Abstract
Objective
Decreased reciprocal inhibition (RI) of motor neurons may contribute to spasticity after stroke. However, decreased RI is not a uniform observation among stroke survivors, suggesting that this spinal circuit may be influenced by other stroke-related characteristics. The purpose of this study was to measure RI post-stroke and to examine the relationship between RI and other features of stroke.
Methods
RI was examined in 15 stroke survivors (PAR) and 10 control subjects by quantifying the effect of peroneal nerve stimulation on soleus H-reflex amplitude. The relationship between RI and age, time post-stroke, lesion side, walking velocity, Fugl-Meyer, Ashworth, and Achilles reflex scores was examined.
Results
RI was absent and replaced by reciprocal facilitation in 10 of 15 PAR individuals. Reciprocal facilitation was associated with low Fugl-Meyer scores and slow walking velocities but not with hyperactive Achilles tendon reflexes. There was no relationship between RI or reciprocal facilitation and time post-stroke, lesion side, or Ashworth score.
Conclusions
Decreased RI is not a uniform finding post-stroke and is more closely related to walking ability and movement impairment than to spasticity.
Significance
Phenomena other than decreased RI may contribute to post-stroke spasticity.
Keywords: Spasticity, Rehabilitation, Hemiparesis, CVA
1. Introduction
Individuals with chronic, post-stroke hemiparesis often display spasticity which is a complex motor disorder characterized by a velocity-dependent increase in muscle response to stretch with exaggerated tendon reflexes, caused by hyperexcitability of the stretch reflex (Lance, 1979). The mechanisms underlying spasticity post-stroke have not been fully elucidated, but prior work suggests that decreased reciprocal inhibition (RI) of motor neurons may make an important contribution. In neurologically intact individuals, Group Ia mediated RI contributes to the suppression of antagonist muscle activity during movement (Tanaka, 1974; Crone et al., 1987; Crone and Nielsen, 1989; Crone, 1993; Yanagisawa et al., 1976). However, Crone and colleagues have provided convincing demonstrations of reduced transmission in the RI pathway after stroke (Crone et al., 2000, 2003). They used the technique of Hultborn et al. (1987) whereby soleus (SO) H-reflexes were conditioned by peroneal nerve stimulation and conditioning-induced suppression of H-reflexes was indicative of RI of SO motor neurons. None of the stroke survivors examined displayed RI. Instead, all six subjects displayed pronounced conditioning-induced facilitation of SO H-reflexes, which we refer to here as reciprocal facilitation. In a single subject examined longitudinally, RI was absent 3 weeks post-stroke, and reciprocal facilitation appeared 2 weeks later, coincident with the appearance of clinical signs of spasticity. While causality could not be established, the authors suggested that decreased RI may be a mechanism underlying spasticity.
While the work of Crone and colleagues (Crone et al., 2000, 2003) provides compelling evidence for decreased RI post-stroke, others have not reported such unambiguous findings. Okuma and Lee (1996) failed to show a significant decrease in RI in a sample of sixteen stroke survivors, and they detected reciprocal facilitation in only two subjects. Moreover, they showed enhanced RI in stroke survivors with good recovery. Cramp et al. (2000) showed decreased RI, but not reciprocal facilitation, of SO motor neurons in the paretic as compared to the non-paretic limb of stroke survivors at 1 month post-stroke. Five months later, RI was increased in the paretic as compared to the non-paretic leg. Finally, Yanagisawa et al. (1976) showed mixed results in eleven individuals with stroke. Three subjects showed reciprocal facilitation; two showed RI, and six showed no response to conditioning.
Collectively, these observations suggest that decreased RI and/or reciprocal facilitation is not a uniform observation among stroke survivors and that the excitably of the RI pathway must be influenced by stroke-related characteristics. Hence, the purpose of the present study was to examine Group Ia mediated RI of SO motor neurons in people with chronic, post-stroke hemiparesis and to explain the relationship between RI and other features of stroke. We hypothesize that, if decreased RI makes an important contribution to post-stroke spasticity, then the absence of RI and/or the presence of reciprocal facilitation would be more strongly associated with clinical manifestations of spasticity as compared to other stroke-related impairments.
2. Materials and methods
2.1. Subjects
Fifteen individuals with chronic post-stroke hemiparesis (PAR) and 10 neurologically intact (NI) individuals participated. The mean (±SE) age of PAR and NI individuals was 54.9 (±3.3) and 44.5 (±3.9) years, respectively. These values were not significantly different (P = 0.060). However, because the PAR group tended to be older than the NI group and because previous work suggests that reciprocal inhibition (RI) changes with age (Kido et al., 2004a), we accounted for age in statistical analyses. There were 8 females in the PAR group and 6 females in the NI group. PAR individuals had sustained a single unilateral cortical or subcortical stroke at least 1.2 years prior to testing, and the mean (±SE) time since stroke was 8.6 (±2.1) years. There were 5 subjects with right and 10 subjects with left hemiparesis (see Table 1). No subjects had taken any anti-spasticity medications for at least 3 months prior to testing. NI individuals had no signs or history of stroke or other neurological impairment. All subjects participated voluntarily after providing written informed consent as approved by the Institutional Review Board at Marquette University.
Table 1.
