Abstract
Innate immunity activation largely depends on recognition of microorganism structures by Pattern Recognition Receptors (PRRs). PRR downstream signaling results in production of pro- and anti-inflammatory cytokines and other mediators. Moreover, PRR engagement in antigen-presenting cells initiates the activation of adaptive immunity. Recent reports suggest that for the activation of innate immune responses and initiation of adaptive immunity, synergistic effects between two or more PRRs are necessary. No systematic analysis of the interaction between the major PRR pathways were performed to date. In this study, a systematical analysis of the interactions between PRR signaling pathways was performed. PBMCs derived from 10 healthy volunteers were stimulated with either a single PRR ligand or a combination of two PRR ligands. Known ligands for the major PRR families were used: Toll-like receptors (TLRs), C-type lectin receptors (CLRs), NOD-like receptors (NLRs), and RigI-helicases. After 24 h of incubation, production of tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), IL-6, and IL-10 was measured in supernatants by enzyme-linked immunosorbent assay (ELISA). The consistency of the PRR interactions (both inhibitory and synergistic) between the various individuals was assessed. A number of PRR-dependent signaling interactions were found to be consistent, both between individuals and with regard to multiple cytokines. The combinations of TLR2 and NOD2, TLR5 and NOD2, TLR5 and TLR3, and TLR5 and TLR9 acted as synergistic combinations. Surprisingly, inhibitory interactions between TLR4 and TLR2, TLR4 and Dectin-1, and TLR2 and TLR9 as well as TLR3 and TLR2 were observed. These consistent signaling interactions between PRR combinations may represent promising targets for immunomodulation and vaccine adjuvant development.
INTRODUCTION
The first step in mounting an appropriate host defense to infection is the recognition of pathogens by the innate immune system. This recognition is mediated through so-called pattern recognition receptors (PRRs) expressed on the cell membrane of cells of the innate immune system (1, 2). The recognition of pathogens by PRRs leads on the one hand to the activation of inflammation, or innate host defense, and on the other hand to the initiation of adaptive immunity, and eventually to immunological memory, as pursued in vaccination strategies.
PRRs recognize Pathogen-Associated Molecular Patterns (PAMPs), conserved motifs derived from a broad spectrum of pathogens, including fungi, bacteria, parasites, and viruses (1, 2). Hence, these receptors can provide highly specific recognition of a vast range of microbes (3). Recognition of a PAMP by a PRR results in the activation of the innate immune system via several intracellular signaling pathways that are described in more detail elsewhere (4–6). Several major families of PRRs have been described to date, including the Toll-like receptors (TLRs), C-type lectin receptors (CLRs), NOD-like receptors (NLRs), and RigI-helicases (7–9). TLRs and CLRs are present on the cell membrane and in endosomes, while NLRs and RigI-helicases are intracellular microbial sensors (2, 10).
Important adaptor molecules of the TLR intracellular pathways are MyD88, TRIF, TRAM, and MAL/TIRAP (11). Other adaptor molecules such as Syk and Raf-1 (in the case of CLRs) or Rip2/RICK (in the case of some NLRs such as NOD1/NOD2) are also involved in intracellular signaling (12, 13). PRRs recruit one or more of these adaptor molecules in order to provide specific signaling (5). Activation of signaling pathways ultimately leads to the production of pro- and/or anti-inflammatory cytokines, such as those mediated by the transcription factors nuclear factor κB (NF-κB) and activating factor 1 (AP1), that induce production of inflammatory cytokines and shape the subsequent adaptive immune response (7, 14, 15).
Previous studies have indicated that some PRRs are able to interact with each other, thereby modulating the magnitude and/or type of cytokine production (4, 16–18), with synergistic or inhibitory effects or both. However, whether these interactions are generally embedded in the innate immune system and biologically conserved between different individuals is not known. The identification of the most consistent PRR interactions would provide insight into the interplay of the signaling pathways that modulate cytokine responses in the majority of the individuals in a population, with important consequences for the design of vaccine adjuvants. In this study, we investigated signaling interactions between several PRRs in order to identify biologically conserved signaling in cytokine responses of innate immune cells. Ultimately, this could lead to combinations of PRR agonists that can be used as a vaccine adjuvant.
MATERIALS AND METHODS
Volunteers.
Blood samples were collected from 10 healthy volunteers. After informed consent was obtained, blood was collected into 10-ml EDTA tubes (BD, Plymouth, United Kingdom).
