Abstract
One hundred and eleven golf courses from 39 counties in the Carolinas were surveyed for plant-parasitic nematodes. Species diversity within habitats was analyzed with five diversity indices including Diversity index (H’), Evenness (J’), Richness (SR), Dominance (λ) and Diversity (H2). The results revealed a remarkably high diversity of 24 nematode species belonging to 19 genera and 11 families. Of those, 23 species were found in SC, 19 species in NC, and 18 species were detected in both states. Helicotylenchus dihystera, Mesocriconema xenoplax, Hoplolaimus galeatus, Tylenchorhynchus claytoni, Belonolaimus longicaudatus, Meloidogyne graminis and Paratrichodorus minor were the most prevalent and abundant species in golf course turfgrasses in both states. Twelve species were new records of plant parasitic nematodes in turfgrasses in both NC and SC. The results also revealed effects of different habitats on diversity of nematode species in turfgrass ecosystem. H’ and SR values were higher in SC than in NC. H’, J’ and H2 values were significantly higher in sandy than in clay soil in NC, but no significant differences between sand and clay soil were detected in SC or in pooled data from both states. There were no significant differences for all indices among the management zones (putting green, fairway and tee) in NC. However, in SC and pooled data, H’, SR and H2 were significantly higher in putting greens than in fairways and tees. Significant differences from different grass species (bermudagrass, creeping bentgrass and zoysiagrass) were detected only in H’, which was significantly higher in zoysiagrass than in bentgrass or bermudagrass in NC. In pooled data, H’ was significantly higher in zoysiagrass samples than in creeping bentgrass samples but was not significantly different from bermudagrass samples.
Keywords: North Carolina, South Carolina, detection, distribution, diversity, ecology, golf course, identification, plant-parasitic nematode, turfgrass
Turfgrasses are among the most widely planted ornamental plants in the world, serving important functions in soil stabilization and providing safe surfaces for recreational activities. In the United States, there are currently over 19,000 golf courses (http://www.thegolfcourses.net), including 451 in South Carolina (SC) (http://www.mapsofworld.com/usa/states/south-carolina/south-carolina-golf-courses.html) and 644 in North Carolina (NC) (http://www.mapsofworld.com/usa/states/north-carolina/north-carolina-golfcourses.html). However, maintenance of golf courses is becoming more challenging due to economic pressures and changes in the availability of pesticides. Of all turfgrass pests, nematodes are probably the least understood and most often overlooked. Due to lack of understanding of the nematodes associated with turfgrasses and their impact on turfgrass health, it is very difficult to accurately diagnose nematode problems. More research is needed in this area so that nematode problems can be effectively diagnosed and managed in turfgrass systems.
Over the past 40 years, numerous research papers have been published on plant-parasitic nematodes associated with turfgrass worldwide, including the US (Sikora et al., 2001; Lucas et al., 1974; Crow and Walker, 2003; Hixson et al., 2004; Karssen et al., 2004; Crow, 2005; Mitkowski, 2007), Canada (Yu et al., 1998; Mitkowski and Jackson, 2003; Bélair et al., 2006; Simard et al., 2008, 2009), Argentina (Echeverría and Chaves, 1998), Germany, Sweden and Norway (Magnusson and Hammeraas, 1997; Knuth, 1998), Belgium (Viaene et al., 2007; Vandenbossche et al., 2011), and Israel (Oka et al. 2004).
In the United States, 38 species in 23 genera of plant-parasitic nematodes associated with turfgrasses have been recorded so far. Despite the importance of turfgrasses and the prevalence of nematode problems in NC and SC turfgrasses, little research has been carried out on the nematodes in this region. In SC, species from genera of Mesocriconema, Helicotylenchus, Trichodorus, Meloidogyne, Hoplolaimus and Belonolaimus are known to be problematic in warm and cool season turfgrasses (http://www.clemson.edu/extension/horticulture/turf/pest_guidelines/2011_pest_guidelines/nematodes_2011.pdf). Lucas et al. (1974) showed that Mesocriconema ornata, Helicotylenchus dihystera, Trichodorus christiei, Meloidogyne sp., Tylenchorhynchus claytoni, Hoplolaimus galeatus and B. longicaudatus were the common plant-parasitic nematodes; and Pratylenchus zeae, Xiphinema americanum and Paratylenchus sp. were occasionally found in soil samples from golf greens in NC. Little detailed, systematic and updated information about the species, occurrence, population levels and distribution of turf nematodes in NC and SC has been presented since this initial work in 1974.
In recent years, diversity indices have become common tools for describing the diversity and distribution of plant-parasitic nematodes. Useful indices include Shannon diversity index (H’), Evenness (J’), Richness (SR), Dominance (λ) and Diversity (H2). In conjunction with proper sampling strategies, these indices can be used to make inferences on the influence of soil type, host species composition, or other factors on nematode populations. A study on diversity and distribution of nematode communities in grassland from Romania, and a survey of plant-parasitic nematodes in natural and semi-natural mountain grassland in southern Spain were undertaken (Popvici and Ciobanu, 2000; Talavera and Navas, 2002). The related results revealed that index H’ did not differ significantly between the different types of grasslands (Popvici and Ciobanu, 2000), and there were no significant differences between mountainous areas (Talavera and Navas, 2002). However, our knowledge of the diversity of nematodes associated with golf grasses is very scarce compared with agricultural crops.
The purpose of this study was to determine species diversity, incidence and distribution of plant-parasitic nematodes associated with golf course turfgrasses in NC and SC to determine how grass species, soil type, and management zone influence the distribution of plant-parasitic nematodes.