Subject characteristics.
| Subject id | Age (yrs) | Time since stroke (yrs) | Lesion side | FM score | Walking velocity (m/s) | Reflex score Achilles | Ashworth score Ankle | Short latency Max response | Long latency Max response |
|---|---|---|---|---|---|---|---|---|---|
| S46 | 52 | 1.2 | R | 69 | 0.23 | 4+ | 1 | 112.1 | 90.1 |
| S41 | 61 | 1.4 | L | 54 | 0.45 | 4+ | 4 | 113.8 | 94.5 |
| S19 | 64 | 6.6 | R | 91 | 0.85 | 3+ | 1 | 127.2 | 86.0 |
| S14 | 55 | 31.4 | R | 79 | 0.93 | 3+ | 2 | 107.6 | 64.9 |
| S25 | 51 | 3.2 | R | 76 | 0.49 | 3+ | 2 | 119.0 | 94.5 |
| S15 | 44 | 7.6 | R | 70 | 0.39 | 3+ | 2 | 101.3 | 73.7 |
| S03 | 47 | 6.5 | L | 77 | 0.87 | 2+ | 2 | 109.5 | 91.4 |
| S43 | 65 | 1.8 | L | 74 | 0.13 | 2+ | 3 | 107.4 | 100.6 |
| S42 | 76 | 12.3 | L | 73 | 0.97 | 1+ | 2 | 117.3 | 88.2 |
| S10 | 63 | 4.6 | R | 65 | 0.39 | 1+ | 0 | 160.0 | 70.8 |
| S01 | 62 | 7.4 | R | 91 | 1.11 | 4+ | 1 | 80.6 | 75.0 |
| S24 | 52 | 16.3 | L | 91 | 1.08 | 4+ | 1 | 75.5 | 81.6 |
| S34 | 57 | 4.9 | R | 88 | 0.58 | 4+ | 2 | 89.8 | 87.7 |
| S44 | 19 | 17.7 | R | 85 | 1.15 | 4+ | 3 | 67.4 | 78.0 |
| S29 | 55 | 6.5 | R | 84 | 0.81 | 4+ | 1 | 90.1 | 91.5 |
| Mean | 54.9 | 8.6 | … | 78 | 0.7 | 3.1 | 1.9 | 105.2 | 84.6 |
| SE | 3.3 | 2.1 | … | 2.8 | 0.1 | 0.3 | 0.3 | 5.9 | 2.6 |
L = left, R = right, FM = lower extremity Fugl-Meyer score. The maximum response to short latency conditioning is show in percent unconditioned H-reflex.
2.2. Equipment
Bipolar surface electrodes (Delsys, Inc. 10 mm length, 1 mm width, 1 cm inter electrode distance) were used to record EMG from the SO and tibialis anterior (TA). EMG signals were amplified 10× at the electrode site before remote differential amplification (common mode rejection ratio 92 dB, gain range 100–10,000 times, frequency response 20–450 Hz). Data were sampled online at 2000 Hz via a 16-bit analog to digital converter. Tibial and peroneal nerve stimulations were delivered with constant current stimulators and isolation units (Digitimer DSA7, current range 50 μA–200 mA, total output capability 400 V). All stimulation pulses were 1 ms in duration.
2.3. Procedures and protocol
PAR individuals underwent the lower limb portion of the Fugl-Meyer test (Fugl-Meyer et al., 1975) for assessment of global lower extremity motor function (maximum possible score = 96) and performed the 8 m timed walk test (Bohannon, 1986) for assessment of walking velocity. Ashworth scores (Ashworth, 1964) were completed on the paretic ankle (normal tone = 0) by slowly moving the joint through available range of motion. Achilles tendon reflexes were also recorded (DeMyer, 2004) (normal reflexes = 2+). All clinical tests were performed by a licensed physical therapist prior to electrophysiological testing.
Before placing the stimulating and recording electrodes, the skin at each electrode site was gently abraded and cleaned with alcohol. Surface EMG electrodes were placed over the distal half of the SO and proximal half of the TA of the right leg of NI and the paretic leg of PAR individuals. A common reference electrode was placed over the distal tibia just proximal to the medial malleolus. Bipolar stimulating electrodes (Ambu, Neuroline 715) were placed over the popliteal fossa to stimulate the tibial nerve and over the caput fibulae to stimulate the peroneal nerve. The cathode was placed proximally. Effort was made to place stimulating electrodes in such a way as to avoid activation of neighboring muscles. The specificity of electrode positioning was checked repeatedly during the experiment. Adhesive tape was used to secure the electrodes. After all the electrodes were positioned, subjects were seated comfortably with the hip, knee and ankle at 120°, 160° and 110° respectively, and were asked to remain still during testing.
Inhibition of SO motor neurons was examined according to the method of Crone (Crone et al., 2003) whereby SO H-reflexes were conditioned with peroneal nerve stimulation at various inter-stimulus intervals (ISIs). Previous studies have shown that, when the SO H-reflex is conditioned by peroneal nerve stimulation at ISIs of 2–4 ms, the observed H-reflex depression can be attributed to RI of SO motor neurons (Hultborn et al., 1987). SO H-reflex depression is also evident at ISIs > 5 ms. This depression, referred to as D1 inhibition, is believed to be caused by presynaptic inhibition of Group Ia afferents converging on SO motor neurons (Tanaka, 1974; Mizuno et al., 1971).
