Abstract
Desiccation tolerance plays an important role in the overwinter survival of the foliar nematode Aphelenchoides fragariae. Survival rates of A. fragariae were compared with those of the anhydrobiotic soil-dwelling nematode Aphelenchus avenae after desiccation (90% RH), cold (4°C) and osmotic (500 mM sucrose) stress treatments. A. fragariae formed aggregates during desiccation and showed higher survival rates than A. avenae under desiccation and osmotic stress. Analysis of transcripts with Illumina RNA-seq indicated that glutaredoxin and other antioxidant-related genes were up-regulated under desiccation stress. Quantitative RT-PCR demonstrated 2.8 fold and 1.3 fold up-regulation of a glutaredoxin gene under desiccated and osmotic stress, respectively, suggesting the participation of antioxidant mechanisms in desiccation tolerance of A. fragariae.
Keywords: Aphelenchoides fragariae, desiccation, cold stress, osmotic stress, glutaredoxin, survival rate
The foliar nematode Aphelenchoides fragariae infects aerial parts of plants, causing serious damage in nurseries, greenhouses, and landscapes (Jagdale and Grewal, 2006). The nematodes overwinter in soil, dormant crowns, and abscised leaves and migrate to new leaves in the spring (Jagdale and Grewal, 2006). Foliar nematodes can enter into anhydrobiosis in the soil and resume activity when free moisture is available, and it is likely that desiccation tolerance plays an important role in overwinter survival. This ability to survive desiccation may render the nematode difficult to eliminate even with repeated nematicide applications, although this has not been explicitly tested in A. fragariae.
Several nematode species are known to survive under extreme desiccation by entering an anhydrobiotic stage during which metabolism and aging are suspended (Cooper and Van Gundy, 1971). Viable nematodes have been found in dry desert soil (Frechman et al., 1975) and dry Antarctic valleys (Treonis and Wall, 2005). Some nematodes can survive in an anhydrobiotic state for many years: the plant-parasitic nematode Ditylenchus dipsaci has been reported to survive for 23 years under dry storage (Fielding, 1951).
Mechanisms of desiccation tolerance have been studied in a small number of nematode species, including Aphelenchus avenae (Burnell and Tunnacliffe, 2011). This species belongs to the same taxonomic order as the foliar nematode and is a good comparative model for desiccation tolerance studies. Several genes have been associated with desiccation and osmotic tolerance in A. avenae, including late embryogenesis abundant proteins (Browne et al., 2002; Ingram and Bartels, 1996), trehalose phosphate synthase (Goyal et al., 2005) anhydrin (Browne et al., 2004), and glutaredoxin (Browne et al., 2004). In this study, we (1) measured the survival of foliar nematodes under different stress conditions using A. avenae as a comparative model and (2) examined changes in gene expression associated with desiccation in A. fragariae, focusing initially on glutaredoxin, a thioltransferase that protects against oxidative stress in A. avenae.
Materials and Methods
Nematode materials and stress treatments: The Aphelenchoides fragariae used in these experiments were obtained from the Clemson University Plant-parasitic Nematode Collection, maintained and cultured in vitro on Cylindrocladium sp. grown in Potato Dextrose Agar (PDA, HiMedia laboratories, India) under laboratory conditions for over 25 years. The origin of the isolate is uncertain. Nematodes were extracted using the Baermann funnel technique (Baermann, 1917), washed three times with sterilized tap water and exposed to one of three stress treatments.
For the desiccation treatment, approximately 50,000 nematodes in 20 ml of sterilized tap water were vacuum filtered onto a 4.7 cm Nuclepore membrane with 5-μm pores (Whatman, NJ). The membrane with nematodes was transferred to an uncovered petri dish on a ceramic holder in an air-tight glass chamber with 72% glycerol solution in the bottom. A MicroRHTemp Data Logger (Madgetech, NH) was placed on the ceramic holder to collect relative humidity (RH) and temperature data. The chamber was incubated at room temperature (23 ± 2°C) for 24 h.
For the cold treatment, approximately 500 nematodes in sterilized tap water were placed in a small petri dish and incubated at 4 °C for 24 h. For the osmotic stress treatment, approximately 500 nematodes were transferred to a beaker with 20 ml of 500 mM sucrose solution and incubated at room temperature for 24 h. Five hundred A. fragariae in sterilized tap water were incubated at room temperature for 24 h as a control for all the stress treatments.
After 24 h of exposure to the three stress conditions, nematodes were transferred to sterilized tap water and allowed to rehydrate for 24 h. Survival was then evaluated by checking motility with physical stimulation under a stereoscope in 100 arbitrarily-selected individuals. The experiment was performed concurrently and in identical manner with Aphelenchus avenae and was repeated three times. The A. avenae culture was obtained from the Clemson University Plant-parasitic Nematode Collection, maintained in vitro on Cryphonectria sp. grown in Potato Dextrose Agar since 1989 and originally isolated from soil around soybean plants in Florence, South Carolina.