Material and Methods
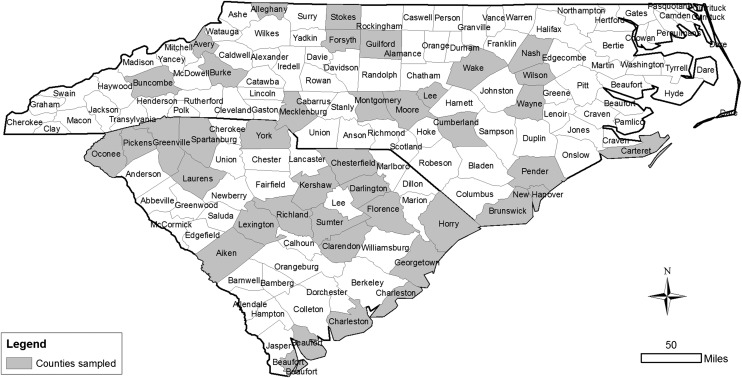
Soil sampling: A total of 282 soil samples were collected from 111 golf courses in 39 counties of NC and SC during the summer of 2011 (Fig. 1). Sampling locations were selected to represent the different grass species [hybrid bermudagrass (Cynodon dactylon × C. transvaalensis), creeping bentgrass (Agrostis stolonifera) and zoysiagrass (Zoysia matrella and Zoysia japonica)], soil textures (sandy and clay which did not include sandy clay and clay loam, etc.) and management zones (putting greens, fairways, and tees). Preference was given to locations that had previously experienced or reported problems with plant-parasitic nematodes, but some exploratory sampling was also done in areas where no nematode problems had been previously reported. Each sample consisted of 12 soil cores (1.5 cm diam. × 20 cm deep) sampled at roughly equal intervals in a zig-zag pattern across an area of 1000 m2 or less. Soil samples were placed in sealed plastic bags, which were then placed in sample boxes and stored at 4°C before analysis to minimize changes in nematode populations.
Fig. 1.
Map of North Carolina and South Carolina showing 39 sampled counties (shaded).
Nematode extraction: Nematodes were extracted from soil samples by a combination of elutriation (Byrd et al., 1976) and centrifugation (Jenkins, 1964) methods. A 500-cm3 subsample was taken from each composite and assayed for enumeration and identification of plant-parasitic nematodes. These tests were carried out by the Nematode Assay Section of the NCDA&CS Agronomic Division.
Nematode identification and counting: Nematodes were identified and counted under the inverted microscope. For further species identification, nematodes were picked, killed by heating (70°C), then fixed in FG solution (1 ml glycerol, 10ml formalin, and 80ml distilled water). Specimens were dehydrated slowly into 100% glycerol and mounted on slides (Southey, 1970). Measurements were performed with the aid of a drawing tube using a LEICA DM 2500 microscope and a stage micrometer. Morphometric data were collected and analyzed using Excel software as described by Ye (1996). In addition to morphological identification, most of the species were characterized by sequencing of the near full-length small subunit (SSU) and internal transcribed spacer region subunit 1 (ITS1) of the rDNA locus in a separate study.
Data analysis: Nematode diversity and incidence were assessed by calculating prevalence, mean intensity, and maximum density (Boag, 1993). Prevalence was defined as the number of samples having a particular nematode species divided by the number of samples examined, expressed as a percentage. Relative abundance was calculated as the total number of individuals of a particular nematode species per 500 cm3 soil in all the samples divided by the number of samples including those with zero counts for that species. Mean intensity was defined as the number of individuals of a particular nematode species per 500 cm3 soil in the positive samples divided by the number of positive samples, and maximum density was determined as the maximum number of individuals of a particular nematode species per 500 cm3 soil recovered from a sample. Several diversity indices were also calculated according to Yeates and Bird (1994):
 |
Richness SR = (s-1) ln N
Evenness J’ = H’/H’max
Simpson’s dominance λ = Σ pi2
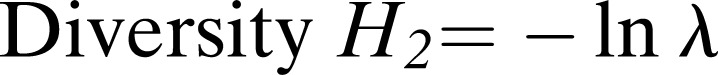 |
Where s = number of taxa (species) in the sample; N = total number of nematodes identified in the sample; pi = proportion of individuals of taxa i in the total population; H’max = ln s.
Differences in the diversity indices among various factors (grass species, management zone, and soil type) were analyzed by Duncan’s multiple range test (p< 0.05) in SAS 9.2 (SAS Institute, Cary, NC, USA).
Results
Survey: Twenty-four species of plant-parasitic nematodes in 19 genera of 11 families were identified from the 282 soil samples collected from 111 golf courses in 39 counties in NC and SC. Detailed nematode morphological identification and a new species description were published in earlier papers (Zeng et al., 2012a, b). Among those nematodes, 23 species were found in SC, 19 in NC, and 18 species in both states (Tables 1-2). The following species are new records of plant-parasitic nematodes associated with turfgrasses: 12 species (Cactodera sp., Dolichodorus heterocephalus, Hemicaloosia graminis, Hemicriconemoides wessoni, Hemicycliophora conida, Heterodera sp., Longidorus paralongicaudatus, Loofia thienemanni, Ogma floridense, Paratrichodorus allius, Pratylenchus penetrans, Xiphinema bakeri) in both NC and SC; 12 species (Cactodera sp., Dolichodorus heterocephalus, Hemicaloosia graminis, Heterodera sp., Hemicycliophora conida, Longidorus paralongicaudatus, Loofia thienemanni, Ogma floridense, Paratrichodorus allius, Pratylenchus penetrans, Scutellonema brachyurum and Tylenchorhynchus claytoni) in SC; and 10 species (Hemicaloosia graminis, Hemicriconemoides wessoni, Hemicycliophora conida, Mesocriconema xenoplax, M. curvatum, M. sphaerocephala, Ogma floridense, Paratrichodorus allius, Pratylenchus penetrans and Xiphinema bakeri) in NC.
