Abstract
The capacity of the osteoclast (OC) to resorb bone is dictated by cytoskeletal organization which, in turn, emanates from signals derived from the αvβ3 integrin and c-Fms. Syk is key to these signals and, in other cells, this tyrosine kinase exerts its effects via intermediaries including the SLP-adaptors, SLP-76 and BLNK. Thus, we asked if these two SLP proteins regulate OC function. We find BLNK-deficient OCs are normal, while cytoskeletal organization of those lacking SLP-76 is delayed, thus modestly reducing bone resorption, in vitro. Cytoskeletal organization and bone resorption are more profoundly arrested in cultured OCs deficient in BLNK and SLP-76 (double knockout; DKO). On the other hand, stimulated bone resorption, in vivo, is inhibited approximately 40% in either SLP-76−/− or DKO mice. This observation, taken with the fact that DKO OCs are rescued by retroviral transduction of only SLP-76, indicates that SLP-76 is the dominant SLP-family member in the resorptive process. We also find SLP-76 is phosphorylated in a Syk-dependent manner. Furthermore, in the absence of the adaptor protein, integrin-mediated phosphorylation of Vav3, the OC cytoskeleton-organizing guanine nucleotide exchange factor (GEF), is abrogated. In keeping with a central role of SLP-76/Vav3 association in osteoclastic resorption, retroviral transduction of SLP-76, in which the Vav binding site is disrupted (3YF), fails to normalize the cytoskeleton of DKO OCs and the cell’s resorptive capacity. Finally, c-Fms-activated Syk also exerts its OC cytoskeleton-organizing effect in a SLP-76/Vav3-dependent manner.
Keywords: Cells-Monocytes/Macrophages, Diseases-Rheumatoid Arthritis, Molecules-Adhesion Molecules, Processes-Signal Transduction
Introduction
Osteoclasts (OCs), polykaryons of the myeloid lineage, are the exclusive cells responsible for bone resorption and the targets of anti-resorptive therapies used in the treatment of osteoporosis and inflammatory osteolysis. The OC creates a sealing zone at the site of attachment to bone, permitting localized secretion of proteases into an acidic microenvironment needed for skeletal degradation. Formation of the sealing zone requires cytoskeletal rearrangement which creates an actin ring-like structure (1). The αvβ3 integrin plays a central role in the process of actin ring formation and we have focused on delineating the mechanism of αvβ3 signaling in OCs.
Since the OC is of hematopoietic lineage, it is not surprising that it shares signaling pathways with other immune cells, such as those initiated by the T-cell receptor (TCR). Analogous to the TCR, αvβ3 occupancy induces rapid phosphorylation of Src- and Syk-family tyrosine kinases essential for actin ring formation and OC function (2).
Src-homology 2-containing leukocyte adaptor proteins (SLP) are expressed throughout the hematopoietic lineage and phosphorylated by Syk family kinases in multiple cell types (3). SLP-76 is the dominant family member expressed in T-cells and involved in TCR signaling. SLP-76 also regulates signal transduction in platelets, mast cells, dendritic cells, and neutrophils, while SLP-65/BLNK (B-cell linker) mediates B-cell receptor (BCR) signaling (3). In both T- and B-cells, these adaptor proteins are phosphorylated by Syk family kinases after receptor engagement, allowing for assembly of a multimeric complex through three structural domains. The N-terminal acidic-rich region contains three phospho-inducible tyrosines (Y112, Y128, and Y145), which are binding sites for the GEF, Vav, the adaptor, Nck, and Tec kinases. The central proline-rich region associates with PLCγ and the Grb2 family member, Gads, via an RxxK motif. In the C-terminus, the adaptor ADAP (adhesion and degranulation-promoting adaptor protein) and HPK1 kinase bind via the SH2 domain (3). The function of this scaffolding complex was first characterized in the context of T-cell development as T cells in SLP-76−/− mice fail to mobilize calcium or induce NF-AT and AP1 gene regulation following TCR engagement and are arrested in the double negative (DN) three stage (DN3) CD4−CD8− (4–6).
In addition to its role in T-cell development, SLP-76 impacts the cytoskeleton in many cells. It is required, for example, to form the immune synapse in T-cells, a process dependent on cytoskeletal remodeling (7). SLP-76 is also involved in platelet spreading on fibrinogen, a dynamic cytoskeletal event regulated by the αIIbβ3 integrin (8–10).
We find that OCs lacking Syk and Vav3 fail to spread normally and do not form actin rings, reflecting a disorganized cytoskeleton. As a result, Syk- or Vav3- deficient OCs are unable to efficiently resorb bone (2, 11). We demonstrate that SLP-76 is the key SLP family member in the OC, promoting cytoskeletal organization and resorptive function by linking Syk to Vav3 in an αvβ3- and c-Fms-dependent manner.
Materials and Methods
Mice
BLNK−/−, SLP-76+/−, Syk+/−, LAT−/−, and Gads−/− mice were previously described (4, 12–15). BLNK mice on a C57BL/6 background were purchased from Jackson Labs; Syk+/− mice were a gift from Dr. Victor Tybulewicz (National Institute for Medical Research, London) and are on a 129.sv background; C57BL/6 LAT−/− mice were a gift from Dr. Larry Samelson (National Institutes of Health, Bethesda MD); C57BL/6 Gads mice were provided by Andrew Chan (Genentech). SLP-76+/− were backcrossed to C57BL/6 background >10 generations. All mice were housed in the animal facility at Washington University School of Medicine and were maintained according to the guidelines set by the Association for Assessment and Accreditation of Laboratory Animal Care. All animal studies were approved by the Animal Studies Committee of Washington University School of Medicine.
To overcome the perinatal lethality of SLP-76−/−, DKO, and Syk−/− mice, we used a bone marrow transplantation strategy to generate radiation chimeras. Embryos were collected from heterozygous Syk+/−, SLP-76+/−, or SLP-76+/−BLNK−/− breeding pairs at E16-20; fetal liver cells were isolated from and injected into the tail vein of lethally irradiated (1000Gy) wild-type C57/BL6 recipient mice. Chimeras were used for in vivo experiments or as a source of bone marrow derived macrophages, following 4 weeks marrow reconstitution.
In vivo studies utilized radiation chimeras generated from SLP-76−/− and SLP-76+/? littermates or SLP-76+/?/BLNK−/− (control) and SLP-76−/−/BLNK−/− (DKO) littermates (n=4–5 in each group). 10 μg of PTH(1-34) (Bachem) in 25 μl of buffer (0.1% BSA 0.1mM HCl) or buffer alone was injected supracalvarially every six hours for four days. Mice were sacrificed and calvaria were isolated for histology. Fasting serum was collected and analyzed for CTx using the RatLaps ELISA in duplicate. (Immunodiagnostic Systems Inc). Three calvarial sections from each mouse were stained for TRAP and analyzed histomorphometrically at 16x using the Osteomeasure software.
Macrophage Isolation and Osteoclast Culture
Following sacrifice by CO2 inhalation, the long bones from mice were isolated and flushed with α-MEM. Whole bone marrow was cultured on bacterial plastic, which promotes growth of monocyte cells and prevents adherence of mesenchymal cells. The cells were cultured with α-MEM containing 10% inactivated fetal bovine serum, 100 IU/ml penicillin, 100μg/ml streptomycin, (α-10 media) supplemented with 1:10 CMG (conditioned media supernatant containing recombinant M-CSF (16)). On day four of culture, the remaining cells, which have been selected for as a pure population of macrophages, were lifted using 1x tryspin/EDTA and re-plated at 5×103 cells in a tissue-culture treated plastic 96-well plate in α-10 media supplemented with 100ng/ml GST-RANKL and 1:50 CMG and cultured over time (3–6 days) and stained for TRAP (Sigma-Aldrich). In Figure S2, 50ng/ml purified RANKL (Peprotech) and 10 ng/ml M-CSF (R & D Systems) was used as previously described (17). For actin ring and pit resorptive assays, cells were cultured as described above on bovine bone slices. Actin rings were analyzed using Alexa-488 phalloidin (Invitrogen) after fixation in 4% paraformaldehyde and permeablization with 0.1% TritonX-100. For resorptive pits, the cells were removed from the bone slices by mechanical agitation and incubated with peroxidase conjugated wheat germ agglutinin (WGA) (Sigma-Aldrich) and stained with 3,3′-diaminobenzidine (Sigma-Aldrich).