Genes induced by desiccation: The transcriptome of the foliar nematode was sequenced in a separate project using Illumina RNAseq technology. Approximately 5,000 nematodes of each of the treatments described above were used for RNA extraction, using the PureLink RNA mini Kit (Ambion, CA) and following the manufacturer’s instructions. Total RNAs were treated with RNase-Free DNase set (Qiagen, Maryland, USA) to remove DNA contamination. Total RNA purity and degradation were checked on a 2% agarose gel before proceeding. RNA quality and integrity were verified using a 2100 Bioanalyzer with RNA nano chip (Aligent, CA). The samples from all the treatments had RNA Integrity Number (RIN) above 6.0 and concentration above 150 ng/μl. Illumina sequencing using HiSeq 2000 was performed by the David H. Murdock Research Institute (Kannapolis, NC, http://www.dhmri.org/home.html). The samples for sequencing were prepared using the TruSeq RNA sample preparation kit (Illumina, San Diego, USA). Briefly, the poly-T oligo-attached magnetic beads were used to purify mRNA from total RNA. After purification, mRNAs were fragmented into small pieces using diavalent cations. The small pieces of mRNA were synthesized into first strand cDNA using reverse transcriptase and random primers. The second strand cDNA synthesis was conducted using DNA Polymerase I and RNase H. Following double strand cDNA synthesis, the cDNA went through end-repair process, single ‘A’ base was added to the 3’ ends, then the adaptors were ligated to the cDNA fragments. The cDNA fragments were purified and enriched with PCR to generate final libraries for sequencing. Libraries of the four treatments were ‘tagged’ using different oligonucleotides. The final cDNA libraries were sequenced via Illumina HiSeq 2000 in one lane. The read length was 100 bp, and only one end of the libraries were sequenced. Quality scores of the sequences were calculated using Illumina Pipeline 1.5.
Raw reads were quality-filtered and trimmed using Prinseq (http://edwards.sdsu.edu/cgi-bin/prinseq/prinseq.cgi) and assembled into transcripts with the Trinity de novo assembler (Grabher et al., 2011). Functional annotation of assembled transcripts was performed with Blast2GO (Conesa et al 2005). Expression levels of Trinity transcripts were quantified with RSEM (http://deweylab.biostat.wisc.edu/rsem/), and genes whose expression differed significantly between treatment conditions were identified with Fisher’s Exact Test implemented in IDEG.6 (http://telethon.bio.unipd.it/bioinfo/IDEG6/index.html) with a Bonferroni correction for multiple comparisons (familywise error rate = 0.05). Genes with a 5-fold or greater significant difference in expression between desiccated and control nematodes were considered to be strongly differentially expressed. Enrichment analysis was performed in Blast2GO to determine whether specific gene ontology terms were over- or under-represented in the strongly differentially-expressed gene set.
Glutaredoxin sequence analysis: A local BLAST database of Trinity-assembled transcripts was queried with the A. avenae glutaredoxin sequence Aav-glx (AY340999.1) to identify A. fragariae glutaredoxin homologs. Seven contigs were found share high similarity with Aav-glx, and two contigs (Af-glx-1 and Af-glx-2) were further analyzed and submitted to Genbank with accession numbers JN881463 (E-value=4e−31) and JN881464 (E-value=6e−17), respectively. Open reading frames (ORF) for Af-glx-1 and Af-glx-2 sequences were located using ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html), and predicted protein sequences were analyzed via the Conserved Domains database of NCBI (Marchler-Bauer et al., 2011). Signal peptides were predicted via SignalP (Petersen et al., 2011). A protein sequence alignment of predicted Af-GLX-1 and Af-GLX-2 was created in ClustalW2 (Larkin et al., 2007) and displayed in Jalview (Waterhouse et al., 2009).
RNA extraction and quantitative reverse transcription PCR (qRT-PCR): Gene-specific primers for the amplification of glutaredoxin were designed based on the sequence of Af-glx-1, using Primer 3 (Rozen and Skaletsky, 2000), with an expected amplicon size of 183 bp. Approximately 5,000 foliar nematodes were treated under the three stress conditions described above (desiccation, cold and osmotic stress) for 24 h. Approximately 5,000 nematodes suspended in sterilized tap water and incubated at room temperature for 24 h were used as the control.