Data concerning prevalence, relative abundance, mean intensity, and maximum density of nematodes are presented in Table 1. The most prevalent nematodes on golf courses in both states were Mesocriconema xenoplax (69.9%), Helicotylenchus dihystera (60.3%), Belonolaimus longicaudatus (46.5%), Paratrichodorus minor (42.9%), Meloidogyne graminis (36.9%), Hoplolaimus galeatus (35.1%), and Tylenchorhynchus claytoni (25.2%). These seven species were also most prevalent on golf course putting greens, but in a different order of occurrance: Mesocriconema xenoplax (70.8%), Helicotylenchus dihystera (43.8%), B. longicaudatus (40.6%), Hoplolaimus galeatus (33.3%), P. minor (33.3%), T. claytoni (28.1%), and Meloidogyne graminis (27.1%) (Table 1).
Table 1.
Frequency of occurrence and population density of plant-parasitic nematodes from golf courses in North and South Carolina.
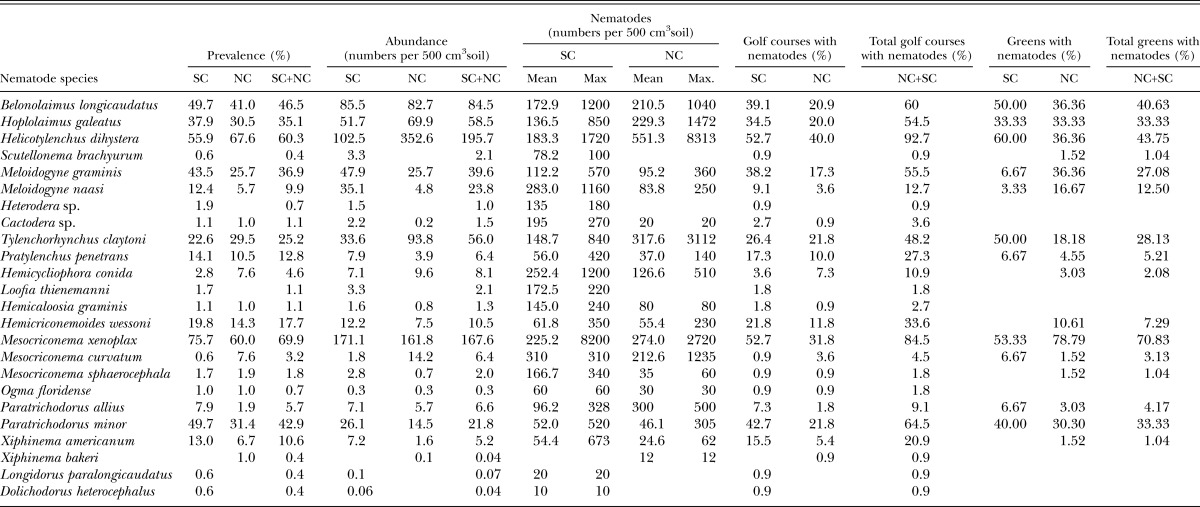
The most abundant species wereHelicotylenchus dihystera (196 per 500 cm3 of soil), Mesocriconema xenoplax (168), B. longicaudatus (85), Hoplolaimus galeatus (59), T. claytoni (56), Meloidogyne graminis (40), M. naasi (24), and P. minor (22) (Table1).
Other species found occasionally at high densities were Mesocriconema curvatum (maximum density 1235 in NC), Hemicycliophora conida (1200 in SC; 510 in NC), Xiphinema americanum (673 in SC), Pratylenchus penetrans (420 in SC), Hemicriconemoides wessoni (350 in SC), Mesocriconema sphaerocephala (340 in SC), and Paratrichodorus allius (328 in SC; 500 in NC) (Table 1).
The species with the highest mean intensities and maximum densities were B. longicaudatus (173 and 1200 in SC respectively; 211 and 1040 in NC), Helicotylenchus dihystera (173 and 1200 in SC; 211 and 1040 in NC), Hoplolaimus galeatus (137 and 850 in SC; 230 and 1472 in NC), Mesocriconema xenoplax (225 and 8200 in SC; 274 and 2720 in NC), T. claytoni (149 and 840 in SC; 318 and 3112 in NC), Meloidogyne naasi (283 and 1160 in SC), and Hemicycliophora conida (252 and 1200 in SC; 127 and 510 in NC) (Table 1).
The total numbers of nematode species recovered were greater in SC (23 species) than in NC (19). In addition, the numbers of species exceeding 10% prevalence (11 in SC vs 9 in NC), relative abundance exceeding 30 nematodes per 500 cm3 of soil (7 vs 5), mean densities exceeding 100 individuals (15 vs 9) and maximum densities exceeding 500 individuals (11 vs 9) were all greater in SC than in NC (Table 1).
