Abstract
Microorganisms produce volatile organic compounds (VOCs) which mediate interactions with other organisms and may be the basis for the development of new methods to control plant-parasitic nematodes that damage coffee plants. In the present work, 35 fungal isolates were isolated from coffee plant rhizosphere, Meloidogyne exigua eggs and egg masses. Most of the fungal isolates belonged to the genus Fusarium and presented in vitro antagonism classified as mutual exclusion and parasitism against the nematode-predator fungus Arthrobotrys conoides (isolated from coffee roots). These results and the stronger activity of VOCs against this fungus by 12 endophytic bacteria may account for the failure of A. conoides to reduce plant-parasitic nematodes in coffee fields. VOCs from 13 fungal isolates caused more than 40% immobility to Meloidogyne incognita second stage juveniles (J2), and those of three isolates (two Fusarium oxysporum isolates and an F. solani isolate) also led to 88-96% J2 mortality. M. incognita J2 infectivity decreased as a function of increased exposure time to F. oxysporum isolate 21 VOCs. Gas chromatography-mass spectrometry (GC-MS) analysis lead to the detection of 38 VOCs produced by F. oxysporum is. 21 culture. Only five were present in amounts above 1% of the total: dioctyl disulfide (it may also be 2-propyldecan-1-ol or 1-(2-hydroxyethoxy) tridecane); caryophyllene; 4-methyl-2,6-di-tert-butylphenol; and acoradiene. One of them was not identified. Volatiles toxic to nematodes make a difference among interacting microorganisms in coffee rhizosphere defining an additional attribute of a biocontrol agent against plant-parasitic nematodes.
Keywords: biological control, Meloidogyne exigua, Fusarium, antagonism
The plant parasitic nematode Meloidogyne incognita causes loss of many crops worldwide (Luc et al., 2005). M. incognita’s hosts include coffee which is also attacked by M. exigua, the most widespread species in Brazil (Campos and Villain, 2005). The largest incidence of Meloidogyne exigua in coffee plantations is in southern of Minas Gerais State, Brazil (Castro et al., 2008), where about 25% of all Brazilian coffee is grown (Conab, 2012). Fungi associated with Meloidogyne sp. egg masses include nematode antagonistic fungi that have been extensively studied for their potential as biological control agents (Rodriguez-Kabana and Morgan-Jones, 1988; Meyer et al., 1990). Nematode pathogenic fungi can attack nematode eggs, capture the second stage juveniles and colonize females bodies of Meloidogyne spp. (Coimbra et al., 1999; Mizobutsi et al., 2000; Siddiqui and Mahmood, 1996). In addition to direct parasitism of the nematode, fungal and bacteria growth in the soil, produce both volatile and non-volatile substances that are toxic to nematodes. Those water soluble molecules have been studies in artificial cultivated organisms (Amaral et al., 2003; Oliveira et al., 2009). Lately more studies have been directed to the volatile group of molecules produced by the soil microflora (Gu et al., 2007; Zou et al., 2007), which were lost over the years of research with filtrates from cultured microorganism because inappropriate methodology had been employed for volatile search (Campos et al., 2010).
Despite the importance of volatile organic compounds (VOCs) produced by microorganisms for their interaction with the environment (Campos et al., 2010), the vast majority of studies examining the efflux of VOCs from terrestrial ecosystems have focused on the production of these substances by plants (Kesselmeier and Staudt, 1999). According to Knudsen and Gershenzon (2006), plants can produce more than 1700 VOCs, among which are chemicals that play important roles in plant defense, reproduction, interaction (plant to plant), and abiotic stress (Dudareva et al., 2006). Only a few studies have investigated the production of these substances by soil bacteria and fungi (McAfee and Taylor, 1999; Ezra and Strobel, 2003; Left and Fierer, 2008). Even scarcer are the studies on the role of VOCs on rhizosphere organisms such as fungi, bacteria and nematodes, especially on the interaction among them in the same site (Gu et al., 2007; Zou et al., 2007; Fernando et al., 2005).
The VOCs produced by soil bacteria inhibit spore germination and mycelial growth of two common soil fungi, Paecilomyces lilacinus and Pochonia chlamidosporia. Consequently, the study of the influence of these substances on the interaction between fungi, bacteria and plant-parasitic nematodes may generate relevant information for the development of bionematicides or to explain the failure of nematode-control by microorganism introduction in field settings (Zou et al., 2007).
The aim of the present work was to explore interactions of organisms present in the coffee plant and rhizosphere. To accomplish this, the following objectives were established: (1) isolate rhizosphere fungi from different sites of M. exigua infested coffee plants and study the possible antagonism between some of them found in coffee rhizosphere, (2) estimate the possible nematicidal activities of VOCs produced by fungi isolated from coffee rhizosphere sites on second stage juveniles (J2) of M. incognita and on host infectivity of M. incognita J2 exposed to VOCs of selected fungus, (3) determine the effect of bacterial VOCs on selected fungi isolated from M. exigua infested coffee rhizosphere, (4) estimate the body energy loss (lipid) from J2 exposed to VOCs of selected fungus and (5) identify potential fungal and nematicidal VOCs through the use of headspace-solid-phase microextraction followed by gas chromatography-mass spectrometric analysis.
Materials and Methods
Fungus isolation and storage - Meloidogyne exigua egg masses: Egg masses from M. exigua-infested coffee roots collected in the field, were placed onto 0.028 mm sieves in petri dishes filled with distilled water. Seven days later, the egg masses covered by fungal mycelia were transferred to petri dishes containing water-agar that were sealed and maintained at 25°C in darkness. After two weeks, fungal colonies that were similar by observation under a microscope were transferred to petri dishes containing malt medium to obtain pure cultures which were incubated and stored.
Meloidogyne exigua eggs: Field coffee roots infected with the nematode were cut into 1-2 cm fragments and eggs were released according to the Hussey and Barker (1973) procedure. Those eggs showing adhered fungal hyphae when observed under a microscope were transferred to petri dishes with 2 % water-agar and placed at 25°C in darkness. After 15 days, fungal colonies which were similar by observation under a microscope were transferred to petri dishes containing malt medium to obtain pure cultures which were incubated and stored.
Soil: A sample of 10 g of soil adhered to rootlets from the coffee rhizosphere was added to 200 ml distilled water to afford a mixture that was stirred for 10 min. After decanting for 2 min, the supernatant was discharged and the precipitate was suspended in water and filtrated through 1.0 mm, 0.7 mm, 0.5 mm, and 0.21 mm aperture sieves. The residue held by the 0.21 mm sieve was placed onto filter paper to eliminate excess water, and poured onto petri dishes containing corn meal agar (CMA) medium plus the bactericide chloramphenicol. After incubation at 25°C in darkness, the fungal colonies were transferred to petri plates containing malt medium, incubated and stored.