PBMC isolation and stimulation with PRR ligands.
Peripheral blood mononuclear cells (PBMCs) were isolated as described previously (19). Briefly, a PBMC fraction was obtained by differential centrifugation over Ficoll-Paque. PBMCs (5 × 105 cells per well) were incubated for 24 h at 37°C in round-bottom 96-well plates (Cellstar; Greiner Bio-one, Alphen a/d Rijn, the Netherlands) with a single ultrapure PRR ligand or a combination of two. RPMI 1640 Dutch modification culture medium (Sigma-Aldrich, Zwijndrecht, the Netherlands) was used, which was supplemented with 1% gentamicin, 1% l-glutamine, and 1% pyruvate (Life Technologies, Nieuwerkerk, the Netherlands). The concentrations of the different ultrapure ligands used are based on literature research and experience in our laboratory and are described in Table 1. These concentrations give a robust but nonmaximal response of most cytokines if used as a single ligand to stimulate PBMCs. This allows detection of increases in cytokine production upon combination with another ligand. Therefore, the concentrations used are suitable to study synergistic or inhibitory effects.
Table 1.
Ultrapure PRR ligands used in stimulation experiments with PBMCs
| PRR | Ligand | Concn | Origin | Median cytokine production |
|---|---|---|---|---|
| TLR2 | Pam3Cys | 10 μg/ml | EMC Microcollections, Tuebingen,Germany | IL-1β, 445 pg/ml; TNF-α, 270 pg/ml; IL-6, 5,462 pg/ml; IL-10, 145.4 pg/ml |
| TLR3 | Poly(I·C) | 50 μg/ml | Invivogen, San Diego, CA | IL-1β, 39 pg/ml; TNF-α, 143.5 pg/ml; IL-6, 236.5 pg/ml; IL-10, 15.7 pg/ml |
| TLR4 | E. coli LPS (O55,B5) | 1 ng/ml | Sigma-Aldrich, St. Louis, MO (further purified as described previously [20]) | IL-1β, 525 pg/ml; TNF-α, 315.5 pg/ml; IL-6, 9,200 pg/ml; IL-10, 279 pg/ml |
| TLR5 | Flagellin | 1 μg/ml | Invivogen, San Diego, CA | IL-1β, 247 pg/ml; TNF-α, 145.5 pg/ml; IL-6, 5,775 pg/ml; IL-10, 139.2 pg/ml |
| TLR9 | CpG | 10 μg/ml | Invivogen, San Diego, CA | IL-1β, 39 pg/ml; TNF-α, 143.5 pg/ml; IL-6, 236.5 pg/ml; IL-10, 15.7 pg/ml |
| NOD2 | Muramyldipeptide (MDP) | 10 μg/ml | Sigma-Aldrich, Buchs, Switzerland | IL-1β, 39 pg/ml; TNF-α, 78 pg/ml; IL-6, 121.2 pg/ml; IL-10, 7 pg/ml |
| Dectin-1 | β-Glucan | 10 μg/ml | Kindly provided by G. D. Brown, University of Aberdeen, United Kingdom | IL-1β, 39 pg/ml; TNF-α, 78 pg/ml; IL-6, 17.3 pg/ml; IL-10, 7 pg/ml |
| Mannose receptor (MR) | Mannan | 10 μg/ml | Sigma-Aldrich, Buchs, Switzerland | IL-1β, 39 pg/ml; TNF-α, 78 pg/ml; IL-6, 15.6 pg/ml; IL-10, 4.7 pg/ml |
After stimulation, the plates were centrifuged (8 min, 1,700 rpm, room temperature [RT]) and the supernatants were collected and stored at −20°C until analysis. The stimulation experiments were performed in duplicate, and supernatants from duplicate wells were pooled for cytokine determination using enzyme-linked immunosorbent assays (ELISA). Each combination of ligands was used with PBMCs of 10 different volunteers.
ELISA.
Cytokine concentrations in culture supernatants were determined using commercially available ELISA kits for tumor necrosis alpha (TNF-α) and interleukin-1 beta (IL-1β) (R&D Systems, Abingdon, Oxfordshire, United Kingdom) and IL-10 and IL-6 (Pelikine Compact; Sanquin Reagents, Amsterdam, the Netherlands). The measurements were performed according to the manufacturer's instructions.
Calculations and statistical analysis.