Occurrence, frequency and total numbers of plant-parasitic nematodes among different grass species, management zones, and soil types are presented in Table 2. The numbers of species recovered in bermudagrass were greater than in creeping bentgrass and zoysiagrass in both states. In bermudagrass, a greater number of species occurred in SC than in NC (21 species and 15, respectively); and B. longicaudatus, P. minor, Meloidogyne graminis, Mesocriconema xenoplax, Helicotylenchus dihystera, and Hoplolaimus galeatus were most prevalent species in SC. A similar species profile was observed in bermudagrass in NC, except that prevalence of H. galeatus was relatively low. In zoysiagrass, the number of nematode species identified was greater in NC (10 species) than in SC (7 species). B. longicaudatus, P. minor, Meloidogyne graminis and Mesocriconema xenoplax were the most prevalent species in SC; these species plus Helicotylenchus dihystera and Hoplolaimus galeatus were most prevalent in NC. In creeping bentgrass, in addition to B. longicaudatus, H. dihystera, M. xenoplax and P. minor, T. claytoni was most prevalent in NC. A similar profile was observed, except that B. longicaudatus was less prevalent in SC (Table 2).
Table 2.
Prevalence of plant-parasitic nematodes in different habitats of golf courses in North and South Carolina.
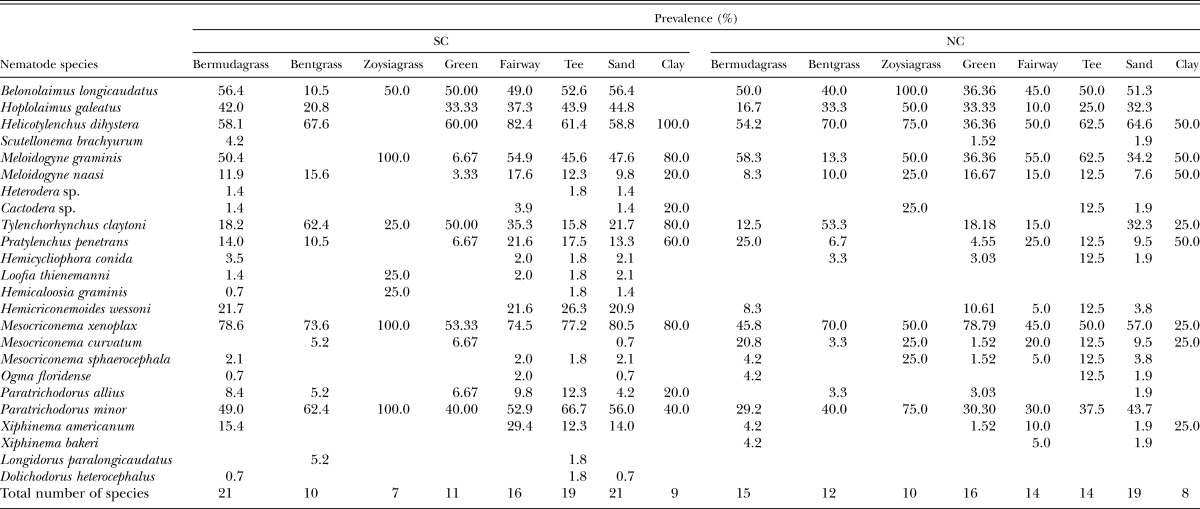
In putting greens, the number of nematode species identified were greater in NC (16 species) than in SC (11), lower in golf course tees in NC (14) than in SC (19), but similar in fairways across both states (16 in SC, 14 in NC).B. longicaudatus, P. minor, Mesocriconema xenoplax, Helicotylenchus dihystera were prevalent species in all management zones in NC and SC. H. galeatus was prevalent in all management zones in SC but only in putting greens in NC. Meloidogyne graminis was dominant in all management zones in NC but only in fairways and tees in SC. T. claytoni was prevalent only in SC putting greens and fairways (Table 2).
The total number of nematode species identified in sandy soil was greater than in clay soil in both states. However, there was no clear difference between NC and SC in the number of species identified in different soil types. In sandy soils, B. longicaudatus, P. minor, Mesocriconema xenoplax, H. dihystera, H. galeatus, Meloidogyne graminis and T. claytoni were prevalent in both states. In clay soils, H. dihystera, M. graminis and P. penetrans were prevalent species in both states, but T. claytoni, M. xenoplax and P. minor were prevalent only in SC, and M. nassi was only in NC (Table 2).
Distribution: The distribution of nematodes on golf courses in NC and SC is shown in Table 3. The most widespread species over the two states were P. minor (19 counties in SC; 16 in NC), Meloidogyne graminis (18 in SC; 12 in NC), Mesocriconema xenoplax (19 in SC; 13 in NC), H. dihystera (18 in SC; 18 in NC), T. claytoni (14 in SC; 12 in NC), B. longicaudatus (11 in SC; 10 in NC), H. galeatus (13 in SC; 11 in NC) and P. penetrans (15 in SC; 9 in NC) (Table 3).
Table 3.
Distribution of plant-parasitic nematodes in North and South Carolina.

Some species were not evenly distributed among the two states. For example, Hemicriconemoides wessoni was documented in 10 counties in SC but only 5 counties in NC, and X. americanum in 9 counties in SC but only 4 counties in NC. Other species were found only in one state. For instance, Scutellonema brachyurum, Heterodera sp., Loofia thienemanni, Longidorus paralongicaudatus and Dolichodorus heterocephalus were only found in SC, whereas X. bakeri was only found in NC (Table 3).
Nematode diversity: The effects of different habitats on nematode biodiversity are presented in Tables 4–7. Of the five indices reflecting biodiversity, Diversity (H’), Richness (SR) and Dominance (λ) values were significantly different between NC and SC (Table 4). H’ and SR were higher in SC than in NC, while λ was higher in NC (Table 4).
Table 4.
Diversity of plant-parasitic nematodes in North and South Carolina.