Roots: M. exigua-infested roots obtained from coffee plants in the field were cut into small pieces, washed carefully with sterilized distilled water, transferred to a beaker containing 400 ml sterilized distilled water, and slowly stirred for 30 min. After filtration through 0.075 and 0.025 mm sieves, the residue held by the 0.025 mm sieve was washed with water and centrifugated for 2 min at 2816 g. After discharging the supernatant, the residue was dispersed with a Drigalski loop on water-agar (WA)-containing petri dishes, which were sealed and incubated at 25°C in the dark. Daily, a search for fungal colonies in the WA was carried out under a microscope. Selected fungal colonies were transferred to malt medium, incubated at 25°C and identified. All fungal strains were morphologically identified to the genus or species level if possible (Domsch et al., 1980; Barnett and Hunter, 1987).
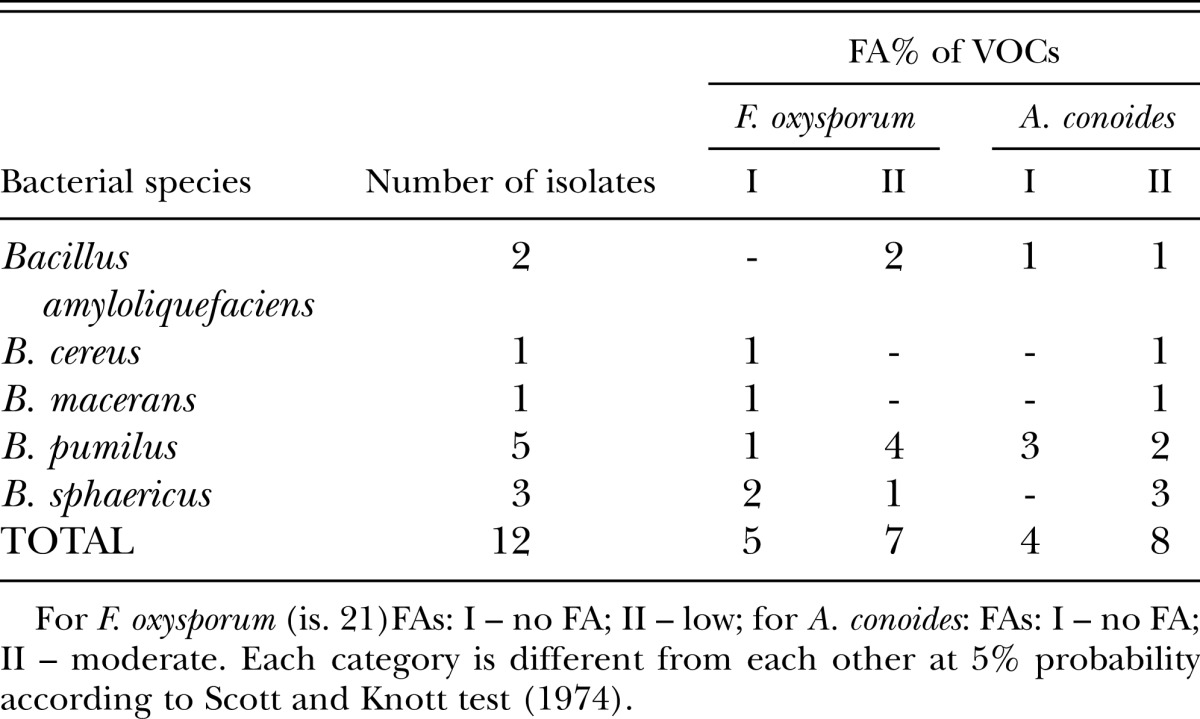
Bacterial isolates: This work was carried out with bacterial strains previously isolated from tomato and red pepper stems (Silva et al., 2008), which are on deposit at the Department of Phytopathology - Federal University of Lavras, MG, Brazil. After a previous screening (unpub. data) a total of 12 bacterial isolates belonging to six species were examined for their potential to produce fungicidal VOC (Table 2). These isolates were incubated in peptone-glycerol medium (20.0 ml peptone, 10.0 ml glycerol, 1.5 g K2HPO4, 1 liter distilled water) and stored at -80°C. Prior to the experiments, the stock cultures were streaked onto tryptic soy agar medium (TSA) (Difco Laboratories, Detroit, MI) and incubated at 28°C for 24 hr.
Table 2.
Bacterial isolates with fungicidal activities (FA) caused by volatile organic compounds (VOCs) against Fusarium oxysporum (is. 21) and Arthrobotrys conoides.
Nematodes: M. incognita J2 were used to assess the in vitro activity (J2 mortality and mobility), and the assessment of lipid body content and host plant infectivity using J2 exposed to fungal VOCs. M. incognita was cultured on tomato plants in a greenhouse. After three months, eggs were obtained from galled roots by the Hussey and Barker (1973) procedure. Eggs were placed in a hatching chamber and nematode J2 were collected and used for the tests.
In vitro antagonism assay: Thirty-five fungal isolates obtained as described above (Table 1) were tested in duplicate for activity against the nematode predator fungus A. conoides which was isolated from coffee root surface (Table 1). A. conoides and the isolates tested were point inoculated 3 cm distant from each other on 6 cm diameter petri dishes. The test was performed at 15°C on malt agar medium and the observations were made 12 days after the inoculation. Interactions between the developing colonies were classified as follows: 1) no interaction: colonies growing over each other; 2) mutual exclusion: colony growth stops when rims of colonies touch; 3) parasitism: the isolate tested destroys A. conoides.
