Abstract Abstract
A new species of Nicon Kinberg, 1866 from the east Pacific coast of Ecuador is described. The new species is characterized by a long, thin dorsal ligule on median and posterior parapodia and infracicular sesquigomph falcigers in the neuropodia. A key to all species of Nicon is provided.
Keywords: Annelida, polychaetes, Nereididae, intertidal, Ecuador, taxonomy, systematics
Introduction
Ecuador possesses a great variety of coastal environments resulting in a high diversity of marine species; however, taxonomic studies on marine invertebrates are few, especially in the case of the polychaetes. In Ecuador (excluding the Galapagos), only 29 families, 53 genera and 75 species of polychaetes have been recorded. Hartman (1939) was the first to report on the polychaetes from Ecuador and described four new species and ten new records from Puna and Santa Clara Islands (Guayas Province). Later, Cruz et al. (1980) provided four new records from benthic samples collected on the Estero Salado, Guayaquil Gulf. In the same Gulf, 29 species of polychaetes were identified by Villamar (1983). Villamar (1989) later reported marine species at Canal del Morro and Jambeli in the Guayaquil Gulf. Villamar and Cruz (2007) reported three taxa for Ecuador from the intertidal zone of Monteverde (Guayas Province). A new species of Australonuphis, used as fishing bait, was described by de León-González et al. (2008) in Santa Elena Bay (Guayas Province). In northern Ecuador very little is known about the polychaete fauna and only one ecological study has been carried out by Villamar (2006) in the intertidal zones of Manabi and Esmeraldas Provinces. In that paper he reported 27 species, of which 14 constituted new records for Ecuador. More recently, Trovant et al. (2012) reported 12 new species records in the Bunche and Cabo San Francisco intertidal sandy beaches of northern Ecuador (Esmeraldas Province).
The importance of the family Nereididae is manifested by their high diversity and abundance in all marine substrates, occurring in all oceans from the supralittoral to the abyssal zone. This family includes 44 genera and approximately 460 valid species (de León-González, 2009). Nicon is one of the least species rich genera of Nereididae. The genus was first described by Kinberg, (1866) for six species, Nicon pictus, Nicon tahitianus, Nicon maculata, Nicon eugeniae, Nicon loxechini and Nicon virgini, none of which were figured. In this paper, a new species of Nicon is described. It is characterized by having an elongate notopodial dorsal ligule, resembling a long cirrus on median and posterior parapodia, as well by the presence of sesquigomph falcigers in the neuropodia.
Material and methods
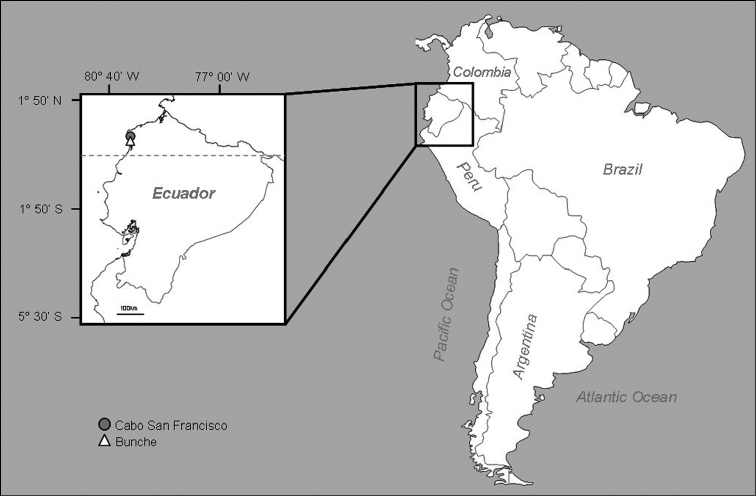
Samples were collected in March 2009 (dry season) in the intertidal zone of two sandy beaches located in the Esmeraldas Province, northern Ecuador (Fig. 1). Bunche beach (0°37'55"N, 80°02'14"W) is a protected area characterized as a low energy beach, with soft sloping banks and very fine particle sand, and Cabo San Francisco beach (0°39'11"N, 80°04'10"W) is characterized as a high energy environment, subjected to frequent and severe storms, with high slopes. Fresh-water discharges affect both beaches. Sediment samples were sieved through a 1mm mesh. Specimens were fixed in 10% formalin and later preserved in 70% ethanol. Terminology of parapodial structures was taken from Bakken and Wilson (2005). Type material has been deposited in the Natural History Museum of Los Angeles County, Allan Hancock Foundation Polychaete Collection (LACM-AHF), and the Polychaetological Collection of the Universidad Autónoma de Nuevo León (UANL).