Total RNA from nematodes in each treatment was extracted using the RNeasy Mini kit (Qiagen, MD, USA) following the instructions of the manufacturer. On-column DNase digestion was performed during the RNA extraction using the RNase-Free DNase set (Qiagen, MD, USA). Quantitative reverse transcription PCR (qRT-PCR) reactions were set up in 25 μl following the instructions of the QuantiTect SYBR Green RT-PCR kit (Qiagen, MD, USA) on a Stratagene Mx3000P QRT-PCR system, using 0.25 μl of the total RNA from each treatment as a template. Quantitative RT- PCR parameters included initial reverse transcription at 50°C for 30 min, initial denaturation at 95°C for 15 min, followed by 40 cycles of 94°C for 15 s, 50°C for 30 s, and 72°C 30 s (at the end of the this step, fluorescence data were collected). The dissociation curve (melting curve) was performed at the end of the program from 60°C to 90°C. Partial 28S ribosomal RNA gene (154 bp) was included for all samples as a reference for normalization of products. The primers used in the qRT-PCR were glx-F: 5’-ATT AGA TGG CTT GGG CTT CTT G-3’, glx-R: 5’-TTG AGA TTG AAG ACC GCA AAG A -3’; and 28S-F: 5’-AGT GGG ACA CTT GGT GTC TGT GA-3’, 28S-R: 5’-TCT GAC TTC GTC CTG TTC GGG CA-3’. Four replicates were included from each treatment. The standard curve method was used to quantify differences in expression. A dilution series of the control hydration nematode total RNA was used to create standard curves for both the 28S and glutaredoxin gene. Threshold (Ct) values from each sample were converted to relative quantities based on the standard curve. The expression levels of Af-glx-1 were normalized to expression of the 28S ribosomal gene and separately computed in each stress condition. All amplicons were treated with ExoSAP-IT (Affymetrix, Santa Clara, CA) following the instructions of the manufacturer and sequenced at the Clemson University Genomic Institute.
Statistical analysis: Nematode survivorship under the three stress treatments was calculated relative to the control for each species, assuming the control as 100% survival. Differences in survivorship between the two species were analyzed by one-way ANOVA with JMP 9 (SAS Institute. Cary, NC). Significant differences in survivorhsip between species within each stress treatment were determined by Student’s t-test (P < 0.05).
Results
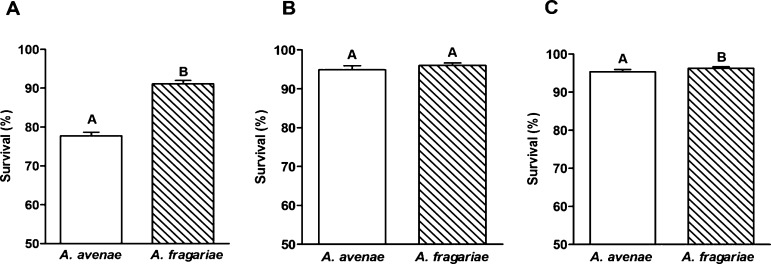
Nematode survival under stress treatments: Aphelenchoides fragariae and A. avenae formed aggregates or ‘nematode wool’ on the membrane in the desiccation stress treatments. Nematode body fluid was transparent immediately after rehydration and became darker after a few hours of rehydration. A 30 minute lag phase (i.e. the period of time before activity is resumed after rehydration) was observed in A. fragariae. Two hours later, 90% of A. fragariae were motile. In contrast, A. avenae exhibited a lag phase of two hours, and required four hours to achieve 90% motility after rehydration. The survival rate of A. fragariae (91.09%) was significantly higher than that of A. avenae (77.73%) under desiccation stress (Fig. 1). The same trend was evident for osmotic stress. No difference in survivorship was found between the two species under cold stress (> 90 % survival in both).
Fig. 1.
Survivorship of Aphelenchoides fragariae and Aphelenchus avenae under (A) desiccation stress, (B) cold stress and (C) osmotic stress. All data were normalized to the untreated control. Different letters indicate significant differences (P < 0.05) between species within the same stress treatment (Student’s t-t test).
