31101742), the China Modern Agriculture Research System (CARS-42), and the National Science Foundation for Distinguished Young Scholars of China (grant 31225026).
Footnotes
Published ahead of print 18 January 2013
REFERENCES
- 1. Bastawde KB. 1992. Xylan structure, microbial xylanases, and their mode of action. World J. Microbiol. Biotechnol. 8:353–368 [DOI] [PubMed] [Google Scholar]
- 2. Saha BC. 2003. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 30:279–291 [DOI] [PubMed] [Google Scholar]
- 3. Sun Y, Cheng J. 2002. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour. Technol. 83:1–11 [DOI] [PubMed] [Google Scholar]
- 4. Biely P. 1985. Microbial xylanolytic systems. Trends Biotechnol. 3:286–290 [Google Scholar]
- 5. Viikari L, Sundquist J, Kettunen J. 1991. Xylanase enzymes promote pulp bleaching. Paper Timber 73:384–389 [Google Scholar]
- 6. Aspinall GO. 1980. Chemistry of cell wall polysaccharides, p 473–500 In Preiss J. (ed), The biochemistry of plants (a comprehensive treatise), vol 3. Carbohydrates: structure and function. Academic Press, New York, NY [Google Scholar]
- 7. Bocchini DA, Alves-Prado HF, Baida LC, Roberto IC, Gomes E, Da-Silva R. 2002. Optimization of xylanase production by Bacillus circulans D1 in submerged fermentation using response surface methodology. Process Biochem. 38:727–731 [Google Scholar]
- 8. Ravanal MC, Callegari E, Eyzaguirre J. 2010. Novel bifunctional α-l-arabinofuranosidase/xylobiohydrolase (ABF3) from Penicillium purpurogenum. Appl. Environ. Microbiol. 76:5247–5253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van den Broek LAM, Lloyd RM, Beldman G, Verdoes JC, McCleary BV, Voragen AGJ. 2005. Cloning and characterization of arabinoxylan arabinofuranohydrolase-D3 (AXHd3) from Bifidobacterium adolescentis DSM20083. Appl. Microbiol. Biotechnol. 67:641–647 [DOI] [PubMed] [Google Scholar]
- 10. Alvira P, Negro MJ, Ballesteros M. 2011. Effect of endoxylanase and α-l-arabinofuranosidase supplementation on the enzymatic hydrolysis of steam exploded wheat straw. Bioresour. Technol. 102:4552–4558 [DOI] [PubMed] [Google Scholar]
- 11. Guerfali M, Gargouri A, Belghith H. 2011. Catalytic properties of Talaromyces thermophilus α-l-arabinofuranosidase and its synergistic action with immobilized endo-β-1,4-xylanase. J. Mol. Catal. B Enzym. 68:192–199 [Google Scholar]
- 12. Numan MT, Bhosle NB. 2006. α-l-Arabinofuranosidases: the potential applications in biotechnology. J. Ind. Microbiol. Biotechnol. 33:247–260 [DOI] [PubMed] [Google Scholar]
- 13. Raweesri P, Riangrungrojana P, Pinphanichakarn P. 2008. α-l-Arabinofuranosidase from Streptomyces sp. PC22: purification, characterization and its synergistic action with xylanolytic enzymes in the degradation of xylan and agricultural residues. Bioresour. Technol. 99:8981–8986 [DOI] [PubMed] [Google Scholar]
- 14. Sørensen HR, Meyer AS, Pedersen S. 2003. Enzymatic hydrolysis of water-soluble wheat arabinoxylan. 1. Synergy between α-l-arabinofuranosidases, endo-1,4-β-xylanases, and β-xylosidase activities. Biotechnol. Bioeng. 81:726–731 [DOI] [PubMed] [Google Scholar]
- 15. Shi P, Tian J, Yuan T, Liu X, Huang H, Bai Y, Yang P, Chen X, Wu N, Yao B. 2010. Paenibacillus sp. strain E18 bifunctional xylanase-glucanase with a single catalytic domain. Appl. Environ. Microbiol. 76:3620–3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu YG, Whittier RF. 1995. Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25:674–681 [DOI] [PubMed] [Google Scholar]
- 17. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 18. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 19. Manin C, Shareek F, Morosoli R, Kluepfel D. 1994. Purification and characterization of an α-l-arabinofuranosidase from Streptomyces lividan 66 and DNA sequence of the gene (abfA). Biochem. J. 302:443–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shi P, Li N, Yang P, Wang Y, Luo H, Bai Y, Yao B. 2010. Gene cloning, expression, and characterization of a family 51 α-l-arabinofuranosidase from Streptomyces sp. S9. Appl. Biochem. Biotechnol. 162:707–718 [DOI] [PubMed] [Google Scholar]
