Abstract
A novel entomopathogenic nematode species, Heterorhabditidoides rugaoensis n. sp. RG081015, collected from Rugao, China, is described. The new species is morphologically very similar to H. chongmingensis but can be distinguished from it on the basis of some morphological characteristics, combined with molecular data and a cross-hybridization test. Males of the new species can be recognized on the basis of body length averaging 1396.2 μm; lateral field with one ridge; metastome isoglottoid with one hemispherical swellings comprised of two to three well-developed warts; asymmetric spicules; peloderan bursa. In IJs, EP = 134.5 μm; ES = 149.3 μm; tail length = 82.5 μm; and a = 20.5. Hermaphroditic females have four to five lateral ridges. The 18S rDNA and ITS sequences of the two nematodes share 99% and 98% identity, respectively. Phylogenetic trees of 18S rDNA and ITS indicate that the new species is most closely related to H. chongmingensis; thus, the two nematodes belong to the same genus. Failure of cross-hybridization between them indicates that nematode strain RG081015 is a novel species and is described herein as H. rugaoensis n. sp. The LC50 of the novel species against Galleria mellonella were 24.35 IJs / ml within 48 hours of infection. Morphological characteristics, genetic similarity analyses, and phylogenetic relationships provide strong evidence that some species of Oscheius/Insectivora-group should be reassigned to the genus Heterorhabditidoides.
Keywords: Entomopathogenic nematode, Heterorhabditidoides rugaoensis n. sp., Insectivora-group, new nematode species, pathogenicity, phylogeny, Oscheius, taxonomy
Entomopathogenic nematodes (EPNs) are effective biological control agents for many important economic pests; therefore, EPN resources have been gaining attention in the field of biocontrol research (Gaugler and Kaya, 1990; Ehlers, 1996; Mannion et al., 2000; Nguyen et al., 2008; Stock et al., 2009; Shapiro-Ilan et al., 2012). More than 100 laboratories in 70 countries worldwide are conducting research on these beneficial organisms. Recognition of new strains and novel species has increased rapidly with the development and improvement of isolation and identification technology (Gaugler, 2002; Qiu et al., 2005; Kaya et al., 2006; Campos-Herrera et al., 2009; Edgington et al., 2010; Malan et al., 2011, Kanga et al., 2012). As of early 2012, 78 valid EPN species have been identified. 63 of which belong to the family Steinernematidae and 15 to Heterorhabditidae. According to the revised criteria for EPNs by Dillman et al. (2012), the other two species of the family Rhabditidae, Heterorhabditidoides chongmingensis (Zhang et al. 2008), the type species of genus Heterorhabditidoides, and Heterorhabditidoides (Oscheius) carolinensis (Ye et al. 2010), should belong to entomopathogenic nematodes (Torres-Barragan and Cardoza, 2010; Dillman et al., 2012). In contrast to Steinernematidae and Heterorhabditidae, EPNs of Heterorhabditidoides carry specific mutualistic bacteria belonging to the genus Serratia in their intestine or on their cuticle (Kaya and Gaugler, 1993; Zhang et al., 2009; Torres-Barragan et al., 2011).
There are some controvesies about the status of the genus Heterorhabditidoides. Zhang et al. (2008) defined the genus Heterorhabditidoides and described the type species Heterorhabditidoides chongmingensis, whereas Ye et al. (2010) and Liu et al. (2012) considered the genus Heterorhabditidoides as a junior synonym of Oscheius. The status of Heterorhabditidoides and its relationship with Oscheius will be treated in the Discussion.
During a survey on EPNs in Jiangsu Province, a novel EPN strain RG081015, belonging to Heterorhabditidoides was discovered. Morphological characteristics combined with molecular data suggested that strain RG081015 is a novel species, described herein as H. rugaoensis n. sp.
Materials And Methods
Nematode source: The new species H. rugaoensis n. sp. was recovered from two soil samples collected from Rugao, Jiangsu Province, China, using the Galleria mellonella baiting method (Bedding and Akhurst, 1975; Zhang et al., 2008). One strain, RG081015, was used as the type population for description. Last-instar G. mellonella larvae were placed in 250-mL plastic containers (five larvae per container) with moistened soil from the collected samples and kept at room temperature (20 ± 3 °C) (Stock et al., 1999). Water was added to the samples periodically to keep the soil moistened during baiting. G. mellonella larvae were checked every day, and each killed larva was replaced by a fresh one. After 7 days, dead insects were thoroughly rinsed in distilled water and placed in modified White traps (Kaya and Stock, 1997) until the emergence of third-stage infective juveniles (IJs or dauer juveniles). IJs were identified by their emergence time and morphological characters.
Nematode culture: Twenty G. mellonella larvae were exposed to approximately 1000 IJs at 20 ± 3 °C in a Petri dish (60 × 15 mm) lined with two moistened filter papers. The dead insects were immediately transferred to a White trap and incubated at 20 ± 3 °C until IJs emerged (White, 1927). First-generation males and females, and hermaphroditic females were obtained by dissecting last-instar G. mellonella larvae after infection with IJs 3–4 and 5–7 days later, respectively.
Light microscopy: Twenty hermaphroditic nematodes, 20 first-generation males and females, and 20 IJs were randomly selected from different G. mellonella cadavers. One male was selected as the holotype of this new species. All nematodes were examined live and then heat-killed on glass slides at 60 °C in Ringer’s solution. The heat-killed nematodes were then fixed in triethanolamine–formalin (TAF) fixative solution (Kaya and Stock, 1997) and transferred to anhydrous glycerin for mounting (Seinhorst, 1959). Examination and measurements were performed using an Axio Imager A1 microscope (Carl Zeiss).
Scanning electron microscopy: For scanning electron microscopic (SEM) examination, nematodes of different developmental stages were fixed in 3% glutaraldehyde buffer at pH 7.2 for at least 48 hr at 4–8 °C (Nguyen and Smart, 1995). They were then post-fixed with 2% osmium tetroxide solution for 12 hr at 25 °C, dehydrated in a graded ethanol series, critical point-dried with liquid CO2, mounted on SEM stubs, and coated with gold (Nguyen and Smart, 1995). Samples were examined using a Hitachi S-3000N scanning electron microscope (Hitachi).
Cross-hybridization test: Reproductive compatibility of H. rugaoensis n. sp. (RG081015) and H. chongmingensis (DZ0503CMFT) were tested using G. mellonella hemolymph by the method reported by Nguyen and Duncan (2002).
Polymerase chain reaction (PCR) amplification and sequencing: DNA extraction and PCR amplification were performed according to the methods described by Zhang et al. (2008). Primers used for amplification and sequencing of the internal transcribed spacer (ITS) and 18S rDNA of the new species were those reported by Vrain et al. (1992) and Liu et al. (1997), respectively. The ITS primers were 5′-TTG ATT ACG TCC CTG CCC TTT-3′ (forward) and 5′-TTT CAC TCG CCG TTA CTA AGG-3′ (reverse). The 18S rDNA primers were 5′-GGT GAA ACT GCG AAC GGC TCA-3′ (forward) and 5′-CCG GTT CAA GCC ATT GCG ATT-3′ (reverse). PCR products were purified and sequenced by the Beijing Genomics Institute (Shenzhen, China).