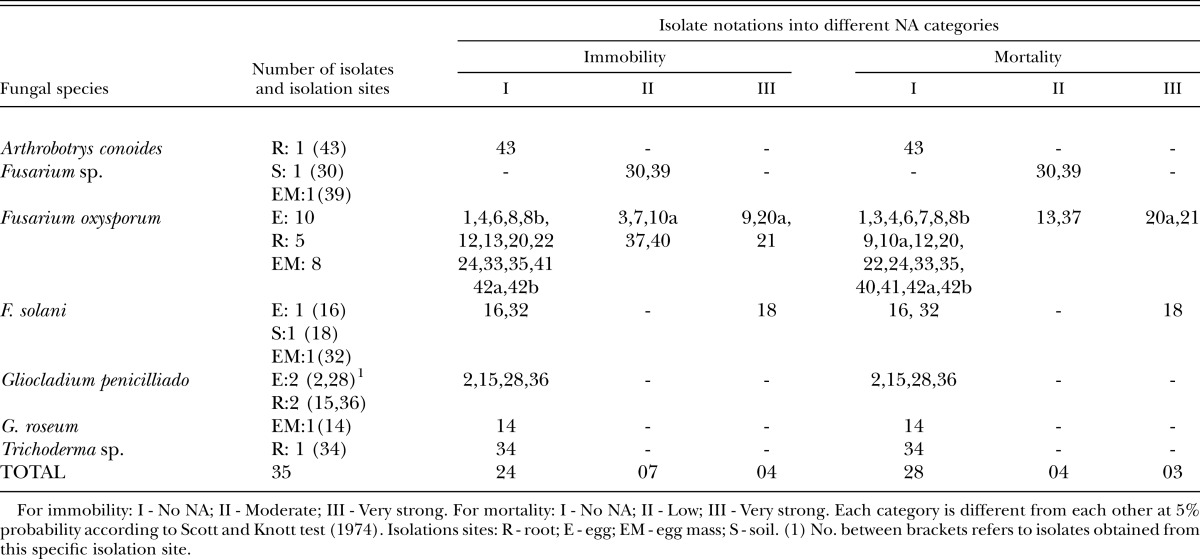
Table 1.
Fungal isolates (identified by sequential numbers) with nematicidal and nematostatic activities (NA) caused by volatile organic compounds against second stage juveniles of Meloidogyne incognita.
Fungicide activity (FA) of bacterial VOCs: The nematode predator fungus A. conoides (isolated from coffee roots), F. oxysporum - isolate (is.) 21 (the most prevalent species on coffee soil rhizosphere) (Table 1) and endophytic bacterial strains obtained from tomato and pepper plants (Table 2) were used in a bioassay designed to allow only volatile compounds from bacteria (Bacillus spp.) to be the cause of fungal (A. conoides and F. oxysporum is. 21) mycelial growth inhibition. Bacterial suspensions (300 μl) obtained by cultivation at 28°C for 24 h, were poured onto one half of a two-compartmented petri plate containing TSA medium. A 5 mm round plug taken from the border of a newly grown A. conoides or F. oxysporum colony was placed on the surface of the other half of the petri plate which contained malt agar medium. All plates were wrapped with two layers of Parafilm and incubated at 25°C in the dark. At 24 h intervals, the linear growth of the filamentous fungi was measured from the edge of the inoculum plugs until the colony reached the rim of the plate. Fungicidal activities (FA) due to bacterial VOCs were presented as percentage of mycelium growth reduction compared to control (TSA medium) with no bacteria.
In vitro nematicidal and nematostatic activities of fungal VOCs (J2 immobility and mortality tests): Nematicidal and nematostatic activities (NA) of fungal VOC were evaluated according to the method of Fernando et al. (2005) with some modifications. Briefly, each fungus was inoculated on one half of two-compartmented petri plate that contained malt agar medium. When the fungus colony reached 4.5 cm diameter, about 200 M. incognita J2 were added onto the other half, which contained a layer of WA. As a control, the same amount of malt medium, without fungus inoculation, was poured into one compartment and the experiment was done in completed randomized design. There were four replicates for each treatment. Plates were immediately wrapped with Parafilm to prevent the escape of the volatiles and incubated at 25 °C in the dark for 72 hr. Mobile and non-mobile J2 were counted under a microscope. Then 150 μL J2 suspensions were pipette into wells of polypropylene plate and completed the volume with 150 μL of distilled water. After 24 h, non-mobile J2 were considered dead (mortality). Data were transformed into percentage (%= 100 x dead or immobile J2/total number of J2) before the statistical calculations. The experiment was repeated twice.
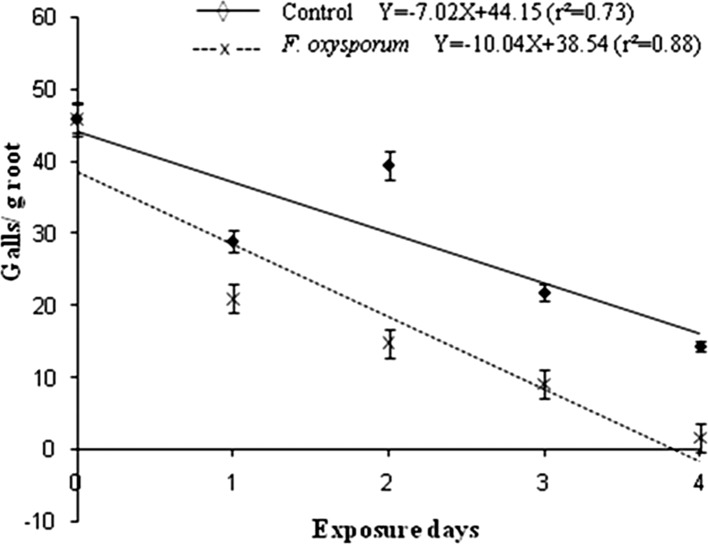
Infectivity of Meloidogyne incognita J2 on tomato after exposure to Fusarium oxysporum (is. 21) VOCs: F. oxysporum (is. 21) was selected to carry out this experiment, since it caused increased immobility and mortality of M. incognita J2 in the in vitro experiment described above (Table 1). The setup described in vitro assay was also used to expose 350 M. incognita J2 to fungal VOCs for 24, 48, 72 or 96 h. As a control, water substituted for the fungus. About 300 J2 (50 J2 were kept for the lipid content test which is described below) for each period of exposition were inoculated with a micropipette on twenty five-day old tomato seedlings through four holes (1.5 cm deep) in the substrate around the plant base. Seedlings were arranged in a randomized block design with four replicates and placed in a temperature controlled room at 28 °C with a photoperiod of 12 h. Thirty days after J2 inoculation, root systems were harvested, washed gently, weighted, and galls were counted. Galls per gram of roots were calculated after dividing the total numbers per root weight.