The criteria according to which interactions between two specific signaling pathways downstream of activated PRRs were considered synergistic or inhibitory were defined before the start of the experiments. A “synergistic effect” was defined as a cytokine response to a combination of ligands that was at least 1.5-fold higher than the sum of the cytokine responses induced by each of the individual ligands. An “inhibitory effect” was defined as a cytokine response to a combination of ligands that was less than or equal to 0.75-fold of the sum of the cytokine responses to each of the individual ligands and lower than the cytokine response to a single ligand or to both of the ligands. The definitions of both synergy and inhibition are further explained in Fig. 1. All other patterns were designated “no effect/additive effect.”
Fig 1.
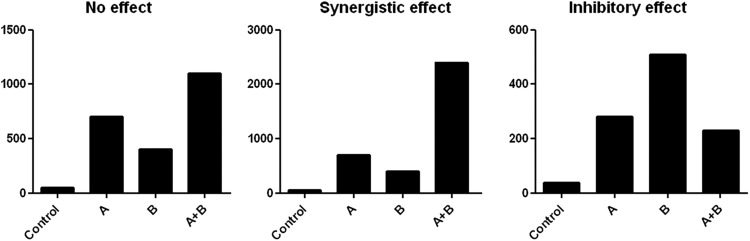
Interaction classifications. On the y axis, production of a cytokine is depicted. On the x axis, the ligands (A and B) used to stimulate the PBMCs are depicted; PBMCs were stimulated with either a single ligand or a combination of two PRR ligands. To identify a synergistic effect, the cytokine production after stimulation with both ligands combined should be higher than the combined values of the two single effects. An inhibitory effect was defined as a cytokine response to a combination of ligands that was maximally 0.75-fold of the sum of the cytokine responses to either of the individual ligands and lower than the cytokine response to a single ligand or to both of the ligands. A cytokine production of the combination of stimuli higher than production of each of the single stimulations, but not higher than the sum of the two, is considered additive and is thus classified as “no effect/additive effect.”
An effect was considered present (biologically conserved) in the majority of the healthy volunteers tested if a type of interaction was demonstrated in at least 7 of the 10 subjects. If there was more variation between subjects, it was defined as a “variable effect.”
The biologically conserved interactions (depicted in red and blue in Fig. 2) were analyzed statistically using Wilcoxon matched-pair tests. Sums of cytokine concentrations after single-ligand stimulations were compared to cytokine concentrations after combined stimulations. Differences were considered significant if P < 0.05.
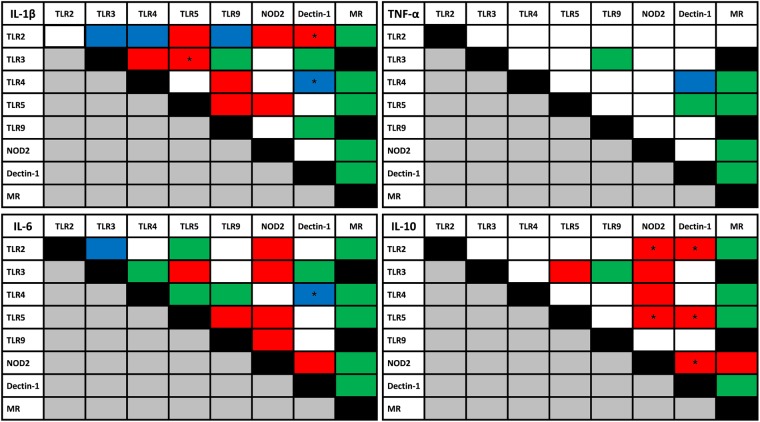
Fig 2.
Overview of interaction PRRs. Color indicates type of interaction in at least 7 of 10 of the healthy volunteers. Data represent results of an ELISA on culture supernatants after 24 h of stimulation at 37°C with combinations of ligands as specified in Table 1. *, P < 0.05 (Wilcoxon matched-pair test, comparison of cytokine production upon stimulation with both ligands with sum of cytokine production values upon stimulation with each of the ligands separately). Red, synergistic effect; green, no effect/additive effect; blue, inhibitory effect; white, variable effect; black, experiment not performed.
RESULTS
Interaction studies.
The interactions between different PRR ligands are depicted in Fig. 2. An inhibitory effect for comparisons of the production of IL-1β, IL-6, and TNF-α between TLR4 and Dectin-1 (significant for IL-1β and IL-6) as well as TLR3 and TLR2 was found for IL-1β and IL-6. Similar effects were found for combinations of TLR4 and TLR2 or TLR2 and TLR9 induction of IL-1β production.