For pre-osteoclasts integrin signaling, 5×106 BMMs were re-plated in 15cm plates in α-10 and 100ng/ml GST-RANKL and 1:50 CMG. After three to four days, the cells were serum and cytokine starved for four hours, lifted with 0.5%EDTA in PBS, washed in α-MEM and plated on plastic coated with 5μg/ml vitronectin (Millipore) blocked with BSA, or maintained in suspension for 30 minutes. For M-CSF signaling, 1.5×106 BMMs were plated in 10cm plates in α-10 with 100ng/ml GST-RANKL and 1:50 CMG for three days, serum and cytokine starved for eight hours and stimulated with 100ng/ml human recombinant M-CSF or vehicle for five minutes.
Plasmids and Retroviral Transduction
The SLP-76-pMX-IRES-BSR was sub-cloned from a SLP-76 plasmid, previously described (18), into pMX-IRES-BSR by introducing the BamHI 5′ and XhoI 3′ restriction sites with an HA-tag at the 3′ end. The 3YF and R448K mutations were generated by mutating SLP-76-pMX vector using site-directed mutagenesis (Stratagene). The Δ157-222 construct was created by standard molecular biology techniques. 8μg of the pMX retroviral vector was transfected into plat-E packaging cell line (19) using the Transfectol reagent (GeneChoice) according to the manufacturer’s instructions. The viral supernatant was collected 48 hours post-transfection and added to BMMs in α-10 with 1:10 CMG and 4μg/ul polybrene (Sigma-Aldrich). After 24hr incubation with the virus, transduced cells were selected using 1μg/ml blasticidin (Calbiochem) for three days and plated for osteoclast precursors as described above. Using the constructs described above, GFP was tagged to the N-terminus of WT-SLP-76 and mutants and inserted into the pMX retroviral vector.
RT-PCR
Reverse Transcription-PCR—Total RNA was isolated using RNeasy kits (Qiagen). First-strand cDNA was generated from 1 μg of total RNA using the SuperScript first-strand synthesis system for reverse transcription-PCR (Invitrogen)as instructed by the manufacturer. PCR for DC-STAMP levels was performed with 1μl of diluted cDNA reaction mixture using Taq polymerase and primers 5′ - CCA CGA AGC CCT AGC TGG CT - 3′ and 5′ - CCA GTG CCA GCC GCA ATC AA - 3′ in a volume of 50 μl for 30 amplification cycles (94°, 58°, and 72°C).
Western Blotting and Immunoprecipitation
Cells were washed with ice-cold PBS and lysed in RIPA buffer containing 20mM Tris, pH 7.5; 150mM NaCl; 1mM EDTA; 1mM EGTA; 1% Triton-X-100, 2.5mM sodium pyrophosphate, 1mM β-glycerophosphate; 1mM sodium orthovanadate, 1mM sodium fluoride and 1x protease inhibitor cocktail (Roche). After 10-minute incubation on ice, lysates were cleared of debris by 10 min centrifugation at 14000 rpm. Thirty to forty micrograms were subjected to 8% SDS-PAGE and transferred onto a PVDF membrane. Following blocking with 0.1% casein in PBS, primary antibodies were incubated overnight at 4 degrees and probed with fluorescent-labeled secondary antibodies (Jackson) and detected on the Odyssey Infrared Imaging System (LI-COR Biosciences). For immunoprecipitation studies, 750μg cleared lysates were incubated with 2μg of primary antibody for overnight at four degrees, followed by three hour incubation with Protein A or Protein G sepharose beads. Washed beads were resuspended in 2X SDS loading buffer, boiled for five minutes, centrifuged and subjected to SDS-PAGE gel.
Primary antibodies include: Rabbit anti-Vav3, mouse anti-phosphotyrosine mAB 4G10 (Millipore); Rabbit anti-SLP-76, rabbit anti-β3 integrin, and rabbit anti-BLNK (Cell Signaling); Rabbit anti-Syk (N-19), mouse anti-Vav, mouse anti-NFAT (Santa Cruz); mAb 327, mouse anti-Src provided by Dr. Andrey Shaw, Washington University, sheep-anti SLP-76, was previously described (20).
Rac Assays
Rac Pull-down (Pierce): Cell lysates were prepared according to manufacturer’s instructions. 500 μg of protein was subjected to GST-PAK pull-down (Pierce). Following multiple washes, the beads were resuspended in 2x SDS-loading buffer and analyzed on a 12% SDS-PAGE gel. After transfer, the membrane was immunoblotted for Rac1. Rac G-LISA (Cytoskeleton Inc.): Cell lysates from suspended and adhered cells were prepared using the manufacturer’s lysis buffer. 12.5 μg of protein per well was used in the assay, which was performed according to instructions. Each sample was tested in triplicate.
Statistics
For in vivo studies comparing histological analysis and CTx values between four genotypes, a one way analysis of variance was peformed with Tukey post hoc between group comparisons. All significant values (p<0.05) are reported. The remaining statistics used a student’s t-test, comparing mutant/deficient cells to the wild-type/control group. Cell culture and biochemical data are representative figures from experiments performed in at least duplicate or triplicate. Data are presented as mean +/− S.D.
Results
SLP-76 and BLNK are phosphorylated in OCs by integrin occupancy in a Syk-dependent manner
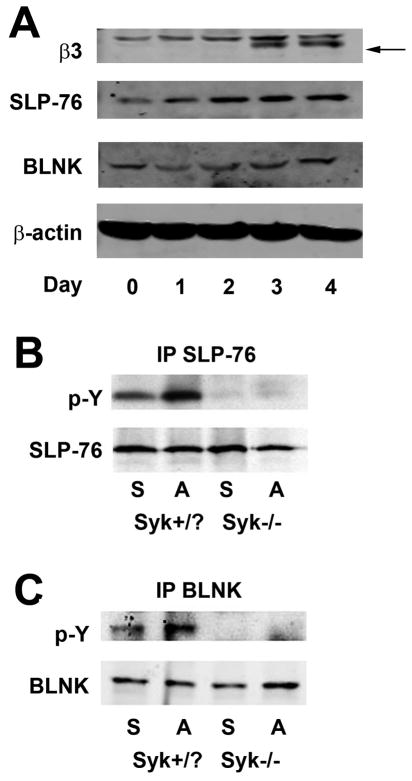
To determine the impact of OC differentiation on expression of SLP-adaptor proteins we cultured wild-type (WT) bone marrow macrophages (BMMs) with M-CSF and RANKL (receptor activator of NF-κB ligand). Immunoblot of cell lysates prepared daily, for five days, reveals that SLP-76 and BLNK are present in all stages of OC differentiation (Figure 1A).
Figure 1. SLP-76 and BLNK are phosphorylated, in OCs, by integrin occupancy in a Syk-dependent manner.
(A) Wild-type BMMs, cultured with M-CSF and RANKL, with time, were lysed and the lysate immunoblotted for SLP-76 and BLNK. β3 integrin subunit (arrow) serves as a marker of OC differentiation. (B and C) Syk+/? and Syk−/− BMMs were cultured with M-CSF and RANKL for four days to generate pre-OCs. The cells were serum and cytokine starved, lifted with 0.5% EDTA and either re-plated on vitronectin-coated plates or maintained in suspension. Cleared cell lysates from adhered (A) or suspended (S) cells were immunoprecipitated for either SLP-76 (B) or BLNK (C) and the immunoprecipitates were immunoblotted for phosphotyrosine and SLP-76 or BLNK.