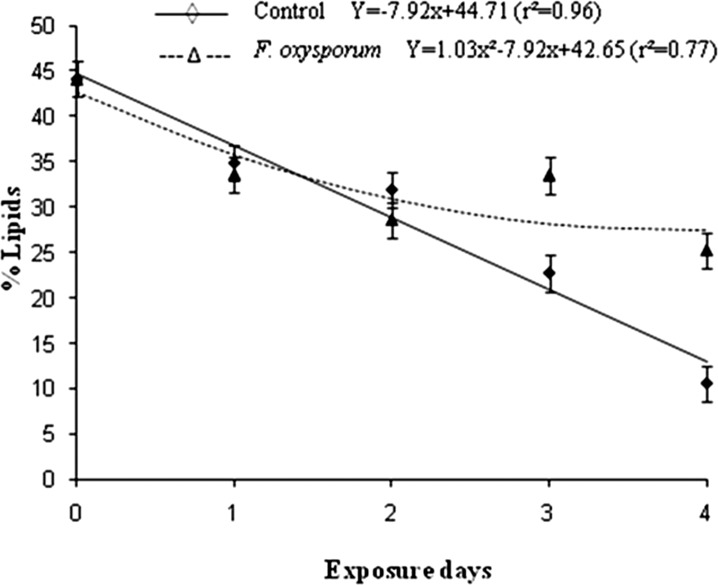
Meloidogyne incognita J2 lipid content after exposure to Fusarium oxysporum (is. 21) VOCs: The 50 exposed J2 to F. oxysporum is. 21 VOCs over time used in this test were essentially the same as those used in the infectivity test. J2 body lipid content was determined according to the method of Christophers et al. (1997) with some modifications, after exposure to fungal VOC for 24, 48, 72 or 96 h, or water (control) as described above. Briefly, Oil Red O stain (3.0 ml of a 0.5 % solution) was added to an aqueous J2 suspension and heated in a water bath at 60°C for 20 min. After cooling to room temperature, the suspension containing stained J2 was centrifugated for 3 min at 1416 g. The supernatant was eliminated and 1.5 ml of a glycerin: water solution (1:1) was added to the J2 suspension. Twenty randomly selected J2 were mounted on a microscope slide with glycerin and photographed. The red-stained area of the J2 body, corresponding to lipids, and the full area of the nematode body were calculated by analyzing the photographs with the “Image Tools for Windows” software, version 3.0. Measurement of the red-stained area allowed us to infer lipid percentage in relations to the full J2 body area. There were four replications for each treatment.
Analysis of Fusarium oxysporum VOC by gas chromatography/mass spectrometry (GC/MS) - Headspace-solid-phase microextraction (HS-SPME): F. oxysporum (is. 21) was selected to carry out this analysis since VOCs produced by this fungus increased M. incognita J2 immobility and mortality, and decreased J2 infectivity (Table 1 and Fig. 3). Fungus-culture plugs from recently grown colonies were inserted into vials containing liquid malt medium and shaking-incubated at 25°C for seven days at 80 rpm. The suspension was filtered through a 0.22 μm membrane. Aliquots (9 ml) of the filtered fungal culture were transferred to 80 × 28 mm (39 mL internal volume) sterilized SupelcoTM SPME glass vials sealed with silicone septas and stored at 0-4 °C. The volatiles were collected on a 100 μm fused silica-non-bonded polydimethyl siloxane (PDMS) SupelcoTM Fiber Core. The fiber was introduced into the headspace of each vial using a SupelcoTM Solid-Phase Micro Extraction Fiber Manual Holder. After insertion of the fiber, the vial was warmed in a 45°C water bath for 30 min. Each extraction was performed and immediately analyzed as described below. Malt culture medium (no fungal culture) was also extracted using this procedure for comparison. There were four replications for each treatment and the experiment was repeated twice.
Fig. 3.
Tomato galls per gram of roots caused by second stage juveniles of Meloidogyne incognita after exposure to Fusarium oxysporum is. 21 volatile organic compound (VOC) and control (without VOC exposure).
Gas Chromatography-Mass Spectrometry (GC-MS) analysis of HS-SPME samples: After each extraction as described above, the SPME fiber was inserted for 3 min in the injector of a Shimadzu Gas Chromatograph (Model GC-2010)-Mass Spectrometer (Model QP2010) running GCMS-Solution Release 2.30 Software. Analysis conditions: injector temp.: 220 °C, injection mode: splitless, sampling time: 1.5 min, flow control mode: linear velocity, press.: 64.7 kPa, total flow: 16.3 ml/min, column: DB-5MS (30 m × 0.25 mm × 0.25 μm), carrier gas: helium, column flow: 1.21 ml/min, linear velocity: 39.7 cm/s, split ratio after sampling time: 10, oven temp.: 40.0°C (5 min), rate 5.0°C/min to 280°C (48 min), 280°C (15 min), equilibrium time: 2.0 min, ion source temp: 250°C, interface temp.: 280°C, solvent cut time: 1.50 min, detector gain mode: relative, detector gain: 0.0 kV, threshold: 1000, acquisition mode: scan, interval: 0.30 s, scan speed: 1250, m/z range: 50.00-400.00. Substances were identified by comparison of their mass spectra to Wiley 7.0, NIST 12 and NIST 62 mass spectral libraries.
Data analysis and statistics: Data were analyzed using analysis of variance (ANOVA). Nematicidal and nematostatic activities - NA (mortality and immobility), and fungicidal activity - FA (mycelial growth diameter) were calculated as the means of four replicates. VOC categories were built based on grouping analysis done by Scott and Knott test (1974) at 5% probability, then defining: for immobility NA≤17.25 (I), 30.50≤NA≤47.00 (II), and NA≥68.50 (III), were, respectively, considered to have no NA (I), moderate (II) and very strong (III); for mortality NA≤12.25 (I), 22.25≤NA≤34.25 (II), and NA≥68.50 (III) were considered to have no NA (I), low (II) and very strong (III) VOCs activities, respectively. The categories established for F. oxysporum FA were FA≤5.23 (I), 10.47≤FA≤23.26 (II) considered to have no FA (I) and low (II); for A. conoides FA were 13.04≤FA≤13.30 (I), 20.13≤FA≤30.43 (II), considered to have no NA (I), moderate (II), VOC activities. In galls per gram of root (infectivity test) and percentage lipid (body lipid content) data regression analysis were used.
Results
Fungus isolation and in vitro antagonism: Thirty-five fungal isolates comprising five different species (Table 1), with three isolates identified only to genus level, were obtained from coffee rhizosphere. Twenty-three of them were F. oxysporum isolates, five were isolated from coffee roots (isolates 3, 13, 24, 33 and 35), eight were isolated from M. exigua egg mass (isolates 6, 7, 8, 8b, 9, 10a, 20 and 20a) and ten were obtained from M. exigua eggs (isolates 1, 4, 12, 21, 22, 37, 40, 41, 42a and 42b). Other genera were also isolated: Arthrobotrys, Gliocladium and Trichoderma, and other species of Fusarium (F. solani – 3 isolates). Although most of the 35 fungal isolates were associated with coffee plant roots or nematode eggs, species belonging to the Fusarium genus were found most frequently in M. exigua eggs and egg masses.