Sequences analysis and phylogenetic relationships: The ITS and 18S rDNA sequences of EPNs and related nematodes were used for taxonomic and phylogenetic analyses. Sequences from the new species were obtained in this study, while those from others were obtained from GenBank. Sequences were assembled using Sequencing Analysis 3.0 and aligned with ClustalX (Thompson et al., 1997) under the default alignment parameters. Using MEGA version 4 (Tamura et al., 2007), molecular phylogenetic relationships between the nematodes were estimated by neighbor-joining (NJ) method with the Kimura 2-parameter model. Relative support for clades was assessed by bootstrap analysis (Felsenstein, 1985) with 1000 replicates.
Isolation and identification of the symbiotic bacteria of H. rugaoensis n. sp.: The symbiotic bacteria strains were isolated from IJs of H. rugaoensis n. sp. by two methods. The first method was to crush approximately 100 surface-sterilized IJs followed by streaking the product on nutrient-bromothymol blue agar plates (NBTA, nutrient agar, 0.0025% bromothymol blue, and 0.004% triphenyltetrazolium chloride medium) and incubated at 28 °C for 48 hours (Akhurst, 1980, Zhang et al., 2008). Surface sterilization of IJs was conducted by method developed by Akhurst (1980) as follows. 100 IJs were surface-sterilized by immersion in 0.1 % (w/v) merthiolate for 2 to 3 hr, washed three times in sterile water, then suspended in YS broth (NH4H2PO4, 0.5 g; K2HPO4, 0.5 g; MgSO4. 7H2O, 0.2 g; NaCl, 5 g; yeast extract, 5 g; water, 1 1; Dye, 1968) and macerated in a tissue homogenizer. The macerate was spread on nutrient agar (NA) and 0.025 % (w/v) bromothymol blue (NBTA). The second method was to streak onto NBTA plates a drop of hemolymph harvested from insects parasitized by nematodes 24–48 hours. Genomic DNA of the symbiotic bacteria was prepared following the method of Marmur (1961). Primers used for amplification and sequencing of 16S rDNA of the symbiotic bacteria strains were 5’-AGA GTT TGA TCC TGG CTC AG-3’ (forward) and 5’-TAC CTT GTT ACG ACT TCA CCC CA-3’ (reverse; Brosius 1978). PCR amplification of the 16S rRNA gene was performed as described by Li et al. (2007). PCR products were purified and sequenced by the Beijing Genomics Institute (Shenzhen, China). Identification analysis of 16S rDNA sequences of strain DR186 with all described bacteria type strains were conducted using EzTaxon Server version 2.1 (Chun et al., 2007).
Pathogenicity of the new nematode against the insect host: The pathogenicity of H. rugaoensis n. sp. against the fifth stage larva of Galleria mellonella was estimated using the methods described by Bonifassi et al. (1999) with a small modification. Pathogenicity of IJs was tested by exposure to last-instar larvae of G. mellonella. Zero (control), 10 , 20, 40, 80, 160, 320, 640 IJs in 1.2 ml water were placed onto filter paper in Petri dishes (10 cm diameter) with 10 last-instar G. mellonella larvae as targets, and incubated at 25 °C for 72 hours. Each treatment was replicated three times. Larval mortality was recorded every 12 hours, and then analyzed with one-way ANOVA. Significant ANOVAs (P < 0.05) were subjected to Tukey HSD tests. All of the original data passing these tests were applied to estimate the larval mortality means varying with treatment time and IJs concentration, using Graphpad prim v.5.01 (2007). Original data was arcsine-square-root transformed, and tested for normality with one-sample Kolmogorov-Smirnov tests. We then estimated the lethal concentration needed to kill 50% of the infected insects (LC50) with probit analysis. All statistical analyses were conducted with SPSS v.20.0 (IBM 2011).
Pathogenicity of Aposymbiotic (symbiont-free) nematode IJs of the new species against the insect host: Aposymbiotic IJs of the new nematodes were obtained according to the methods described by Sicard et al. (2004). Sodium hypochloride (final concentration of 10%, w/v) was added into sterile Ringer solution (NaCl 0.9%, w/v) with grinding of 40 first-generation mature females during 18 min to disinfect the surface of the eggs of the new nematodes. Disinfected eggs were then rinsed twice with sterile Ringer’s solution and transferred to ‘liver-agar’ plates for incubation at 25 °C. Three weeks later, axenic (i.e. grown without any germs) IJs were obtained from these plates, which were then available for further experimentation. Sterility of the IJs was assessed by inoculating nutrient broth tubes with samples from the liver plates. Aposymbiotic nematodes were produced by infesting insects with axenic IJs using the aforementioned methods for wild-type nematode production. The IJs emerging from these infestations were not axenic as they came from insect cadavers containing their own microflora. However, they were aposymbiotic because they grew within the insect in the absence of Serratia and did not obtain any saprophytic bacteria from the insect in their intestines (Sicard et al., 2003). Pathogenicity tests were performed according to the aforementioned methods with a small modification (number of treated nematodes was 320 IJs), in which wild-type IJs and axenic IJs added with symbiotic bacteria strain DR186 were used as control.
Results
Description (Table 1, Figs. 1–4): Morphometric measurements of the holotype and all developmental stages of the new EPN species are given in Table 1.
Table 1.
Morphometrics (μm) of Heterorhabditidoides rugaoensis n. sp., presented as the mean ± SD and the range.