Figure 1.
Map of Ecuador indicating the sampling sites, Bunche and Cabo San Francisco Beaches.
Results
Systematics. Class Polychaeta Grube, 1850. Order Phyllodocida Örsted, 1843. Family Nereididae Lamarck, 1818
Nicon
Kinberg, 1866 emended
http://species-id.net/wiki/Nicon
Type species.
Nicon maculata Kinberg, 1866.
Diagnosis.
Prostomium pyriform to subpyriform, with two pairs of eyespots, paired frontal antennae and biarticulate palps. Four pairs of tentacular cirri with distinct cirrophores, smooth or articulated. Parapodia of first two chaetigers subbiramous, notopodium represented by a single ligule with dorsal cirri at its base. Subsequent notopodia with dorsal and ventral ligules with or without a small notopodial prechaetal lobe decreasing in far posterior parapodia. Neuropodia with superior and inferior prechaetal lobes, digitiform or conical postchaetal lobe present or absent along body, and a ventral ligule which can be reduced in posterior parapodia; ventral cirri short, tapered. All notochaetae homogomph spinigers; neurochaetae homogomph, heterogomph or sesquigomph falcigers, may be accompanied by homogomph and heterogomph spinigers, and simple chaetae. Pygidium with paired anal cirri. Pharynx with paired mandibles, without paragnaths or papillae.
Remarks.
This generic diagnosis was modified from Pettibone (1971), Wu and Sun (1979) and Hutchings and Reid (1990). Some important characteristics were not included by Pettibone (1971) because at that time she recognized Nicon maculata as the only member of the genus. Later on, Wu and Sun (1979) and Hutchings and Reid (1990) expanded the genus diagnosis including characters of recently described species such as Nicon japonicus Imajima, 1972, Nicon yaguinae Fauchald, 1972, Nicon sinica Wu & Sun, 1979 and Nicon rotunda Hutchings & Reid, 1990. Some new characters included in the present diagnosis are the presence-absence of a notopodial prechaetal lobe, and the occurrence of neuropodial sesquigomph falcigers.
Nicon orensanzi sp. n.
urn:lsid:zoobank.org:act:149CDBF9-ACD0-4D7E-8407-BD8ADF3955C6
http://species-id.net/wiki/Nicon_orensanzi
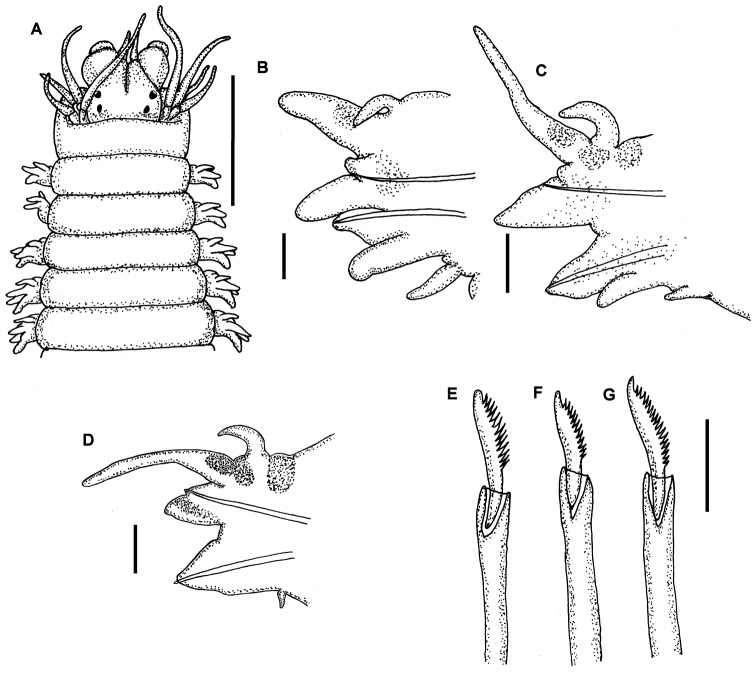
Figure 2.
Nicon orensanzi sp. n. Holotype. A Anterior end, dorsal view B Parapodium 10, anterior view C Parapodium 25, anterior view D Parapodium 60, anterior view E–G. Infracicular sesquigomph falcigers of parapodia 10, 25 and 50 respectively. Scale bars: A= 1 mm; B–D= 100 µ; E–G= 30µ.