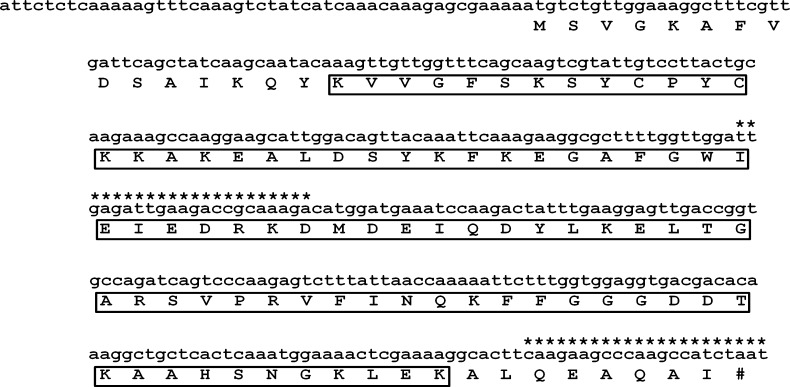
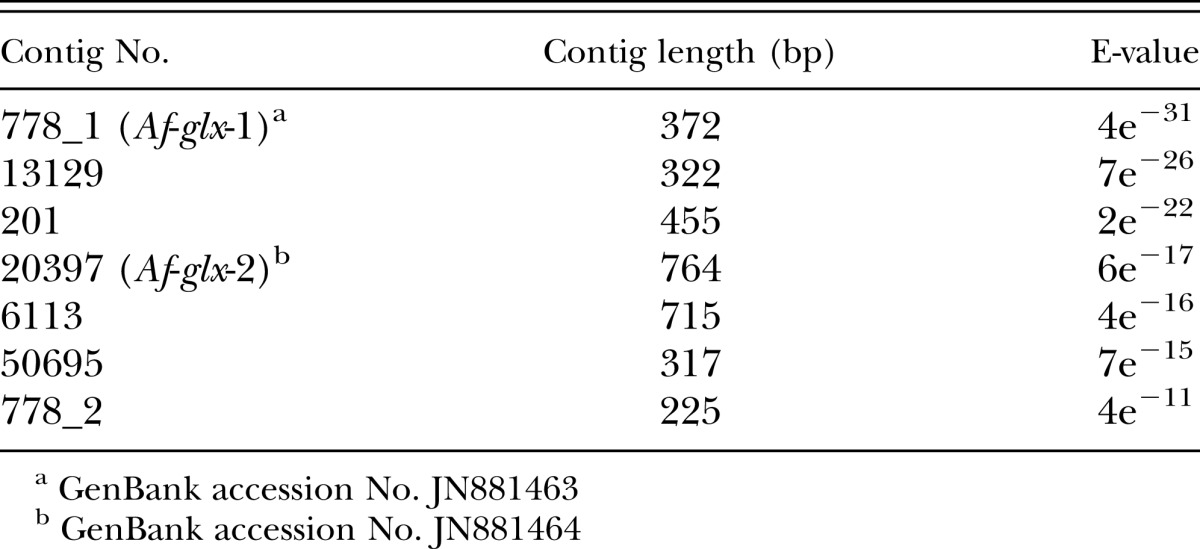
Characterization of Af-glx: Seven A. fragariae assembled transcripts from the RNA-seq experiment were similar (E< 10−10) to the glutaredoxin gene of A. avenae (Aav-glx Table 2), two of which were alternatively-spliced isoforms of the same gene (contig 778_1 and contig 778_2). Two transcripts were selected for further characterization: Af-glx-1 (JN881463, with the lowest E-value in Table 2) and Af-glx-2 (JN881464, with the longest contig in Table 2 ). The ORF of Af-glx-1 spans 47 to 371 bp and encodes a protein of 107 aa (Fig. 2) with a molecular weight of 12.011 kDa and an isoelectric point (PI) 8.89. The ORF of Af-glx-2 spans 76 to 744 bp and encodes protein of 232 aa, with a molecular weight of 26.053 kDa and a PI of 9.02. The Af-GLX-1 protein has a glutaredoxin (GRX) domain from 16 to 96 aa (Fig. 2); Af-GLX-2 has a GRX domain from 127 to 212 aa and a Selenoprotein S domain from 4 aa to 94 aa. Af-GLX-1 aligns with Af-GLX-2 along its entire GRX domain (Fig. 3). While the two putative polypeptide sequences are not identical, they both have RSVP and GGDD conserved glutathione binding sites, as well as cysteine and serine catalytic residues.
Table 2.
Expression levels of selected genes from a desiccation-up-regulated gene set of Aphelenchoides fragariae (see Supplementary Table 1 for list of all genes significantly up-regulated >5-fold in desiccated A. fragariae).
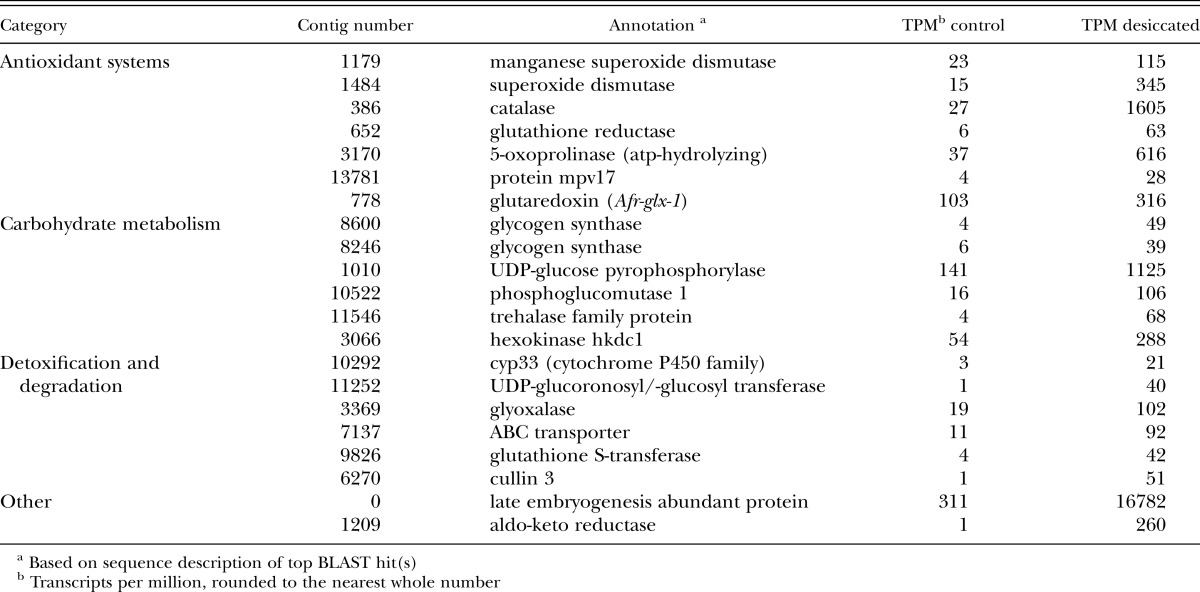
Fig. 2.