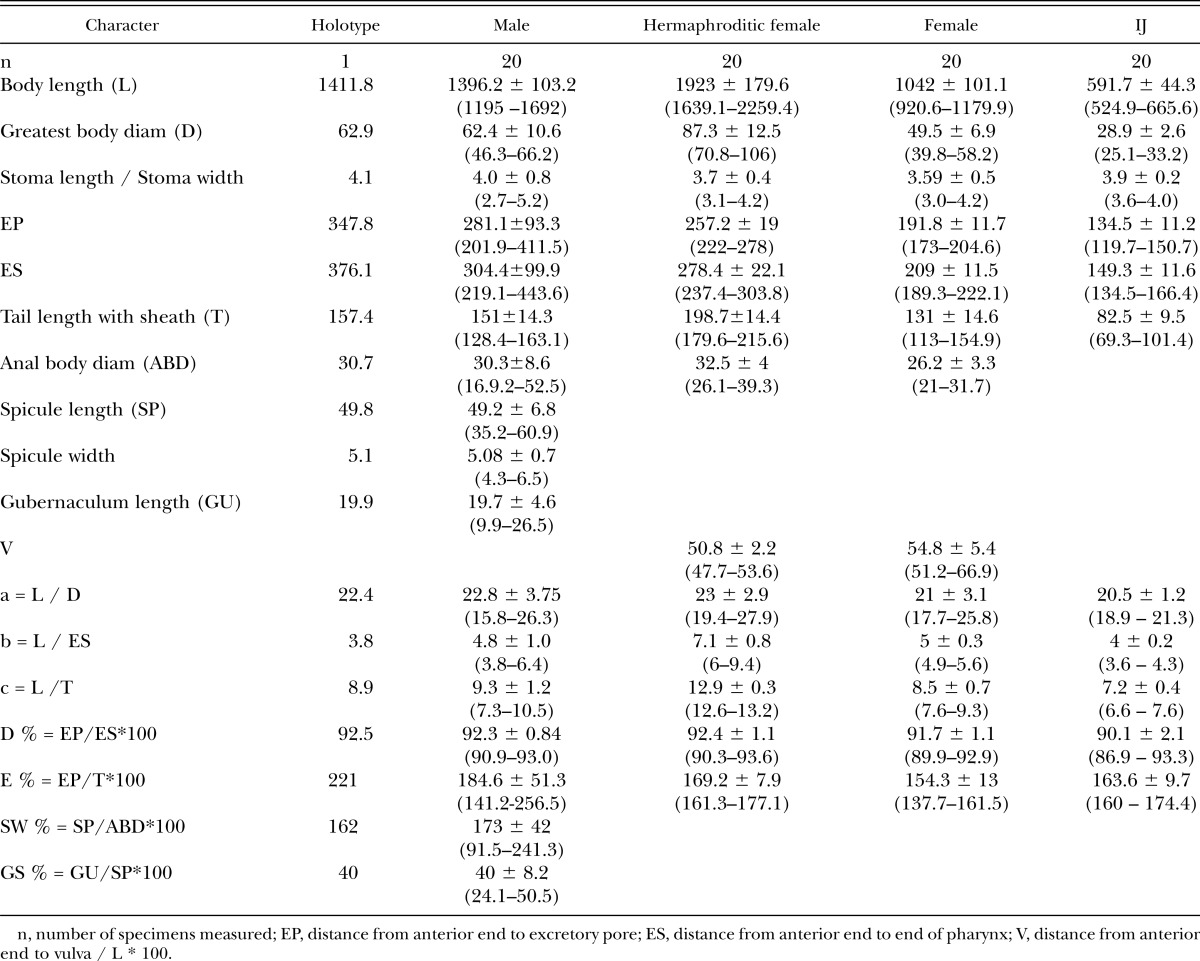
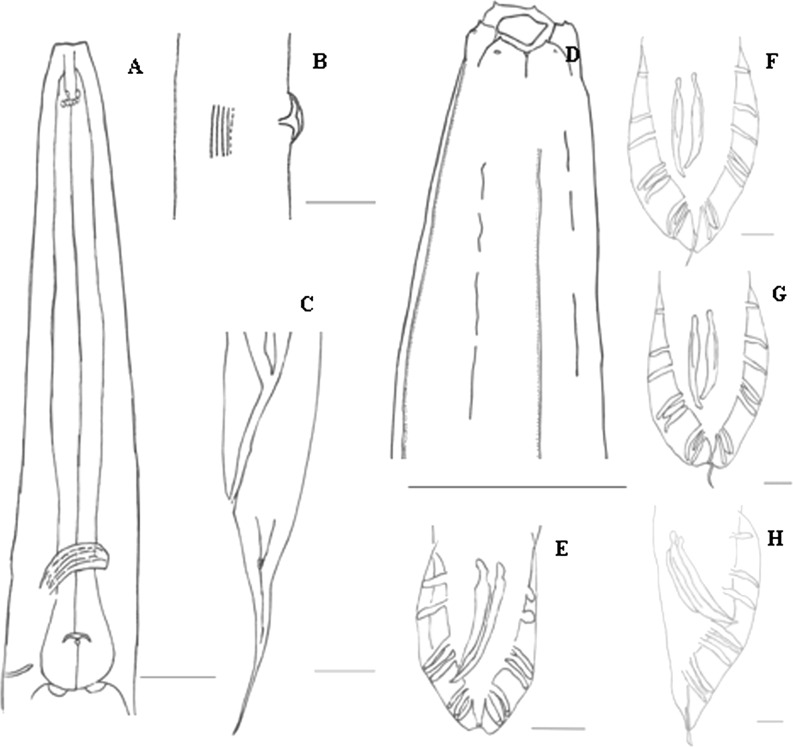
Fig. 1.
Heterorhabditidoides rugaoensis n. sp. A: Mouth and pharynx of amphimictic female, B: Lateral view of vulva region and lateral field of hermaphroditic female , C: Lateral view of hermaphroditic female tail region showing rectum, phasmid and lateral ridges, D: Head, anterior and lateral ridges of male, E-H: Various view of male tails with spicules and bursal papillae. Scale bars: A-F and G 20μm, H 10μm.
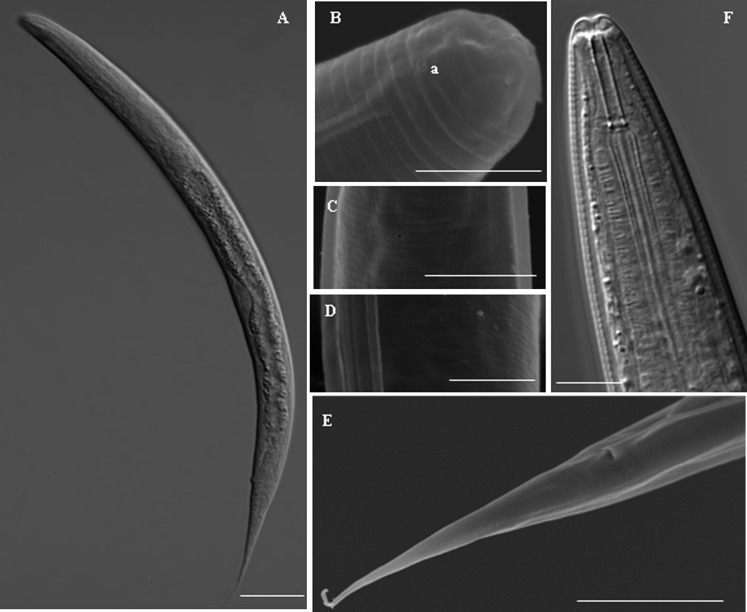
Fig. 4.
Morphology of juvenile. A: Overall shape of juvenile killed by heat, B: Anterior of juvenile, C: Excretory pore of juvenile, D: Lateral lines of juvenile, E: Tail and cloaca of juvenile, F: Anterior of juvenile under light microscope. Scale bars: A 100μm, B and D 5μm, C and F 10μm, E 20μm.
Males (Figs.1, 2): Males have a J-shaped body when killed by heat. The head shows no constriction, and has six separate well-developed lips, each with one or two conical terminal papillae. Two pore-like amphids are arranged on the lateral sides of the two opposite lips with one conical terminal papilla each. The lateral fields have one longitudinal ridge and two discontinious strips. Bursa ridges are well- developed, and the spicules are paired, separate, often asymmetrical, and slightly curved ventrally. They possess a tubular stoma, with a width approximately 1.5 times the head width, with well-cuticularized cheilorhabdions. A metastome isoglottoid with one hemispherical swelling comprised of two or three developed warts. Another three to six well-developed warts are observed at the bottom of metastome. The males have a pharynx with a cylindrical corpus. The metacorpus is swollen and the esophageal collar is very long. The isthmus is distinct with a globose basal bulb and a prominent valve. The nerve ring surrounding the isthmus is located posterior to it, with the cardia protruding into the intestine and the excretory pore usually located near the middle of the basal bulb. The testis is monorchic and reflexed. The gubernaculum is boat-shaped (lateral view) and ventrally curved, and its length approximately 40% of the spicule length. The cuticula of the bursa peloderan extends on both sides and surrounds the male cloaca. Papilla formula of the bursa is 1, 2, 3, 3, the number of papillae in the terminal group is constant, and often retains the fourth juvenile tail sheath, giving it a pseudopeloderan appearance. The average spicule length is 49 (min. 35, max. 61) μm. The head of the spicule tip is round, crochet-needle-shaped. A bristle-like pair of sensilla are posterolateral to the cloacal opening. The males have a small, pointed tail.