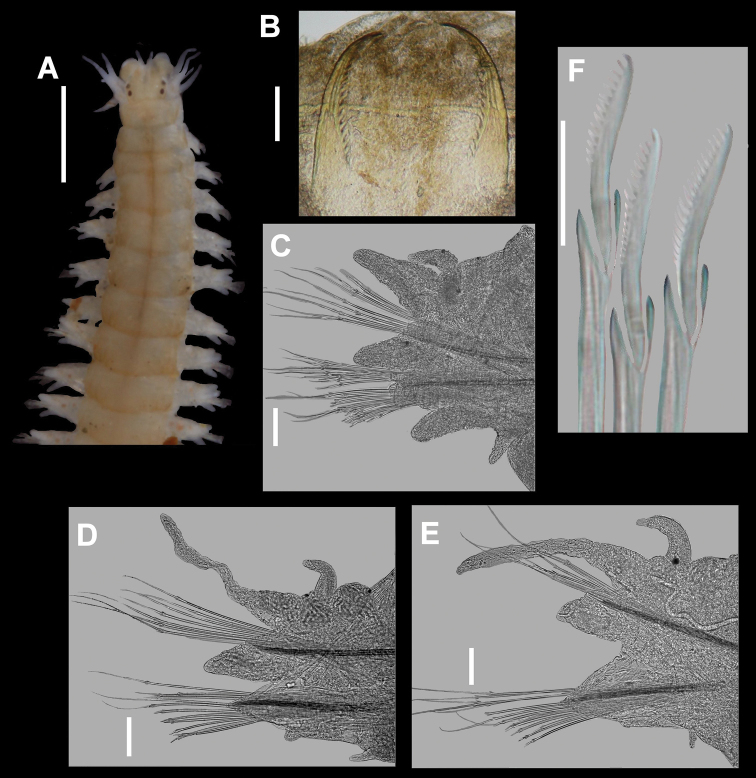
Figure 3.
Nicon orensanzi sp. n. Paratype (UANL 7840). A Anterior end, dorsal view B Mandibles; Holotype (LACM) C Parapodium 9, anterior view D Parapodium 29, anterior view E Parapodium 62, anterior view F Infracicular sesquigomph falcigers of parapodium 62. Scale bars: A= 1 mm; B= 0.1 mm; C–E= 100 µ; F= 30µ.
Material examined.
Holotype (LACM-AHF 4999), Paratype (LACM-AHF 5000) and Paratype (UANL 7840) collected at Bunche beach (0°39'01.98"N, 80°03'55.01"W), Esmeraldas Province, Ecuador, March 21 2009, coll. Berenice Trovant and Santiago Tineo. Additional material: seven anterior fragments, same data as holotype; two complete specimens and three anterior fragments, Cabo San Francisco beach (0°38'16.35"N, 80°3'14.07"W), Esmeraldas Province, Ecuador, March 20 2009, coll. Berenice Trovant and Santiago Tineo.
Description.
Holotype incomplete posteriorly, with 85 chaetigers, 19mm long, 1.4mm wide. Prostomium pyriform, with frontal cleft extending to middle of prostomium. Two pairs of eyespots in trapezoidal arrangement, anterior pair slightly larger, with lenses. Pair of small cirriform antennae extending slightly beyond palps. Palps biarticulate, globose, with subspherical palpostyles. Peristomium longer than next segment, with four pairs of short tentacular cirri, longest reaching chaetiger two (Figs 2A, 3A). Pharynx lacking papillae or paragnaths, armed with pair of toothed mandibles (Fig. 3B). Anterior notopodia with short cirriform dorsal cirri, subtriangular dorsal ligule, and subulate notopodial ventral ligule. Small triangular prechaetal lobe, restricted to limited number of anterior chaetigers, reducing in size posteriorly, last present about chaetigers 28-30. Anterior neuropodia with superior and inferior lobe, subulate ventral ligule, ventral cirrus with inflated base (Figs 2B, 3C), postchaetal neuropodial lobe subulate, present in first 18 chaetigers, not visible in anterior view. Median and posterior notopodia with dorsal ligule long cirrus-like; prechaetal lobe absent, notopodial ventral ligule triangular, decreasing in size in posterior chaetigers. Median and posterior neuropodia with superior and inferior lobes poorly defined, neuropodial postchaetal lobe absent, neuropodial ventral ligule subulate, decreasing in size in posterior chaetigers until disappearing completely, ventral cirri cirriform, shorter than dorsal one (Figs 2C–D, 3D–E). All notochaetae homogomph spinigers, with long, thin blades. Anterior supracicular neurochaetae 6 long-bladed homogomph spinigers superiorly; 6 short-bladed heterogomph spinigers inferiorly. Anterior infracicular chaetae homogomph spinigers with long blade, and sesquigomph falcigers with anterior part ending in a blunt tooth (Fig. 2E). Median and posterior supracicular neurochaetae with long-bladed homogomph spinigers. Infracicular neurochaetae with a few homogomph spinigers superiorly, and sesquigomph falcigers inferiorly, anterior end sharper (Figs 2F–G, 3F). Pygidium lacking in holotype, with terminal anus and two thin lateral cirri on others specimens.