- 21. Miller GL. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31:426–428 [Google Scholar]
- 22. Shao W, Xue Y, Wu A, Kataeva I, Pei J, Wu H, Wiegel J. 2011. Characterization of a novel β-xylosidase, XylC, from Thermoanaerobacterium saccharolyticum JW/SL-YS485. Appl. Environ. Microbiol. 77:719–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lineweaver H, Burk D. 1934. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 56:658–666 [Google Scholar]
- 24. Yang P, Shi P, Wang Y, Bai Y, Meng K, Luo H, Yuan T, Yao B. 2007. Cloning and overexpression of a Paenibacillus β-glucanase in Pichia pastoris: purification and characterization of the recombinant enzyme. J. Microbiol. Biotechnol. 17:58–66 [PubMed] [Google Scholar]
- 25. Matsuo N, Kaneko S, Kuno A, Kobayashi H, Kusakabe I. 2000. Purification, characterization and gene cloning of two α-l-arabinofuranosidases from Streptomyces chartreusis GS901. Biochem. J. 346:9–15 [PMC free article] [PubMed] [Google Scholar]
- 26. Adelsberger H, Hertel C, Glawischnig E, Zverlov VV, Schwarz WH. 2004. Enzyme system of Clostridium stercorarium for hydrolysis of arabinoxylan: reconstitution of the in vivo system from recombinant enzymes. Microbiology 150:2257–2266 [DOI] [PubMed] [Google Scholar]
- 27. Kim YA, Yoon KH. 2010. Characterization of a Paenibacillus woosongensis β-xylosidase/α-arabinofuranosidase produced by recombinant Escherichia coli. J. Microbiol. Biotechnol. 20:1711–1716 [PubMed] [Google Scholar]
- 28. Utt EA, Eddy CK, Keshav KF, Ingram LO. 1991. Sequencing and expression of the Butyrivibrio fibrisolvens xylB gene encoding a novel bifunctional protein with β-d-xylosidase and α-l-arabinofuranosidase activities. Appl. Environ. Microbiol. 57:1227–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beldman G, Schols HA, Pitson SM, Searl-van Leeuwen MJF, Voragen AG. 1997. Arabinans and arabinan degrading enzymes. Adv. Macromol. Carbohydr. Res. 1:1–64 [Google Scholar]
- 30. Lagaert S, Pollet A, Delcour JA, Lavigne R, Courtin CM, Volckaert G. 2010. Substrate specificity of three recombinant α-l-arabinofuranosidases from Bifidobacterium adolescentis and their divergent action on arabinoxylan and arabinoxylan oligosaccharides. Biochem. Biophys. Res. Commun. 402:644–650 [DOI] [PubMed] [Google Scholar]
- 31. Ichinose H, Yoshida M, Fujimoto Z, Kaneko S. 2008. Characterization of a modular enzyme of exo-1,5-α-l-arabinofuranosidase and arabinan binding module from Streptomyces avermitilis NBRC14893. Appl. Microbiol. Biotechnol. 80:399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou J, Bao L, Chang L, Zhou Y, Lu H. 2012. Biochemical and kinetic characterization of GH43 β-d-xylosidase/α-l-arabinofuranosidase and GH30 α-l-arabinofuranosidase/β-d-xylosidase from rumen metagenome. J. Ind. Microbiol. Biotechnol. 39:143–152 [DOI] [PubMed] [Google Scholar]
- 33. Rahman AKMS, Sugitani N, Hatsu M, Takamizawa K. 2003. A role of xylanase, α-l-arabinofuranosidase, and xylosidase in xylan degradation. Can. J. Microbiol. 49:58–64 [DOI] [PubMed] [Google Scholar]
- 34. Wagschal K, Heng C, Lee CC, Robertson GH, Orts WJ, Wong DW. 2009. Purification and characterization of a glycoside hydrolase family 43 β-xylosidase from Geobacillus thermoleovorans IT-08. Appl. Biochem. Biotechnol. 155:304–313 [DOI] [PubMed] [Google Scholar]
- 35. Kormelink FJM, Voragen AGJ. 1993. Degradation of different [(glucurono)arabino] xylans by a combination of purified xylan-degrading enzymes. Appl. Microbiol. Biotechnol. 38:688–695 [Google Scholar]