The experiment began with supra-maximal activation of the tibial nerve to elicit the maximum SO M-wave (Mmax) after which stimulation intensity was adjusted with the goal of eliciting SO H-reflexes that were approximately 10% of Mmax. Subsequent analysis revealed that unconditioned H-reflexes were, on average (±SD), 13 (±3)% of M-max in the NI group and 17 (±4)% of M-max in the PAR group. There was no relationship between unconditioned H-reflex amplitude and response to conditioning (R2 = 0.000196, P = 0.95). Moreover, Crone et al. (1985) have shown that RI is not affected by these small differences in H-reflex size. H-reflexes were elicited 10 s apart to avoid rate sensitive depression (Schindler-Ivens and Shields, 2000). When a small M-wave preceded the H-reflex, we also monitored its amplitude to ensure that tibial nerve stimulation remained constant. Peroneal nerve stimulation was used to condition SO H-reflexes at ISIs of 0, 1, 2, 3, 4, 6, 10, 20 and 30 ms. The intensity of peroneal nerve stimulation was maintained at 1.2 times the motor threshold of TA. The order in which ISIs were presented was randomly determined for each subject. For each ISI, approximately 60 pulses were elicited in a single block. Each block contained approximately 30 conditioned and 30 unconditioned pulses delivered in random order.
2.4. Data analysis and statistics
After measuring the peak to peak (P–P) amplitude of all H-reflexes, each conditioned H-reflex was expressed as a percent of the mean of the unconditioned H-reflexes. For every subject, the mean (±SE) of these normalized values was computed at each ISI and plotted to obtain a time course of the effect of peroneal nerve stimulation on the amplitude of the H-reflex. Group time courses for PAR and NI groups were obtained by averaging responses to conditioning across subjects at each ISI. Consistent with previous studies, two-tailed, single sample t-tests were applied to determine whether there was a significant effect of conditioning at each ISI within each subject and within each group (Crone et al., 1987, 2003; Crone and Nielsen, 1989; Petersen et al., 1998).
To further assess the magnitude of reciprocal inhibition between groups we examined each subject’s data at the short latency ISIs (2–4 ms) and found the ISI with the largest significant deviation from the unconditioned values. If no ISI reached statistical significance, then the ISI with the maximum deviation from the unconditioned values was used. The same was done for D1 inhibition. We took this approach because it allowed us to obtain a single value for short and a single value for long latency inhibition that could be compared between groups and used for correlation and regression. Moreover, we were concerned that the group time course plots might obscure the effects of conditioning, as not all subjects displayed effects of conditioning at the same ISI. The mean (±SE) of these values was computed for the PAR and NI group. Single group t-tests were used to determine whether the maximum response to conditioning was significantly different from zero in each group. Analysis of covariance (ANCOVA) was applied with age as a cofactor to determine whether there was a significant between-group (PAR versus NI) effect of the maximum response to short latency conditioning.
Pearson correlation coefficients were used to examine relationships between the maximum response to short and long latency conditioning and clinical measures, which included Fugl-Meyer score, walking velocity, Ashworth score, Achilles tendon reflex score, age, and time post-stroke. Any clinical measure that was significantly correlated with response to short latency conditioning was entered into a forward stepwise regression model to identify those factors that made a significant contribution to predicting the maximum response to short latency conditioning (P < 0.05 for entry, P > 0.10 for removal). Pearson correlation coefficients were also used to examine the relationship between RI and D1 inhibition in PAR and NI groups. A chi-square test was used to examine the effect of lesion side. Unless otherwise noted, all effects were considered significant at P < 0.05.
3. Results
All PAR individuals displayed stroke-related movement impairments. As shown in Table 1, the group mean (±SE) for the lower extremity Fugl-Meyer score was 78 (±2.8). Mean (±SE) walking velocity was 0.7 (±0.1) m/s. Eleven PAR subjects displayed hyperactive Achilles tendon reflexes on the paretic side as evidenced by values >2+. All but one PAR individual had abnormally increased muscle tone at the ankle as shown by Ashworth scores > 0.
3.1. Group responses to short and long latency conditioning
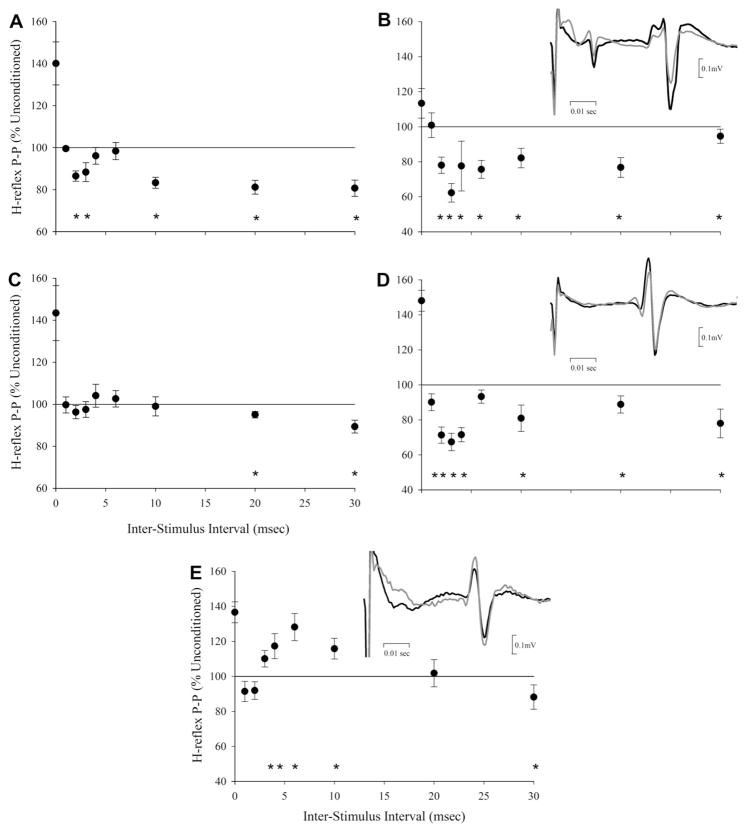
As shown in Fig. 1A and Table 2, the NI group displayed significant SO H-reflex inhibition in response to conditioning with peroneal nerve stimulation. There were two periods of H-reflex inhibition. The first occurred at ISIs of 2 and 3 ms; the second period occurred at ISIs of 10, 20, and 30 ms. All NI individuals displayed H-reflex inhibition in response to short and long latency conditioning. See Fig. 1B for representative example. In 7 individuals short latency inhibition reached statistical significance, and in 9 NI subjects, long latency inhibition was statistically significant. The remaining subjects, whose responses did not reach statistical significance, showed similar patterns of inhibition.