The combination of TLR5 and TLR9 resulted in synergistic effects for IL-1β and IL-6 production. Furthermore, synergistic effects on IL-1β, IL-6, and IL-10 production were observed for the combinations TLR2 and NOD2 (significant for IL-10), TLR5 and NOD2 (significant for IL-10), and TLR5 and TLR3 (significant for IL-1β). A synergistic effect was also found for IL-10 and IL-6 production after stimulation of a combination of TLR3 and NOD2 or NOD2 and Dectin-1 (significant for IL-10). Stimulation of TLR5 and Dectin-1 resulted in a synergistic effect on IL-10 production, but other cytokines showed only variable effects.
Of interest, the synergistic effects of TLR3 and TLR5 and of TLR5 and TLR9 stimulation mentioned above were observed in at least 9 of the healthy volunteers for IL-1β (significant) and IL-10 production and for IL-1β production (significant), respectively (Fig. 3).
Fig 3.
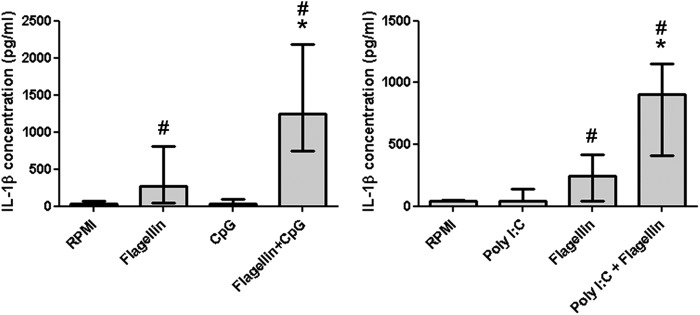
Flagellin interactions. PBMCs were stimulated for 24 h with the indicated ligand or combinations of ligands, and cytokines were measured in the supernatants by ELISA (n = 10 volunteers). The bars indicate a synergistic interaction of flagellin with CpG or poly(I·C) for the production of IL-1β. Data are expressed as medians with interquartile ranges. *, significantly different (P < 0.05) from stimulation with single ligands and the sum of both single ligands; #, significantly different (P < 0.05) from no-ligand (RPMI) results (Wilcoxon matched-pair test).
Several PRRs did not interact with others. In particular, mannose-receptor-dependent induction of cytokine production seemed independent of activation of other PRRs.
TLR2 and TLR4 interaction.
The results of our study indicate an inhibitory effect between TLR2 and TLR4 for the production of IL-1β. This is in contrast with previous studies (18, 21–25). In order to study the interaction between TLR2 and TLR4 in more detail, a dose-response experiment with lipopolysaccharide (LPS) and Pam3Cys was performed. Increasing concentrations of LPS or Pam3Cys resulted in increased IL-1β and TNF-α production, but the combination of the two ligands showed an inhibitory effect (Fig. 4), confirming our initial results.
Fig 4.
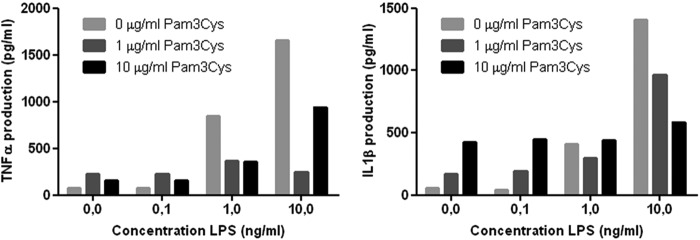
Dose response. LPS plus Pam3Cys PBMCs were stimulated for 24 h with the indicated ligand or combinations of ligands, and cytokines were measured in the supernatants by ELISA (n = 2 volunteers). The bars indicate different concentrations of Pam3Cys, while increasing concentrations of LPS are depicted on the x axis. Increasing concentrations of LPS and Pam3Cys resulted in increased IL-1β and TNF-α production for single ligands, but no synergistic production of either IL-1β or TNF-α was observed when LPS and Pam3Cys were combined. Data are depicted as medians.
Interindividual variation.
In this study, the interactions between PRRs were highly variable between subjects. To illustrate the interindividual variation in PRR signaling interactions, the interaction between NOD2 and TLR4 for the production of TNF-α in all 10 healthy volunteers is depicted in Fig. 5. In most volunteers, muramyl dipeptide (MDP)-induced TNF-α production was around 100 to 200 pg/ml. LPS stimulation generally resulted in higher levels of TNF-α than MDP stimulation. The response to the combination of the two ligands was highly variable between subjects, and no effects/additive effects, inhibitory effects, or synergistic effects on TNF-α production were observed in the different volunteers.