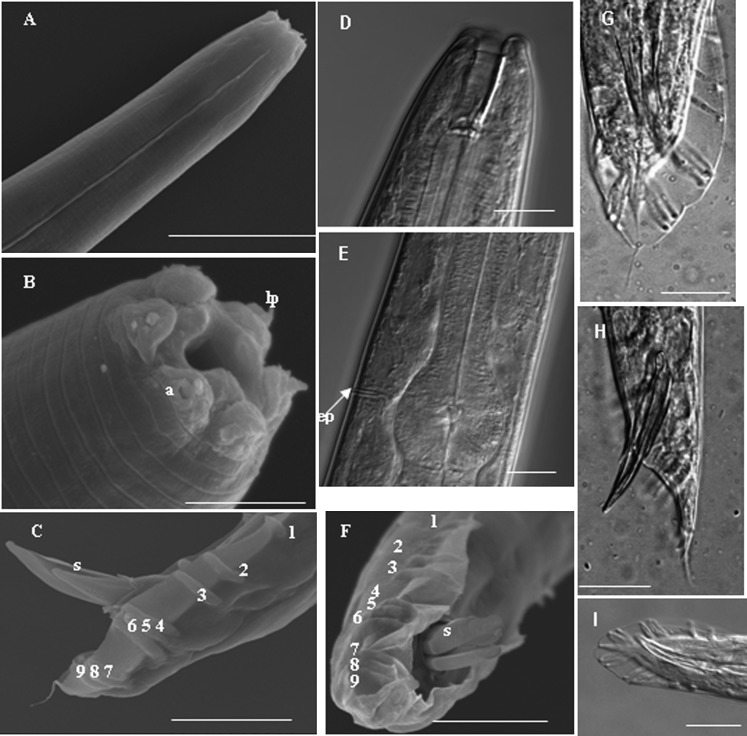
Fig. 2.
Morphology of male nematode. A: Anterior showing lateral ridges, B: Head showing six lips, labial papillae (lp) and amphidial opening (a) , C and F-I: Various view of male tails with spicules and bursal papillae, D: Anterior of male, E: Basal bulb and excretory pore (ep) of male. Scale bars: A and F-I 20μm, B 5μm , C 30μm, D and E 10μm.
Hermaphroditic females (Table 1, Figs. 1, 3): Hermaphroditic females have a C-shaped body when killed by heat. The cuticle is well-annulated. The lateral field at midbody has four to five ridges that gradually lead into one ridge near the anus, and the excretory pore is circular. The labial region has six well-developed lips, two pore-like amphids, and 6 + 4 separated cephalic papillae; the mouth is trigonal in face view. The stoma is tubular, and the cheilorhabdions are well cuticularized. These females possess a metastome isoglottoid with one hemispherical swelling and three to seven warts. The isthmus is long and distinguishable, and the nerve ring surrounding the isthmus is located posterior to it. The valve of the basal bulb is prominent. Gonads are didelphic, amphidelphic, and reflexed. The vulva is a transverse slit situated on a protruding area usually near midbody (V = 47.74–53.62%). It is protected by two symmetrical cuticular flaps, and the vagina is short. The tail is approximately three times longer than the body width at the anus and has a conoid or post-anal swelling with a pointed terminus.
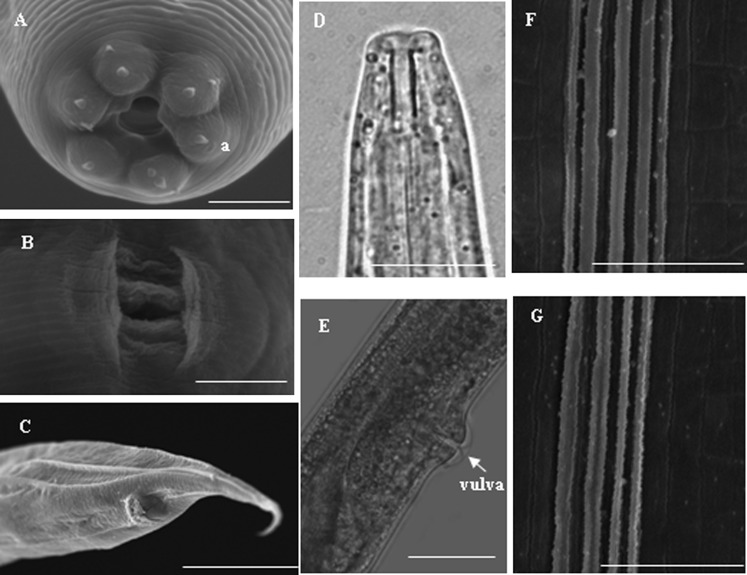
Fig. 3.
Morphology of hermaphroditic and amphimictic female. A: Head of hermaphroditic female, B: Vulva pattern of hermaphroditic female, C: Tail of amphimictic female, D: Anterior of amphimictic female, E: Lateral view of vulva of amphimictic female, F: Lateral field at mid body of hermaphroditic female with four ridges, G Lateral field at mid body of hermaphroditic female with four ridges. Scale bars: A, B, F and G 5 μm, C 30 μm, D 10 μm, E 20 μm.
Amphimictic females (Table 1, Figs. 1, 3): Amphimictic females are similar to hermaphroditic females but are smaller. The reproductive system is amphidelphic and reflexed. The vulva is a transverse slit situated on a protruding area. It is covered with exudates after mating.
IJs(Table 1, Fig. 4): IJs have an elongated C-shaped body when killed by heat (Fig. 4A). Their cuticles are well-annulated. The lateral field at midbody has two longitudinal ridges that form a very deep groove and gradually lead into one ridge near the anus (Figs. 4B–4E). The tail is very long and pointed and covered with a sheath. It is approximately five times longer than the body width at the anus and has one phasmid. The head has no prominent dorsal tooth (Fig. 4B) and has a triangular slit. The labial region has six labial papillae, lacking visible cephalic papillae, and the mouth is closed (Fig. 4B). One slit-like amphid is arranged behind the labial field (Fig. 4B). The stoma is tubular, and the cheilorhabdions are not cuticularized. The metarhabdions show no hemispherical swellings; instead, two well-developed warts are observed. The metastome isoglottoid has three to seven warts.
Type host and locality: The natural host remains unknown. The nematode was recovered by baiting with G. mellonella larvae from soil samples collected from Rugao, Jiangsu Province, P. R. China.
Type material: Paratypes slides of five males (T-6182p to T-6186p), five hermaphroditic females (T-6187p to T-6191p), five amphimictic females (T-6192p to T-6196p), and five IJs (T-6197p to T-6201p) were deposited in the United States Department of Agriculture Nematode Collection (USDANC), Beltsville Maryland. Holotype and additional specimens were deposited in the laboratory of the Department of Zoology, College of Life Sciences of Nanjing Agricultural University, Nanjing, P. R. China.
Cross-hybridization test: Males and females of H. rugaoensis n. sp. did not interbreed with H. chongmingensis, although males and females of each isolate mated and produced offspring normally. The reproductive incompatibility of H. rugaoensis n. sp. and H. chongmingensis indicated that they do not belong to the same species.