Fig. 1.
The time course of responses to H-reflex conditioning with peroneal nerve stimulation. (A) Group data from NI subjects. (B) Representative data from one NI individual. (C) Group data from PAR subjects. (D) Representative data from one PAR individual displaying short latency inhibition. (E) Representative data from one PAR individual displaying short latency facilitation. Symbols represent mean (±SE). Asterisks represent significant changes in H-reflex peak-to-peak amplitude for conditioned as compared to unconditioned responses. Insets are representative examples of conditioned (gray) and unconditioned (black) H-reflexes. Data in the insets are pulled from the 3 ms, 2 ms, and 4 ms ISIs in B, D, and E respectively.
Table 2.
Group responses to short and long latency conditioning.
| Short latency
|
Long latency
|
||||||
|---|---|---|---|---|---|---|---|
| 2 ms | 3 ms | 4 ms | 10 ms | 20 ms | 30 ms | ||
| NI | Mean (±SE) | 86.5 (2.4) | 88.3 (4.5) | 96.1 (4.0) | 83.3 (±2.6) | 81.2 (±3.2) | 80.7 (±3.8) |
| P-value | <0.001 | 0.029 | 0.355 | <0.001 | <0.001 | <0.001 | |
| PAR | Mean (±SE) | 96.2 (3.1) | 97.5 (3.8) | 104.1 (5.4) | 99.02 (4.5) | 95.0 (1.5) | 89.4 (3.1) |
| P-value | 0.249 | 0.516 | 0.466 | 0.832 | 0.005 | 0.004 | |
Mean (±SE) is shown in percent unconditioned H-reflex. NI = neurologically intact, PAR = paretic. Significant effects are represented in bold.
In the PAR group there was no significant change in H-reflex amplitude in response to short latency conditioning (Fig. 1C and Table 2). At the long latency ISIs, the PAR group showed significant H-reflex inhibition at the 20 and 30 ms ISIs, but not at the 10 ms ISI. The absence of any group effect of short latency conditioning in PAR individuals was a consequence of varied responses to short latency conditioning. Five PAR individuals showed RI, and 10 PAR subjects showed reciprocal facilitation. Representative examples of PAR “inhibitors” and “facilitators” are shown in Fig. 1D and E, respectively. Responses to conditioning were statistically significant in 10 PAR individuals. The remaining subjects showed similar patterns of inhibition or facilitation.
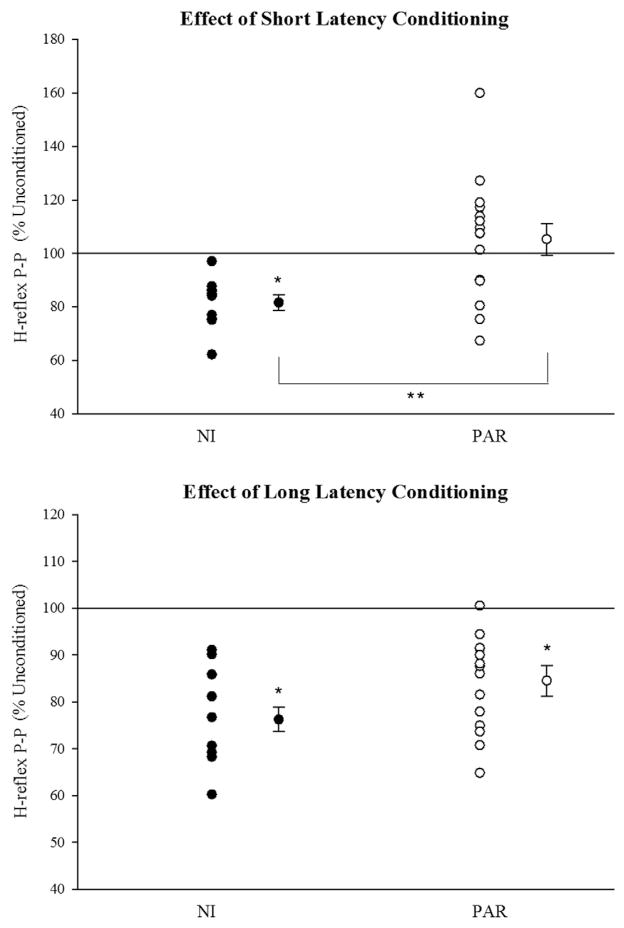
Between-group differences in short and long latency conditioning are further exemplified in Fig. 2 which displays group means (±SE) and individual values for the maximum response to short (top) and long (bottom) latency conditioning. Individual values for PAR subjects are also provided in Table 1. Short latency conditioned H-reflexes were significantly smaller than unconditioned H-reflexes in the NI group (P = 0.006) but not in the PAR group (P = 0.390). Moreover, the maximum response to short latency conditioning was always inhibitory in the NI group; whereas, in the PAR group, some subjects showed inhibition and others showed facilitation. There was a significant between-group difference (NI versus PAR) in maximum response to short latency conditioning even after accounting for between-group differences in age (ANCOVA P = 0.04 group effect, P = 0.02 age effect). Maximum long latency inhibition was significantly different from zero in the NI and PAR group (P < 0.001). There was no significant between-group difference (NI versus PAR) in maximum response to long latency conditioning after accounting for between-group differences in age (ANCOVA P = 0.220 group effect, P = 0.122 age effect).