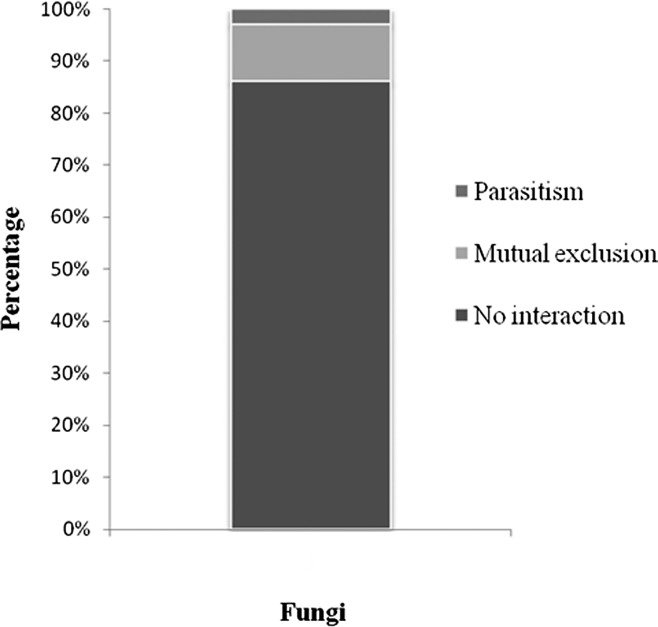
As Arthrobotrys conoides is a predatory fungus, the antagonism of rhizosphere microflora to this fungus may reduce its effectiveness as a biological control agent. Thereafter, in vitro antagonism assay was carried out to evaluate the interaction between A. conoides and all 34 fungus isolates. Among all the isolated fungi used in the in vitro antagonism assay, only Fusarium species showed some type of antagonistic effect against A. conoides isolated from coffee roots (Fig. 1). Isolates no. 40, 30, and 22 caused mutual exclusion, while no. 10a presented parasitism.
Fig. 1.
Frequency (%) of in vitro antagonism against Arthrobotrys conoides of fungi isolated from Meloidogyne exigua eggs and egg mass, soil and coffee roots.
Bacterial VOC active against Arthrobotrys conoides and Fusarium oxysporum (is. 21): The bacterial species used in this assay is commonly found as plant endophytes and rhizosphere organism and have demonstrated efficacy on nematode control in previous assay (data not present). As candidates of biological control agent, their VOC antagonisms to other plant rhizosphere inhabitants are of relevance. VOCs antagonism from rhizosphere bacteria may reduce the effectiveness of A. conoides as biological control agent and change the coffee rhizosphere fungus species equilibrium since F. oxysporum is. 21 is the most prevalent in coffee rhizosphere. Among the 12 bacterial isolates screened for the production of antifungal volatiles, eight of them moderately inhibited A. conoides micelial growth (from 20.13 % to 30.43 %). However, only seven isolates produced VOC able to marginally reduce F. oxysporum mycelial growth (from 10.47% to 23.26%) which was less affected than that of A. conoides (Table 2), suggesting that A. conoides will suffer more bacterial antagonism than F. oxysporum is. 21.
Influence of fungal VOC on motility, mortality and lipid content of M. incognita J2: To test the effect of VOC on nematodes, M. incognita were used since M. exigua move very slowly and were difficult to assay. VOC from fungi isolates showed diverse effects (none, low, moderate, and very strong) on M. incognita J2. F. oxysporum isolates 20a and 21 being the most active. Both of them caused very strong immobility and mortality to the nematode. The isolate 9 caused very strong immobility (over 68.50 %), but no J2 mortality. Isolate 18 (F. solani) also caused very strong immobility and mortality. All the other isolates afforded moderate to no mortality (Table 1). Then among Fusarium spp. isolates the VOC effect can be either nematostatic (immobility) or nematicidal (mortality).
Regarding the lipid content of M. incognita J2 exposed to VOCs produced by F. oxysporum (isolate 21), the values decreased similarly to that observed for the control until the second day. Thereafter, control values continued to decrease, while the values for the fungal volatiles tended to remain unchanged (Fig. 2), then saving energy.
Fig. 2.
Lipid contend (%) of second stage juveniles of Meloidogyne incognita after exposure to volatile organic compounds (VOCs) produced by Fusarium oxysporum is. 21 overtime and by the control (without VOCs exposure).
Infectivity of Meloidogyne incognita J2 on tomato plantlets after exposure to VOCs produced by Fusarium oxysporum (is.21): The F. oxysporum (is. 21) VOCs may reduce the M. exigua field inoculum capacity in coffee rhizosphere or brings about the importance of this fungus to this reduction. Exposure of M. incognita J2 to VOCs produced by F. oxysporum (isolate 21) decreased the nematode infectivity on tomato over time as compared to the controls (Fig. 3), demonstrating VOCs damage to the nematode.
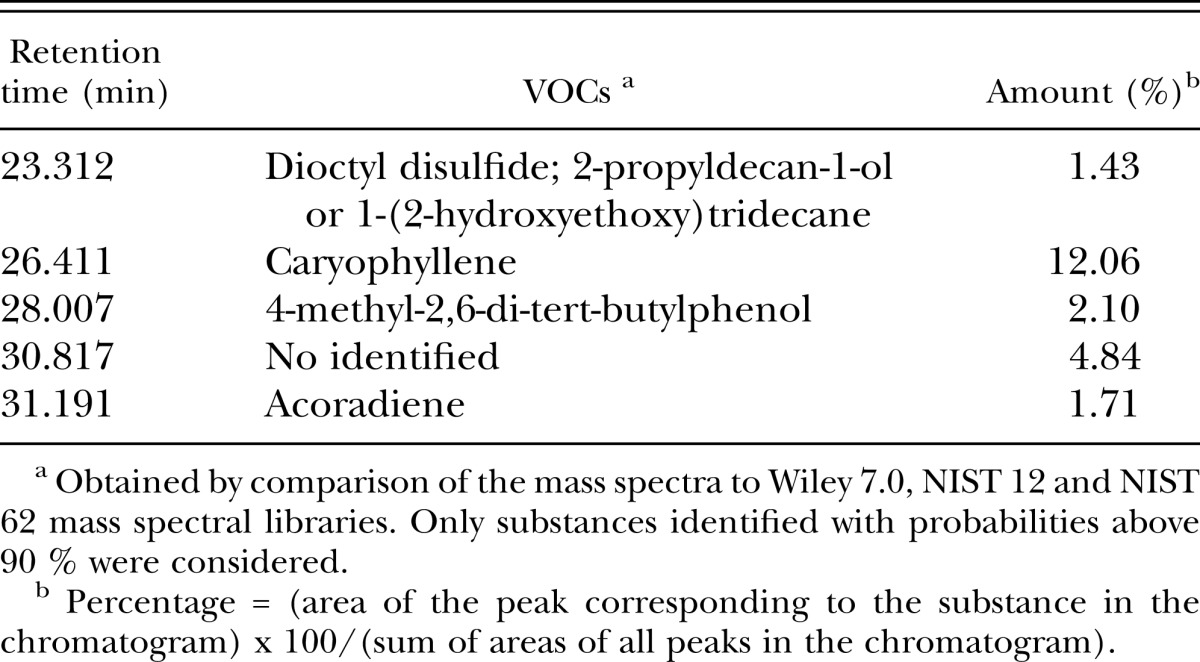
Identification of VOCs produced by F. oxysporum (is.21) using GC-MS: Analysis by GC/MS of the volatiles produced by F. oxysporum (is. 21) and G. penicilliado (is. 2) revealed the presence of 57 and 87 substances, respectively. Only 18 peaks in the chromatogram obtained for both fungi (F. oxysporum is. 21 and G. penicilliado is. 2) presented the same retention time and base peak in the corresponding mass spectra. None of the peaks in the chromatogram of F. oxysporum is. 21 presented the same retention time and base peak observed for VOCs from liquid malt medium. Among the 38 VOCs produced exclusively by F. oxysporum, only five were present in amounts above 1 % (Table 3). Caryophyllene, 4-methyl-2,6-di-tert-butylphenol and one substance not identified occurred in most amount.