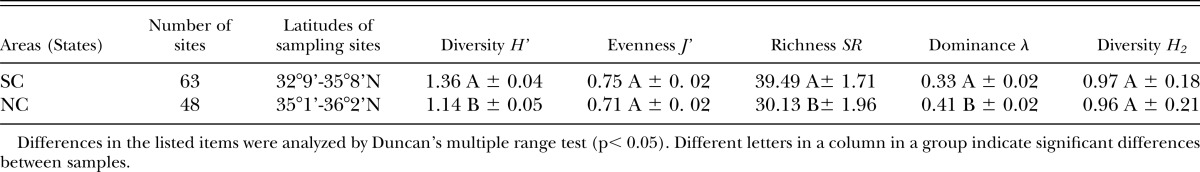
Table 5.
Effect of soil type on nematode diversity in North and South Carolina.
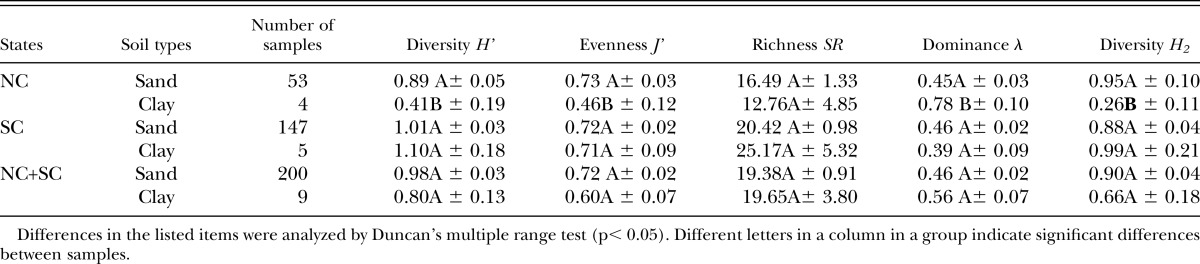
Table 6.
Effect of management zones on nematode diversity in North and South Carolina.
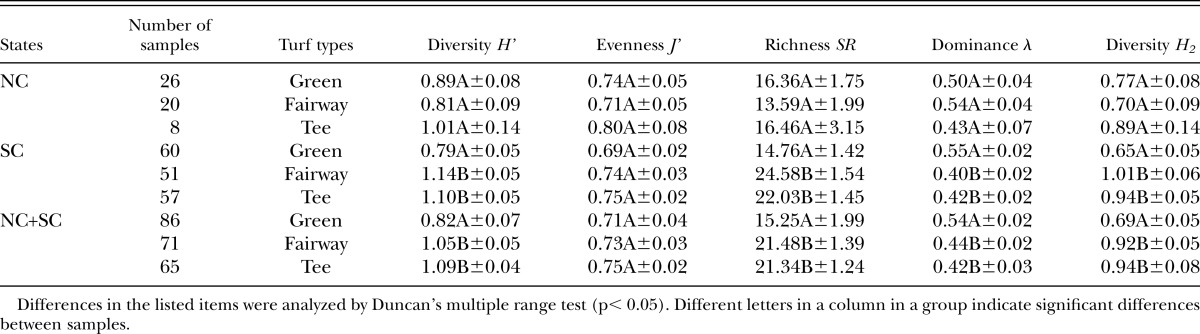
Table 7.
Effect of grass species on nematode diversity in North and South Carolina.
Nematode biodiversity as influenced by soil type presented in Tables 5 shows that the indices H’, λ, J’ and H2 were significantly different between sandy and clay soil in NC. H’, J’ and H2 are significantly higher in sandy samples than in clay ones, but λ was significantly higher in clay samples, indicating dominance by a smaller number of species. However, no significant differences between sandy and clay soil were detected in SC or in pooled data from both states (Table 5).
The effects of management zone on nematode diversity indices are shown in Tables 6. There were no significant differences among the management zones in NC. However, in SC, H’, SR and H2 were significantly higher in putting greens than in fairways and tees. Pooled data from NC and SC showed similar differences for H’, λ, SR and H2 (Table 6).
Comparing samples from different grass species (Tables 7), significant differences were detected only in Diversity (H’), which was significantly higher in zoysiagrass than in bentgrass or bermudagrass in NC. In pooled data from NC and SC, H’ was significantly higher in zoysiagrass samples than in creeping bentgrass samples but was not significantly different from bermudagrass samples.
Discussion
Twenty-four species of plant-parasitic nematodes were identified among 282 soil samples collected from 111 golf courses in NC and SC. Of those nematodes, 23 and 19 species were detected in SC and NC, respectively. Eighteen species were documented in both states (Tables 1–2). Of the 38 nematode species previously reported from turfgrasses in the USA, 65.8% of them were documented in the current study. These results revealed a great diversity of nematode species associated with golf course turfgrasses in NC and SC.
The overall nematode populations in NC and SC were very similar; 75% of species were present in both adjacent states. However, some differences between the two states were observed. For example, Heterodera sp., Loofia thienemanni, Longidorus paralongicaudatus and D. heterocephalus were found only in SC, but Xiphinema bakeri only in NC. In addition, the numbers of species that exceeded 10% prevalence (11 in SC vs 9 in NC), relative abundance exceeding 30 nematodes per 500 cm3 of soil (7 vs 5), mean densities exceeding 100 nematodes (15 vs 9) and maximum densities exceeding 500 nematodes (11 vs 9) were all greater in SC than in NC.
The results in this survey indicated that Helicotylenchus dihystera, Mesocriconema xenoplax, Hoplolaimus galeatus, T. claytoni, B. longicaudatus, Meloidogyne graminis and P. minor were not only most prevalent but also the most abundant species, especially in putting greens in both states (Table 1). Similar results were reported in NC (Lucas et al., 1974) and SC(http://www.clemson.edu/extension/horticulture/turf/pest_guidelines/2011_pest_guidelines/nematodes_2011.pdf), Florida (http://edis.ifas.ufl.edu/pdffiles/IN/IN12400.pdf), New York (Murdoch et al., 1978), Kansas (Todd and Tisserat, 1990), and Oklahoma (Walker et al., 2002). This revealed the wide distribution and important role of these species in the dynamics and structure of the turfgrass nematode community.