Fig. 2.
Maximum response to short and long latency conditioning. Mean (±SE) values are shown for each group. Single and double asterisks represent significant within and between group effects, respectively. Individual responses are shown to the left of the mean data and represent the mean of the maximum short latency response observed for each subject. NI = neurologically intact, PAR = paretic.
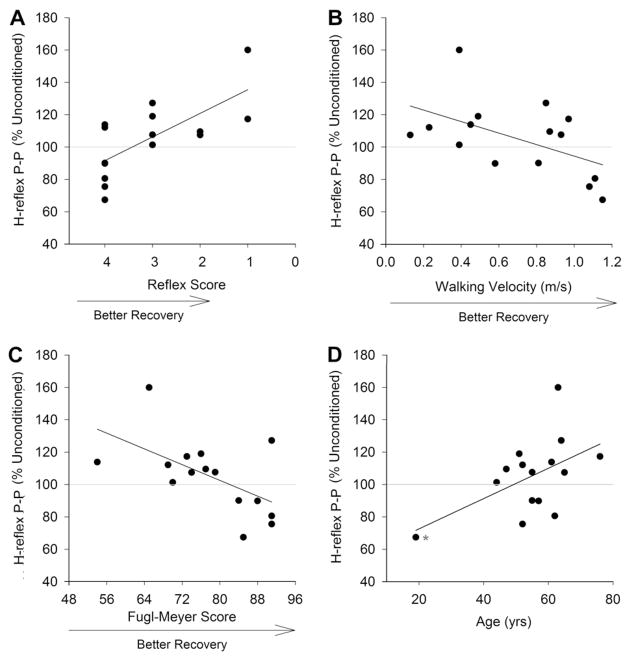
3.2. Relationship between response to short latency conditioning and clinical measures
As shown in Table 3, maximum responses to short latency conditioning were significantly correlated with Achilles reflex scores, Fugl-Meyer scores, walking velocity, and age. The age effect was driven by one highly influential outlier (indicated with an asterisk in Fig. 3D), and when this point was removed, age was not associated with response to conditioning (R = 0.30, P = 0.30). There was no significant association between response to short latency conditioning and Ashworth score or time since stroke. When the four significantly correlated clinical measures were entered into regression analysis, only Achilles reflex score (P = 0.004) and walking velocity (P = 0.043) made a significant contribution the prediction of response to conditioning as describe by the following equation:
where R is the magnitude of the maximum short latency response to conditioning in percent of unconditioned H-reflex amplitude, Rx the Achilles reflex score, and W is the walking velocity.
Table 3.
Correlations between clinical measures and maximum response to short latency conditioning.
| R | P-value | |
|---|---|---|
| Achilles reflex score | 0.700 | 0.004 |
| Age | 0.522 | 0.046 |
| Ashworth score | −0.249 | 0.370 |
| FM score | −0.571 | 0.026 |
| Time post-stroke | −0.320 | 0.244 |
| Walking velocity | −0.519 | 0.047 |
FM = lower extremity Fugl-Meyer score, R = Pearson correlation coefficient. Significant effects are represented in bold.
Fig. 3.
Relationship between response to conditioning and Achilles reflex scores (A), walking velocity (B), lower extremity Fugl-Meyer score (C), and age (D). Each point represents a different paretic individual except in A where there are two subjects with a reflex score of 4 and H-reflex peak-to-peak amplitude of approximately 90% of unconditioned. These dots cannot be distinguished from each other. The asterisk in D is an outlier. See text for details.
The overall regression model was significant at P = 0.002 and R2 = 0.643. As shown in Fig. 3, normal (2+) and hypo-active (1+) Achilles reflex scores were always associated with reciprocal facilitation; whereas, faster walking velocity was associated with RI. Fugl-Meyer score dropped out of the regression model, likely because walking velocity and Fugl-Meyer scores were directly related (r = 0.653, P = 0.008).
There was no significant correlation between D1 inhibition at any individual ISI and any of the clinical measures examined (P ≥ 0.160). When maximum response to long latency conditioning was used, there was a significant inverse relationship between the magnitude of inhibition and time post-stroke (r = −0.665, P = 0.007), suggesting more D1 inhibition with increasing time post-stroke. There was no significant correlation between the magnitude of short and long latency inhibition in the PAR (P ≥ 0.263) or NI (P ≥ 0.137) group.