Table 3.
Major volatile organic compounds (VOCs) produced by Fusarium oxysporum (is. 21), quantified and identified by gas chromatography-mass spectrometry.
Discussion
The isolation and identification of fungi carried out in the present study are in agreement with the literature, since all of these microorganisms belong to genera known to contain species associated with nematode eggs, cysts or egg masses (Rodrigues-Kabana and Morgan Jones, 1988; Crump, 1991; Naves and Campos, 1991). Analogously, other research groups have described the isolation of F. oxysporum and F. solani from soil and nematode cysts (Crump, 1987; Meyer et al., 1990). Specifically from coffee rhizosphere, Ribeiro and Campos (1993), reported the presence of the fungi Penicillium sp. in coffee roots infected by M. exigua.
Since Fusarium spp. accounted for the largest number (83%) of fungal species found in the M. exigua-infested coffee rhizosphere (Table 1), their mutual exclusion and parasitic interactions with A. conoides (Figure 1) may account for the lack of competitive power of A. conoides as a biocontrol agent against Meloidogyne sp. in the field. Likewise, strong antagonism has been found against Pochonia chlamydosporia – a nematode biocontrol agent – by Trichoderma harzianum by in vitro antagonism test (Kok et al., 2001). Further studies on the antagonistic effect of fungi and bacterial VOC should include the species A. robusta and A. irregulars which are the basic active ingredients of Royal 300 and Royal 350 (Cayrol et al., 1978; Cayrol and Frankowski, 1979). This will provide information on competitive capacity of these biocontrol species against Fusarium spp and bacteria in soil once those bionematicides (Royal 300 and 350) are applied in the field. Besides, Fusarium spp may become a biological agent once has been shown that F. moniliforme produces a non-volatile toxic metabolite to M. exigua (Amaral, et al., 2003).
Analogously, some bacterial VOCs presented moderate activity against A. conoides, which can also contribute to the inefficiency of this fungus to control nematodes in coffee fields. Although this is the first time the activity of bacterial VOCs against A. conoides is reported, other research groups have already described the antifungal properties of such substances against Paecillomyces lilacinus, P. chlamydosporia (Zou et al., 2007) and against plant-pathogenic fungi (Fernando et al., 2005).
Some of the fungal VOCs in the present study, especially those produced by two isolates of F. oxysporum (isolates 20a and 21) and one of F. solani (isolate 18), caused very strong J2 mortality, revealing nematicidal effect. In fact, the killing action of F. oxysporum VOCs was proved by infectivity (galls) decrease when the M. incognita J2 exposed to isolate 21 were inoculated in tomato plants. Consequently, some of the molecules presented in these VOCs may, possibly, become structural models for future fumigant nematicides if the compounds can be identified. Even the VOCs that caused high in vitro immobility of M. incognita J2 may also be useful for the development of new products for the control of nematodes, since similar behavior has been observed for some commercial organophosphate and organocarbamate nematicides (Sikora et al., 2005). Furthermore, these results are consistent with the reports by Riga et al. (2008), since the authors described the immobility and mortality effects of Muscodor albus VOCs on M. chitwoodi, Paratrichodorus allius, and Pratylenchus penetrans. Our study is the first to characterize the nematicidal activity of fungal volatiles from rhizosphere Fusarium species against plant parasitic nematodes.
The stable body energy content of J2 exposed to F. oxysporum VOC over time in contrast to control indicates reduced energy spending, also confirmed by exposed J2 death. To prove that M. incognita J2 were dead after VOC exposure, the lipid body energy was estimated. It is already known that in juvenile nematodes exposed to pesticides which retard their movement without killing them, the lipid energy is exhausted and infectivity decreases (Andaló et al., 2008).
Comparison of the mass spectrum of the substance detected more intensely in the VOC from F. oxysporum (is. 21) and not in the control is a means to identify compounds possibly antagonistic to M. incognita. Although no test to evaluate the individual activity of these VOC was carried out in the present work, all compounds with differential abundance are considered to be potentially antagonistic to nematodes and fungi. Even so, several interesting compounds were identified. For example, the mass spectral libraries used in the present work suggest one substance corresponds to caryophyllene (91 % probability). This compound is a natural bicyclic sesquiterpene that is a constituent of many essential oils (Gertsch et al., 2008; Ormeño et al. 2008) and produced by fungi in the Fusarium genus (Jelén et al., 1995). It is usually found as a mixture with isocaryophyllene (the cis double bond isomer) and α-humulene (obsolete name: α-caryophyllene), an open-ring isomer. Larvae of the leaf beetle Diabrotica virgifera virgifera feeding on maize roots induce the production of caryophyllene, which is attractive to the entomopathogenic nematode Heterorhabditis megidis in the laboratory and field (Rasmann et al., 2005). However, this compound presented no toxicity against the pine wood nematode (Bursaphelenchus xylophilus) (Park et al., 2007). Meyer et al. (2008) demonstrated the nematicidal activity against Meloidogyne incognita by the caryophyllene-rich essential oil from Syzygium aromaticum (L.) Merr and Perry. Also worth mention is the toxicity of the essential oil of Aloysia gratissima to Meloidogyne spp., since caryophyllene oxide is one of the major components (12.06%) of this product (Duschatzky et al., 2004).