Aphelenchoides fragariae glutaredoxin Af-glx-1 cDNA sequence (GenBank accession JN881463) and predicted polypeptide. The open reading frame spans nucleotides 47 to 371. The glutaredoxin (GRX) domain is boxed (position 16 to 99). The primers designed for quantitative reverse-transcription polymerase chain reaction (qRT-PCR) are indicated with asterisks and amplify a fragment of 183 bp.
Fig. 3.
Sequence alignment of two predicted Aphelenchoides fragariae glutaredoxin proteins, Af-GLX-1 and Af-GLX-2. Af-GLX-1 aligned to Af-GLX-2 from position 112 to the end. Boxed amino acids are glutathione (GSH) binding sites, and arrows indicate catalytic residues of glutaredoxin.
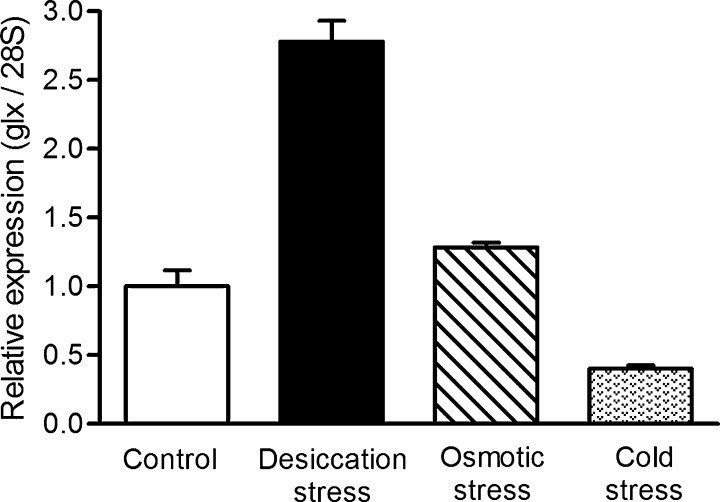
Differential expression of Af-glx-1 under stress treatments: The qRT-PCR standard curves of the Af-glx-1and 28S genes had r2 of 0.995 and 0.998, respectively. Melting curves of the 28S gene and the Af-glx-1 gene were identical for all samples. Relative quantities were computed based on the standard curves (28S, y=-3.554logx+3.9 and glx, y=-3.322logx+17.47, y: relative quantity; x: Ct value). Expression of Af-glx-1 under desiccation stress was 2.8 fold higher than in the untreated control (Fig. 4), and minor up-regulation (1.3 fold-change) of Af-glx-1 was also detected under osmotic stress. Af-glx-1 was down-regulated (0.4 fold-change) under cold stress. Af-glx-1 expression was also quantified in the RNA-seq transcriptome experiment. In this case, its expression was 3.2 times higher in desiccated nematodes compared to controls (Table 1).
Fig. 4.
Differential expression of glutaredoxin Af-glx-1 of Aphelenchoides fragariae under desiccation, cold, and osmotic stress treatments. Differential gene expression was measured by quantitative reverse-transcription polymerase chain reaction (qRT-PCR), and expression levels of Af-glx1 were normalized to the 28S ribosomal gene. Relative expression was computed separately for each stress treatment (4 replicates per treatment). Error bars indicate the standard deviation.
Table 1.
Aphelenchoides fragariae transcripts showing significant similarity to a glutaredoxin gene from Aphelenchus avenae, Aav-glx (NCBI accession No.AY340999.1).
Genes expression changes under desiccation: A number of additional transcripts involved in antioxidant metabolism and the detoxification of reactive oxygen species (ROS) were differentially expressed in the RNA-seq transcriptome experiment. Two superoxide dismutase genes, a glutathione reductase gene and a catalase gene were significantly up-regulated in response to desiccation (Table 1). Also upregulated were 5-oxoprolinase, a component of the gamma-glutamyl cycle that synthesizes glutathione, mpv17, a mitochondrial inner membrane protein thought to be involved in ROS metabolism, and a number of genes involved in the removal of xenobiotics and damaged proteins. Several enzymes central to carbohydrate metabolism were also up-regulated in desiccated nematodes: two glycogen synthase isoforms, UDP-glucose pyrophosphorylase, phosphoglucomutase, hexokinase and trehalase. A putative late embryogenesis abundant protein homolog increased 53-fold in expression under desiccation and was one of the most highly-expressed transcripts in the desiccated transcriptome (over 16000 transcripts per million).