4. Discussion
Our data indicate that Group Ia mediated RI of SO motor neurons is absent and replaced by reciprocal facilitation in some but not all individuals with chronic post-stroke hemiparesis. In this sample of 15 stroke survivors, 10 displayed reciprocal facilitation and 5 displayed RI. Reciprocal facilitation was related to stroke-related impairment, but not in the way that we hypothesized. Stroke survivors with reciprocal facilitation were more likely than those with RI to have poor movement ability as measured by slower walking velocities and lower Fugl-Meyer scores. However, individuals with reciprocal facilitation were not more likely to have hyperactive Achilles tendon reflexes. All the individuals with RI had Achilles tendon reflex scores of 4+, which is an abnormal response characterized by very brisk reflexes and/or 1–3 beats of clonus (DeMyer, 2004). Reflex scores of 4+ were also the highest scores recorded in this study. In contrast, all but 2 individuals with reciprocal facilitation had Achilles tendon scores less than 4+. Collectively, these observations suggest that reciprocal facilitation of SO motor neurons is not a uniform finding across chronic stroke survivors and that it is more closely related to walking ability and movement impairment than to spasticity. Hence, decreased RI may not be the mechanism underlying post-stroke spasticity.
The observations reported here are different from those of Crone et al. (2000, 2003) who showed that 6 of 6 hemiparetic stroke survivors had pronounced reciprocal facilitation of SO H-reflexes and that, in a single subject examined over time, reciprocal facilitation appeared at approximately the same time as clinical manifestations of spasticity. In comparison to the work of Crone and colleagues, our data are more closely aligned with that of Yanagisawa et al. (1976) who showed a mixed response to SO H-reflex conditioning with peroneal nerve stimulation. These investigators identified 3 stroke survivors with reciprocal facilitation, 2 with RI, and 6 with no response to conditioning. Mixed responses to stimulation have also been reported by Okuma and Lee (1996) who detected reciprocal facilitation in 2 of 16 stroke survivors examined; the remaining 14 individuals displayed reduced RI or no response to conditioning.
Non-uniform responses to conditioning within studies and disparate findings among studies suggest that Group Ia mediated RI is not affected in the same way for all stroke survivors. This observation suggests that the excitably of the RI pathway must be influenced by stroke-related characteristics or that RI influences recovery. Previous reports suggest that RI post-stroke is related to ankle muscle strength. Yanagisawa and Okuma (Yanagisawa et al., 1976) showed that individuals with no RI or with reciprocal facilitation tended to have poorer ankle muscle strength, particularly in the TA, as compared to those with RI. Okuma and Lee (1996) showed that the magnitude of RI observed in stroke survivors increased with increasing TA muscle strength. Our data extend these observations by demonstrating that RI is related, not only to muscle strength, but also to the ability to produce isolated, single joint movements of the lower limb. The Fugl-Meyer test awards some points for the ability to produce strong movements in flexion and extension synergies. Importantly, however, scores increase as subjects are able to move out of synergy and produce isolated knee flexion and ankle dorsiflexion. The PAR individuals with RI had an average (±SE) Fugl-Meyer synergy score of 19.6 (±1.3) out of a maximum possible score of 22; the subjects with facilitation had a score of 13.6 (±1.1). These data suggest that individuals with RI had superior ability, in comparison to those with facilitation, to isolate movement at a single joint, particularly at the ankle. Perhaps isolated joint movement is possible in some stroke survivors because of the presence of descending control of RI. Indeed, Group Ia mediated RI suppresses antagonist muscle activity to allow unopposed activation of desired muscles (Tanaka, 1974; Crone et al., 1987; Crone and Nielsen, 1989; Crone, 1993; Yanagisawa et al., 1976). This process is controlled, in part, by the motor cortex. During voluntary movement, axons from the motor cortex make direct connections to spinal motor neurons and send collaterals to Ia inhibitory interneurons, minimizing antagonist muscle activation (Jankowska et al., 1976). Perhaps stroke survivors with less cortical damage have better cortical control over RI, resulting in better unidirectional, isolated joint movement. Alternatively, better movement may enhance RI.
We also observed that responses to conditioning were related to walking velocity. Individuals with faster walking velocities tended to display RI, and those with slower walking velocities tended to have reciprocal facilitation. It is difficult to identify a direct, uncomplicated link between Group Ia mediated RI of SO motor neurons and walking because this task involves simultaneous control of numerous joints and muscles and is influenced by descending commands and sensory feedback mediated at multiple sites in the nervous system. Moreover, previous work in able-bodied individuals has shown that, unlike H-reflexes and presynaptic inhibition, RI is not modulated across the gait cycle (Capaday et al., 1990; Kido et al., 2004b). Rather, RI is strongly dependent on background muscle activity. Hence, an indirect link between RI and walking ability is more likely than a direct link. Strong, isolated activation of the TA may induce RI of the SO, facilitate toe clearance, and lead to a safer and more effective gait pattern. In turn, walking may become faster and more functional, increasing subjects’ exposure to challenging locomotor experiences and physical activity. Indeed, Crone et al. (1985) have shown that the magnitude of RI is directly related to physical training. With respect to each of these possible links between RI and clinical presentation, further study is required. The data available to date cannot establish a causal relationship between any of these variables, nor can it determine whether RI is enhanced by more effective moment and physical training or whether better movement and physical training is a consequence of strong RI.