Type locality.
Bunche beach, Esmeraldas Province, Ecuador
Distribution.
This species is only known from Bunche and Cabo San Francisco beaches, Esmeraldas Province, Ecuador.
Discussion.
Of the six species originally included in the genus Nicon by Kinberg (1866) two have been transferred to other genera (Nicon eugeniae, currently Nereis eugeniae from Strait of Magellan and Nicon loxochini, currently Platynereis magalhensis from Strait of Magellan) and three species are considered indeterminable due to their incomplete descriptions and the poor condition of the available syntypes (Nicon maculata from La Plata, Argentina, Nicon pictus from Brazil, Nicon tahitianus from Tahiti, and Nicon virgini from Strait of Magellan) (Pettibone, 1971). Of these species, only Nicon maculata is considered valid at the present time. No type species was designated by Kinberg. Hartman (1949) designated Nicon pictus as the type species, even though she did not provide a diagnosis or figures. Pettibone (1971) later revised the genus and designated Nicon maculata as the type species. Currently this genus consists of ten species: Nicon maculata Kinberg, 1866 from La Plata, Argentina, Nicon moniloceras (Hartman, 1940) from Catalina Island, USA, Nicon aestuarensis Knox, 1951 from New Zealand, Nicon polaris Hartman, 1967 from the Antarctic peninsula, Nicon abyssalis Hartman, 1967 from the Antarctic peninsula, Nicon japonicus Imajima, 1972 from Japan, Nicon yaquinae Fauchald, 1977 from off the Oregon coast, USA, Nicon sinica Wu & Sun, 1979 from the Yellow Sea, Nicon rotunda Hutchings & Reid, 1990 from Australia, and Nicon pettibonae de León-González & Salazar-Vallejo, 2003 from the Loyalty Islands, New Caledonia. Pettibone (1971) also considered that Nicon abyssalis and Nicon polaris had doubtful generic affinities with Nicon; however, we believe that Nicon abyssalis possesses the generic characters of Nicon and therefore should be included in the genus. Nicon polaris was described based on an epitoke; however, the possession of an expanded elytra-shaped dorsal cirrus in the chaetiger 7 makes it doubtful that it belongs to Nicon; a similar structure is found in Kainonereis, currently a genus in inquirenda described from an epitokous stage by Chamberlin (1919).
Species of Nicon may be separated into two groups based on the presence or absence of notopodial prechaetal lobes. Those species with a notopodial prechaetal lobe are: Nicon aestuarensis, Nicon japonicus, Nicon polaris, Nicon rotunda, and Nicon sinica; while Nicon abyssalis, Nicon maculata, Nicon moniloceras, Nicon pettibonae and Nicon yaquinae lack a superior notopodial lobe. Some important characteristics of Nicon species are listed in Table 1.
Table 1.
Diagnostic features of the species of Nicon (modified from Hutchings and Reid 1990). Abbreviations: TC= chaetiger number reached by longest tentacular cirri, ho sp= homogomph spinigers, he sp= heterogomph spinigers, ho f= homogomph falcigers, he f= heterogomph falciger, sf= sesquigomph falciger, DL= dorsal ligule, PL= Prechaetal lobe, ST= Subtriangular, SU= Subulate; DI= Digitate, CI= Cirriform, CO= Conical, E= Elongated.