In T-cells, the Syk-family kinase, Zap-70, phosphorylates SLP-76 after TCR engagement. Similarly, in B-cells, Syk phosphorylates BLNK following BCR ligation (21, 22). Given that Syk is activated by occupied αvβ3 ligation (2), we asked if SLP-76 is also phosphorylated by the integrin, in OCs. To this end, control BMMs, containing at least one wild-type Syk allele (Syk+/?), were cultured with M-CSF and RANKL for three days to generate pre-fusion osteoclasts (pre-OCs), which express the αvβ3 integrin (Figure 1A). Pre-OCs were serum- and cytokine-starved, lifted and either re-plated on the αvβ3 substrate, vitronectin, or maintained in suspension. As an additional control, plates coated with native collagen, which does not bind αvβ3, yielded similar results as those maintained in suspension (data not shown). Immunoprecipitates of SLP-76 and BLNK from suspension or adhesion cell lysates demonstrate that SLP-76 and BLNK are phosphorylated in an integrin-dependent manner (Figure 1B and C). Moreover, integrin-mediated SLP-76 phosphorylation requires Syk, as pre-OCs lacking the tyrosine kinase do not phosphorylate the adaptor protein when plated on vitronectin (Figure 1B and C). Thus, SLP-76 and BLNK are Syk substrates in αvβ3-initiated signaling in OCs.
SLP-76 is required for OC function but not differentiation
In vivo, OCs and bone mass of BLNK−/− mice are indistinguishable from their heterozygous and WT littermates (Figure S1). Similarly, BLNK-deficient OCs spread normally, form actin rings, and are capable of normal bone degradation, in vitro.
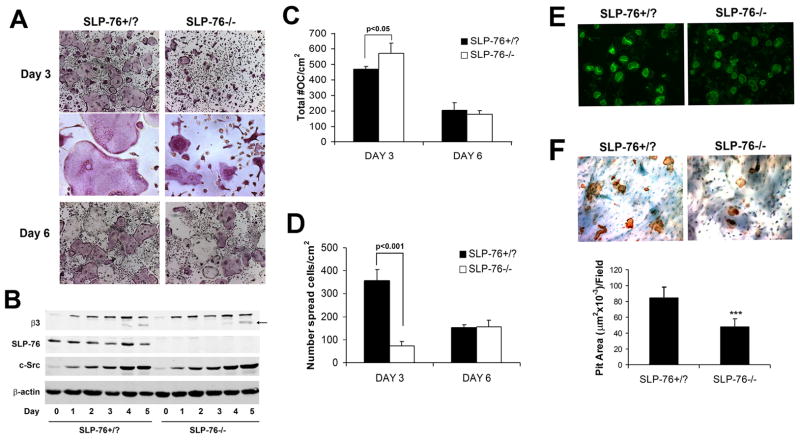
In contrast to BLNK−/− mice, which are born in Mendelian ratio, global deletion of SLP-76 generates few viable pups. In fact, we recovered one knockout animal in 250 mice produced from heterozygous breeding pairs, a yield precluding meaningful morphological or biochemical evaluation of the skeleton. To circumvent this problem we intravenously injected E16-20 SLP-76−/− or SLP-76+/? (control) fetal liver cells into lethally irradiated WT mice. Following marrow reconstitution, BMMs were isolated from the radiation chimeras and differentiated into OCs in M-CSF and RANKL. Both SLP-76−/− and control precursors form TRAP-expressing multinucleated cells by day 3 (Figure 2A). Confirming normal OC differentiation, SLP-76−/− and control cells express c-Src and the β3 integrin subunit equally during osteoclastogenic culture (Figure 2B) and do not generate reduced numbers of multinucleated TRAP positive cells (Figure 2C). On the other hand, whereas day 3 SLP-76+/? OCs are indistinguishable from those derived from naïve WT mice, the majority of those lacking SLP-76 fail to spread, and exhibit a morphology similar to Syk−/− cells, which are smaller and have a crenated appearance (2) (Figure 2A). In fact, the modestly increased number of SLP-76-deficient OCs at this juncture (Figure 2C) may reflect available space in light of attenuated spreading. Unlike their Syk−/− counterparts, however, the phenotype of SLP-76−/− OCs resolves with time, mirroring WT by day 6, as manifest by TRAP staining (Figure 2A), number (Figure 2C), spreading (Figure 2D) and capacity to form actin rings (Figure 2E). In fact, the populations of mutant and control OC numbers are similarly reduced by day 6, likely reflecting equivalent apoptosis of terminally differentiated cells (23) (Figure 2A, 2C and 2D). Despite unimpeded differentiation of the mutant cells and their normalization of spreading with time, the quantity of bone resorbed during six days of osteoclastogenesis is reduced 43% without SLP-76 (Figure 2F). Hence, SLP-76 does not mediate OC maturation, but its absence impairs OC function.
Figure 2. SLP-76-deficient BMMs differentiate into OCs normally but exhibit delayed spreading and reduced resorptive capacity.
(A) SLP-76+/? and SLP-76−/− BMMs, isolated from radiation chimera marrow, were cultured in M-CSF and RANKL for 3 or 6 days. The cells were stained for TRAP activity (top and bottom panels, magnification 40x, middle panel 200x). (B) SLP-76+/? and SLP-76−/− BMMs were cultured in M-CSF and RANKL, with time. Lysates were immunoblotted for the OC differentiation markers, β3 integrin subunit and c-Src; β-actin serves as a loading control. (C, D) Quantification of C) total and D) spread OCs in (A). (E) SLP-76+/− and SLP-76−/− BMMs were cultured on bone slices, with M-CSF and RANKL, for six days. The resultant OCs were labeled with FITC-phalloidin to visualize actin rings (top panel) (200x). They were then removed and resorptive pits stained with peroxidase-conjugated WGA followed by 3-3′-diaminobenzidine (bottom panel) (200x). (F) Quantification of resorptive pit area in (D). (***p<0.001)
BLNK compensates for absence of SLP-76, in vitro
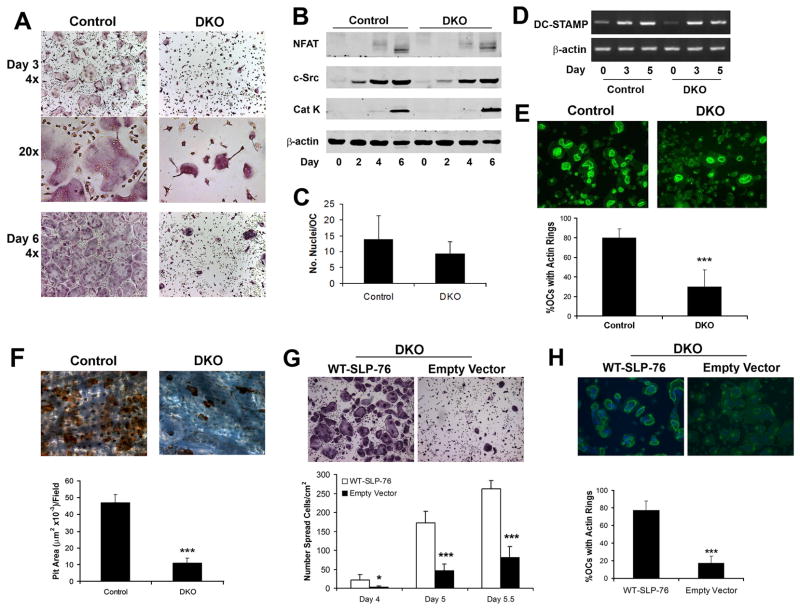
SLP-76 is a Syk target, but the spreading defect of OCs lacking the tyrosine kinase is more prolonged than of those deficient in the adaptor protein (2). This observation raised the possibility that BLNK partially compensates for the loss of SLP-76 in linking Syk to the OC cytoskeleton. We tested this hypothesis by generating SLP-76−/−/BLNK−/− (DKO) embryos and injecting their liver cells into irradiated wild-type hosts. Chimerism was confirmed by western blot analysis and quantitative RT-PCR demonstrating less than 2% SLP-76 remaining in wild-type mice transplanted with SLP-76 deficient bone marrow (data not shown). Based on the fact that BLNK deficiency, alone, does not impact the OC (Figure S1), we used SLP-76+/?/BLNK−/−littermates as control (“Control” in Figures 3,4,6). Similar to those deleted of only SLP-76, BMMs lacking both SLP proteins differentiate into multinucleated, TRAP positive cells which express osteoclastogenic markers normally, but form smaller OCs with a “crenated” appearance (Figure 3A and B). Unlike SLP-76−/− cells (Figure 2A), however, prolonged exposure to M-CSF and RANKL does not rescue “crenated” DKO OCs, indicating that BLNK compensates partially for absence of SLP-76 (compare Figures 2A and Figure 3A).