Perhaps the most surprising aspect of this study was the relationship between RI and Achilles tendon reflex excitability. We expected that reciprocal facilitation would be most evident in people with hyperreflexia, consistent with the suggestion that absent RI and/or reciprocal facilitation may contribute to spasticity (Crone et al., 2000, 2003). While some individuals with reciprocal facilitation had hyperactive Achilles tendon reflexes, many did not. In contrast, all subjects with RI had 4+ reflex scores, which were the highest values recorded. Because of this dissociation between hyperactive Achilles tendon reflexes and reciprocal facilitation, our data suggest that reciprocal facilitation does not cause hyperexcitable tendon reflexes. Moreover, because the Achilles tendon reflex is a measure of spasticity, these observations also suggest that spasticity is not caused by reciprocal facilitation.
Indeed, these conclusions challenge current understanding that reciprocal facilitation or reduced RI makes an important contribution to spasticity. Therefore, let us consider these conclusions more carefully. It could be argued that the Achilles tendon reflex is not an appropriate measure of spasticity. We do not believe this to be the case. Lance (1979) defined spasticity as a complex motor disorder characterized by a velocity-dependent increase in muscle resistance to passive stretch with exaggerated tendon jerks, caused by hyperexcitability of the stretch reflex. The Achilles tendon reflex assesses the net excitability of the pathway between stretch-sensitive muscle spindle afferents and spinal motor neurons, with the fastest component of the response representing the Group Ia-mediated, monosynaptic component of the stretch reflex. Hence, using the Lance definition to define spasticity, elevated Achilles tendon reflexes are an appropriate measure of the condition. Indeed, we cannot rule out all subjective influences on Achilles tendon reflex testing, as this procedure relies on a clinician’s manual dexterity to provide a tap, tactile skills to appraise the briskness of the response, and experience to determine whether responses are different from normal. Moreover, the Achilles tendon reflex is influenced by muscle properties as well as central processing of sensory signals. Future studies that aim to further examine the relationship between RI and spasticity might consider using mechanized measures of stretch reflex excitability that may be more objective and quantitative than manual approaches and may be able to distinguish between neural and muscular contributions to elevated stretch-induced muscle responses to stretch. Nevertheless, inadequacies in clinical reflex assessment cannot explain a systematic elevation of Achilles reflex scores in subjects with RI, as we saw here. Furthermore, all the clinical testing, including reflex testing, was done before RI testing. The individual performing the clinical tests was not the same person who did RI testing; therefore, bias could not have emerged from prior knowledge of either test result.
Given that the Achilles tendon reflex is an appropriate measure of spasticity, what do these findings reveal about the mechanisms underlying spasticity? As indicated above, our data suggest that contrary to previous suggestions reciprocal facilitation does not cause hyperexcitable tendon reflexes or spasticity. It might be tempting to conclude, albeit based on correlational data, that RI could be the cause of spasticity. However, we think this is unlikely, as we can think of no neurophysiological explanation as to how an intact inhibitory circuit (i.e. RI) could contribute to elevated reflexes or spasticity. Hence, we are left to conclude that spasticity must be caused by mechanisms other than reciprocal facilitation and/or impaired RI that affect stretch reflex excitability. Recall, that the stretch reflex examines the net excitably of the pathway between muscle spindle afferents and spinal motor neurons, and that this pathway is affected by numerous central and peripheral factors that include, but are not limited to RI. Such influences include motor neuron excitability, gamma drive, and presynaptic inhibition of Group Ia afferents. Numerous studies completed over more than 30 years have examined a number of spinal circuits that influence stretch reflexes and that could contribute to spasticity (see Nielsen et al. (2007) for review). In addition to RI, these studies provide evidence for contributions from abnormal plateau potentials, autogenic Group Ib inhibition, and presynaptic inhibition. Here, we also provide evidence for impaired presynaptic inhibition in people post-stoke, as we showed that D1 inhibition occurred at 20 and 30 ms ISIs, but not at 10 ms like in control subjects. D1 inhibition has been attributed to presynaptic inhibition of Group Ia afferents (Tanaka, 1974; Mizuno et al., 1971). While these data suggest that presynaptic inhibition could contribute to spasticity, if absent presynaptic inhibition were a powerful contributor, one would expect to detect impairment of this pathway at all the long latency ISIs examined and with maximum D1 inhibition, which was not the case. Moreover, one would expect decreased presynaptic inhibition, as measured by D1 inhibition, to be associated with hyperactive Achilles tendon reflexes. However, D1 inhibition was not significantly correlated with any clinical measure examined, except time post-stroke. Hence, impaired presynaptic inhibition cannot explain the clinical manifestations of spasticity any better than reciprocal facilitation. Of interest, the correlation between D1 inhibition and time post-stroke suggest that presynaptic inhibition may continue to improve many years after stroke.
Nielsen et al. (2007) have suggested that spasticity may not be caused by a single mechanism but by several changes in spinal circuitry and descending drive that interact in complex ways to produce this condition. Thus, a reductionist approach, like we and many others have used, may be limited in its usefulness for understanding the cause of spasticity. This multiple, co-occurring mechanism hypothesis may explain our results. Perhaps pathways involving RI interact with abnormal plateau potentials, autogenic Group Ib inhibition, impaired presynaptic inhibition, descending commands, and other influences on the stretch reflex pathway to cause the clinical manifestations of spasticity. Future studies should examine multiple possible contributors to spasticity and their interactions to test this hypothesis.