Discussion
Water loss is a challenge for plant-parasitic nematodes. They may experience periods when they are outside of host tissues and exposed to dry soil, and foliar nematodes risk exposure to air as they climb to infect host stems and foliage. The ability of plant parasitic nematodes to withstand desiccation varies among genera and species. Larvae and pre-adults of reniform nematodes Rotylenchulus reniformis were unable to survive direct short-term exposure to 97% relative humidity (Womersley and Ching, 1989), whereas, at the same relative humidity, the soil dwelling nematode A. avenae can survive in anhydrobiotic aggregates (Crowe and Madin, 1975). Aggregation is a group behavioral mechanism that decreases surface exposure to the environment and is widely observed in desiccated plant parasitic nematodes (Barrett, 1991). Aphelenchoides fragariae exhibited greater survivorship under desiccation than A. avenae in our study.
A lag phase in motility following rehydration was observed for both species. We did not directly measure the time at which 50% of the nematodes became active after rehydration (T50). However, based on the observation of motility every 30 min, we estimate that the T50 of A. avenae was between 2 h and 3 h, whereas that of A. fragariae was between 1 h and 2 h. Wharton and Aalders (1999) demonstrated that the length of the lag phase was strongly related to the duration of desiccation but not to the final relative humidity, which suggests that longer desiccation might extend the lag phase.
Both species showed high survival under the cold stress treatment. Cold tolerance is thought to be enhanced by exposure to desiccation: nematodes exposed to desiccation before experiencing sub-zero temperatures may lose freezable water from their bodies, allowing them to survive in an anhydrobiotic state under freezing conditions (Wharton, 2011). We did not test nematode survival under the combination of desiccation and cold stress, which may have produced results different from either stress alone.
In addition to tolerating desiccation well, Aphelenchoides fragariae also has the ability to survive under osmotic stress. As in plants, nematode response mechanisms to osmotic stress and drought stress are likely to overlap (Ingram and Bartels, 1996). These may include production of antioxidants and stress-protective proteins, changes in primary metabolites, and synthesis of compatible solutes (Ingram and Bartels, 1996).
When water is withdrawn from the cell interior, the concentration of ions and solutes increases, leading ultimately to the production of reactive oxygen species (ROS). ROS produced during desiccation (Kranner and Birtić, 2005) and osmotic stress oxidatively damage proteins, increasing their susceptibility to unfolding and aggregation (Burnell and Tunnacliffe, 2011). Glutaredoxin is an important member of cell redox system that reactivates oxidized proteins by reducing the mixed disulfide bonds that form during oxidative damage. Survival of bacteria from dry soils is highly dependent on mechanisms that decrease protein oxidation during desiccation (Fredrickson et al., 2008), and glutaredoxin genes are induced in yeast (Saccharomyces cerevisiae) in response to heat, oxidative and osmotic stress (Grant et al., 2000). The up-regulation of Af-glx-1 under desiccation and osmotic stress in our study suggests that both stresses elicit an antioxidant response in the nematode. This hypothesis is strengthened by the concomitant up-regulation of a host of other antioxidant genes, including superoxide dismutase and catalase.
The two glutaredoxins examined in our study are both categorized as Class I glutaredoxins on the basis of their active site sequences (Couturier et al., 2009), although they differ in the number of cysteine catalytic residues (two in AF-GLX-1, one in AF-GLX-2). AF-GLX-2 contains both a glutaredoxin domain at the C terminus and a Selenoprotein S domain at the N terminus, a structure that has also been reported for a glutaredoxin of Ascaris suum (ADY43354; Wang et al., 2011). Af-glx-2 was not expressed in control or desiccated A. fragariae reared on fungus in the present study, but it was detected in A. fragariae feeding on plant material in the RNA-seq transcriptome experiment (data not shown).
ROS damage to proteins and other cellular components necessitates detoxification and/or degradation of the damaged molecules. In our study, enhanced degradation of damaged proteins via the proteasomal pathway was suggested by a 53-fold up-regulation of cullin 3, a component of ubiquitin-protein ligase complexes that target proteins for degradation. Furthermore, key enzymes in the detoxification pathway for xenobiotics and oxidatively-damaged molecules were also up-regulated. The detoxification process proceeds in three phases (Barrett, 2011), and enzymes commonly associated with each phase were present in the desiccation up-regulated gene set: cytochrome P450 (bioactivation); UDP-glucoronosyl/-glucosyl transferase, glutathione-S-transferase and glyoxylase 1 (conjugation); and an ATP binding cassette (ABC) transporter (elimination). We also observed a 260-fold increase in the expression of aldo-keto reductase, a monomeric NADPH-dependent oxidoreductase whose mammalian homologs are capable of detoxifying lipid peroxidation products that result from oxidative damage to membranes (Bartels, 2001).
