Abstract
Egg protein ovotransferrin derived peptides (IRW and IQW) can attenuate tumor necrosis factor (TNF) induced inflammatory responses and oxidative stress in endothelial cells. The present study investigates the structural requirements and molecular mechanisms underlying these events. Whereas IRW significantly inhibited TNF-induced up-regulation of intercellular cell adhesion molecule-I (ICAM-1) and vascular cell adhesion molecule-I (VCAM-1), IQW could inhibit only the up-regulation of ICAM-1. The anti-inflammatory effects of these peptides appeared to be mediated by the nuclear factor-κB (NF-κB) pathway, which was differentially regulated by IRW and IQW. Both IRW and IQW exhibited antioxidant effects as shown by reduction of TNF-induced superoxide generation. The structural integrity of these peptides was essential for their activities, because dipeptides or the combination of constituent amino acids did not exhibit the same effect. This study demonstrated the significance of the structural integrity of these two tripeptides in attenuating endothelial inflammation and oxidative stress, indicating their potential as nutraceuticals.
Keywords: bioactive peptides, egg protein, ovotransferrin, endothelial cells, inflammation, superoxide
Introduction
Endothelial cells are the major regulator of vascular tone. Endothelial dysfunction manifests as one of the common features of cardiovascular diseases (CVDs), the leading cause of morbidity and mortality worldwide.1−3 Vascular inflammation and oxidative stress are two key factors that lead to endothelial dysfunction.
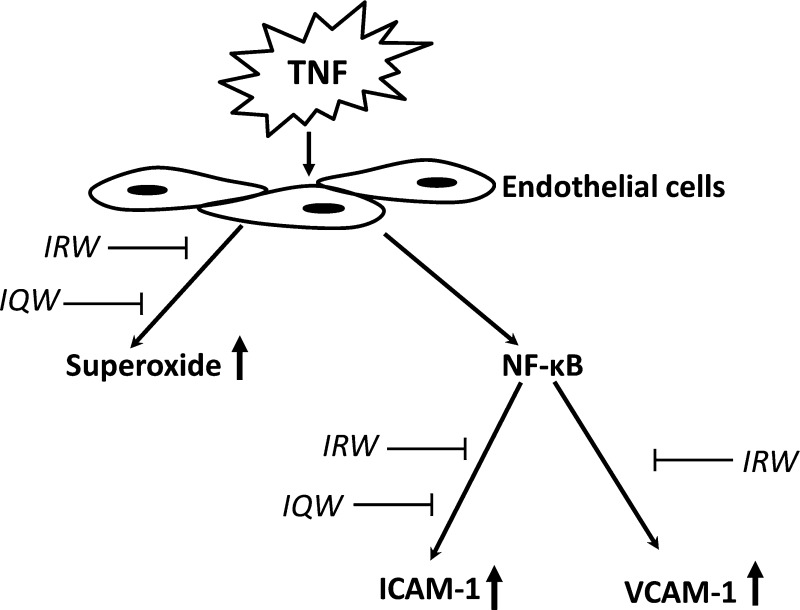
Tumor necrosis factor (TNF), a pro-inflammatory cytokine, participates in the inflammatory response and plays an important role in the development of atherosclerotic lesions.4,5 TNF-activated endothelial cells up-regulate the expression of adhesion molecules intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), which is important in the onset and progression of vascular inflammation.6 TNF-induced up-regulation of adhesion molecules is mediated through the transcription factor nuclear factor-κB (NF-κB) pathway.4,5 TNF also increases the production of superoxide (O2–) through activation of NADPH oxidase.7 Increased O2– production is responsible for impaired bioavailability of nitric oxide (NO) and endothelial vasodilator dysfunction, which may lead to hypertension.8 Subsequently, O•2– can also increase cytoplasmic levels of H2O2 that can activate NF-κB,9 resulting in a pro-inflammatory shift in the endothelial gene expression, endothelial activation, and increased leukocyte recruitment to the endothelium that accelerates the development of atherosclerotic lesions.9−11 Therefore, the targeting of TNF-induced inflammation and oxidative stress provides a strategy for controlling vascular diseases such as atherosclerosis and hypertension.
Due to the unavoidable side effects of synthetic drugs, there is an increasing interest in the search for novel bioactive food components, such as angiotensin converting enzyme (ACE) inhibitory peptides, for the prevention and treatment of CVDs.12 Many food-derived compounds, such as bioactive peptides, are known to possess a wide range of bioactivities including antimicrobial, anticarcinogenic, anti-inflammatory, antioxidant, and antihypertensive effects.13,14 IRW, characterized through an integrated quantitative structure and activity relationship (QSAR) and bioinformatics approach from egg white protein ovotransferrin,15 was found to exhibit an anti-inflammatory effect through the NF-κB pathway by blocking the nuclear translocation of p65.16 IQW, another potent ACE inhibitory peptide differing in only one amino acid residue from IRW, was also derived from ovotransferrin. However, the antioxidant and anti-inflammatory effects and the underlying mechanism of IQW have not been studied. In addition, the structural requirements of these peptides for the anti-inflammatory and antioxidant properties are not known. Therefore, the objectives of the present study were to investigate the structure and activity relationships of IRW and IQW and to examine the underlying molecular mechanisms of their antioxidant and anti-inflammatory activities.
Materials and Methods
Reagents and Antibodies
Dulbecco’s phosphate-buffered saline (PBS), M199 medium with phenol red, porcine gelatin, dithiothreitol (DTT), catalase, and polyethylene glycol-conjugated superoxide dismutase (PEG-SOD) were bought from Sigma Chemical Co. (St. Louis, MO, USA). Oligofectamine, Optimem1, M199 medium without phenol red, and fetal bovine serum (FBS) were obtained from Gibco/Invitrogen (Carlsbad, CA, USA). Type 1 collagenase was purchased from Worthington Biochemical Corp. (Lakewood, NJ, USA). Triton X-100 and endothelial cell growth supplement (ECGS) were obtained from VWR International (West Chester, PA, USA). Both IRW and IQW were synthesized and supplied by GenScript Corp. (Piscataway, NJ, USA), and their purity (>95%) was verified by HPLC-MS/MS. All other chemicals and reagents were of the analytical grade.