Some species with lower relative abundances were found occasionally at high densities, including Mesocriconema curvatum, M. sphaerocephala, Hemicycliophora conida, X. americanum, Pratylenchus penetrans, Hemicriconemoides wessoni, and Paratrichodorus allius (Table 1). However, the relationships between nematode density and impact on turfgrass growth are not yet clear even though both NC and SC employ general damage thresholds for the common genera. Further study of these species is necessary to better understand the ecological processes involving plant-parasitic nematodes in turfgrass ecosystems.
Previous studies showed that nematodes from the genera Belonolaimus, Mesocriconema, Helicotylenchus, Hemicycliophora, Hoplolaimus, Paratrichodorus, Pratylenchus, Meloidogyne, Trichodorus, and Tylenchorhynchus were associated with both warm- and cool-season grasses (Lucas et al., 1974; Sikora et al., 2001; Walker et al., 2002). Similar results were found in this study: Belonolaimus longicaudatus, Paratrichodorus minor, Mesocriconema xenoplax, Helicotylenchus dihystera, Hoplolaimus galeatus, Pratylenchus penetrans, Meloidogyne naasi and Tylenchorhynchus claytoni were found in both bermudagrass and creeping bentgrass. It appears that these most common nematode species are not host specific. In addition, Brodie and Burton (1967) and Nutter and Christie (1958) showed that warm-season hybrid bermudagrass putting greens were susceptible to a number of plant-parasitic nematodes. Findings were similar in this study. The total numbers of species identified were greater in bermudagrass (21 species in SC, 15 in NC) than in creeping bentgrass (10 species in SC, 12 in NC) and zoysiagrass (7 species in SC, 11 in NC) in both states. This result revealed bermudagrass to be a more favorable host than creeping bentgrass and zoysiagrass. In creeping bentgrass, the most prevalent nematodes in NC include B. longicaudatus, H. dihystera, M. xenoplax, P. minor and T. claytoni. A similar result was observed in SC, but B. longicaudatus was less prevalent. This discrepancy could be explained by the fact that very little creeping bentgrass is grown in the southeastern part (Coastal Plain) of SC where temperatures are very warm, making its culture difficult. Most of the bentgrass samples from SC came from the Piedmont or Mountain/Valley regions. The scarcity of sandy soil in those regions could account for the apparent lower prevalence of B. longicaudatus.
Most of the plant-parasitic nematodes that damage turfgrasses favor sandy soil (Crow, 2005). The results in the present study confirmed this fact. The total number of species identified in sandy soils was greater than in clay soils in both states. Seven species were prevalent in sandy soils (B. longicaudatus, Paratrichodorus minor, Mesocriconema xenoplax, Helicotylenchus dihystera, Hoplolaimus galeatus, Meloidogyne graminis and T. claytoni), but only three species (H. dihystera, M. graminis and P. penetrans) were prevalent in clay soils. Particularly, B. longicaudatus and H. galeatus were only found in sandy soil samples from both states, indicating that they are very sensitive to soil texture. This finding is consistent with a previous report that B. longicaudatus is limited to soils with over 80% sand content (Robbins and Barker, 1974), and it is the dominant and most severely damaging nematode species in golf courses, especially on putting greens that are constructed of over 90% sand content (Crow, 2005).
Indices such as H’, J’, SR, λ and H2 are useful tools for describing biodiversity and analyzing the impact of certain factors on species distribution. Popvici and Ciobanu (2000) showed that the H’ of nematode communities did not differ significantly between the different types of grasslands. Similar results were showed by Talavera and Navas (2002). However, clear relationships between the diversity of nematodes and soil texture, climate, plant species, pesticides, and management practices in grasslands were indicated by Yeates and Bongers (1999). Similar results were found in the present study, which revealed effects of different habitats (soil texture, grass species and management zone) on diversity of nematode species in turfgrass ecosystems. H’, J’, λ and H2 values were significantly different between sand and clay soil in NC, being significantly higher (except for λ) in sandy soils than in clay soils, indicating that a greater diversity and more even distribution of the prevalent species occurred in sandy than in clay soil. However, no significant differences between sandy and clay soil were observed in SC or in pooled data.
With regard to the effects of grass species, there were no significant differences among bermudagrass, bentgrass and zoysiagrass for all indices in SC nor for J’, λ, SR and H2 either in NC or in pooled data. However, for H’, there was significant difference between zoysiagrass and other species in NC and between zoysiagrass and bentgrass in pooled data. H’ values were greater in zoysiagrass than in other species. There appeared to be higher diversity of species in zoysiagrass than in others.
Concerning the effects of the management zones, there were no significant differences for all indices among the putting green, fairway and tee in NC. However, in SC and pooled data from both states, H’, SR and H2 values were significantly higher in fairways and tees than in greens, thus indicating a lower diversity of species in greens.