Enhanced synthesis of the non-reducing disaccharide trehalose has frequently been reported in anhydrobiotic nematodes, where it is thought to act as a compatible solute, maintaining protein confirmation and membrane stability during desiccation (Perry and Moens, 2011; Madin and Crowe, 1975). Up-regulation of trehalose-6-phosphate-synthase (and down-regulation of glycogen synthase, which draws on the same pool of intermediary metabolites) has previously been reported in desiccated Plectus murrayi (Adhikari et al., 2009). We identified three trehalose-6-phosphate-synthase genes from control and desiccated A. fragariae, all of which showed a non-significant trend toward increased expression under desiccation (average fold-change 2.2, data not shown). Interestingly, we did measure significant increases in the expression of trehalase, which hydrolyzes trehalose to glucose, and in glycogen synthase, which synthesizes glycogen from UDP-glucose. Also up-regulated were the three enzymes that link trehalose hydrolysis with glycogen synthesis through the transformation of glucose to UDP glucose: hexokinase, phosphoglucomutase and UDP-glucose pyrophosphorylase (Behm, 1997). Glycogen levels typically fall during desiccation and increase again as nematodes rehydrate (see, for example, Gal et al. 2001). It is possible that brief hydration of our nematodes prior to or during RNA extraction activated a transcriptional switch from trehalose to glycogen synthesis. At minimum, it is clear that the trehalose/glycogen metabolic network was profoundly affected by changes in nematode water status.
One of the most abundantly expressed mRNAs in the desiccated A. fragariae transcriptome was a putative late embryogenesis abundant (LEA) protein with significant homology to lea-1 of C. elegans. LEA proteins accumulate to high levels during desiccation stress in both plants and animals, where they stabilize proteins and prevent their aggregation (Goyal et al., 2005).
In conclusion, the foliar nematode A. fragariae has the ability to survive under desiccation and osmotic stress, which may help explain the overwintering survival ability of this nematode in dry soil, abscised leaves and dormant buds. Glutaredoxin genes have been identified from the A. fragariae transcriptome, and up-regulation of glutaredoxin has been detected under desiccation and osmotic stresses, suggesting that antioxidant mechanisms underlie A. fragariae tolerance to water loss. Transcriptome data indicate marked up-regulation of cellular antioxidant and detoxification systems, as well as shifts in carbohydrate metabolism in response to desiccation.
Literature Cited
- Adhikari BN, Wall DH, Adams BJ. Desiccation survival in an Antarctic nematode: molecular analysis using expressed sequenced tags. BMC Genomics. 2009;10:69. doi: 10.1186/1471-2164-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baermann G. 1917 Eine einfache Methode zur Auffindung von Ancylostomum (Nematoden) Larven in Erdproben. Geneeskd Tijdschr Ned Indie 57. [Google Scholar]
- Barrett J. Anhydrobiotic nematodes. Agricultural Zoology Review. 1991;4:161–176. [Google Scholar]
- Barrett J. 2011 Biochemistry of survival. Pp. 282–310 in Molecular and Physiological Basis of Nematode Survival. CAB International, Wallingford, UK. [Google Scholar]
- Bartels D. 2001 Targeting detoxification pathways: an efficient approach to obtain plants With multiple stress tolerance? Trends in Plant Science 7:284–286. [Google Scholar]
- Behm CA. The role of trehalose in the physiology of nematodes. International Journal for Parasitology. 1997;27:215–229. doi: 10.1016/s0020-7519(96)00151-8. [DOI] [PubMed] [Google Scholar]
- Browne J, Tunnacliffe A, Burnell A. Anhydrobiosis: Plant desiccation gene found in a nematode. Nature. 2002;416:38. doi: 10.1038/416038a. [DOI] [PubMed] [Google Scholar]
- Browne JA, Dolan KM, Tyson T, Goyal K, Tunnacliffe A, Burnell AM. Dehydration-specific induction of hydrophilic protein genes in the anhydrobiotic nematode Aphelenchus avenae. Eukaryotic Cell. 2004;3:966–975. doi: 10.1128/EC.3.4.966-975.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell AM, Tunnacliffe A. 2011 Gene induction and Desiccation stress. Pp.126–156 in R. N. Perry, and D. A. Wharton, eds. Molecular and physiological basis of Nematode survival. Wallington: CABI. [Google Scholar]
- Conesa A, Götz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- Cooper AFJ, Van Gundy SD. 1971 Senescence, quiescence and cryptobiosis. Pp. 297–318 in B. M, Zuckerman, W. F. Mai, and R. A. Rohde, eds. Plant parasitic nematodes. New York: Academic Press. [Google Scholar]
- Couturier J, Jacquot JP, Rouhier N. Evolution and diversity of glutaredoxins in photosynthetic organisms. Cell and Molecular Life Sciences. 2009;66:2539–2557. doi: 10.1007/s00018-009-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe JH, Madin KAC. Anhydrobiosis in nematodes: evaporative water loss and survival. The Journal of Experimental Zoology. 1975;193:323–334. [Google Scholar]