Endothelial Cell Culture
Human umbilical vein endothelial cells (HUVECs) were isolated from human umbilical cords obtained from the Royal Alexandra Hospital (Edmonton, AB, Canada).17−21 HUVECs are a widely used model for studying the vascular endothelium.20 The protocol was approved by the University of Alberta Ethics Committee, and the investigation also conformed to the principles outlined in the Declaration of Helsinki and also Title 45, U.S. Code of Federal Regulations, Part 46, Protection of Human Subjects, revised November 13, 2001, effective December 13, 2001. All subjects provided informed consent before inclusion in this study. Following collection of umbilical cords, the umbilical vein was first flushed with PBS to remove blood clots, and then HUVECs were isolated using a type 1 collagenase containing buffer. The cells were grown in a humidified atmosphere at 37 °C with 5% CO2/95% air in M199 medium with phenol red supplemented by 20% FBS as well as l-glutamine (Gibco/Invitrogen), penicillin–streptomycin (Life Technologies, Carlsbad, CA, USA), and 1% endothelial cell growth supplement (ECGS, from VWR International). We have previously confirmed the endothelial nature of these cells by staining for the endothelium-specific marker, von Willebrand’s factor (vWF).22
Experimental Protocols and Treatments
Second-passage confluent HUVEC monolayers were used in this study. Cells grown in 48-well plates (80–100 K cells/well) were treated with bioactive tripeptides (IRW, IQW), their respective dipeptides (IR, RW, IQ, and QW) or amino acids (I, R, Q, and W, individually or in combination) for 20 h. Cells were then treated with TNF (5 ng/mL) for different time periods for different experimental paradigms as described in the subsequent sections.
Adhesion Molecule Expression
Expression of the adhesion molecules (ICAM-1 and VCAM-1) was determined through Western blot technique. After pretreatment with peptides/amino acid, cells were stimulated for 4 h with TNF. The cells were lysed using boiling hot Laemmli’s buffer containing 0.2% Triton X-100 and DTT as a reducing agent. Samples were then run in a 9% SDS-PAGE, and the protein bands of interest were detected by specific antibodies. Bands for VCAM-1 (rabbit polyclonal antibody from Santa Cruz Biotechnologies, Santa Cruz, CA, USA) and ICAM-1 (mouse monoclonal antibody from Santa Cruz Biotechnologies) were normalized to α-tubulin (rabbit polyclonal antibody from Abcam, Cambridge, MA, USA). Anti-tubulin was used at 0.4 μg/mL, whereas all others were used at 1 μg/mL. Goat anti-rabbit and donkey anti-mouse fluorochrome-conjugated secondary antibodies were purchased from Licor (Licor Biosciences, Lincoln, NE, USA). The protein bands were detected by a Licor Odyssey Bio-Imager and analyzed by densitometry using corresponding software (Licor Biosciences). Samples generated from one particular umbilical cord were run on the same gel. Cell lysates from untreated cells were loaded on every gel, and all data were expressed as fold change over the corresponding untreated control.
Superoxide Detection
Endothelial superoxide generation was measured by staining with dihydroethidium (DHE) similarly to our previous work.18 Cells were pretreated with peptides/amino acids and then followed by a 1 h TNF simulation. DHE is cell permeable and reacts with superoxide to yield ethidium, which binds to nuclear DNA and generates nuclear fluorescence.23 Following stimulation with TNF, with or without pretreatment with the peptide, HUVEC monolayers were washed once and incubated for 30 min at room temperature with 10 μmol/L DHE in Q-medium (phenol red free M199 with 1% FBS). After a 30 min incubation period, cells were washed once and fluorescence was visualized in an Olympus IX81 fluorescent microscope (Carson Scientific Imaging Group, Ontario, Canada) using Slidebook 2D, 3D Timelapse Imaging Software (Intelligent Imaging Innovations Inc., Denver, CO, USA). For each data point, images from three randomly chosen fields were taken. The total fluorescence intensity and the number of cells in each field were noted, and the mean fluorescence intensity per cell (MFI/cell) was determined as previously described.18 Superoxide generation was measured as fold increase in MFI/cell over the untreated control.
NF-κB Activity Detection
NF-κB activity was determined by nuclear translocation of p65 and p50, in the presence of the bioactive peptides [IRW and IQW (50 μmol/L)]. HUVEC monolayers were pretreated with bioactive peptides for 20 h, followed by a 30 min TNF simulation. Then the cells were fixed in 4% formalin, permeabilized with 0.1% Triton X-100, and immunostained using overnight incubation with antibodies against p50 (rabbit polyclonal antibody from Santa Cruz Biotechnologies) and p65 (mouse monoclonal antibody from Santa Cruz Biotechnologies). Cells were treated with fluorescent-labeled anti-rabbit and/or anti-mouse secondary antibodies (Molecular Probes, Eugene, OR, USA) for 30 min in the dark. Nuclei were stained with the Hoechst33342 nuclear dye from Molecular Probes. Cells were visualized under an Olympus IX81 fluorescent microscope (Carson Scientific Imaging Group) using Slidebook 2D, 3D Timelapse Imaging Software (Intelligent Imaging Innovations Inc.). All images have been presented in (×100) magnification.
Statistical Analysis
All data presented were the mean value ± SEM of four to eight independent experiments using HUVECs isolated from different umbilical cords for all experiments. Data were expressed as fold change over the untreated control. One-way analysis of variance (one-way ANOVA) with Tukey’s post hoc test was performed for multiple comparisons. A repeated-measure test was used whenever applicable. Differences were considered to be significant with a P value of <0.05.