According to comparisons to the mass spectral libraries, another substance possibly present in the VOC from F. oxysporum is 4-methyl-2,6-di-tert-butylphenol (95 % probability). Although no report on the nematicidal activity of this substance, also known as butylated hydroxytoluene (BHT), was found in the literature, this phenol is moderately toxic to molluscs, daphnids and fish, with EC50s of 1 to 20 mg/L (Aherns, 2008). This lipophilic (fat-soluble) organic compound is primarily used as an antioxidant food additive (E number E321) as well as an antioxidant additive in cosmetics (Branen, 1975). Apparently, no fungus has ever produced this substance, suggesting that this is the first report on the production of BHT by a fungus.
Regarding acoradiene (91 % probability), which corresponded to 1.71% of the VOC produced by F. oxysporum is. 21, the identification of this substance was also tentative, since it relied only on the comparison to the mass spectral libraries. No report on its activity against nematodes was found in the literature. Similarly to caryophyllenes, this substance is a natural compound produced by fungi of the Fusarium genus (Jelén et al., 1995).
The isolate 21 of F. oxysporum may become a control agent as well as the VOC molecules produced by it, which present potential for the development of new commercial products for the control of plant-parasitic nematodes. Analogously, other research groups have also pointed out the F. oxysporum potential for biocontrol agents (Kerry and Evans, 1996). On the other hand F. oxysporum is also reported as a coffee pathogen (Cardoso, 1986), especially when forming a disease complex with M. incognita and M. arabicida (Negron and Acosta, 1989; Betrand et al., 2000). Consequently, further studies should be carefully carried out to determine the real potential of F. oxysporum (is. 21) and the metabolites (and analogues) produced by this fungus for the control of Meloidogyne spp. in coffee fields.
Acknowledgments
The authors gratefully acknowledge the Brazilian financial supports provided by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Literature Cited
- Amaral DR, Oliveira FER, Oliveira DF, Campos VP, Silva GH. Purificação de metabólito produzido por Fusarium moniliforme tóxico a Meloidogyne exigua. Summa Phytopathologica. 2003;29(1):25–29. [Google Scholar]
- Andaló V, Maximiniano C, Campos VP, Moino A., Jr Efeito de filtrados entomobacterianos sobre juvenis de Meloidogyne spp. Nematologia Brasileira. 2008;31:186–194. [Google Scholar]
- Aherns M. Review of organic chemical of potential environmental concern in use in Auckland. Auckland Regional Council Technical Report 2008–2028. 2008 [Google Scholar]
- Barnett HL, Hunter BB. 1987 Illustrated genera of imperfect fungi. 4 ed. New York, Macmillan Publishing Company. [Google Scholar]
- Betrand B, Nunez C, Sarah JL. Disease complex in coffee involving Meloidogyne arabicida and Fusarium oxysporum. Plant Pathology. 2000;49:383–388. [Google Scholar]
- Branen AL. Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. Journal of the American Oil chemistry Society. 1975;52(2):59–63. doi: 10.1007/BF02901825. [DOI] [PubMed] [Google Scholar]
- Campos VP, Pinho RSC, Freire ES. Volatiles produced by interacting microorganisms potentially useful for the control of plant pathogens. Ciência e Agrotecnologia. 2010;34:525–535. [Google Scholar]
- Campos VP, Villain L. 2005 Nematodes parasites of coffee and cocoa. Pp. 529–579 in Luc, M., Sikora, R. A. and Bridge, J. (eds). Plant Parasitic nematodes in Subtropical and Tropical Agriculture, 2nd ed. Wallingford: CAB International Publishing. [Google Scholar]
- Cardoso RML. Ocorrência da murcha vascular do cafeeiro (Coffea arabica) no estado do Paraná-Brasil, induzida por Fusarium oxysporum f.sp. coffeae. Fitopatologia Brasileira. 1986;11:753–760. [Google Scholar]
- Castro JMC, Campos VP, Pozza EA, Naves RL, Andrade Junior VC, Dutra MR, Coimbra JL, Maximiniano C, Silva JRC. Levantamento de fitonematóides em cafezais do sul de Minas Gerais. Nematologia Brasileira. 2008;32:56–64. [Google Scholar]
- Cayrol JC, Frankowski JP, Laniece A, D’Hardemare G, Talon JP. Contre les nematódes en champignonniére. Mise au point d’une méthode de lutte biologique a l’aide d’un hyphomycete prédateur: Arthrobotrys robusta souche antipolis (Royal 300) Revue Horticole. 1978;184:23–30. [Google Scholar]
- Cayrol JC, Frankowski JP. Une méthode de lutte biologique contre lês nématodes à galles des racines appartenat au genre Meloidogyne. Revue Horticole. 1979;193:15–23. [Google Scholar]
- Christophers AEP, Patel MN, Benson JA, Saka VW, Evans AAF, Wright DJ. A rapid field-laboratory biossay to assess the infectivity of Meloidogyne spp. second stage juveniles. Nematologica. 1997;43(1):117–120. [Google Scholar]
- Coimbra JL, Campos VP, Souza RM. Isolamento e parasitismo de fungos de fêmeas de Meloidogyne javanica e Meloidogyne incognita. Nematologia Brasileira. 1999;23(1):25–33. [Google Scholar]
- Companhia Nacional de Abastecimento, 2012. http://www.conab.gov.br/OlalaCMS/uploads/arquivos/12_09_06_10_10_21_boletim_cafe_-_setembro_2012.pdf.