- Fielding MJ. Observations on the length of dormancy in certain plant infecting nematodes. Proc Helminth Soc Wash. 1951;18:110–112. [Google Scholar]
- Frechman DW, Kaplan DT, Van gundy SD. A Comparison of techniques for extraction and study of anhydrobiotic nematodes from dry soils. Journal of Nematol. 1975;9:176–181. [PMC free article] [PubMed] [Google Scholar]
- Fredrickson JK, Li SM, Gaidamakova EK, Matrosova VY, Zhai M, Sulloway HM, Scholten JC, Brown MG, Balkwill DL, Daly MJ. 2008 doi: 10.1038/ismej.2007.116. Protein oxidation: key to bacterial desiccation resistance? The ISME Journal 2:393–403. [DOI] [PubMed] [Google Scholar]
- Gal TZ, Solomon A, Glazer I, Koltai H. Alterations in the levels of glycogen and glycogen synthase transcripts during desiccation in the insect-killing nematode Steinernema faltiae IS-6. Journal of Parasitology 87L. 2001:725–732. doi: 10.1645/0022-3395(2001)087[0725:AITLOG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Goyal K, Walton LJ, Tunnacliffe A. LEA proteins prevent protein aggregation due to water stress. Biochemistry Journal. 2005;388:151–157. doi: 10.1042/BJ20041931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal K, Browne JA, Burnell AM, Tunnacliffe A. Dehydration-induced tps gene transcripts from an anhydrobiotic nematode contain novel spliced leaders and encode atypical GT-20 family proteins. Biochimie. 2005;87:565–574. doi: 10.1016/j.biochi.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Grabher MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnology. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant CM, Luikenhuis S, Beckhouse A, Soderbergh M, Dawes IW. Differential regulation of glutaredoxin gene expression in response to stress conditions in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 2000;1490:33–42. doi: 10.1016/s0167-4781(99)00234-1. [DOI] [PubMed] [Google Scholar]
- Ingram J, Bartels D. The molecuar basis of dehydration tolerance in plants. Annual Review of Plant Biology. 1996;47:337–403. doi: 10.1146/annurev.arplant.47.1.377. [DOI] [PubMed] [Google Scholar]
- Jagdale GB, Grewal PS. Infection behavior and overwintering survival of foliar nematodes, Aphelenchoides fragariae, on hosta. Journal of Nematology. 2006;38:130–136. [PMC free article] [PubMed] [Google Scholar]
- Kranner I, Birtić S. A modulating role for antioxidants in desiccation tolerance. Integrative and Comparative Biology. 2005;45:451–460. doi: 10.1093/icb/45.5.734. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Madin KAC, Crowe JH. Anhydrobiosis in nematodes: Carbohydrate and lipid metabolism during dehydration. Journal of Experimental Zoology. 1975;193:335–342. [Google Scholar]
- Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Lu F, Marchler GH, Mullokandov M, Omelchenko MV, Robertson CL, Song JS, Thanki N, Yamashita RA, Zhang D, Zhang N, Zheng C, Bryant SH. CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acids Research. 2011;39:225–229. doi: 10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RN, Moens M. 2011 Survival of parasitic nematodes outside the host. Pp. 1–27 in Molecular and Physiological Basis of Nematode Survival. CAB International, Wallingford, UK. [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nature Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods in Molecular Biology. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Treonis AM, Wall DH. Soil nematodes and desiccation survival in the extreme arid environment of the antarctic dry valleys. Integrative and Comparative Biology. 2005;45:741–750. doi: 10.1093/icb/45.5.741. [DOI] [PubMed] [Google Scholar]
- Wang J, Czech B, Crunk A, Wallace A, Mitreva M, Hannon GJ, Davis RE. Deep small RNA sequencing from the nematode Ascaris reveals conservation, functional diversification, and novel developmental profiles. Genome Research. 2001;21:1462–1477. doi: 10.1101/gr.121426.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse AM, Procterm JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2 - a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton DA. 2011 Cold tolerance. Pp. 182–204 in R. N. Perry, and D. A. Wharton, eds. Molecular and physiological basis of nematodes survival. Wallingford:CABI. [Google Scholar]
- Wharton DA, Aalders O. Desiccation stress and recovery in the anhydrobiotic nematode Ditylenchus dipsaci (Nematoda: Anguinidae) European Journal of Entomology. 1999;96:199–203. [Google Scholar]
- Womersley C, Ching C. Natural dehydration regimes as a prerequisite for the successful induction of anhydrobiosis in the nematode Rotylenchulus reniformis. The Journal of Experimental Biology. 1989;143:359–72. doi: 10.1242/jeb.143.1.359. [DOI] [PubMed] [Google Scholar]