Results
Effects of Peptides and Respective Amino Acids on ICAM-1 Expression
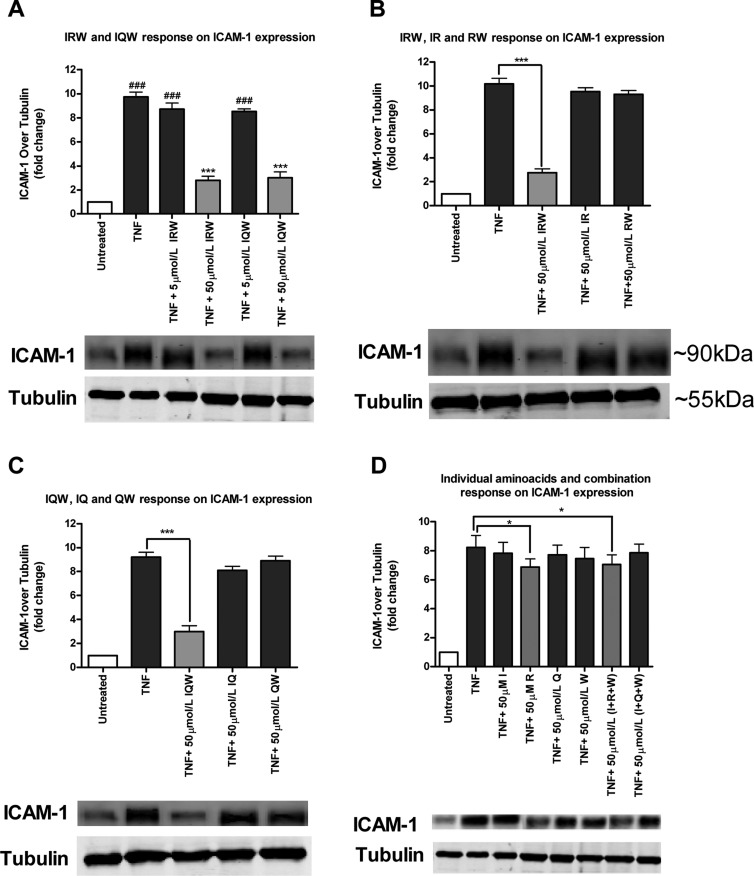
Time course study showed that pretreatment of either tripeptide for 20 h, but not for 4 and 8 h, could significantly suppress TNF-mediated ICAM-1 and VCAM expression (Supplementary Figure 1 in the Supporting Information); therefore, pretreatment of peptide for 20 h was determined. TNF stimulation increased ICAM-1 expression that was inhibited by both IRW and IQW (50 μmol/L) (Figure 1A). Interestingly, when cells were pretreated with respective 50 μmol/L dipeptides (IR and RW) of IRW, it was observed that both dipeptides had no effect on TNF-simulated ICAM-1 expression, but the parent tripeptide IRW (50 μmol/L) could significantly inhibit TNF-induced ICAM-1 expression (Figure 1B). Similarly, pretreatment of dipeptides (IQ and QW) at 50 μmol/L had no effect on TNF-simulated ICAM-1 expression, but the intact IQW (50 μmol/L) could significantly inhibit the TNF-simulated ICAM-1 expression (Figure 1C). When the HUVEC monolayers were pretreated with 50 μmol/L constituent amino acids individually or in combination, then amino acid arginine (R) and its combination of (I+R+W) showed a minor decrease, but the remaining did not exert any effect on TNF-induced increased expression of ICAM-1 (Figure 1D).
Figure 1.
Effects of bioactive peptides and their derivatives on TNF-induced ICAM-1 expression: (A) bioactive peptides (IRW and IQW); (B) respective dipeptides of IRW (IR and RW); (C) respective dipeptides of IQW (IQ and QW); (D) individual amino acids (I, R, Q, and W); peptide sequence combination of respective amino acids (I+R+W and I+Q+W). Confluent HUVEC monolayers were pretreated for 20 h with peptides and amino acids prior to 4 h of incubation with 5 ng/mL TNF. ICAM-1 protein levels are expressed as fold increase over the untreated control. Bars represent mean values (mean ± SEM, n = 8 independent experiments). Representative Western blots are shown below. (###) P < 0.001, (##) P < 0.01, and (#) P < 0.05, compared to untreated control; (∗∗∗) P < 0.001, (∗∗) P < 0.01, (∗) P < 0.05, compared to TNF alone. Bar graph having lighter color indicates that a particular treatment has a significant effect compared with TNF alone.
Effects of Peptides and Respective Amino Acids on VCAM-1 Expression
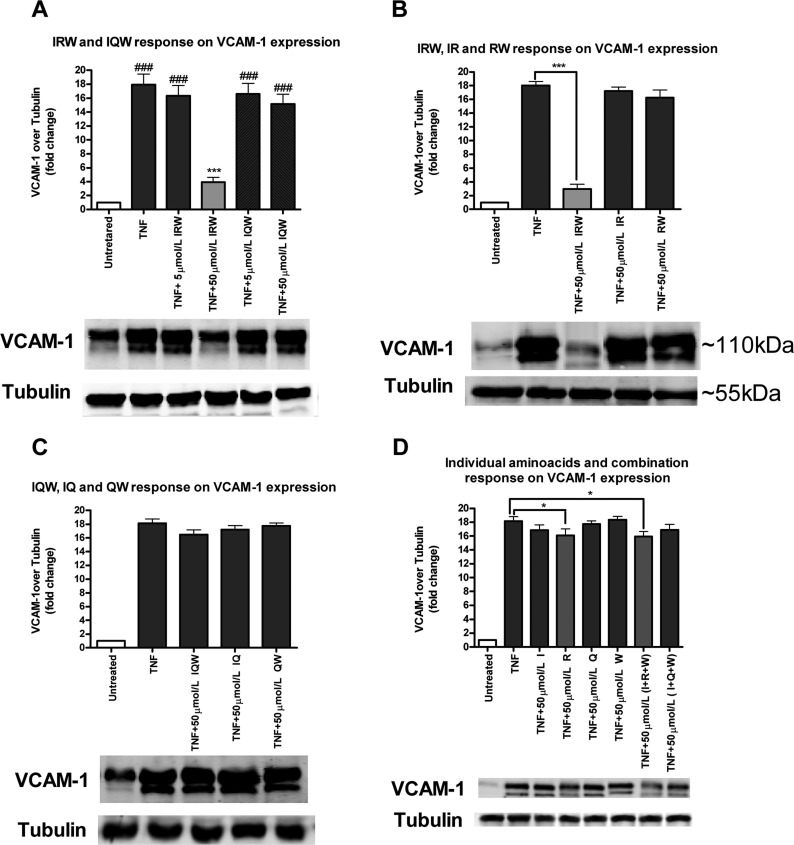
Similar to ICAM-1, TNF simulation significantly increased the expression of VCAM-1 in endothelial cells. Pretreatment with IRW (50 μmol/L) significantly reduced the TNF-simulated increased expression of VCAM-1, but surprisingly IQW did not exert any effect, in contrast to the findings with ICAM-1 (Figure 2A). In addition, pretreatment with 50 μmol/L dipeptides (IR, RW, IQ, and QW) and 50 μmol/L amino acids individually or in combination did not exert any effect on TNF-simulated VCAM-1 expression (Figure 2B,C). But similarly to ICAM-1, amino acid arginine (R) and its combination of (I+R+W) showed a minor decrease in TNF-induced increased expression of VCAM-1 (Figure 2D).