Figure 3. DKO OCs normally differentiate but are dysfunctional.
(A–B) Control (SLP-76+/− BLNK−/−) and DKO BMMs were cultured in M-CSF and RANKL, with time, fixed and (A) stained for TRAP activity (top and bottom panels, 40x, middle panel, 200x) or (B) immunoblotted for c-Src, NF-AT1c and cleaved cathepsin K (Cat K) as markers of OC differentiation. β-actin serves as loading control. (C) The number of nuclei per OC was quantified in day 5 TRAP stained cultures. (D) DKO and control BMMs were cultured in M-CSF and RANKL, with time. DC-STAMP mRNA was assessed by RT-PCR. Actin serves as loading control. (E, F) Control and DKO BMMs were cultured on bone in RANKL and M-CSF for five days. (E) The cells were fixed and labeled with FITC-phalloidin to visualize actin rings or (F) removed and the resorptive pits stained with WGA-peroxidase-conjugated lectin (200x). Resorptive area per field was histomophometrically determined. (G, H) DKO BMMs were transduced with WT-SLP-76 or empty vector. Following selection with blasticidin the transduced cells were plated on (G) plastic or (H) bone in M-CSF and RANKL. Five days later, the number of (G) spread OCs and (H) the percentage of OCs exhibiting actin rings were determined. (*p<0.05 and ***p<0.001)
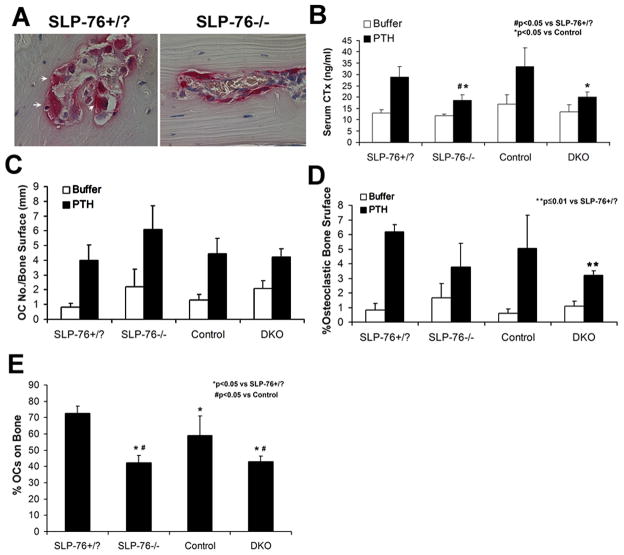
Figure 4. SLP-76 deficient OCs mobilize in response to PTH but fail to normalize bone resorption, in vivo.
(A) SLP-76+/? and SLP-76−/− mice were supracalvarially injected with PTH, 4 times a day. After 4 days histological sections of calvaria stained for TRAP activity (red reaction product) (600x). (B) CTx content of serum collected at sacrifice of SLP-76+/?, SLP-76−/, control (SLP-76+/?BLNK−/−) and DKO mice. (N=4–5 in each group). (C) The number of OCs per millimeter bone surface was quantified histomorphometrically. (D) The percent of bone surface covered by OCs was quantified histomorphometrically. (E) The percent of OCs on bone was determined histomorphometrically.
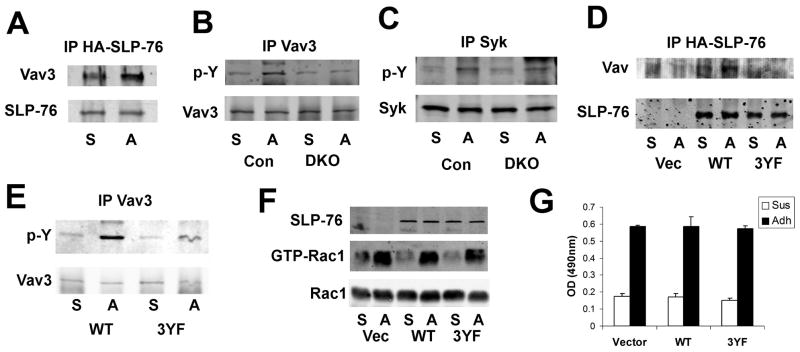
Figure 6. SLP-76 links Syk to Vav3 following integrin engagement in the OC.
BMMs were differentiated into pre-OCs by 3 days culture with M-CSF and RANKL. They were serum and cytokine starved, lifted, and either maintained in suspension (S) or adhered to vitronectin (A). (A) HA-immunoprecipitates of DKO pre-OCs transduced with HA-tagged SLP-76, immunoblotted for Vav and SLP-76, as loading control. (B–C) Lysates of DKO and control (SLP-76+/?BLNK−/−) pre-OCs were immunoprecipitated with an anti-Vav3 (B) or anti-Syk (C) antibody. The immunoprecipitates were immunoblotted for phosphotyrosine and Vav3 (B) or Syk (C) as loading controls. (D–G) DKO BMMs were transduced with WT or 3YF-SLP-76 constructs or empty vector prior to three-day culture with M-CSF and RANKL. (D) Lysates were immunoprecipitated with an anti-HA antibody. Immunoprecipitates were immunoblotted for Vav and SLP-76. (E) Lysates of transduced DKO pre-OCs were immunoprecipitated with an anti-Vav3 Immunoprecipitates were immunoblotted for phosphotyrosine and Vav3. (F–G) Lysates of transduced DKO pre-OCs were (F) subjected to GST-PAK pull-down, followed by Rac1 immunoblot or (G) used to quantitate GTP-Rac1/2 by ELISA. In (F), Rac1 serves as loading control.
OCs enlarge by fusing with their counterparts raising the possibility that the relatively small size of the DKO cells represents attenuated fusion. In this circumstance, one would expect a relative paucity of nuclei in mutant OCs. We find, however, such is not the case (Figure 3C). Furthermore, expression of DC-STAMP mRNA, whose product is involved in cell fusion, is temporally indistinguishable in DKO and control BMMs undergoing osteoclastogenesis (Figure 3D). Hence, the DKO OC phenotype does not represent attenuated fusion but likely reflects dysfunctional cytoskeletal organization. Consistent with this posture, DKO OC actin ring formation is compromised (Figure 3E) and resorption pit excavation is reduced more than occurs with deletion of only SLP-76 (Figure 3F). Confirming their phenotypic abnormalities reflect absence of SLP-76, DKO OCs retrovirally reconstituted with WT-SLP-76 spread normally and generate abundant actin rings (Figure 3G and H). Hence, BLNK partially compensates for the absence of SLP-76 in vitro and SLP-76 fully normalizes BLNK-deficient OCs.