In turfgrass ecosystems, fluctuations or distribution differences of nematode populations are common (Davis et al., 1994; Peacock et al., 2005; Jordan and Mitkowski, 2006; Settle et al., 2006; Westerdahl and Harivandi, 2007; Giat et al., 2008; McGroary et al., 2009). McGroary et al. (2009) reported that seasonal fluctuations in B. longicaudatus and root growth of bermudagrass varied among locations and years. Settle et al. (2006) indicated that populations of H. galeatus in a naturally infested experimental putting green of creeping bentgrass increased from late spring through midsummer, declined in August, and increased again in the fall. Jordan and Mitkowski (2006) showed that there was a significant effect of season on population level of Mesocriconema, Tylenchorynchus and Heterodera juveniles in the sampling year. Such seasonal fluctuations probably lead to changes of nematode structure in the turfgrass community. In this study, soil samples were collected only in the summer of 2011. Further study on dynamics of various important nematodes over the course of several seasons or even of several continuous years could prove invaluable for the effective management of turf nematodes. This study provides a baseline of information on species diversity, incidence and distribution of plant-parasitic nematodes associated with golf course turfgrasses in NC and SC.
Acknowledgments
The authors thank Dr. Bode A. Olukolu from laboratory of maze disease, Department of Plant Pathology, North Carolina State University (NCSU) for SAS analysis of diversity data, Dr. Hanafy Fouly from Pee Dee Research & Education Center, Clemson University for his input in this project and John Royals from Central Piedmont Community College for collecting SC soil samples. This work was supported by a Rounds-4-Research grant from the Carolinas Golf Course Superintendents Association. Financial support was also provided by Bayer Environmental Science, Quali-Pro, and Syngenta Lawn and Garden. The first author received a grant focused on developing flower industrial system from Guangdong Department of Agriculture, China, and a visiting scholar fellowship from Zhongkai University of Agriculture and Engineering.
Literature Cited
- Bélair G, Simard L, Eisenback JD. First report of the barley root-knot nematode Meloidogyne naasi infecting annual bluegrass on a golf course in Quebec, Canada. Plant Disease. 2006;90:1109. doi: 10.1094/PD-90-1109A. [DOI] [PubMed] [Google Scholar]
- Boag B. Standardization of ecological terms in Nematology. Fundamental and Applied Nematology. 1993;16:190–191. [Google Scholar]
- Brodie BB, Burton GW. Nematode population reduction and growth response of bermuda turf as influenced by organic pesticide applications. Plant Disease Reporter. 1967;51:562–566. [Google Scholar]
- Byrd DW, Jr, Barker KR, Ferris H, Nusbaum CJ, Griffin WE, Small RH, Stone CA. Two semi-automatic elutriators for extracting nematodes and certain fungi from soil. Journal of Nematolology. 1976;8:206–212. [PMC free article] [PubMed] [Google Scholar]
- Crow WT. Plant parasitic nematodes on golf course turf. Outlooks on Pest Management. February. 2005:10–15. [Google Scholar]
- Crow WT, Walker NR. Diagnosis of Peltamigratus christiei, a plant-parasitic nematode associated with warm-season turfgrasses in the Southern United States. Plant Health Progress. 2003:1–8. [Google Scholar]
- Davis RF, Wilkinson HT, Noel GR. Vertical distribution of three nematode genera in a bentgrass putting green in central Illinois. Journal of Nematology. 1994;26:518–521. [PMC free article] [PubMed] [Google Scholar]
- Echeverría MM, Chaves EJ. Identification of Meloidogyne naasi Franklin, 1965 from Argentina. Nematologica. 1998;44:219–220. [Google Scholar]
- Giat E, Kaspi R, Anderson CA, Westerdahl BB. Seasonal population dynamics of the plant-parasitic nematode, Anguina pacificae on golf course putting greens in California. Journal of Nematology. 2008;40:252–257. [Google Scholar]
- Hixson AC, Crow WT, McSorley R, Trenholm LE. Host status of ‘SeaIsle 1’ seashore paspalum (Paspalum vaginatum) to Belonolaimus longicaudatus and Hoplolaimus galeatus. Journal of Nematology. 2004;36:493–498. [PMC free article] [PubMed] [Google Scholar]
- Jenkins WR. A rapid centrifugal-floatation technique for separating nematodes from soil. Plant Disease Reporter. 1964;48:692. [Google Scholar]
- Jordan KS, Mitkowski NA. Population dynamics of plant-parasitic nematodes in golf course greens turf in Southern New England. Plant Disease. 2006;90:501–505. doi: 10.1094/PD-90-0501. [DOI] [PubMed] [Google Scholar]
- Karssen G, Bolk RJ, Van Aelst AC, van den Beld I, Kox LFF, Korthals G, Molendijk L, Zijlstra C, Van Hoof R, Cook R. Description of Meloidogyne minor n. sp. (Nematoda: Meloidogynidae), a root-knot nematode associated with yellow patch disease in golf courses. Nematology. 2004;6:59–72. [Google Scholar]
- Knuth P. Schaden in einem Sportsrasen durch das Würzengallenälchen Meloidogyne naasi in Baden-Württemberg. Nachrichtenblatt des Deutschen Pflanzenschutzdienstes. 1998;12:305–307. [Google Scholar]
- Lucas LT, Blake CT, Barker KR. Nematodes associated with bentgrass and bermudagrass golf greens in North Carolina. Plant Disease Reporter. 1974;58:822–824. [Google Scholar]