Figure 2.
Effects of bioactive peptides and their derivatives on TNF-induced VCAM-1 expression: (A) bioactive peptides (IRW and IQW); (B) respective dipeptides of IRW (IR and RW); (C) respective dipeptides of IQW (IQ and QW); (D) individual amino acids (I, R, Q, and W); peptide sequence combination of respective amino acids (I+R+W and I+Q+W). Confluent HUVEC monolayers were pretreated for 20 h with peptides and amino acids prior to 4 h of incubation with 5 ng/mL TNF. VCAM-1 protein levels are expressed as fold increase over the untreated control. Bars represent mean values (mean ± SEM, n = 8 independent experiments). Representative Western blots are shown below. (###) P < 0.001, (##) P < 0.01, and (#) P < 0.05, compared to untreated control; (∗∗∗) P < 0.001, (∗∗) P < 0.01, and (∗) P < 0.05, compared to TNF alone. Bar graph having lighter color indicates that a particular treatment has a significant effect compared with TNF alone.
Effects of Peptides and Respective Amino Acids on TNF-Induced Superoxide Generation
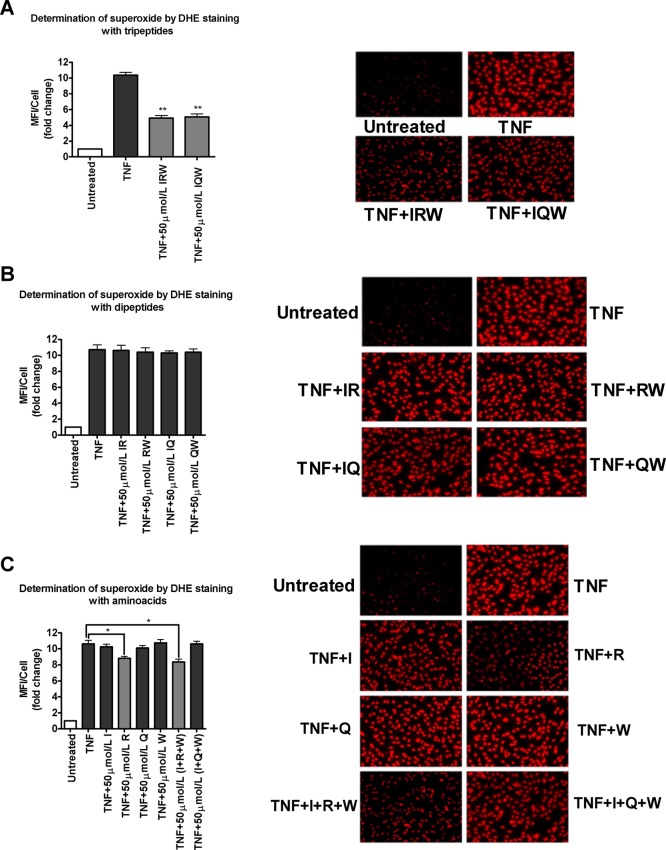
Inflammatory responses are often associated with increased levels of oxidative stress and vice versa. Therefore, the effects of ovotransferrin-derived bioactive peptides (IRW and IQW), their respective dipeptides (IR, RW, IQ, and QW), and amino acids (I, R, Q, and W) on TNF-stimulated superoxide production were studied. Our results showed that both IRW and IQW at 50 μmol/L significantly reduced TNF-simulated superoxide generation (Figure 3A). However, the pretreatment with 50 μmol/L dipeptides (IR, RW, IQ, and QW) did not exhibit a significant effect on TNF-induced superoxide production (Figure 3B). On the other hand, respective amino acids (I, R, Q, and W) treated individually or in combination also did not exert any effect on TNF-induced superoxide generation except that arginine (R) and its combination (I+R+W) exerted a minor effect, which indicates that the presence of free arginine could exhibit a minor reduction in TNF-induced superoxide production (Figure 3C).
Figure 3.
Effect of bioactive peptides and their derivatives on TNF-induced endothelial superoxide generation: (A) bioactive peptides (IRW and IQW); (B) respective dipeptides of IRW (IR and RW) and IQW (IQ and QW); (C) individual amino acids (I, R, Q, and W); peptide sequence combination of constituent amino acids (I+R+W and I+Q+W). Confluent HUVEC monolayers were pretreated for 20 h with 50 mol/L peptide and amino acids prior to 1 h of incubation with 5 ng/mL TNF. A representative set of images are shown. Data were calculated as MFI/cell and expressed as fold increase over the untreated control. Bars represent mean values (mean ± SEM, n = 8 independent experiments). (###) P < 0.001 and (#) P < 0.05, compared to untreated control; (∗∗) P < 0.01 and (∗) P < 0.05, compared to TNF alone. Bar graph having lighter color indicates that a particular treatment has a significant effect compared with TNF alone.
Effects of Arginine on ICAM-1 and VCAM-1 Expression
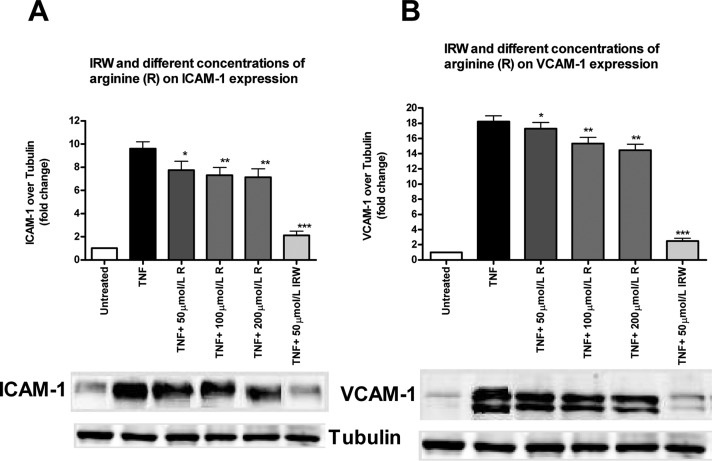
Because arginine alone showed a minor but significant anti-inflammatory effect (Figures 1D and 2D), we further examined the effect of increasing concentrations of arginine on TNF-induced inflammatory molecule expression. We found that none of the concentrations (50, 100, and 200 μmol/L) of arginine used could elicit the same extent of anti-inflammatory responses as that of IRW (50 μmol/L), suggesting the significance of the integrity of the tripeptide (IRW) for the full extent of the anti-inflammatory effect observed (Figure 4).