Our capacity to generate DKO OCs stands in contrast to the failure of Shinohara et al to do so (17). Because the M-CSF and RANKL used in this study is generated in our laboratory, we determined if the discrepancy reflects reagent differences. Thus, we cultured DKO and control BMMs with commercial, recombinant RANKL and M-CSF in concentrations used by Shinohara et al (17). As shown in Figure S2, DKO OCs are generated in these conditions and are indistinguishable from those illustrated in Figure 3A.
Absence of SLP-76 compromises OC function in vivo
To determine the impact of the adaptor protein on bone resorption in vivo, we supracalvarially injected parathyroid hormone 1–34 (PTH), four times daily for four days, into radiation chimera mice with SLP-76+/? or SLP-76−/− OC genotypes. Similar to our in vitro observations, OCs are recruited in the absence of SLP-76, in vivo. On the other hand, SLP-76−/− OCs are generally smaller and more elongated than normal cells (Figure 4A). We repeated this exercise using DKO radiation chimeras and littermate controls, observing similar results. Confirming impaired bone resorption, PTH-enhancement of serum collagen fragments (CTx) is blunted in SLP-76−/− and DKO mice (Figure 4B). In contrast to the in vitro compensatory properties of BLNK, however, reduction of PTH-induced bone resorption is equivalent in SLP-76−/− and DKO mice. On the other hand, the total numbers of OCs bearing SLP-76−/− or DKO mutations are not reduced indicating suppressed resorption in these animals reflects OC dysfunction rather than recruitment (Figure 4C). Buttressing this conclusion, the proportion SLP-76−/− or DKO OCs juxtaposed to bone and/or the percentage of bone surface in contact with such mutant OCs are reduced in PTH-treated animals (Figure 4D and E). Interestingly, the percent SLP-76+/?/BLNK−/− (control) OCs on bone is slightly, but significantly, reduced relative to SLP-76+/? cells with intact BLNK. While the significance of this observation is obscure, it raises the possibility that deletion of three SLP family alleles impacts OC/bone attachment.
Gads and LAT are dispensable for OC spreading and function
In TCR-mediated signaling, SLP-76 is a component of a plasma membrane-residing quaternary complex including LAT (Linker of Activated T-cells), PLC-γ1 and Gads, a Grb2 family member containing SH2 and SH3 domains. Membrane-associated LAT is phosphorylated by Syk following receptor engagement and recruits SLP-76 through its interaction with Gads (14, 24). Gads is constitutively bound to SLP-76 through its SH3 domain and recognizes LAT via the Gads SH2 domain only after phosphorylation (3, 25). In contrast to the TCR, however, αIIbβ3 integrin-stimulated platelet cytoskeletal organization, activated via SLP-76 phosphorylation, is unimpeded in the absence of LAT or Gads (8–10).
Like αIIbβ3 in the platelet, SLP-76-mediated OC activation is independent of LAT and Gads. Specifically, LAT−/− and Gads−/− OCs spread normally and resorb bone as effectively as wild-type cells (Figure S3A, C, D and F). Moreover, integrin-mediated SLP-76 phosphorylation is intact in LAT−/− and Gads−/− OCs (Figure S3B and E). Lastly, a SLP-76 mutant (R237A) specifically disrupting its association with Gads and family member, Grb2 (26), rescues the DKO phenotype as effectively as WT-SLP-76 (Figure S3G). Thus, it is unlikely that the normal phenotype of Gads null cells reflects compensation by related proteins. Activation of SLP-76 in response to integrin activation in OCs is, therefore, LAT- and Gads- independent and mirrors αIIbβ3 signaling in the platelet rather than the TCR.
SLP-76 N-terminal tyrosine and proline-rich regions organize the OC cytoskeleton
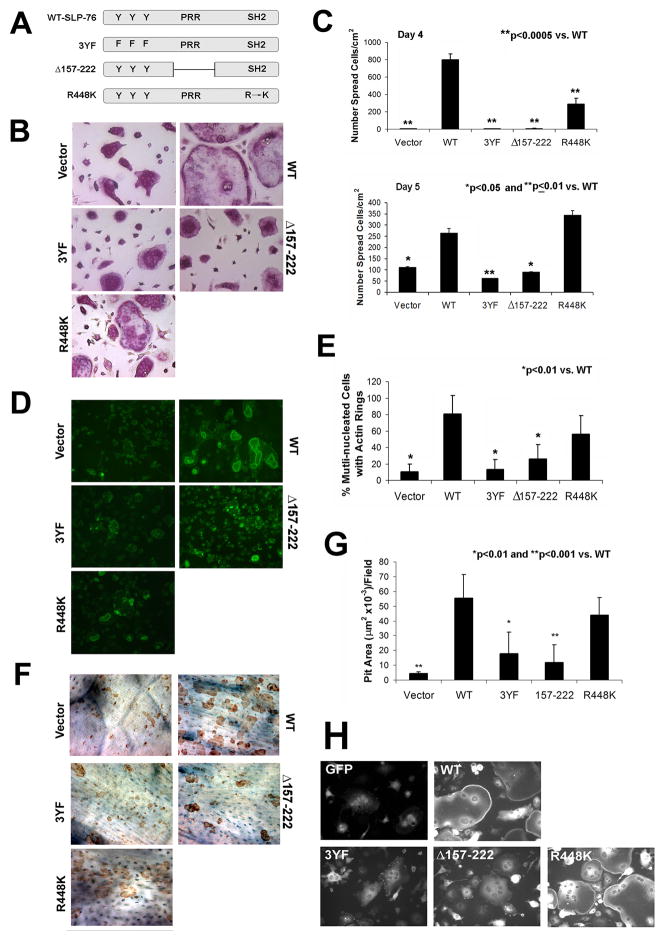
To identify associated proteins mediating SLP-76-stimulated cytoskeletal organization, we mutated its N-terminal phosphotyrosine (3YF) and proline-rich regions (Δ157-222), and the SH2 motif (R448K) (Figure 5A). DKO BMMs were transduced with WT-SLP-76, the mutant constructs, or empty vector and differentiated into mature OCs on plastic or bone. In contrast to WT transductants, DKO OCs expressing 3YF or Δ157-222-SLP-76 are unable to normalize spreading after prolonged culture with M-CSF and RANKL (Figure 5B and C). Interestingly, R448K-SLP-76, which specifically disrupts the ability of the SH2 domain to bind phosphotyrosines, partially rescues the DKO cytoskeleton (Figure 5B). While their spreading on day four is compromised, DKO OCs expressing R448K-SLP-76 are more akin to WT on day 5 although morphological differences remain (Figure 5B and C). Confirming partial rescue, the SH2 domain, but not 3YF or Δ157-222-SLP-76, mutant, yields OCs with actin ring formation similar to WT (Figure 5D and E). DKO cells expressing mutations in the N-terminal and the proline-rich regions also fail to effectively degrade bone whereas those transduced with R448K-SLP-76 are normal in this regard (Figure 5F and G).
Figure 5. The tyrosine and proline rich regions of SLP-76 mediate OC spreading and function.
(A) Retroviral SLP-76 constructs used: WT tyrosine to phenylalanine mutation (3YF), proline-rich region (PRR) deletion (Δ157-222), and SH2 point mutation (R448K). (B–G) DKO BMMs were transduced with the four SLP-76 constructs or empty vector. (B) Following selection, the cells were plated with M-CSF and RANKL on plastic. After 5 days, OCs were stained for TRAP activity. (C) The number of spread cells in (B) was quantified on days 4 and 5. (D) Actin ring formation by transduced cells, generated on bone, visualized by FITC-phalloidin staining. (E) The percentage of cells exhibiting actin rings on day 5 was quantified. (F) After removal of OCs generated on bone, resorptive lacunae were stained with peroxidase-conjugated WGA and 3-3′-diaminobenzidine and (G) Resorptive pit area was quantified histomorphometrically. (H) DKO BMMs were transduced with GFP alone (empty vector), GFP-WT-SLP-76 or GFP-tagged mutant constructs. Cells were cultured on glass coverslips with M-CSF and RANKL for five days to generate OCs. The cells were examined by fluorescent microscopy to determine GFP distribution. (*p<0.05, **p<0.01, ***p<0.001 vs WT) All images were acquired at 200x magnification.