Molecular characterization: The ITS rDNA region of H. rugaoensis n. sp. is characterized by a total length of 807 bp: ITS1 = 418 bp, 5.8S rDNA = 154 bp, and ITS2 = 235 bp (being shorter by two nucleotides than that of H. chongmingensis). The ITS1 sequence length of the novel species is identical to that of H. chongmingensis, but longer than those of heterorhabditids and Pellioditis, and shorter than that of Oscheius (Table 2). The 5.8S rDNA sequence length of H. rugaoensis n. sp. is the same as those of H. chongmingensis, the Insectivora-group of Oscheius, Pellioditis and heterorhabditids, but longer than that of the Dolichura- group of Oscheius (Table 2). The 5.8S rDNA of Heterorhabditidoides shows 94% sequence identity with the Oscheius/Insectivora-group, but only 76-77% identity with the Oscheiu/Dolichura-group, mostly less than with Pellioditis or heterorhabditids (with 90% and 89%, respectively. Table 2).
Table 2.
Sequence length, similarity and composition of 5.8S rDNA and ITS1 regions of species of Heterorhabditidoides rugaoensis n. sp. and its closer relatives.
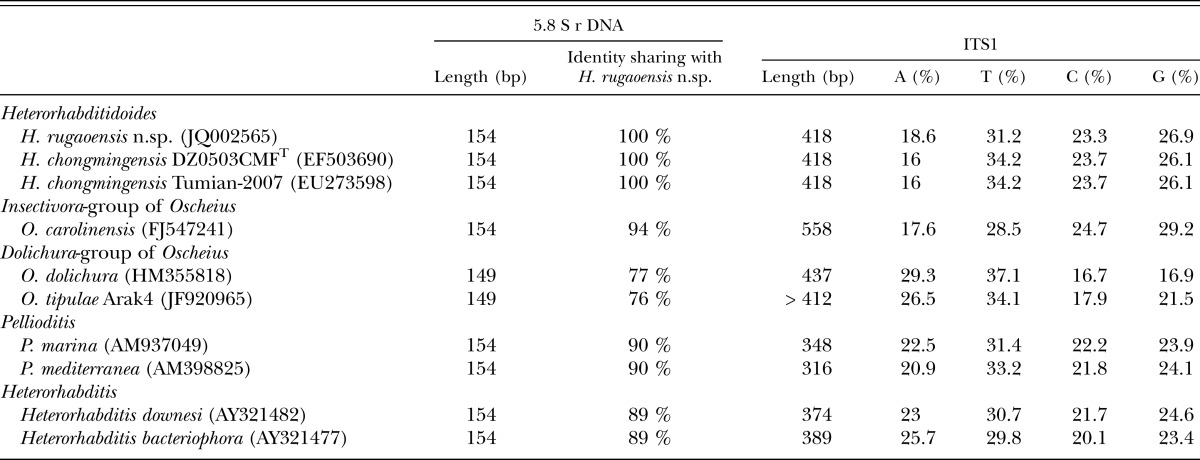
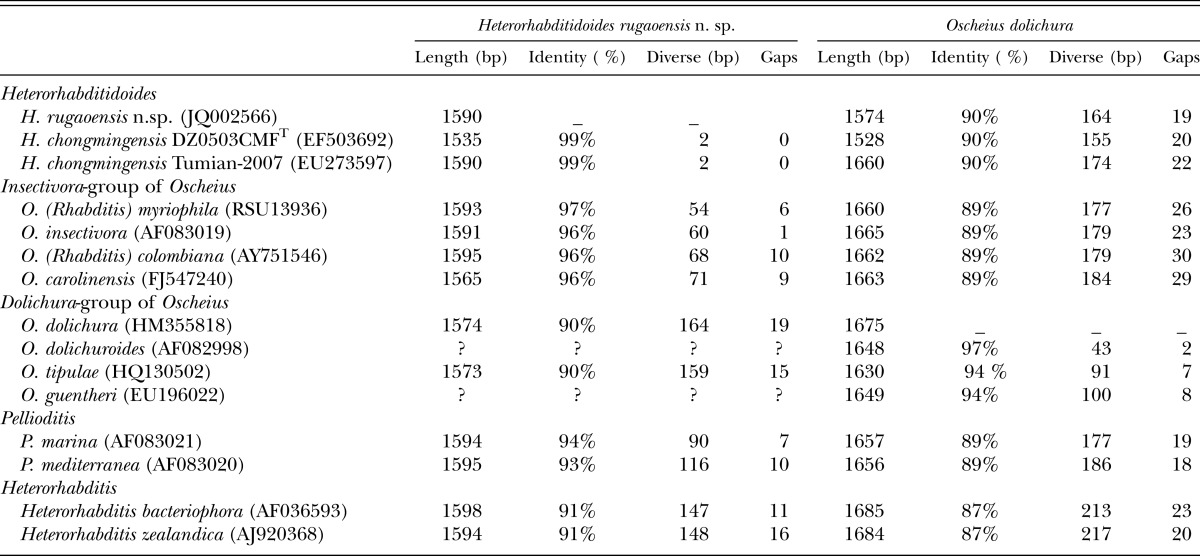
The length of 18S rDNA of the new nematode species is 1590 bp and comprises 422 A, 433 T, 325 C, and 410 G. As per a BLAST search, the 18S rDNA sequence of the new nematode species is closest to that of H. chongmingensis at 99% identity, than to that of the Oscheius/Insectivora-group at 96-97% identity (Table 3). The 18S rDNA of Heterorhabditidoides shows 90% sequence identity with the Oscheius/Dolichura-group, less than that of Heterorhabditidoides with Pellioditis or heterorhabditids (with 93% and 91%, respectively. Table 3).
Table 3.
BLAST results of of 18S rDNA of Heterorhabditidoides rugaoensis n. sp. and Oscheius dolichura showing identity between the two species and their closer relatives.
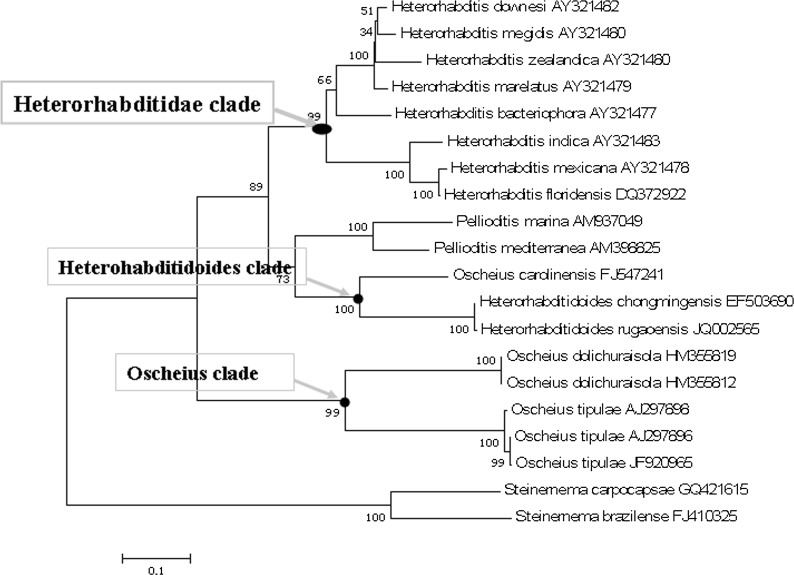
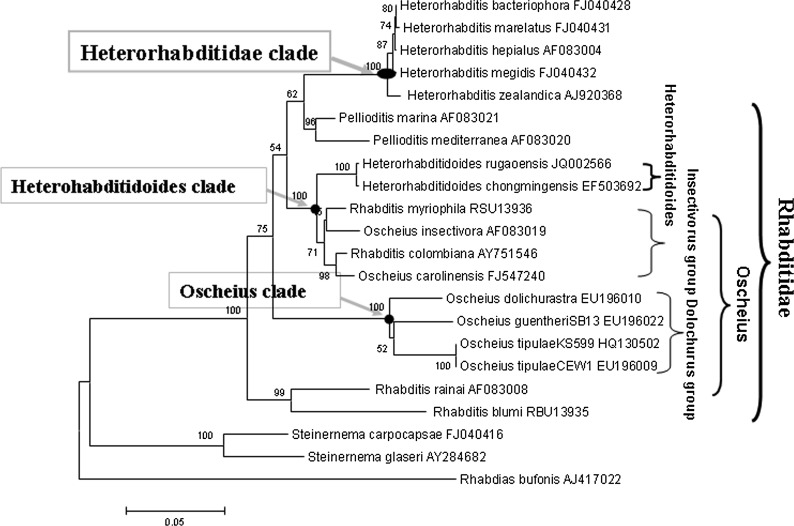
Phylogenetic reconstruction based on ITS and 18S rDNA sequence data indicates that H. rugaoensis n. sp. and H. chongmingensis form a monophyletic group, indicating that the new species belongs to the genus Heterorhabditidoides (Figs. 5,6). The closer relationship between Heterorhabditidoides and the Oscheius/Insectivora-group is at the most significant level with a bootstrap value of 100%.