- Crump DH. Effect of time sampling, method of isolation and age of nematode on the species of fungi isolated from females of Heterodera schachtii and H. avenae. Revue Nematologie. 1987;10(3):369–373. [Google Scholar]
- Crump DH. Fungal species isolated from beet, cereal and potato cyst nematodes. Bulletim IOBC/WPRS. 1991;14:58–64. [Google Scholar]
- Domsch KH, Gams W, Anderson TH. 1980 Compendium of soil fungi, vol. 1.London, Academic Press. [Google Scholar]
- Dudareva N, Negre F, Nagegowda DA, Orlova I. Plant volatiles: Recent advances and future perspectives. 2006 Critical Reviews in Plant Sciences 25:417:440. [Google Scholar]
- Duschatzky CB, Martinez AN, Almeida NV, Bonivardo SL. Nematicidal activity of the essential oils of several Argentina plants against the root-knot nematode. Journal of Essential Oil Research. 2004;16(6):626–628. [Google Scholar]
- Ezra D, Strobel GA. Effect of substrate on the bioactivity of volatile antimicrobials produced by Muscodor albus. Plant Science. 2003;165(6):1229–1238. [Google Scholar]
- Fernando WGD, Ramarathnam R, Krishnamoorthy A, Sauchuk SC. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biology & Biochemistry. 2005;37:955–964. [Google Scholar]
- Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ, Altmann KH, Karsak M, Zimmer A. Beta-caryophyllene is a dietary cannabinoid. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(26):9099–9104. doi: 10.1073/pnas.0803601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu YQ, Mo MH, Zhou JP, Zou CS, Zhang KQ. Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biology & Biochemistry. 2007;39:2567–2575. [Google Scholar]
- Hussey RS, Barker KR. A comparison of methods for collecting inocula of Meloidogyne spp. including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Jelén HH, Mirocha CJ, Wasowicz E, Kamiński E. Production of volatile sesquiterpenes by Fusarium sambucinum strains with different abilities to synthesize trichothecenes. Applied and Environmental Microbiology. 1995;61(11):3815–3820. doi: 10.1128/aem.61.11.3815-3820.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerry BR, Evans K. 1996 News strategies for the management of plant-parasitic nematodes. Pp. 134–152 in Hall, R. (ed.) Principles and Practice of Managing Soilborne Plant Pathogens, 1st ed. Saint Paul: APS Press. [Google Scholar]
- Kesselmeier J, Staudt M. Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. Journal of Atmospheric Chemistry. 1999;33(1):23–88. [Google Scholar]
- Knudsen JT, Gershenzon J. 2006 The chemistry diversity of floral scent. Pp. 27–52 in Dudareva, N. and Pichersky, E. (eds).Biology of floral scent. Boca Raton: CRC Press. [Google Scholar]
- Kok CJ, Papert A, Bok-A-Bin CB. Microflora of Meloidogyne egg masses: species composition, population density and effect on the biocontrol agent Verticillium chlamydosporium (Goddard) Nematology. 2001;3(8):729–734. [Google Scholar]
- Left JW, Fierer N. Volatile organic compound (VOC) emissions from soil and litter samples. Soil Biology & Biochemistry. 2008;40(7):1629–1636. [Google Scholar]
- Luc M, Sikora RA, Bridge J. 2005 Plant parasitic nematodes in subtropical and tropical agriculture, 2nd ed. Wallingford: CABI Publishing. [Google Scholar]
- McAfee BJ, Taylor A. A review of the volatile metabolites of fungi found on wood substrates. Natural Toxins. 1999;7:283–303. doi: 10.1002/1522-7189(199911/12)7:6<283::aid-nt70>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Meyer SLF, Huettel RN, Sayre RM. Isolation of fungi from Heterodera glycines and in vitro bioassays for their antagonism to eggs. Journal of Nematology 22(4):532–537. 1990 [PMC free article] [PubMed] [Google Scholar]
- Meyer SLF, Lakshman DK, Zasada IA, Vinyard BT, Chitwood DJ. Dose–response effects of clove oil from Syzygium aromaticum on the root-knot nematode Meloidogyne incognita. Pest Management Science. 2008;434:223–229. doi: 10.1002/ps.1502. [DOI] [PubMed] [Google Scholar]
- Mizobutsi EH, Ferraz S, Ribeiro RCF. Avaliação do parasitismo de diversos isolados fúngicos em ovos de Heterodera glycines e Meloidogyne incognita. Nematologia Brasileira. 2000;24(2):167–172. [Google Scholar]
- Naves RL, Campos VP. Ocorrência de fungos predadores de nematóides no Sul de Minas Gerais e estudo da capacidade predatória e crescimento in vitro de alguns de seus isolados. Nematologia Brasileira. 1991;15(2):152–162. [Google Scholar]
- Negron JA, Acosta N. The Fusarium oxysporum f.sp. coffea – Meloidogyne incognita complex in “Bourbon” coffee. Nematropica. 1989;19:161–168. [Google Scholar]
- Oliveira DF, Carvalho HWP, Nunes AS, Silva GH, Campos VP, Junior HM, Cavalheiro AJ. The activity of amino acids produced by Paenibacillus macerans and from commercial sources against the root-knot nematode Meloidogyne exigua. European Journal of Plant Pathology. 2009;124(1):57–63. [Google Scholar]
- Ormeño E, Baldy V, Ballini C, Fernandez C. Production and diversity of volatile terpenes from plants on calcareous and siliceous soils: effect of soil nutrients. Journal of Chemical Ecology. 2008;34(9):1219–1229. doi: 10.1007/s10886-008-9515-2. [DOI] [PubMed] [Google Scholar]
- Park IK, Kim J, Lee SG, Shin SC. Nematicidal activity of plant essential oils and components from ajowan (Trachyspermum ammi), allspice (Pimenta dioica) and litsea (Litsea cubeba) essential oils against pine wood nematode (Bursaphelenchus xylophilus) Journal of Nematology. 2007;39(3):275–279. [PMC free article] [PubMed] [Google Scholar]
- Rasmann S, Rasmann S, Kollner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TCJ. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature. 2005;434:732–737. doi: 10.1038/nature03451. [DOI] [PubMed] [Google Scholar]
- Ribeiro RCF, Campos VP. Isolamento e identificação e efeito de temperatura no crescimento in vitro de fungos parasitas de ovos de Meloidogyne spp. no sul de Minas Gerais. Nematologia Brasileira. 1993;17:132–138. [Google Scholar]
- Riga E, Lacey LA, Guerra N. Muscodor albus, a potential biocontrol agent against plant-parasitic nematodes of economically important vegetable crops in Washington State, USA. Biological Control. 2008;45(3):380–385. [Google Scholar]
- Rodriguez-Kabana R, Morgan-Jones G. Potential for nematode control by mycofloras endemic in the tropics. Journal of Nematology. 1988;20(2):191–203. [PMC free article] [PubMed] [Google Scholar]
- Scott AJ, Knott M. A cluster analysis method for grouping means in the analysis of variance. Biometrics. 1974;30(3):507–512. [Google Scholar]
- Siddiqui ZA, Mahmood I. Biological control of plant parasitic nematodes by fungi: a review. Bioresource Technology. 1996;58:229–239. [Google Scholar]
- Sikora RA, Bridge J, Starr JL. 2005 Management practices: An overview of integrated nematode technologies. Pp. 793–825 in Luc, M., Sikora, R. A. and Bridge, J. (eds.) Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 2nd ed. Wallingford: CAB International Publishing. [Google Scholar]
- Silva JRC, Souza RM, Zacarone AN, Silva LHCP, Castro AM. Bactérias endofíticas no controle e inibição in vitro de Pseudomonas syringae pv tomato, agente da pinta bacteriana do tomateiro. Ciência e Agrotecnologia. 2008;32(4):1062–1072. [Google Scholar]
- Zou CS, Mo MH, Gu YQ, Zhou JP, Zhang KQ. Possible contributions of volatile-producing bacteria to soil fungistatic. Soil Biology & Biochemistry. 2007;39:2371–2379. [Google Scholar]