Fig 5.
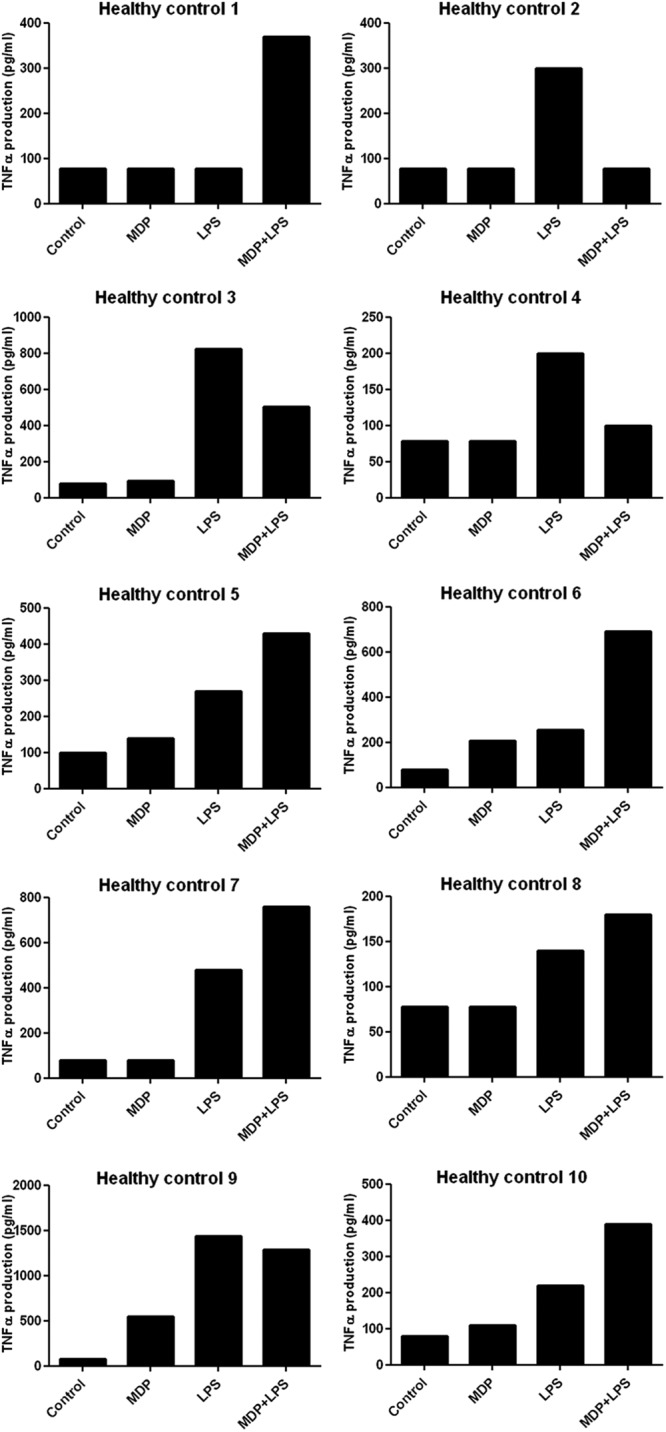
Interindividual variation. Data represent TNF-α production after 24 h of PBMC stimulation with MDP, LPS, or MDP and LPS (n = 10 volunteers). Five individuals exhibited no interaction, three demonstrated an inhibitory effect, and two produced TNF-α in a synergistic manner after combined stimulation.
DISCUSSION
The aims of this study were to systematically investigate the interactions between the best-known PRR pathways on the one hand, and to assess the consistency of these effects in various individuals on the other hand. Knowledge on the consistency of the synergistic combinations of PRR pathways could be used to develop novel vaccine adjuvants that would exert their boosting effect during vaccination in the vast majority of the individuals in a population. This study resulted in the identification of several combinations of PRR ligands that have this potential.
A remarkable, yet not entirely unexpected finding of this study is the large variability between healthy individuals. This variation can be attributed to many factors. One of these is the genetic background of the individuals tested. There are many established variations in genes that are known to influence the function and downstream effects of PRRs, which could naturally influence the cytokine production after stimulation with PRR ligands (26, 27). This interindividual variation has important implications with regard to the design of immunotherapeutic approaches based on PRR-mediated effects. For the development of vaccine adjuvants based on PRR-ligand combinations, only those combinations that result in synergistic cytokine production in the majority of the population are likely to be of therapeutic value.