Fig. 5.
Phylogenetic relationships of RG081015 and other closely related nematodes based on ITS sequences data.
Fig. 6.
Phylogenetic relationships of RG081015 and other closely related nematodes based on 18S rDNA sequences data.
Diagnosis and relationship with H. chongmingensis and with some species in the Oscheius/Insectivora-group: The morphological characteristics of the new species are similar to those of H. chongmingensis DZ0503CMFT; however, some marked differences exist between the two nematodes. Differences are observed in body length, the male ridge pattern of the lateral field, stoma size, metarhabdions, vulva pattern in the female, and amphid arrangement in IJs (Table 1, Figs. 1–4). Because of these features, the newly identified nematode is categorized as a new species belonging to Heterorhabditidoides. Males of the new nematode species can be recognized on the basis of body length averaging 1396.2 μm (min. 1195, max. 1692), asymmetric spicules and a lateral field with one ridge; these features differ from those of H. chongmingensis (Zhang et al. 2008, Liu et al., 2012). The stoma of the males is long and narrow, while that of H. chongmingensis is shorter and wider. The metarhabdions of the new species have one hemispherical swelling comprised of two or three developed warts, whereas those of H. chongmingensis always have two smooth hemispherical swellings, symmetrically arranged on the two sides of the metarhabdions. The bursa pattern of the new species is peloderan, whereas that of H. chongmingensis is pseudopeloderan. The new species can be distinguished from H. chongmingensis DZ0503CMFT on the basis of the body length of IJs (average, 591.7 vs. 428 μm). In IJs of the new species, EP = 134.5 (119.7–150.7) μm and ES = 149.3 (134.5–166.4) μm, both significantly larger than those in IJs of H. chongmingensis DZ0503CMFT. Compared with IJs of Heterorhabditidae and Steinernematidae, IJs of the new species have a head lacking a prominent dorsal tooth and a labial region with six labial papillae. Females of the new species have a vulva pattern different from that of H. chongmingensis DZ0503CMFT females. The female tail of H. rugaoensis n. sp. is approximately three times longer than the body width at the anus, larger than that of H. chongmingensis.
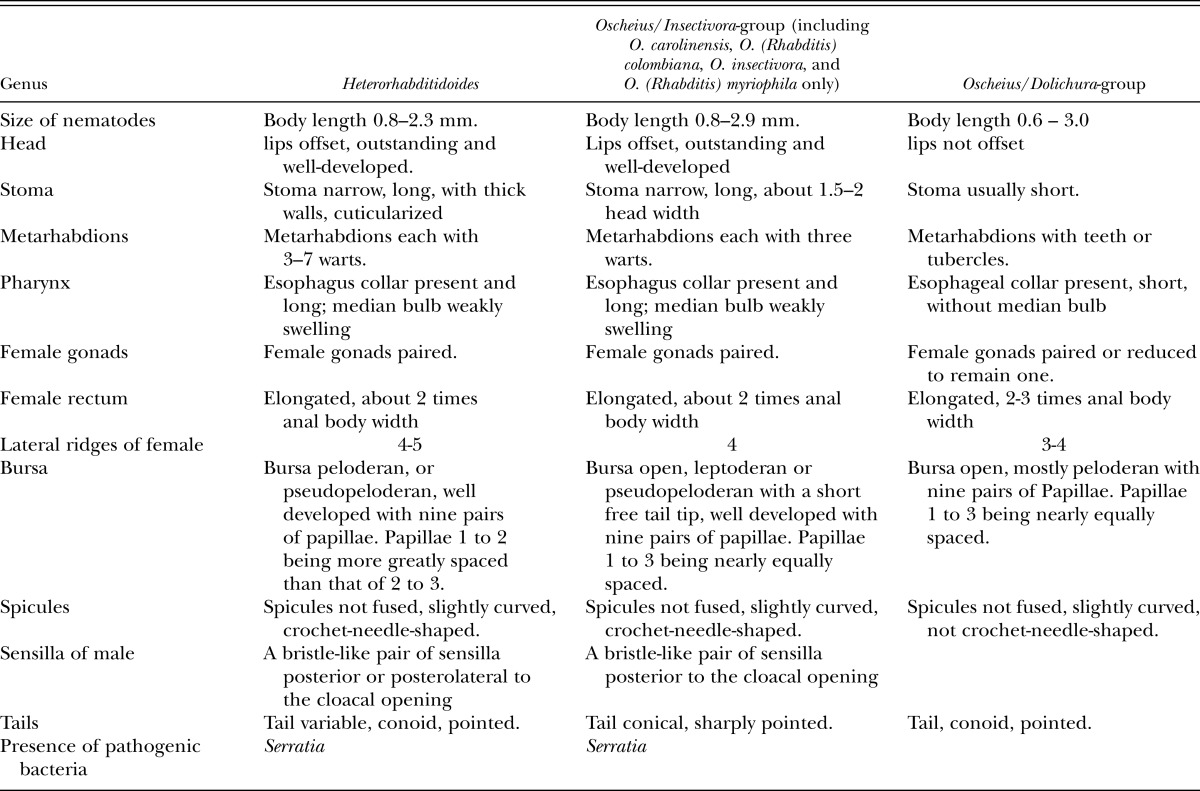
A bristle-like pair of sensillae posterolateral to the cloacal opening, and crochet-needle-shaped spicules of H. rugaoensis n. sp. suggest that the new species has a closer relationship with the Oscheius/Insectivora-group. The new species can be distinguished from other species of the Oscheius /Insectivora-group on the basis of its body length, bursal papillae distance between the first and second rays, bursa pattern, lateral ridges and composition of ITS1 sequences (Tables 2,4). The body lengths of the new species’ adults are longer than those of O. colombianus and O. myriophila, and shorter than those of O. carolinensis and O. insectivorus. For the new species, the distance between the first and second rays of the bursal papillae is greater than those of the aforementioned four species of the Oscheius/Insectivora-group.
Table 4.