Figure 4.
Effect of various concentrations of arginine on TNF-induced ICAM-1 and VCAM-1 expression: (A) various concentrations (50, 100, and 200 mol/L) of arginine (R) and IRW (50 mol/L) on ICAM-1 expression; (B) various concentrations (50, 100, and 200 mol/L) of arginine (R) and IRW (50 mol/L) on VCAM-1 expression. ICAM-1 and VCAM-1 protein levels are expressed as fold increase over the untreated control. Bars represent mean values (mean ± SEM, n = 8 independent experiments). Representative Western blots are shown below. (∗∗∗) P < 0.001, (∗∗) P < 0.01, and (∗) P < 0.05, compared to TNF alone. Bar graph having lighter color indicates that a particular treatment has a significant effect compared with TNF alone.
Effects of Superoxide Dismutase on Adhesion Molecule Expression
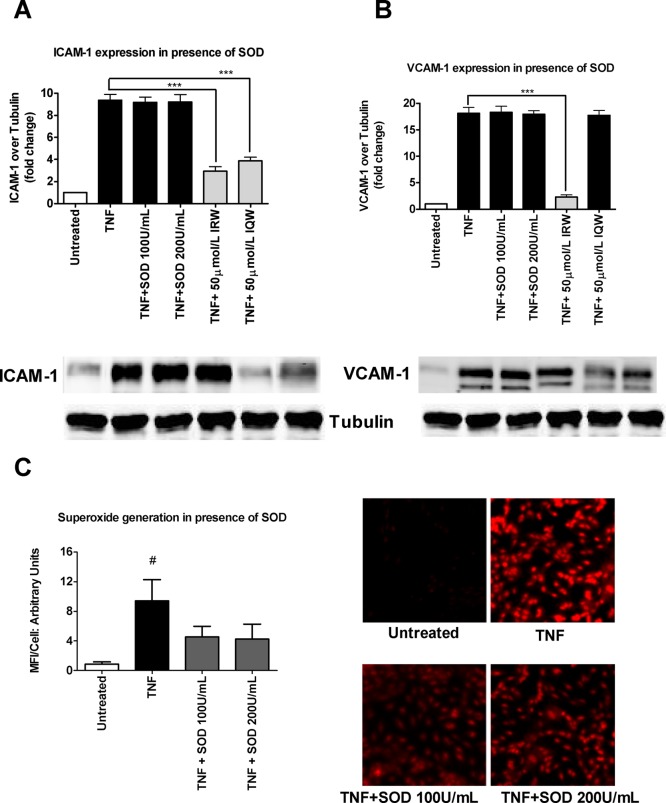
A previous study had shown that inhibition of superoxide can prevent adhesion molecule expression on human aortic endothelial cells.24 We used several different concentrations (100 and 200 U/mL) of cell permeable superoxide dismutase (SOD) to pretreat the HUVEC monolayers prior to TNF stimulation. Interestingly, SOD treatment had no effect on ICAM-1 and VCAM-1 expression (Figure 5A,B). However, the same concentrations of SOD significantly reduced TNF-stimulated superoxide (Figure 5C). SOD possibly generates H2O2 from superoxide, so a combination of catalase with SOD was used to assess the contribution of H2O2. Our results showed that a combination of these two enzymes had no effect on ICAM-1 and VCAM-1 (data not shown). These data suggest that the tripeptides (IRW and IQW) exerted their anti-inflammatory effects independent of their antioxidant properties.
Figure 5.
Effect of various concentrations of superoxide dismutase (SOD) on TNF-induced adhesion molecules expression and superoxide generation: (A) various concentrations of SOD (100 and 200 U/mL) and IRW and IQW (50 mol/L) on ICAM-1 expression; (B) various concentrations of SOD (100 and 200 U/mL) and IRW and IQW (50 mol/L) on VCAM-1 expression. Confluent HUVEC monolayers were pretreated with peptides for 20 h and then treated with SOD (100 and 200 U/ML) for 1 h prior to 4 h of incubation with 5 ng/mL TNF. ICAM-1 and VCAM-1 protein levels are expressed as fold increase over the untreated control. Bars represent mean values (mean ± SEM, n = 4 independent experiments). Representative Western blots are shown below. (∗∗∗) P < 0.001, (∗∗) P < 0.01, and (∗) P < 0.05, compared to TNF alone. (C) Effect of various concentrations of SOD (100 and 200 U/mL) on superoxide generation. Confluent HUVEC monolayers were pretreated for 1 h with SOD (100 and 200 U/ML) prior to 1 h of incubation with 5 ng/mL TNF. A representative set of images are shown. Bars represent mean values (mean ± SEM, n = 4 independent experiments). (#) P < 0.05, compared to untreated control. Bar graph having lighter color indicates that a particular treatment has a significant effect compared with TNF alone.
Effect of TNF Stimulation on NF-κB Translocation and ICAM-1 and VCAM-1 Expression
TNF is able to induce a range of cellular responses via modulation of a number of gene expressions through activation of various nuclear transcription factors, such as NF-κB and AP-1. Cells pretreated with NF-κB inhibitor (BAY 11-7085) almost abolished the TNF-simulated up-regulation of ICAM-1 and VCAM-1 (Figure 6A). Therefore, TNF-induced increased expression of ICAM-1 and VCAM-1 is primarily NF-κB dependent.
Figure 6.
Role of IRW and IQW on TNF induced NF-B activation: (A) confluent HUVEC monolayers were treated with BAY 11-7085 (NF-B inhibitor) for 15 min before 4 h with 5 ng/mL TNF. ICAM-1 and VCAM-1 protein levels are expressed as fold increase over the untreated control. Bars represent mean values (mean ± SEM, n = 4 independent experiments). Representative Western blots are shown below. (##) P < 0.01, compared to untreated control; (∗∗) P < 0.01, compared to TNF alone. (B, C) Confluent HUVEC monolayers were pretreated for 20 h with 50 mol/L IRW and IQW prior to 30 min of incubation with 5 ng/mL TNF. Cells were fixed, permeabilized, and immunostained for p65 (B) and p50 (C). Representative sets of images from six independent experiments are shown. Bar graph having lighter color indicates that a particular treatment has a significant effect compared with TNF alone.