- Magnusson C, Hammeraas B. Nematoder i grasbaner. Växtskyddsnotiser. 1997;61:121–132. [Google Scholar]
- McGroary P, Crow WT, McSorley R, Giblin-Davis RM, Cisar JL. Seasonal fluctuations of Belonolaimus longicadatus in Bermudagrass. Nematropica. 2009;39:99–110. [Google Scholar]
- Mitkowski NA. First report of Subanguina radicicola, the root-gall nematode infecting Poa annua putting greens in Washington State. Plant Disease. 2007;91:905. doi: 10.1094/PDIS-91-7-0905C. [DOI] [PubMed] [Google Scholar]
- Mitkowski NA, Jackson N. Subanguina radicicola, the root-gall nematode infecting Poa annua in New Brunswick, Canada. Plant Disease. 2003;87:1263. doi: 10.1094/PDIS.2003.87.10.1263C. [DOI] [PubMed] [Google Scholar]
- Murdoch CL, Tashiro H, Harrison MB. Plant-parasitic nematodes associated with golf putting-green turf in New York. Plant Disease Reporter. 1978;62:85–87. [Google Scholar]
- Nutter GC, Christie JR. Nematode investigations on putting green turf. Proceedings of the Florida State Horticultural Society. 1958;71:445–449. [Google Scholar]
- Oka Y, Karssen G, Mor M. First report of the root-knot nematode Meloidogyne marylandi on turfgrasses in Israel. Plant Disease. 2004;88:309. doi: 10.1094/PDIS.2004.88.3.309B. [DOI] [PubMed] [Google Scholar]
- Peacock CH, Flanagan MS, Dunn RA. Nematode population dynamics and nematicide efficacy on bermudagrass, Cynodon dactylon (L.) Pers. International Turfgrass Society Research Journal. 2005;10:746–752. [Google Scholar]
- Popovici I, Ciobanu M. Diversity and distribution of nematode communities in grasslands from Romania in relation to vegetation and soil characteristics. Applied Soil Ecology. 2000;14:27–36. [Google Scholar]
- Robbins RT, Barker KR. The effects of soil type, particle size, temperature, and moisture on reproduction of Belonolaimus longicaudatus. Journal of Nematology. 1974;6:1–6. [PMC free article] [PubMed] [Google Scholar]
- Settle DM, Fry JD, Todd TC, Tisserat NA. Population dynamics of the lance nematode (Hoplolaimus galeatus) in creeping bentgrass. Plant Disease. 2006;90:44–50. doi: 10.1094/PD-90-0044. [DOI] [PubMed] [Google Scholar]
- Sikora EJ, Guertal EA, Bowen KL. Plant-parasitic nematodes associated with hybrid bermudagrass and creeping bentgrass putting greens in Alabama. Nematropica. 2001;31:301–305. [Google Scholar]
- Simard L, Bélair G, Miller S. First report of Longidorus breviannulatus associated with damage on creeping bentgrass golf greens in Québec, Canada. Plant Disease. 2009;93:846–847. doi: 10.1094/PDIS-93-8-0846C. [DOI] [PubMed] [Google Scholar]
- Simard L, Bélair G, Powers T, Tremblay N, Dionne J. Incidence and population density of plant-parasitic nematodes on golf courses in Ontario and Quebec, Canada. Journal of Nematology. 2008;40:241–251. [Google Scholar]
- Southey JF. 1970 Laboratory methods for work with plant and soil nematodes. London, UK, Her Majesty’s Stationery Office, 148 pp. [Google Scholar]
- Talavera M, Navas A. Incidence of plant-parasitic nematodes in natural and semi-natural mountain grassland and the host status of some common grass species. Nematology. 2002;4:541–552. [Google Scholar]
- Todd TC, Tisserat NA. Occurrence, spatial distribution, and pathogenicity of some phytoparasitic nematodes on creeping bent grass putting greens in Kansas. Plant Disease. 1990;74:660–663. [Google Scholar]
- Vandenbossche B, Viaene N, De Sutter N, Maes M, Kareeen G, Bert W. Diversity and incidence of plant-parasitic nematodes in Belgian turf grass. Nematology, 2011;13:245–256. [Google Scholar]
- Viaene N, Wiseborn DB, Karssen G. First report of the root-knot nematode Meloidogyne minor on turfgrass in Belgium. Plant Disease. 2007;91:908. doi: 10.1094/PDIS-91-7-0908B. [DOI] [PubMed] [Google Scholar]
- Walker NR, Goad CL, Zhang H, Martin DL. Factors associated with populations of plant-parasitic nematodes in bentgrass putting greens in Oklahoma. Plant Disease. 2002;86:764–768. doi: 10.1094/PDIS.2002.86.7.764. [DOI] [PubMed] [Google Scholar]
- Westerdahl BB, Harivandi MA. Variability in populations of plant parasitic nematodes on turfgrass. Acta Horticulturae. 2007;762:139–142. [Google Scholar]
- Ye W. Applying Microsoft Works spreadsheet in statistics for morphometric data of nematode identification. Afro-Asian Journal of Nematology. 1996;6:203–211. [Google Scholar]
- Yeates GW, Bird AF. Some observations of the influence of agricultural practices on the nematode fauna of some south Australian soils. Fundamental and Applied Nematology. 1994;17:133–145. [Google Scholar]
- Yeates GW, Bongers T. Nematode diversity in agroecosystems. Agriculture, Ecosystems and Environment. 1999;74:113–135. [Google Scholar]
- Yu Q, Potter JW, Gilby G. Plant-parasitic nematodes associated with turfgrass in golf courses in southern Ontario. Canadian Journal of Plant Pathology. 1998;20:304–307. [Google Scholar]
- Zeng Y, Ye W, Tredway L, Martin S, Martin M. Description of Hemicaloosia graminis n. sp., (Nematoda: Caloosiidae) associated with turfgrasses in North and South Carolina, USA. Journal of Nematology. 2012a;44:134–141. [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Ye W, Tredway L, Martin S, Martin M. Taxonomy and morphology of plant-parasitic nematodes associated with turf grasses in North and South Carolinas, USA. Zootaxa. 2012b;3452:1–46. [Google Scholar]