In this respect, in the present report we identify several consistent PRR interactions that were present in the majority of individuals tested. The combinations of ligands most consistently associated with synergistic effects were NOD2 and TLR2, NOD2 and TLR5, TLR3 and TLR5, and TLR5 and TLR9. In particular, the interaction of TLR5 with TLR3 and TLR9 was remarkably consistent, since it was present in at least 9 of the studied volunteers for all the cytokines studied. These combinations could therefore represent candidates for the development of novel vaccine adjuvants. In the pursuit of identifying novel vaccine adjuvants to activate the immune system, synergy for IL-10 production could be disadvantageous, since IL-10 is known to have potent anti-inflammatory effects (28). In that respect, the combination of TLR5 and TLR9 appears also promising, because IL-1β, but not IL-10, is produced in a synergistic manner after engagement of these receptors.
In addition to the synergistic effects of some PRR combinations as detailed above, a number of biologically conserved inhibitory combinations were also documented: TLR4 and Dectin-1, TLR3 and TLR2, TLR2 and TLR9, and TLR4 and TLR2 result in impaired cytokine production. These findings are surprising, as they contradict a number of studies published in the literature suggesting additive or even synergistic effects of ligands for these receptors. This is particularly true for the TLR4-TLR2 costimulation (18, 21–25). We have performed additional experiments to assess the validity of our observation, and a dose-response experiment with LPS and Pam3Cys confirmed our initial findings. The most likely explanation for the difference between our findings and those in previous studies is that we have used primary human PBMCs, and we assessed the interindividual consistency of these stimulations, while previous studies have mostly used either mouse cells or human cell lines (18, 21–25).
Next to these observations regarding the consistency of the biological effect of PRR-ligand combinations, additional important observations can be made. First, the interaction patterns differed substantially between the four measured cytokines. There are relatively more combinations of ligands that exhibit synergism in the production of IL-10, IL-1β, and IL-6 than of TNF-α. These differences could theoretically be due to the existence of (partly) separate signaling pathways for these cytokines. It is also conceivable that a pathway leading to production of one cytokine is more biologically conserved than that leading to another cytokine.
Second, it appears that there are several main “intracellular highways” which comprise PRRs that preferentially interact with each other. Multiple PRRs within one highway effectively influence each other, while PRRs in different highways do not appear to interact. The mannose receptor, for example, appears not to interact with the signaling pathways of other PRRs and thus can be regarded as an isolated highway. Alternatively, our results of mannose-receptor stimulation could have been influenced by the very low number of mannose receptors present on the surface of monocytes (29). TLR4 signaling, although to a lesser extent, also appears to represent an isolated pathway: when PBMCs are stimulated with LPS, a relatively high number of combinations of ligands result in a noninteractive/additive cytokine production. In addition to the mannose-receptor and TLR4 pathways, we can also distinguish a group of receptors which appear to show complex interactions with each other, that may be considered a “TLR2-Dectin-1-NOD2-TLR3-TLR5 highway.” These receptors seem to interact with each other very effectively. TLR9 also seems to be involved in interactions with the intracellular pathways from this group of receptors, but specifically for the induction of IL-1β and IL-6 production, and to a lesser extent for IL-10 and TNF-α production. In particular, cytokine responses through dectin-1 signaling are dependent on these interactions, since stimulation with β-glucan alone results in very limited cytokine production. Therefore, it is possible that dectin-1 signaling is dedicated to the regulation and enforcement of the effect of other PRRs, rather than the activation of the immune system by itself.
Several limitations apply to our study. First of all, the molecular mechanisms behind the interactions described in the present report have not been explored and should be evaluated in future studies. These interactions could be the result of intracellular interactions between signaling pathways, but they could also be regulated via release of soluble regulatory factors such as cytokines. Furthermore, altered expression of receptors by (combinations of) ligands could also confound true interaction effects between these receptors. Future studies that focus on the use of the herein-identified combinations of ligands as vaccine adjuvants should include specific cytokines with specific properties (e.g., IP-10 or interferon [IFN] for the TRIF pathway, or potential indicators for Th1 responses) and detailed data on dendritic cells and T cell responses. Finally, multiple time points could be included in future studies; in particular, information on early responses (4 to 6 h) would be helpful.