| Species | Neuropodial chaetae | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Supracicular | Infracicular | Notopodia | |||||||||||
| TC | ho sp | he sp | ho f | he f | sf | ho sp | he sp | ho f | he f | sf | DL | PL | |
| Nicon abyssalis | 2 | X | X | - | - | - | X | X | - | X | - | CI | - |
| Nicon aestuarensis | 5 | X | X | - | X | - | - | X | - | X | - | ST | X |
| Nicon japonicus | 2 | X | - | - | X | - | - | X | - | X | - | ST | X |
| Nicon maculata | 10 | X | - | - | X | - | X | - | - | X | - | SU | - |
| Nicon moniloceras | 9 | X | - | - | X | - | - | X | - | X | - | DI | - |
| Nicon pettibonae | 5 | X | - | - | X | X | X | - | - | X | X | ST | - |
| Nicon polaris | 5 | X | X | - | X | - | - | X | - | X | - | ST | X |
| Nicon rotunda | 2 | X | - | - | X | - | X | - | X | X | - | ST | X |
| Nicon sinica | 9 | X | - | - | X | - | - | X | - | X | - | CO | X |
| Nicon yaguinae | 2 | X | X | - | X | - | ? | ? | ? | ? | - | ST | - |
| Nicon orensanzi sp. n. | 2 | X | X | - | - | - | X | - | - | - | X | E | X |
Nicon orensanzi sp. n. is a member of the first group but differs in its long, thin notopodial dorsal ligule in median and posterior parapodia. Nicon orensanzi sp. n. and Nicon pettibonae are the only species in the genus with neuropodial infracicular sesquigomph falcigers in all parapodia. These two species differ in the shape of their sesquigomph falcigers, the presence of heterogomph falcigers, and a reduced dorsal ligule in the posterior parapodia of Nicon pettibonae.
Etymology. The new species is dedicated to Dr. José María (Lobo) Orensanz, who has made significant contributions to the taxonomy of polychaetes and has been a mentor to the authors of this paper.
Key to Nicon species
| 1 | Superior notopodial lobe present | 2 |
| – | Superior notopodial lobe absent | 7 |
| 2 | Tentacular cirri short, reaching chaetiger 2 | 3 |
| – | Tentacular cirri reaching chaetiger 5 | 5 |
| 3 | Heterogomph falcigers present on supra- and subacicular fascicle, dorsal ligule subtriangular | 4 |
| – | Heterogomph falcigers absent, with sesquigomph falcigers in infracicular position, dorsal ligule long and thin on median and posterior parapodia | Nicon orensanzi sp. n. |
| 4 | With homogomph falcigers in neuropodial subacicular position | Nicon rotunda |
| – | Homogomph falcigers lacking | Nicon japonica |
| 5 | Tentacular cirri reaching chaetiger 5, dorsal ligule subtriangular | 6 |
| – | Tentacular cirri reaching chaetiger 9, dorsal cirri conical | Nicon sinica |
| 6 | Mandibles with 6 oblique teeth, blade of falcigers short, with a terminal tooth directed downward | Nicon polaris |
| – | Mandibles with up to 10 teeth; blade of falcigers longer, with blunt terminal end | Nicon aestuarensis |
| 7 | Tentacular cirri short, reaching chaetiger 2 | 8 |
| – | Tentacular cirri reaching chaetiger 5 | 9 |
| 8 | Dorsal ligule cirriform , reduced in posterior chaetigers; falcigers with prolonged blade | Nicon abysssalis |
| – | Dorsal ligule subtriangular, similar in size throughout; falcigers with long, anteriorly blunt blade distinctly serrated along inner margin | Nicon yaquinae |
| 9 | Tentacular cirri reaching chaetiger 5; subtriangular dorsal ligule; supra and infracicular sesquigomph falcigers present | Nicon pettibonae |
| – | Tentacular cirri to chaetiger 9–10 | 10 |
| 10 | Longest pair of tentacular cirri partially annulated on distal end; falcigers with long blade, denticulate along inner margin | Nicon maculata |
| – | All tentacular cirri annulated, with cylindrical articles; falcigers with short blades, denticles on proximal inner margin | Nicon moniloceras |
Supplementary Material
Acknowledgements
This publication is part of a work carried out by Berenice Trovant as a requirement for obtaining a MSc degree in ‘Biodiversity in tropical areas and its conservation’ at the Universidad Internacional Menéndez Pelayo (UIMP, Spain), a Masters program funded by the Spanish National Research Council (CSIC, Spain) and carried out at the Universidad Central del Ecuador. We thank to Santiago Tineo for their help in fieldwork. Authors would also like to thank two anonymous reviewers and Chris Glasby whose comments and suggestions were much appreciated and very helpful in improving the manuscript.