SLP-76 activation requires its proximity to the plasma membrane. We therefore asked if the failure of 3YF and Δ157-222-SLP-76 to rescue the OC cytoskeleton is attended by defective cell surface localization. To this end, DKO BMMs were transduced with GFP-tagged WT, 3YF, Δ157-222 and R448K-SLP-76 constructs. The cells were differentiated into OCs on glass coverslips and fixed on day 5. Mirroring their capacity to rescue the DKO cytoskeleton, WT and R448K-SLP-76, but not 3YF nor Δ157-222-SLP-76, localize to the OC surface (Figure 5H).
SLP-76 links Syk to Vav3 following integrin engagement in the OC
Having established that the tyrosine region of SLP-76, which contains its putative Vav binding site, regulates cytoskeletal remodeling, we asked if SLP-76 and Vav3 associate following integrin engagement. Thus, DKO BMMs transduced with HA-tagged SLP-76 were treated with M-CSF and RANKL for three days, lifted and maintained in suspension or re-plated on vitronectin to activate αvβ3. In fact, SLP-76 and Vav3 associate more robustly in vitronectin-adherent cells than those in suspension (Figure 6A). To determine if SLP-76 is proximal to Vav activation in αvβ3 signaling, we immunoblotted Vav3 immunoprecipitates, derived from adherent or suspended DKO and control pre-OCs, with a pan-phosphotyrosine antibody. Absence of the SLP-proteins diminishes integrin-induced Vav3 phosphorylation (Figure 6B), while Syk activation is intact (Figure 6C), establishing that SLP-76 and BLNK are downstream of Syk and proximal to Vav3 in the integrin signaling cascade. Furthermore, adhesion-stimulated Vav3 phosphorylation is reduced in DKO pre-OCs retrovirally expressing 3YF-SLP-76, which attenuates SLP-76/Vav3 association (Figure 6D and E).
As Vav3 is a GEF for Rac, we asked if activity of the small GTPase is also reduced by diminished Vav3 phosphorylation. In fact, Rac activation is equally induced by integrin engagement in DKO cells expressing empty vector, WT-, or 3YF-SLP-76. Therefore, although Vav3 phosphorylation is decreased in DKO OCs, Rac is activated by a parallel mechanism, independent of SLP-76.
SLP-76 regulates the OC cytoskeleton in a c-Fms dependent manner
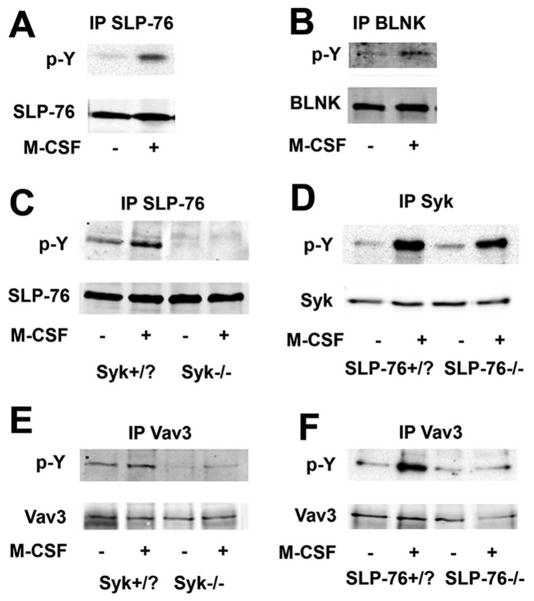
In addition to its capacity to stimulate BMM proliferation, M-CSF promotes organization of the OC cytoskeleton (27–29). Moreover, high-dose M-CSF normalizes the crenated phenotype of β3 integrin-null OCs through a Syk-dependent mechanism (30). Thus, there is commonality in the means by which activated c-Fms and αvβ3 rearrange the OC cytoskeleton. To determine if like the integrin, the cytoskeleton-organizing capacity of c-Fms involves SLP-76, we exposed cytokine-starved pre-OCs to M-CSF for five minutes. Similar to integrin occupancy M-CSF induces Syk (29), SLP-76, and BLNK phosphorylation (Figure 7A and B). Also mirroring αvβ3 signaling, M-CSF-stimulated SLP-76 phosphorylation requires Syk (Figure 7C), while activation of the kinase is independent of SLP-76 (Figure 7D). Finally, previously described M-CSF-mediated Vav3 phosphorylation is abrogated in the absence of either Syk or SLP-76 (11) (Figure 7E and F).
Figure 7. SLP-76 activates cytoskeleton-organizing proteins in an M-CSF-dependent manner, in OCs.
(A, B) WT pre-OCs were serum and cytokine starved and exposed to M-CSF or vehicle for five minutes. Lysates were immunoprecipitated using an (A) anti-SLP-76 or (B) anti-BLNK antibody (B). Immunoprecipitates were immunoblotted for phosphotyrosine. (C) Cytokine-starved Syk+/? or Syk−/− pre-OCs were exposed to M-CSF for five minutes. Lysates were immunoprecipitated with an anti-SLP-76 antibody and immunoprecipitates immunoblotted for phosphotyrosine and SLP-76. (D) SLP-76+/? and SLP-76−/− pre-OCs were treated with M-CSF for five minutes. Lysates were immunoprecipitated using an anti-Syk antibody and the immunoprecipitates were immunoblotted for phosphotyrosine and Syk. (E–F) Vav3 was immunoprecipitated from (E) Syk+/? and Syk−/− or (F) SLP-76+/? and SLP-76−/− cytokine-starved pre-OCs, treated with M-CSF for 5 minutes. The immunoprecipitates were immunoblotted for phosphotyrosine and Vav3.
Discussion
αvβ3-mediated cytoskeletal remodeling requires Syk and Vav3 (2, 11). Because it is activated by Syk in other cells, we posited that SLP-76, which is required for cytoskeletal rearrangement during immune synapse formation and platelet adhesion (7, 9), mediates actin organization in the OC. After confirming that integrin engagement rapidly phosphorylates SLP-76 and BLNK in a Syk-dependent manner, we established modest deficiencies in the spreading and resorptive capacity of SLP-76−/−OCs. Despite the fact that BLNK-deficient OCs are normal, absence of both SLP proteins (DKO) prompts a more severe OC phenotype than does deletion of only SLP-76, indicating that BLNK partially compensates for the loss of SLP-76, in vitro.
In other cells, expression of the two SLP proteins is often mutually exclusive. For example, SLP-76, alone, is present in T-cells and BLNK, in B-cells (3). The macrophage, however, is unique in that both proteins are substantially expressed but their combined deletion does not affect activation of the cell or FcRγ signaling (31). Given that SLP-76 and BLNK are present throughout osteoclastogenesis, we hypothesized that despite SLP-adaptors not functioning in macrophages, they may in OCs. While containing normal osteoclastogenic markers and numbers of nuclei, DKO OCs, in fact have defective cytoskeletons. Our finding that SLP-adaptor proteins do not regulate differentiation or fusion of OCs, but rather their function in response to αvβ3 or c-Fms activation, differs from that of Shinohara et al who fail to generate DKO OCs in vitro (17). While we cannot account for this discrepancy we establish it cannot be attributed to osteoclastogenic reagents. Furthermore, the phenotype of DKO OCs is similar to that attending deletion of other OC integrin-activated, cytoskeleton-organizing proteins. Interestingly, differences in DKO osteoclastogenesis mirrors conflicting observations regarding RANKL-mediated generation of DAP12-deficient OCs which occurs in our hands (29) but not in that of Koga et al (32).