In conclusion, the present report provides new information with regard to the pathway interactions downstream of PRR signaling that lead to the induction of innate host responses and initiate the adaptive immunity. Unraveling these signaling cascades not only is vital for the understanding of the innate immune system in general, but also could help to identify new targets for immunomodulation (3, 30). Furthermore, the consistency of the biological synergistic interactions between several combinations of PRRs may be important to the field of vaccine adjuvant research (18). Robust activation of the innate immune system, subsequently evoking an adaptive immune response involving memory T cells, is vital for the effectiveness of a vaccine. Based on the present data, specific combinations such as TLR5/TLR3 and TLR5/TLR9 appear particularly promising, as their synergistic interactions were biologically conserved in the majority of the individuals tested in the present study population. Therefore, the potential of these combinations of ligands as vaccine adjuvants should be further explored to improve vaccination strategies. Nevertheless, care should be taken that certain ligand combinations do not result in a too-pronounced immune response resulting in tissue damage and organ failure. Hence, future studies should take into account the balance between the immune response required for immunization and potential harmful effects.
ACKNOWLEDGMENTS
T.S.P. and L.A.B.J. were supported within a TI-Pharma network. M.G.N. was supported by a Vici grant of the Netherlands Organization for Scientific Research.
We certify that there is no conflict of interest regarding the material discussed in the manuscript.
Footnotes
Published ahead of print 23 January 2013
REFERENCES
- 1. Bourhis LL, Werts C. 2007. Role of Nods in bacterial infection. Microbes Infect. 9: 629– 636 [DOI] [PubMed] [Google Scholar]
- 2. Shaw MH, Reimer T, Kim YG, Nunez G. 2008. NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr. Opin. Immunol. 20: 377– 382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Testro AG, Visvanathan K. 2009. Toll-like receptors and their role in gastrointestinal disease. J. Gastroenterol. Hepatol. 24: 943– 954 [DOI] [PubMed] [Google Scholar]
- 4. Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, Hellman J. 2007. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J. Immunol. 178: 1164– 1171 [DOI] [PubMed] [Google Scholar]
- 5. Fitzgerald KA, Chen ZJ. 2006. Sorting out Toll signals. Cell 125: 834– 836 [DOI] [PubMed] [Google Scholar]
- 6. Lee MS, Kim YJ. 2007. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu. Rev. Biochem. 76: 447– 480 [DOI] [PubMed] [Google Scholar]
- 7. Kumar H, Kawai T, Akira S. 2009. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 388: 621– 625 [DOI] [PubMed] [Google Scholar]
- 8. Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. U. S. A. 95: 588– 593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Vliet SJ, den Dunnen J, Gringhuis SI, Geijtenbeek TB, van Kooyk Y. 2007. Innate signaling and regulation of Dendritic cell immunity. Curr. Opin. Immunol. 19: 435– 440 [DOI] [PubMed] [Google Scholar]
- 10. Kawai T, Akira S. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7: 131– 137 [DOI] [PubMed] [Google Scholar]
- 11. Jeong E, Lee JY. 2011. Intrinsic and extrinsic regulation of innate immune receptors. Yonsei Med. J. 52: 379– 392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. den Dunnen J, Gringhuis SI, Geijtenbeek TB. 2010. Dusting the sugar fingerprint: C-type lectin signaling in adaptive immunity. Immunol. Lett. 128: 12– 16 [DOI] [PubMed] [Google Scholar]
- 13. Shaw MH, Kamada N, Warner N, Kim YG, Nunez G. 2011. The ever-expanding function of NOD2: autophagy, viral recognition, and T cell activation. Trends Immunol. 32: 73– 79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baek YS, Haas S, Hackstein H, Bein G, Hernandez-Santana M, Lehrach H, Sauer S, Seitz H. 2009. Identification of novel transcriptional regulators involved in macrophage differentiation and activation in U937 cells. BMC Immunol. 10: 18 doi:10.1186/1471-2172-10-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kufer TA, Sansonetti PJ. 2007. Sensing of bacteria: NOD a lonely job. Curr. Opin. Microbiol. 10:62– 69 [DOI] [PubMed] [Google Scholar]