References
- Bakken T, Wilson RS. (2005) Phylogeny of nereidids (Polychaeta, Nereididae) with paragnaths. Zoologica Scripta, 34: 507-547. doi: 10.1111/j.1463-6409.2005.00200.x [DOI] [Google Scholar]
- Chamberlin RV. (1919). The Annelida Polychaeta of the Albatross Tropical Pacific Expedition, 1891–1905. Mem. Mus. Comp. Zool. , Harvard University, 48: 1-514. [Google Scholar]
- Cruz M, de González M, Gualancañay E, Villamar F. (1980) Lista de la Fauna Sublitoral Bentónica del Estero Salado Inferior, Ecuador. Acta Oceanográfica del Pacífico 1 (1): 82-96. [Google Scholar]
- de León-González JA. (2009) Nereididae Lamarck, 1818. In: de León-González JA, Bastida-Zavala JR, Carrera-Parra LF, García-Garza ME, Peña-Rivera A, Salazar-Vallejo SI and Solís-Weiss V. (Eds). Poliquetos (Annelida: Polychaeta) de México y América Tropical. Universidad Autónoma de Nuevo León, Monterrey, México, 30: 325–354.
- de León-González JA, Cornejo-Rodriguez MH, Degraer S. (2008) A new species of Australonuphis (Polychaeta: Onuphidae) from the eastern Pacific. Journal of the Marine Biological Association of the United Kingdom 88: 739-742. doi: 10.1017/S0025315408001252 [DOI] [Google Scholar]
- Hartman O. (1939) Polychaetous Annelids. Part I. Aphroditidae to Pisionidae. Allan Hancock Pacific Expedition 7(1-2)): 1-170. [Google Scholar]
- Hartman O. (1949) The marine annelid erected by Kinberg with notes on some other types in the Swedish State Museum. Arkiv för Zoologi K. Svenska Vetensk, 42A(1): 1–137.
- Hutchings P, Reid A. (1990) The Nereididae (Polychaeta) from Australia. Gymnonereidinae sensu Fitzhugh, 1987: Australonereis, Ceratocephale, Dendronereidides, Gymnonereis, Nicon, Olganereis and Websterinereis. Records of the Australian Museum 42: 69-100. doi: 10.3853/j.0067-1975.42.1990.107 [DOI] [Google Scholar]
- Kinberg JGK. (1866) Anulata nova. K Svanska Vetenskapsakademien, Stockholm. Ofversigt af fürhandlingar 22: 167-179. [Google Scholar]
- Pettibone MH. (1971) Revision of Some Species Referred to Leptonereis, Nicon, and Laeonereis (Polychaeta: Nereididae). Smithsonian Contributions to Zoology, 104: 1-53. doi: 10.5479/si.00810282.104 [DOI] [Google Scholar]
- Trovant B, Elias R, Diez ME, JA de León-González. (2012) New records of polychaetes (Annelida) for northern Ecuador. Marine Biodiversity Records, 5 (e32): 1–8. doi: 10.1017/S1755267211001059 [DOI]
- Villamar F. (1983) Poliquetos del Golfo de Guayaquil. Acta Oceanográfica del Pacífico INOCAR 2 (2): 659-733. [Google Scholar]
- Villamar F. (1989) Estudio de los Poliquetos Bentónicos en el Golfo de Guayaquil, Exterior (Canal del Morro y Jambelí). Acta Oceanográfica del Pacífico INOCAR 5 (1): 34-40. [Google Scholar]
- Villamar F. (2006) Estudio Taxonómico y Distribución de los Poliquetos Bentónicos en la zona del intermareal de las Provincias de Esmeraldas y Manabí (Ecuador). Acta Oceanográfica del Pacífico INOCAR 13 (1): 169–197. [Google Scholar]
- Villamar F, M Cruz. (2007) Poliquetos y Moluscos macrobentónicos de la zona intermareal y submareal en la provincia de Guayas (Monteverde, Ecuador). Acta Oceanográfica del Pacífico INOCAR 14 (1): 147-153. [Google Scholar]
- Wu B, Sun R. (1979) Revision of the genera Nicon and Rullierinereis with descriptions of a new genus Sinonereis (Polychaeta: Nereidae). Oceanic Selections (2): 95–112.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.