Supporting our in vitro observations, PTH-treated SLP-76−/− and DKO radiation chimeras fail to efficiently resorb bone, in vivo. Interestingly, while BLNK partially compensates for absence of SLP-76, in vitro, (compare Figs 2F and 3F), the same does not obtain, in vivo (Figure 4B), at least in the context of PTH stimulation. In this regard, the role of cytokine-dependent hematopoietic cell linker (CLINK), the third SLP family member, is yet to be determined, in the OC (3). As CLINK is expressed only in cytokine-simulated cells, its impact in the complexity of the bone microenvironment may differ from that in cultured OC lineage cells.
Probably reflecting impaired remodeling, radiation chimeras with dysfunctional OCs, such as those lacking Syk, RANK, or Hck/Src, often have normal bone mass (2, 33), Lorenzo, J; unpublished observations), and we find the same obtains in the context of SLP-76 deficiency. On the other hand, the response of such chimeras to stimulated resorption is typically blunted, as we demonstrate in the absence of SLP-76, with or without BLNK. As expected, PTH-treated control animals display a marked increase in OC recruitment and total resorptive activity. DKO and SLP-76−/− radiation chimeras recruit comparable numbers of OCs in response to the hormone. Each fails, however, to increase bone resorption to WT equivalence, indicating that SLP-76, while not involved in OC differentiation, regulates the OC cytoskeleton, and thus, function.
Gads constitutively binds SLP-76 in the TCR. When Syk phosphorylates LAT, it recognizes Gads and the complex brings SLP-76 to the membrane (3). Gads, however, is not required for SLP-76 recruitment as Gads−/− OCs are normal. Additionally, integrin-mediated SLP-76 phosphorylation occurs absent Gads. Because a homologous Grb2 family member may compensate for the loss of Gads, we examined LAT-deficient cells, which also spread normally and are functional. These observations are reminiscent of platelets in which adhesion-dependent signals also do not require the Gads/LAT complex (8, 10, 34). Our data indicate that SLP-76 recruitment to the OC plasma membrane is via the tyrosine and/or the proline region, as mutations within each affects GFP-tagged SLP-76 localization.
Despite the differences in SLP-76 activation in platelets and T-cells, both αIIbβ3 and the TCR regulate Vav3 and cytoskeletal rearrangement (7, 35). Given the common phenotype of OCs lacking SLP-76, Vav3 or Syk, we propose that the adaptor protein links the two others. This hypothesis is supported by the facts that Vav3 contains an SH2 domain that associates with the adaptor’s phosphorylated tyrosines (3). In fact, like Syk, SLP-76 is necessary for optimal Vav3 activation following integrin engagement. Confirming that at least one of the three phosphorylated tyrosines of SLP-76 recognizes Vav3, their mutation to phenylalanine blocks OC spreading and function, as well as phosphorylation of the GEF. Since the 3YF-SLP-76 mutation disrupts other binding partners, such as Tec kinases and Nck, their inactivity possibly contributes to failure of DKO OC rescue. However, the reduction of Vav3 phosphorylation in the DKO cells, coupled with the similar phenotype of Vav3−/− OCs (11), suggests that SLP-76 is functioning, at least, in part through Vav3. Moreover, Tec kinase deficiency impairs osteoclastogenesis via the RANKL signaling pathway, with reduced numbers of TRAP-positive multinucleated cells in culture (17). Since our results are consistent with a defect in cytoskeletal organization and not osteoclastogenesis, it is likely that Tec kinases are not the principal mediators of SLP-76 cytoskeletal effector proteins.
While DKO cells have a disorganized cytoskeleton and reduced Vav3 phosphorylation, their Rac activity is not impaired. As Vav3 was initially identified as a GEF for RhoA, RhoG, and to a lesser extent Rac (36), it is possible that, in the OC, Vav3 is also a GEF for other small GTPases. Because M-CSF-induced RhoA activation is normal in Vav1/Vav3-deficient OCs (11), it is unlikely that RhoA is a target of integrin-activated Vav3. Alternatively, RhoG may play a role in OC cytoskeletal organization as it shares greater amino acid sequence similarity with Rac family members than with Rho proteins and promotes T-cell spreading on fibronectin (36, 37).
c-Fms and αvβ3 activate a number of common cytoskeleton-organizing events in the OC. It is not unexpected, therefore, that the crenated phenotype of β3 integrin-deficient OCs is normalized by culturing cells with high dose M-CSF (30). Consistent with this observation, M-CSF induces Vav3 phosphorylation and GTPase activation (28, 38), which prompted us to examine SLP-76 in the context of the cytokine. M-CSF induces Syk phosphorylation and in consequence, that of SLP-76 and BLNK. Consistent with the integrin pathway, M-CSF-induced Vav3 phosphorylation is abrogated in the absence of SLP-76 while Syk phosphorylation is normal, confirming, once again, SLP-76 is distal to Syk and proximal to Vav3.
Our data suggest a model of αvβ3- and c-Fms-mediated organization of the OC cytoskeleton whereby engagement of either receptor rapidly stimulates c-Src and DAP12, the latter recruiting Syk to its ITAM domain. Syk, which is activated by autophosphorylation in the case of c-Fms, or by c-Src in the context of the integrin, phosphorylates SLP-76 (2, 29). Phosphorylated SLP-76, via its tyrosine-region recognizes Vav3 and forms a complex, which includes small GTPases at the cell surface. SLP-76 is therefore, yet another member of the emerging signaling pathway whereby the OC regulates its cytoskeleton and thus, its unique capacity to resorb bone.
Supplementary Material
Abbreviations
- OC
osteoclast
- DKO
double knockout
- GEF
guanine nucleotide exchange factor
- SLP
Src-homology 2-containing leukocyte adaptor proteins
- BLNK
B-cell linker
- BMMs
bone marrow macrophages
- RANKL
receptor activator of NF-κB ligand
Footnotes
The project described was supported by Shriners’ Hospitals for Chilren Grant Number 8590 (SLT) and Grant Number AR032788 and AR046523 (SLT), AR046852 (FPR), and 1 F30 AG302802 (JLR) from National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Disclosures
The authors declare no conflicts of interest or financial interest.