- 16. Ferwerda G, Meyer-Wentrup F, Kullberg BJ, Netea MG, Adema GJ. 2008. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell. Microbiol. 10: 2058– 2066 [DOI] [PubMed] [Google Scholar]
- 17. Watanabe T, Kitani A, Murray PJ, Strober W. 2004. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat. Immunol. 5: 800– 808 [DOI] [PubMed] [Google Scholar]
- 18. Zhu Q, Egelston C, Vivekanandhan A, Uematsu S, Akira S, Klinman DM, Belyakov IM, Berzofsky JA. 2008. Toll-like receptor ligands synergize through distinct dendritic cell pathways to induce T cell responses: implications for vaccines. Proc. Natl. Acad. Sci. U. S. A. 105: 16260– 16265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kox M, van Velzen JF, Pompe JC, Hoedemaekers CW, van der Hoeven JG, Pickkers P. 2009. GTS-21 inhibits pro-inflammatory cytokine release independent of the Toll-like receptor stimulated via a transcriptional mechanism involving JAK2 activation. Biochem. Pharmacol. 78: 863– 872 [DOI] [PubMed] [Google Scholar]
- 20. Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165: 618– 622 [DOI] [PubMed] [Google Scholar]
- 21. Chapekar MS, Zaremba TG, Kuester RK, Hitchins VM. 1996. Synergistic induction of tumor necrosis factor alpha by bacterial lipopolysaccharide and lipoteichoic acid in combination with polytetrafluoroethylene particles in a murine macrophage cell line RAW 264.7. J. Biomed. Mater. Res. 31: 251– 256 [DOI] [PubMed] [Google Scholar]
- 22. Jung YO, Cho ML, Lee SY, Oh HJ, Park JS, Park MK, Park MJ, Ju JH, Kim SI, Park SH, Kim HY, Min JK. 2009. Synergism of toll-like receptor 2 (TLR2), TLR4, and TLR6 ligation on the production of tumor necrosis factor (TNF)-alpha in a spontaneous arthritis animal model of interleukin (IL)-1 receptor antagonist-deficient mice. Immunol. Lett. 123: 138– 143 [DOI] [PubMed] [Google Scholar]
- 23. Sato S, Nomura F, Kawai T, Takeuchi O, Muhlradt PF, Takeda K, Akira S. 2000. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J. Immunol. 165: 7096– 7101 [DOI] [PubMed] [Google Scholar]
- 24. Shinohara M, Hirata K, Yamashita T, Takaya T, Sasaki N, Shiraki R, Ueyama T, Emoto N, Inoue N, Yokoyama M, Kawashima S. 2007. Local overexpression of toll-like receptors at the vessel wall induces atherosclerotic lesion formation: synergism of TLR2 and TLR4. Arterioscler. Thromb. Vasc. Biol. 27: 2384– 2391 [DOI] [PubMed] [Google Scholar]
- 25. Xu WY, Wang L, Wang HM, Wang YQ, Liang YF, Zhao TT, Wu YZ. 2007. TLR2 and TLR4 agonists synergistically up-regulate SR-A in RAW264.7 through p38. Mol. Immunol. 44: 2315– 2323 [DOI] [PubMed] [Google Scholar]
- 26. Henckaerts L, Nielsen KR, Steffensen R, Van Steen K, Mathieu C, Giulietti A, Wouters PJ, Milants I, Vanhorebeek I, Langouche L, Vermeire S, Rutgeerts P, Thiel S, Wilmer A, Hansen TK, Van den Berghe G. 2009. Polymorphisms in innate immunity genes predispose to bacteremia and death in the medical intensive care unit. Crit. Care Med. 37: 192– 193 [DOI] [PubMed] [Google Scholar]
- 27. Kullberg BJ, Ferwerda G, de Jong DJ, Drenth JP, Joosten LA, Van der Meer JW, Netea MG. 2008. Crohn's disease patients homozygous for the 3020insC NOD2 mutation have a defective NOD2/TLR4 cross-tolerance to intestinal stimuli. Immunology 123: 600– 605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J. 2010. Biology of interleukin-10. Cytokine Growth Factor Rev. 21: 331– 344 [DOI] [PubMed] [Google Scholar]
- 29. Apostolopoulos V, McKenzie IF. 2001. Role of the mannose receptor in the immune response. Curr. Mol. Med. 1: 469– 474 [DOI] [PubMed] [Google Scholar]
- 30. Crespo-Lessmann A, Juarez-Rubio C, Plaza-Moral V. 2010. Role of toll-like receptors in respiratory diseases. Arch. Bronconeumol. 46: 135– 142 (In Spanish) [DOI] [PMC free article] [PubMed] [Google Scholar]