HIGHLIGHTS.
Group Ia mediated reciprocal inhibition of motor neurons is absent and replaced by reciprocal facilitation in some but not all individuals with chronic stroke.
Stroke survivors with reciprocal facilitation were more likely than those with reciprocal inhibition to have poor movement ability but not hyperactive tendon reflexes.
Reciprocal facilitation of motor neurons is more closely related to movement impairment than to spasticity and may not be the mechanism underlying post-stroke spasticity.
Acknowledgments
We would like to thank Jon Wieser and Catharine Relation for their help with data collection. This work was supported by Grant 0630412Z from the American Heart Association (Midwest), and in part, by Grant 1UL1RR031973 from the Clinical and Translational Science Award (CTSI) program of the National Center for Research Resources, National Institutes of Health.
References
- Ashworth B. Preliminary trials of carisoprodol in multiple sclerosis. Practitioner. 1964;192:540–2. [PubMed] [Google Scholar]
- Bohannon RW. Strength of lower limb related to gait velocity and cadence in stroke patients. Physiother Can. 1986;38:204–6. [Google Scholar]
- Capaday C, Cody FW, Stein RB. Reciprocal inhibition of soleus motor output in humans during walking and voluntary tonic activity. J Neurophysiol. 1990;64:607–16. doi: 10.1152/jn.1990.64.2.607. [DOI] [PubMed] [Google Scholar]
- Cramp M, Gill M, Greenwood R, Lehman A, Rothwell J, Scott O. Reciprocal Ia inhibition in the first 6 months following stroke. J Physiol – Lond. 2000;523:233P. [Google Scholar]
- Crone C. Reciprocal inhibition in man. Dan Med Bull. 1993;40:571–81. [PubMed] [Google Scholar]
- Crone C, Hultborn H, Jespersen B. Reciprocal Ia inhibition from the peroneal nerve to soleus motoneurones with special reference to the size of the test reflex. Exp Brain Res. 1985;59:418–22. doi: 10.1007/BF00230924. [DOI] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Jespersen B, Nielsen J. Reciprocal Ia inhibition between ankle flexors and extensors in man. J Physiol. 1987;389:163–85. doi: 10.1113/jphysiol.1987.sp016652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain. 2003;126:495–507. doi: 10.1093/brain/awg036. [DOI] [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Nielsen J. Reciprocal inhibition in hemiplegic patients – a longitudinal study. Suppl Clin Neurophysiol. 2000;53:187–91. doi: 10.1016/s1567-424x(09)70155-2. [DOI] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Spinal mechanisms in man contributing to reciprocal inhibition during voluntary dorsiflexion of the foot. J Physiol. 1989;416:255–72. doi: 10.1113/jphysiol.1989.sp017759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMyer WE. Technique of the neurological examination. 5. New York: McGraw-Hill; 2004. [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Morin C, Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of Ia fibers: a study in man and cat. J Physiol (Lond) 1987;389:729–56. doi: 10.1113/jphysiol.1987.sp016680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Tanaka R. Disynaptic inhibition of spinal motoneurones from the motor cortex in the monkey. J Physiol. 1976;258:467–87. doi: 10.1113/jphysiol.1976.sp011431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido A, Tanaka N, Stein RB. Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol. 2004a;82:238–48. doi: 10.1139/y04-017. [DOI] [PubMed] [Google Scholar]
- Kido A, Tanaka N, Stein RB. Spinal reciprocal inhibition in human locomotion. J Appl Physiol. 2004b;96:1969–77. doi: 10.1152/japplphysiol.01060.2003. [DOI] [PubMed] [Google Scholar]
- Lance J. Symposium synopsis. In: Feldman RG, Young RR, Koella WP, editors. Spasticity: disordered motor control. Chicago: Symposia Specialists, Inc; 1979. pp. 485–94. [Google Scholar]
- Mizuno Y, Tanaka R, Yanagisawa N. Reciprocal group I inhibition on triceps surae motoneurons in man. J Neurophysiol. 1971;34:1010–7. doi: 10.1152/jn.1971.34.6.1010. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity – from a basic science point of view. Acta Physiol (Oxf) 2007;189:171–80. doi: 10.1111/j.1748-1716.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- Okuma Y, Lee RG. Reciprocal inhibition in hemiplegia: correlation with clinical features and recovery. Can J Neurol Sci. 1996;23:15–23. doi: 10.1017/s0317167100039135. [DOI] [PubMed] [Google Scholar]
- Petersen N, Morita H, Nielsen J. Evaluation of reciprocal inhibition of the soleus H-reflex during tonic plantar flexion in man. J Neurosci Methods. 1998;84:1–8. doi: 10.1016/s0165-0270(98)00044-2. [DOI] [PubMed] [Google Scholar]
- Schindler-Ivens S, Shields RK. Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. Exp Brain Res. 2000;133:233–41. doi: 10.1007/s002210000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R. Reciprocal Ia inhibition during voluntary movements in man. Exp Brain Res. 1974;21:529–40. doi: 10.1007/BF00237171. [DOI] [PubMed] [Google Scholar]
- Yanagisawa N, Tanaka R, Ito Z. Reciprocal Ia inhibition in spastic hemiplegia of man. Brain. 1976;99:555–74. doi: 10.1093/brain/99.3.555. [DOI] [PubMed] [Google Scholar]