On activation of the NF-κB pathway, the p65 and p50 homodimer and/or heterodimer proteins are released from the cytosol and then migrate into the cell nucleus, where they interact with the promoter regions of various proteins and up-regulate the expression of inflammatory adhesion molecules such as ICAM-1 and VCAM-1. Thus, nuclear translocation of p65 and p50 is widely used as an index of NF-κB activation. As expected, TNF stimulation caused rapid nuclear translocation of p65 and p50. Pretreatment with IRW, however, abolished the TNF-induced translocation of both p65 and p50 (Figure 6B,C). Surprisingly, pretreatment with IQW restricted only the translocation of p50 but did not affect p65 translocation (Figure 6B, C), possibly accounting for the differential effects observed with these two peptides.
Discussion
CVDs are the number one killer worldwide. In 2008, an estimated 17.3 million people died of CVDs, and this number is expected to reach 23.6 million by 2030.25 Occurrences of CVDs are often linked to diet, leading to an increased interest in using food bioactives as a strategy to reduce the risk of CVDs. Many food components exhibit beneficial effects toward cardiovascular health, such as fruits, vegetable, legumes, cereals, and tea.13 Peptides of food origin are also found to be beneficial against CVDs, such as peptides with blood pressure lowering (ACE inhibitory), cholesterol lowering, antithrombotic, and antioxidant activities. Moreover, some peptides are multifunctional.14 Such peptides can be released during fermentation or digestion of food proteins by proteolytic enzymes, thus exhibiting relevant biological activities. Therefore, “food-derived bioactive peptides” refer to different peptides of plant or animal origin that can exhibit regulatory functions in the human system beyond their nutritional value. As an economically and nutritionally important food commodity, egg is a well-known rich source of many bioactive peptides.26−29 IRW and IQW are two ACE inhibitory peptides characterized previously from egg white protein ovotransferrin.15
Although the beneficial effects of biologically active amino acids or peptides have been suggested, their mechanisms of action have not been fully elucidated.30,31 It is essential to understand the roles of bioactive peptides in cell-mediated pathways. Pro-inflammatory cytokines such as TNF can mediate vascular inflammation and thus play a pivotal role in the pathogenesis of atherosclerosis and its complications. The leukocyte adhesion molecules ICAM-1 and VCAM-1 are the key players of inflammatory responses in leukocyte adhesion and cell signal transduction.32 Both ICAM-1 and VCAM-1 are up-regulated after exposure of vascular endothelial cells to TNF.33 The results showed that TNF treatment significantly increased the expression of both ICAM-1 and VCAM-1 in HUVECs. Our previous study demonstrated that pretreatment with IRW (50 μmol/L) significantly inhibited TNF-induced increased expression of both ICAM and VCAM-1.16 Interestingly, pretreatment with IQW (50 μmol/L), differing by only one amino acid residue from IRW, significantly blocked the TNF-induced increased expression of only ICAM-1 but not VCAM-1. Because both tripeptides have the same N-terminal Ile (I) and C-terminal Trp (W), this difference was possibly due to the presence of a positively charged amino acid of Arg (R) in IRW but not in IQW. In comparison to tripeptides, neither their respective dipeptides (IR, RW, IQ, and QW) nor amino acids (individually or in combination) with the exception of arginine (R) and amino acid combination (I+R+W) affected TNF-induced increased expression of ICAM-1 and VCAM-1. Therefore, our study showed that the integrity of both peptides is essential for inhibiting the TNF-induced increased expression of ICAM-1 and VCAM-1.
The inhibitory effect observed in the TNF-induced increased expression of ICAM-1 and VCAM-1 with various concentrations of arginine (R) and amino acid combination (I+R+W) treatments may be mediated through the nitric oxide synthase (NOS) pathway. l-Arginine is a known substrate for NOS present in the endothelial cells, which can convert l-arginine to l-citrulline and produce NO.34,35 The increased production of NO has previously been shown to attenuate TNF-induced expression of ICAM-1 and VCAM-1.36 In our study, pretreatment of various concentrations of arginine (R) showed only a marginal decrease in TNF-induced expression of ICAM-1 and VCAM-1 compared to almost complete prevention with IRW. This result suggests that the tripeptide IRW possesses a different mechanism of action from arginine (R) alone. Whereas arginine (R) alone contributes to increase NO, IRW appears to act through NF-κB modulation and reduction in oxidative stress.
This study also examined whether IQW could also ameliorate TNF-induced oxidative stress.16 In the present study IQW demonstrated antioxidant effects by inhibiting TNF-induced superoxide generation in endothelial cells. Furthermore, our results demonstrated that both IRW and IQW, but not their respective dipeptides and amino acids with the exception of arginine (R), could significantly reduce the TNF-induced superoxide generation in endothelial cells, similar to the trend observed in the TNF-induced inflammatory response. Whereas oxidative stress can activate pro-inflammatory pathways in endothelial cells, our findings had shown that the presence of SOD did not inhibit TNF-induced ICAM-I and VCAM-1 expression, which indicated that these inflammatory changes are independent of the concomitant increase of superoxide generation. Increased oxidative stress such as generation of superoxide was reported to contribute to the inflammatory changes in the endothelium.32 However, TNF-mediated inflammatory changes are mediated through activation of pro-inflammatory pathways of NF-κB. Both peptides inhibited TNF-induced activation of NF-κB as well as superoxide generation. The differential regulation of NF-κB may explain the different responses observed with the two peptides. It appears that both tripeptides (IRW and IQW) are exerting anti-inflammatory and antioxidative effect in our model system.
Increased TNF signaling can activate various transcription factors such as NF-κB, AP-1, and IRF-1.33−38 NF-κB activation causes enhanced expression of genes whose protein products mediate monocyte binding, monocyte chemotaxis into the subendothelial space, and conversion into macrophages; the monocyte binding is largely mediated through the over-activation of adhesion molecules (e.g., ICAM-1 and VCAM-1).3,11 Anti-inflammatory activity of several food bioactive components was also reported to be associated with the NF-κB pathway. Lunasin, a bioactive peptide isolated from soy protein, was reported to inhibit inflammation in LPS-induced RAW 264.7 macrophage by suppressing the NF-κB pathway.39 Curcumin was also shown to inhibit the TNF-induced nuclear translocation of p65 subunit of NF-κB in human myelomonoblastic leukemia cell line.40 A widely known flavonoid, quercetin, had also shown anti-inflammatory effects in endothelial cells through inhibition of the NF-κB signaling pathway.41 In the previous study we revealed that IRW exhibited an anti-inflammatory effect through the NF-κB pathway by blocking the nuclear translocation of p65;16 in the present study we further revealed that IRW also inhibited the nuclear translocation of p50, indicating that IRW can completely inhibit TNF-induced NF-κB activation and thus exhibit an anti-inflammatory effect. On the other hand, IQW can partially affect the NF-κB activity by blocking the translocation of only p50. These data suggest that VCAM-1 expression might be more dependent on p65 translocation as IQW did not affect p65 translocation and also did not have any effect on VCAM-1 levels.