Characteristics of Heterorhabditidoides, some species of Oscheius/Insectivora-group and Oscheius/Dolichura-group.
Molecular phylogenetic trees based on 18S rDNA and ITS sequences show that the new species is most closely related to H. chongmingensis DZ0503CMFT, suggesting that the two belong to the same genus Heterorhabditidoides. The 18S rDNA sequences of the two nematodes differ by two bases and share 99% identity, while their ITS sequences differ by 12 bases and share 98% identity. The failure of cross-hybridization between the new species and H. chongmingensis DZ0503CMFT supports the assumption of reproductive isolation between them and indicates that the nematode RG081015 is a novel species belonging to Heterorhabditidoides, described herein as H. rugaoensis n. sp.
Isolation and identification of symbiotic bacteria: Two different-colored bacterial colonies were obtained, and two strains, R187 and DR186, were selected from these colonies for further identification. Strain R187 produced a red pigment on NBTA and LB plates, while strain DR186 produced a dark red pigment on the NBTA plate. 16S rDNA sequence analysis showed that strains R187 (GenBank ID: JQ002567) and DR186 (GenBank ID: JQ002568) have very similar 16S rDNA sequences (one base difference), and they were accordingly considered to be two clones of the same species. Identification analysis of strain DR186 with all described bacterial species using 16S rRNA gene sequences data and EzTaxon Server version 2.1 showed that the symbiotic bacterium belongs to the genus Serratia and shares 99.856 %, 99.856 %, and 99.712 % 16S rRNA sequence similarity with its closer relatives S. nematodiphila DZ0503SBS1T, S. marcescens subsp. sakuensis JCM 11315T (Ajithkumar et al. 2003), and S. marcescens subsp. marcescens DSM 30121T, respectively. Numerical taxonomy analysis based on physiological and biochemical properties of those bacterial strains showed that DR186 and R187 have the closest similarity, and the two strains have closer similarity with S. nematodiphila DZ0503SBS1T than them with S. marcescens subsp. sakuensis JCM 11315T or with S. marcescens subsp. marcescens DSM 30121T (unpublished data).
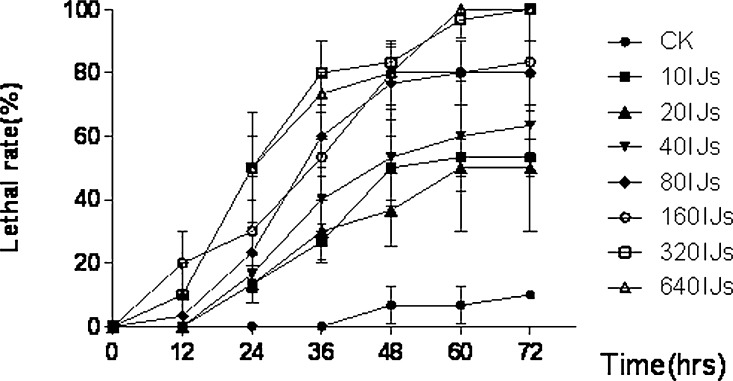
Pathogenicity of the new entomapathogenic nematode symbiont against G. mellonella: Native IJs (harbouring the bacterial symbiont in their intestines) of H. rugaoensis n. sp. have high pathogenicity against G. mellonella, and the lethality rates were positively correlated with nematode concentration and time of infection (Fig. 7). About 284 (188.1–1022.6) IJs/ml could result in 95% larval mortality at 72 hr. The LC50 of the novel species to G. mellonella within 48 hours infection was 24.35 (10.3–53.6) IJs/ml.
Fig. 7.
Lethal rate to Galleria mellonella larvae by IJs of RG081015 varys with time.
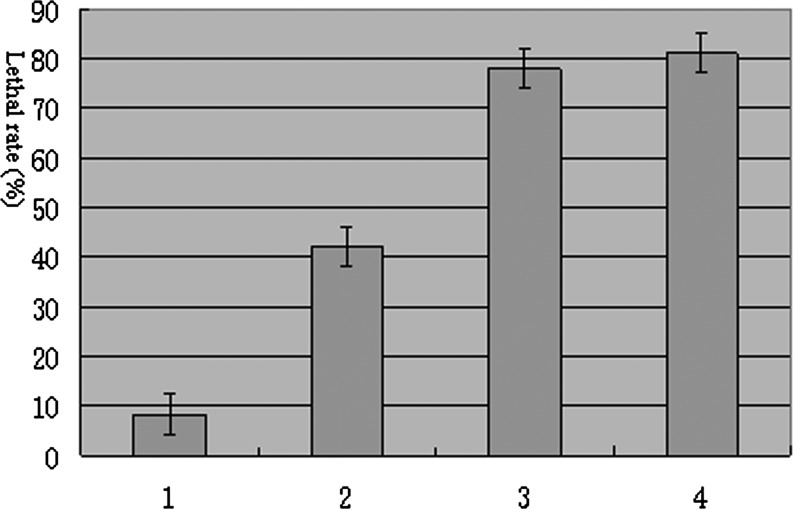
The pathogenicity of the axenic IJs (without bacterial symbiont in their intestines) to G. mellonella larvae was reduced by half, but restored fully when the axenic IJs co-infected the insects together with the bacterial symbiont (Fig. 8). This result indicated that the bacterial symbiont is important for the entomopathogenic activity of Heterorhabditidoides rugaoensis n. sp.
Fig. 8.
Mortality of Galleria mellonella larvae exposed to IJs of RG081015 after 48 hours. 1: Control, 2: axenic IJs, 3: axenic IJs mix with symbiotic bacterium, 4: native IJs. 320 larvae / each, means ± SD (range).
Discussion
This study identified a novel nematode Heterorhabditidoides rugaoensis n. sp. and provided strong evidence for its being entomopathogenic, according the latest interpretation of the criteria for the term entomopathogenic nematodes (Dillman et al., 2012). They killed G. mellonella larvae quickly and efficiently with the aid of their symbiotic bacteria (the 48 hr-LC50 value is 24.35 IJs/ml), and transmitted the bacteria through more than 20 infective generations (unpublished data). The type strain RG081015 of the new nematode was isolated by G. mellonella baiting from a soil sample randomly collected from farmlands near Rugao, being adjacent to Chongming Island where the generic type species Heterorhabditidoides chongmingensis was discovered, but geographically separated from it by the Yangzi River. We hypothesize that geographical isolation has resulted in the existence of reproductive isolation between the two nematodes.
Recently, Oscheius carolinensis and Caenorhabditis briggsae Dougherty and Nigon, 1949, two species of the family Rhabditidae, were found to be pathogenic against pests with the aid of bacterial strains of the genus Serratia (Abebe et al., 2010; Ye et al., 2010; Torres-Barragan et al., 2011). Oscheius carolinensis was confirmed to be an entomopathogenic member, although C. briggsae was not (Dillman et al., 2012). Based on a phylogenetic tree of 18S rDNA sequences, both Ye et al. (2010) and Liu et al. (2012) suggested that Heterorhabditidoides, the first identified entomopathogenic genus in the family Rhabditidae, was a junior synonym of Oscheius, and proposed that the name of the type species of Heterorhabditidoides should be changed to be O. chongmingensis n. comb. However, we argue below that phylogenetic relationships and analyses of morphological and genetic similarity provide convincing evidence that some species of the Insectivora subgroup of Oscheius, including O. colombianus, O. myriophila, O. carolinensis and O. insectivorus, should instead be included in the genus Heterorhabditidoides.