References
- 1.Ross FP, Teitelbaum SL. αvβ3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol Rev. 2005;208:88–105. doi: 10.1111/j.0105-2896.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 2.Zou W, Kitaura H, Reeve J, Long F, Tybulewicz VLJ, Shattil SJ, Ginsberg MH, Ross FP, Teitelbaum SL. Syk, c-Src, the αvβ3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J Cell Biol. 2007;176:877–888. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu JN, Koretzky GA. The SLP-76 family of adapter proteins. Semin Immunol. 2004;16:379–393. doi: 10.1016/j.smim.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Clements JL, Yang B, Ross-Barta SE, Eliason SL, Hrstka RF, Williamson RA, Koretzky GA. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 5.Pivniouk V, Tsitsikov E, Swinton P, Rathbun G, Alt FW, Geha RS. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell. 1998;94:229–238. doi: 10.1016/s0092-8674(00)81422-1. [DOI] [PubMed] [Google Scholar]
- 6.Yablonski D, Kuhne MR, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 7.Bubeck Wardenburg J, Pappu R, Bu JY, Mayer B, Chernoff J, Straus D, Chan AC. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity. 1998;9:607–616. doi: 10.1016/s1074-7613(00)80658-5. [DOI] [PubMed] [Google Scholar]
- 8.Wonerow P, Pearce AC, Vaux DJ, Watson SP. A critical role for phospholipase Cgamma2 in alphaIIbbeta3-mediated platelet spreading. J Biol Chem. 2003;278:37520–37529. doi: 10.1074/jbc.M305077200. [DOI] [PubMed] [Google Scholar]
- 9.Judd BA, Myung PS, Leng L, Obergfell A, Pear WS, Shattil SJ, Koretzky GA. Hematopoietic reconstitution of SLP-76 corrects hemostasis and platelet signaling through alpha IIb beta 3 and collagen receptors. Proc Natl Acad Sci U S A. 2000;97:12056–12061. doi: 10.1073/pnas.97.22.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abtahian F, Bezman N, Clemens R, Sebzda E, Cheng L, Shattil SJ, Kahn ML, Koretzky GA. Evidence for the requirement of ITAM domains but not SLP-76/Gads interaction for integrin signaling in hematopoietic cells. Mol Cell Biol. 2006;26:6936–6949. doi: 10.1128/MCB.01040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faccio R, Teitelbaum SL, Fujikawa K, Chappel J, Zallone A, Tybulewicz VL, Ross FP, Swat W. Vav3 regulates osteoclast function and bone mass. Nat Med. 2005;11:284–290. doi: 10.1038/nm1194. [DOI] [PubMed] [Google Scholar]
- 12.Yoder J, Pham C, Iizuka YM, Kanagawa O, Liu SK, McGlade J, Cheng AM. Requirement for the SLP-76 adaptor GADS in T cell development. Science. 2001;291:1987–1991. doi: 10.1126/science.1057176. [DOI] [PubMed] [Google Scholar]
- 13.Pappu R, Cheng AM, Li B, Gong Q, Chiu C, Griffin N, White M, Sleckman BP, Chan AC. Requirement for B cell linker protein (BLNK) in B cell development. Science. 1999;286:1949–1954. doi: 10.1126/science.286.5446.1949. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, Trible RP, Grinberg A, Tsay HC, Jacobs HM, Kessler CM, Long EO, Love PE, Samelson LE. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 15.Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VL. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 16.Takeshita S, Kaji K, Kudo A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res. 2000;15:1477–1488. doi: 10.1359/jbmr.2000.15.8.1477. [DOI] [PubMed] [Google Scholar]
- 17.Shinohara M, Koga T, Okamoto K, Sakaguchi S, Arai K, Yasuda H, Takai T, Kodama T, Morio T, Geha RS, Kitamura D, Kurosaki T, Ellmeier W, Takayanagi H. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008;132:794–806. doi: 10.1016/j.cell.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 18.Jackman JK, Motto DG, Sun Q, Tanemoto M, Turck CW, Peltz GA, Koretzky GA, Findell PR. Molecular cloning of SLP-76, a 76-kDa tyrosine phosphoprotein associated with Grb2 in T cells. J Biol Chem. 1995;270:7029–7032. doi: 10.1074/jbc.270.13.7029. [DOI] [PubMed] [Google Scholar]
- 19.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- 20.Motto DG, Ross SE, Wu J, Hendricks-Taylor LR, Koretzky GA. Implication of the GRB2-associated phosphoprotein SLP-76 in T cell receptor-mediated interleukin 2 production. J Exp Med. 1996;183:1937–1943. doi: 10.1084/jem.183.4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bubeck Wardenburg J, Fu C, Jackman JK, Flotow H, Wilkinson SE, Williams DH, Johnson R, Kong G, Chan AC, Findell PR. Phosphorylation of SLP-76 by the ZAP-70 protein-tyrosine kinase is required for T-cell receptor function. J Biol Chem. 1996;271:19641–19644. doi: 10.1074/jbc.271.33.19641. [DOI] [PubMed] [Google Scholar]
- 22.Fu C, Turck CW, Kurosaki T, Chan AC. BLNK: a central linker protein in B cell activation. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 23.Bai S, Kitaura H, Zhao H, Chen J, Muller JM, Schule R, Darnay B, Novack DV, Ross FP, Teitelbaum SL. FHL2 inhibits the activated osteoclast in a TRAF6-dependent manner. J Clin Invest. 2005;115:2742–2751. doi: 10.1172/JCI24921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 25.Horejsi V, Zhang W, Schraven B. Transmembrane adaptor proteins: organizers of immunoreceptor signalling. Nat Rev Immunol. 2004;4:603–616. doi: 10.1038/nri1414. [DOI] [PubMed] [Google Scholar]
- 26.Berry DM, Nash P, Liu SK, Pawson T, McGlade CJ. A high-affinity Arg-X-X-Lys SH3 binding motif confers specificity for the interaction between Gads and SLP-76 in T cell signaling. Curr Biol. 2002;12:1336–1341. doi: 10.1016/s0960-9822(02)01038-2. [DOI] [PubMed] [Google Scholar]
- 27.Insogna KL, Sahni M, Grey AB, Tanaka S, Horne WC, Neff L, Mitnick M, Levy JB, Baron R. Colony-stimulating factor-1 induces cytoskeletal reorganization and c-src-dependent tyrosine phosphorylation of selected cellular proteins in rodent osteoclasts. J Clin Invest. 1997;100:2476–2485. doi: 10.1172/JCI119790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faccio R, Takeshita S, Colaianni G, Chappel JC, Zallone A, Teitelbaum SL, Ross FP. M-CSF regulates the cytoskeleton via recruitment of a multimeric signaling complex to c-Fms Tyr-Y559/697/721. J Biol Chem. 2007;282:18991–18999. doi: 10.1074/jbc.M610937200. [DOI] [PubMed] [Google Scholar]
- 29.Zou W, Reeve JL, Liu Y, Teitelbaum SL, Ross FP. DAP12 couples c-Fms activation to the osteoclast cytoskeleton by recruitment of Syk. Mol Cell. 2008;31:422–431. doi: 10.1016/j.molcel.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faccio R, Takeshita S, Zallone A, Ross FP, Teitelbaum SL. c-Fms and the αvβ3 integrin collaborate during osteoclast differentiation. J Clin Invest. 2003;111:749–758. doi: 10.1172/JCI16924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nichols KE, Haines K, Myung PS, Newbrough S, Myers E, Jumaa H, Shedlock DJ, Shen H, Koretzky GA. Macrophage activation and Fcγ receptor-mediated signaling do not require expression of the SLP-76 and SLP-65 adaptors. J Leukoc Biol. 2004;75:541–552. doi: 10.1189/jlb.0703312. [DOI] [PubMed] [Google Scholar]
- 32.Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, taniguchi T, Takayanagi H, Takai T. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 33.Lowell CA, Niwa M, Soriano P, Varmus HE. Deficiency of the Hck and Src tyrosine kinases results in extreme levels of extramedullary hematopoiesis. Blood. 1996;87:1780–1792. [PubMed] [Google Scholar]
- 34.Judd BA, Myung PS, Obergfell A, Myers EE, Cheng AM, Watson SP, Pear WS, Allman D, Shattil SJ, Koretzky GA. Differential requirement for LAT and SLP-76 in GPVI versus T cell receptor signaling. J Exp Med. 2002;195:705–717. doi: 10.1084/jem.20011583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obergfell A, Judd BA, del Pozo MA, Schwartz MA, Koretzky GA, Shattil SJ. The molecular adapter SLP-76 relays signals from platelet integrin alphaIIbbeta3 to the actin cytoskeleton. J Biol Chem. 2001;276:5916–5923. doi: 10.1074/jbc.M010639200. [DOI] [PubMed] [Google Scholar]
- 36.Movilla N, Bustelo XR. Biological and Regulatory Properties of Vav-3, a New Member of the Vav Family of Oncoproteins. Mol Cell Biol. 1999;19:7870–7885. doi: 10.1128/mcb.19.11.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vigorito E, Billadeu DD, Savoy D, McAdam S, Doody G, Fort P, Turner M. RhoG regulates gene expression and the actin cytoskeleton in lymphocytes. Oncogene. 2003;22:330–342. doi: 10.1038/sj.onc.1206116. [DOI] [PubMed] [Google Scholar]
- 38.Sakai H, Chen Y, Itokawa T, Yu KP, Zhu ML, Insogna K. Activated c-Fms recruits Vav and Rac during CSF-1-induced cytoskeletal remodeling and spreading in osteoclasts. Bone. 2006;39:1290–1301. doi: 10.1016/j.bone.2006.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.