Our study demonstrates the anti-inflammatory and antioxidant activities of IRW and IQW, indicating their potential as functional food ingredients or nutraceuticals for the prevention of endothelial dysfunction, a key factor for the development of CVDs. Given the role of inflammatory processes in many other diseases, these peptides may also find usage in the treatment of diverse conditions such as asthma, arthritis, and inflammatory bowel disease. Our study further confirms that the anti-inflammatory effects of IRW and IQW were independent of their antioxidant properties and that the antioxidant effect was not mediated through the endothelial NOS pathway. The structural integrities are essential to exert their activities. Although the underlying anti-inflammatory mechanisms of both peptides were associated with the NF-κB signaling pathway, IRW can inhibit the translocation of both p50 and p65, whereas IQW can suppress only the translocation of p50. It should be noted that the results were derived from in vitro cell experiments, which may deviate from in vivo activity as the effect of digestion and absorption in vivo could also affect their bioavailability.
Acknowledgments
We thank Donna Dawson, Nurse Research Coordinator in the Royal Alexandra Hospital, Edmonton, AB, for providing the human umbilical cords.
Glossary
Abbreviations Used
- ACE
angiotensin converting enzyme-I
- I
isoleucine
- R
arginine
- Q
glutamine
- W
tryptophan
- HUVEC
human umbilical vein endothelial cells
- CVD
cardiovascular diseases
- TNF
tumor necrosis factor
- ICAM-1
intercellular cell adhesion molecule-I
- VCAM-1
vascular cell adhesion molecule-I
- SOD
superoxide dismutase
- NF-κB
nuclear factor κB
- IκB
inhibitor κB
- NADPH oxidase
nicotinamide adenine dinucleotide phosphate oxidase
Supporting Information Available
Additional figure. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
∥ These authors contributed equally to this work.
This work was supported by grants from Natural Sciences and Engineering Research Council (NSERC) of Canada and the Alberta Livestock and Meat Agency (ALMA) to J.W. S.C. is supported by a CIHR postdoctoral fellowship. S.T.D. is an AIHS (Alberta Innovates- Health Solutions) supported AHFMR Scientist and a Tier I Canada Research Chair in Women’s Cardiovascular Health.
The authors declare no competing financial interest.
Supplementary Material
References
- Chockalingam A. World Hypertension Day and global awareness. Can. J. Cardiol. 2008, 24, 441–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt D.; Ganz P. Endothelial function. From vascular biology to clinical applications. Am. J. Cardiol. 2002, 90, 40L–48L. [DOI] [PubMed] [Google Scholar]
- Giannotti G.; Landmesser U. Endothelial dysfunction as an early sign of atherosclerosis. Herz 2007, 32, 568–572. [DOI] [PubMed] [Google Scholar]
- Sprague A. H.; Khalil R. A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Park Y.; Wu J.; Chen X.; Lee S.; Yang J.; Dellsperger K. C.; Zhang C. Role of TNF-α in vascular dysfunction. Clin. Sci. (London) 2009, 116, 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver R. P. Leukocyte–endothelial cell interactions. Curr. Opin. Cell Biol. 1992, 4, 840–849. [DOI] [PubMed] [Google Scholar]
- Pennathur S.; Heinecke J. W. Oxidative stress and endothelial dysfunction in vascular disease. Curr. Diabetes Rep. 2007, 7, 257–264. [DOI] [PubMed] [Google Scholar]
- Muller G.; Morawietz H. Nitric oxide, NAD(P)H oxidase, and atherosclerosis. Antioxid. Redox Signal. 2009, 11, 1711–1731. [DOI] [PubMed] [Google Scholar]
- Rahman I.; Gilmour P. S.; Jimenez L. A.; MacNee W. Oxidative stress and TNF-α induce histone acetylation and NF-κB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Mol. Cell. Biochem. 2002, 234–235, 239–248. [PubMed] [Google Scholar]
- Guzik T. J.; Harrison D. G. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discov. Today 2006, 11, 524–533. [DOI] [PubMed] [Google Scholar]
- Pober J. S.; Min W.; Bradley J. R. Mechanisms of endothelial dysfunction, injury, and death. Annu. Rev. Pathol.–Mech. Dis. 2009, 4, 71–95. [DOI] [PubMed] [Google Scholar]
- Torruco-Uco J. G.; Dominguez-Magana M. A.; Davila-Ortiz G.; Martinez-Ayala A.; Chel-Guerrero L. A.; Betancur-Ancona D. A. Antihypertensive peptides, an alternative for treatment of natural origin: a review. Cienc. Tecnol. Aliment. 2008, 6, 158–168. [Google Scholar]
- Korhonen H.; Pihlanto A. Food-derived bioactive peptides – opportunities for designing future foods. Curr. Pharm. Des. 2003, 9, 1297–1308. [DOI] [PubMed] [Google Scholar]
- Meisel H. Multifunctional peptides encrypted in milk proteins. Biofactors 2004, 21, 55–61. [DOI] [PubMed] [Google Scholar]
- Majumder K.; Wu J. A new approach for identification of novel antihypertensive peptides from egg proteins by QSAR and bioinformatics. Food Res. Int. 2010, 43, 1371–1378. [Google Scholar]
- Huang W.; Chakrabarti S.; Majumder K.; Jiang Y.; Davidge S. T.; Wu J. Egg-derived peptide IRW inhibits TNF-α-induced inflammatory response and oxidative stress in endothelial cells. J. Agric. Food Chem. 2010, 58, 58(20), 10840–10846. [DOI] [PubMed] [Google Scholar]
- Arenas I. A.; Xu Y.; Lopez-Jaramillo P.; Davidge S. T. Angiotensin II-induced MMP-2 release from endothelial cells is mediated by TNF-α. Am. J. Physiol. Cell. Physiol. 2004, 286, C779–C784. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S.; Davidge S. T. High glucose-induced oxidative stress alters estrogen effects on ERα and ERβ in human endothelial cells: reversal by AMPK activator. J. Steroid Biochem. Mol. Biol. 2009, 117, 99–106. [DOI] [PubMed] [Google Scholar]
- Merchant S. J.; Narumiya H.; Zhang Y.; Guilbert L. J.; Davidge S. T. The effects of preeclampsia and oxygen environment on endothelial release of matrix metalloproteinase-2. Hypertens. Pregnancy 2004, 23, 47–60. [DOI] [PubMed] [Google Scholar]
- Narumiya H.; Zhang Y.; Fernandez-Patron C.; Guilbert L. J.; Davidge S. T. Matrix metalloproteinase-2 is elevated in the plasma of women with preeclampsia. Hypertens. Pregnancy 2001, 20, 185–194. [DOI] [PubMed] [Google Scholar]
- Sankaralingam S.; Xu Y.; Sawamura T.; Davidge S. T. Increased lectin-like oxidized low-density lipoprotein receptor-1 expression in the maternal vasculature of women with preeclampsia: role for peroxynitrite. Hypertension 2009, 53, 270–277. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S.; Chan C. K.; Jiang Y.; Davidge S. T. Neuronal nitric oxide synthase regulates endothelial inflammation. J. Leukocyte Biol. 2012, 91, 947–956. [DOI] [PubMed] [Google Scholar]
- Peshavariya H. M.; Dusting G. J.; Selemidis S. Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free Radical Res. 2007, 41, 699–712. [DOI] [PubMed] [Google Scholar]
- Lin S. J.; Shyue S. K.; Hung Y. Y.; Chen Y. H.; Ku H. H.; Chen J. W.; Tam K. B.; Chen Y. L. Superoxide dismutase inhibits the expression of vascular cell adhesion molecule-1 and intracellular cell adhesion molecule-1 induced by tumor necrosis factor-α in human endothelial cells through the JNK p38 pathways. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 334–340. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). Cardiovascular diseases (CVDs); factsheets September 2011; http://www.who.int/mediacentre/factsheets/fs317/en/index.html (accessed April 22, 2012).
- Kovacs-Nolan J.; Phillips M.; Mine Y. Advances in the value of eggs and egg components for human health. J. Agric. Food Chem. 2005, 53, 8421–8431. [DOI] [PubMed] [Google Scholar]
- Miguel M.; Recio I.; Gomez-Ruiz J. A.; Ramos M.; Lopez-Fandino R. Angiotensin I-converting enzyme inhibitory activity of peptides derived from egg white proteins by enzymatic hydrolysis. J. Food Prot. 2004, 67, 1914–1920. [DOI] [PubMed] [Google Scholar]
- Miguel M.; Manso M.; Aleixandre A.; Alonso M. J.; Salaices M.; Lopez-Fandino R. Vascular effects, angiotensin I-converting enzyme (ACE)-inhibitory activity, and anti hypertensive properties of peptides derived from egg white. J. Agric. Food Chem. 2007, 55, 10615–10621. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M.; Fujita H.; Matoba N.; Takenaka Y.; Yamamoto T.; Yamauchi R.; Tsuruki H.; Takahata K. Bioactive peptides derived from food proteins preventing lifestyle-related diseases. Biofactors 2000, 12, 143–146. [DOI] [PubMed] [Google Scholar]
- Phelan M.; Kerins D. The potential role of milk-derived peptides in cardiovascular disease. Food Funct. 2011, 2, 153–167. [DOI] [PubMed] [Google Scholar]
- Quazi R.; Palaniswamy C.; Frishman W. H. The emerging role of apelin in cardiovascular disease and health. Cardiol. Rev. 2009, 17, 283–286. [DOI] [PubMed] [Google Scholar]
- Jacobi J.; Kristal B.; Chezar J.; Shaul S. M.; Sela S. Exogenous superoxide mediators pro-oxidative, proinflammatory and procoagulatory changes in primary endothelial cell cultures. Free Radical Biol. Med. 2005, 39, 1238–1248. [DOI] [PubMed] [Google Scholar]
- Bradley J. R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [DOI] [PubMed] [Google Scholar]
- Sitia S.; Tomasoni L.; Atzeni F.; Ambrosio G.; Cordiano C.; Catapano A.; Tramontana S.; Perticone F.; Naccarato P.; Camici P.; Picano E.; Cortigiani L.; Bevilacqua M.; Milazzo L.; Cusi D.; Barlassina C.; Sarzi-Puttini P.; Turiel M. From endothelial dysfunction to atherosclerosis. Autoimmun. Rev. 2010, 9, 830–834. [DOI] [PubMed] [Google Scholar]
- Luiking Y. C.; Engelen M. P.; Deutz N. E. Regulation of nitric oxide production in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stechmiller J. K.; Childress B.; Cowan L. Arginine supplementation and wound healing. Nutr. Clin. Pract. 2005, 20, 52–61. [DOI] [PubMed] [Google Scholar]
- Waldow T.; Witt W.; Weber E.; Matschke K. Nitric oxide donor-induced persistent inhibition of cell adhesion protein expression and NFκB activation in endothelial cells. Nitric Oxide 2006, 15, 103–113. [DOI] [PubMed] [Google Scholar]
- Chen G.; Goeddel D. V. TNF-R1 signaling: a beautiful pathway. Science 2002, 296, 1634–1635. [DOI] [PubMed] [Google Scholar]
- de Mejia E. G.; Dia V. P. Lunasin and lunasin-like peptides inhibit inflammation through suppression of NF-κB pathway in the macrophage. Peptides 2009, 30, 2388–2398. [DOI] [PubMed] [Google Scholar]
- Singh S.; Aggarwal B. B. Activation of transcription factor NF-κ B is suppressed by curcumin (diferuloylmethane) [corrected]. J. Biol. Chem. 1995, 270, 24995–25000. [DOI] [PubMed] [Google Scholar]
- Al-Shalmani S.; Suri S.; Hughes D. A.; Kroon P. A.; Needs P. W.; Taylor M. A.; Tribolo S.; Wilson V. G. Quercetin and its principal metabolites, but not myricetin, oppose lipopolysaccharide-induced hyporesponsiveness of the porcine isolated coronary artery. Br. J. Pharmacol. 2011, 162, 1485–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.