Accurate phylogenetic analyses of nematodes using 18S rDNA sequences have refined understanding of nematode evolution (Fitch et al., 1995; Blaxter et al., 1998; Chilton et al., 2006; Kiontke et al., 2007; Hwang et al., 2009; Ross et al., 2010; Alguacil et al., 2011). We estimated the phylogenetic tree of Heterorhabditidoides, Oscheius, Heterorhabditidae, Steinernematidae and their closest relatives based on 18S rDNA sequence data. Rhabdias bufonis Schrank, 1788 (a species of Rhabdiasidae, the sister family of families Rhabditidae and Heterorhabditidae ) was selected as the outgroup in order to recover the phylogenetic relationships of those entomopathogenic nematodes. All the species of Heterorhabditidae, Steinernematidae and Heterorhabditidoides respectively form a monophyletic group with the highest bootstap values (100%). By contrast, species of Oscheius were paraphyletic and formed two subclades, the Insectivora group and the Dolichura group, which are consistent with previous results based on morphological characteristics and molecular phylogenetic analysis (Andrássy, 1976; Sudhaus and Hooper, 1994; Stock et al., 2005; Félix, 2006), even considering the results of Ye et al. (2010) and Liu et al. (2012).
The Insectivora subgroup of Oscheius was defined by Andrássy (1976) and elaborated further by Sudhaus and Hooper (1994) and Félix (2006). This group has many distinctive characteristics distinguishing it from the Dolichura group, such as larger size, lips outstanding and well-developed, metarhabdions bearing two to three well-developed warts and three to seven small warts, the lips of papillae 5 and 8 not terminating on the dorsal side of the velum, leptoderan or pseudopeloderan bursa, spicules crochet-needle-shaped, with 154 bp 5.8S rDNA squences etc. These morphological and molecular characteristics are shared with the genus Heterorhabditidoides. Our phylogenetic tree derived from 18S rDNA sequences showed that the monophyletic Insectivora group is the closest sister group of the Heterorhabditidoides group, and those two groups form the sister clade (Heterorhabditidoides clade) of the clade (Heterorhabditidae + Pellioditis). The superclade comprising Heterorhabditidae, Pellioditis, Heterorhabditidoides and the Oscheius/Insectivora-group, is the sister group of the monophyletic Oscheius/Dolichura-group, which suggests that the Heterorhabditidoides clade (including the Oscheius /Insectivora-group) has a closer relationship with Heterorhabditidae and Pellioditis than with the Oscheius/Dolichura group. These results agree with genetic similarity analysis of 18S rDNA based on BLAST, and further supported by length and similarity analysis of 5.8 S rDNA sequences and by ITS phylogenetic analysis. Genetic similarity analysis of 18S rDNA based on BLAST revealed that the Heterorhabditidoides clade shares higher genetic identity with Pellioditis (>93%) and Heterorhabditidae (91%) than with the Oscheius/Dolichura group (90%). In summary, the monophyletic Dolichura group are real members of genus Oscheius and the generic name should be retained. By contrast, four species of the Insectivora group of Oscheius, including the entomopathogenic member Oscheius carolinensis, O. colombianus, O. myriophila and O. insectivorus should be reassigned to the genus Heterorhabditidoides as it is the first identified entomopathogenic nematode genus in the family Rhabditidae. We propose that the aforementioned species of the Insectivora group of Oscheius be renamed as Heterorhabditidoides (Oscheius) carolinensis n. comp., Heterorhabditidoides (Oscheius) insectivora n. comp. Korner, 1954; Heterorhabditidoides (Oscheius) colombiana n. comp. Stock, Caicedo and Calatayud, 2005; and Heterorhabditidoides (Oscheius) myriophila n. comp Poinar,1986.
DR186 and R187, the symbiotic bacterial strains of the new nematode Heterorhabditidoides rugaoensis n. sp., were identified to be two different strains of Serratia nematodiphila based on a 16S rDNA phylogentic tree and genetic identity analysis. To date, the presence of pathogenic bacterial strains of Serratia vectored by IJs is a characteristic of all three entomopathogenic species of the nematode genus Heterorhabditidoides (Zhang et al., 2009; Torres-Barragan et al., 2011). Caenorhabditis briggsae and other species of the family Rhabditidae were also found to harbour pathogenic bacteria of the genus Serratia associated with insect pathogenicity (Abebe et al., 2010). Heterorhabditidoides chongmingensis and Caenorhabditis briggsae need their bacterial partners in order to kill the insect host and reproduce (Zhang et al., 2008; Abebe et al., 2010). In Heterorhabditidoides rugaoensis n. sp., symbiotic bacteria are not required for host killing and reproduction, but their presence can significantly enhance the pathogenicity of the nematode partner against the insect host. Among the three entomopathogenic nematodes of the genus Heterorhabditidoides, H. rugaoensis n. sp. is the most pathogenic against G. mellonella (Zhang et al., 2008; Torres-Barragan et al., 2011). Further studies are needed to reveal the mechanism of the high pathogenicity in this nematode, in order to assess potential applications for pest management.
Literature Cited
- Abebe E, Jumba M, Bonner K, Gray V, Morris K, Thomas WK. An entomopathogenic Caenorhabditis briggsae. J Exp Biol. 2010;213:3223–3229. doi: 10.1242/jeb.043109. [DOI] [PubMed] [Google Scholar]
- Akhurst RJ. Morphological and functional dimorphism in Xenorhabdus spp., bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. J Gen Microbiol. 1980;121:303–309. [Google Scholar]
- Ajithkumar B, Ajithkumar VP, Iriye R, Doi Y, Sakai T. Spore-forming Serratia marcescens subsp. sakuensis subsp. nov., isolated from a domestic wastewater treatment tank. Int J Syst Evol Microbiol. 2003;53:253–258. doi: 10.1099/ijs.0.02158-0. [DOI] [PubMed] [Google Scholar]
- Alguacil MM, Torrecillas E, Lozano Z, Roldán A. Evidence of differences between the communities of arbuscular mycorrhizal fungi colonizing galls and roots of Prunus persica infected by the root-knot nematode Meloidogyne incognita. Appl Environ Microbiol. 2011;77(24):8656–61. doi: 10.1128/AEM.05577-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrássy . 1976. Evolution as a basis for the systematization of Nematodes. London: Pitman Publishing Ltd. [Google Scholar]
- Bedding RA, Akhurst RJ. A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica. 1975;21:109–110. [Google Scholar]
- Blaxter ML, Deley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, Vanfleteren JR, Mackey LY, Dorris M, Frisse LM, Vida JT, Thomas WK. A molecular evolutionary framework for the phylum Nematoda. Nature. 1998;392:71–75. doi: 10.1038/32160. [DOI] [PubMed] [Google Scholar]
- Bonifassi E, Fischer-Le Saux M, Boemare N, Lanois A, Laumond C, Smart G. Gnotobiological study of infective juveniles and symbionts of Steinernema scapterisci: a model to clarify the concept of the natural occurrence of monoxenic associations in entomopathogenic nematodes. J Invert Pathol. 1999;74:164–172. doi: 10.1006/jipa.1999.4866. [DOI] [PubMed] [Google Scholar]
- Brosius J, Palmer JL, Kennedy JP, Noller HF. Complete nucleotide sequence of 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci USA. 1978;75:4810–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Herrera R, Gutiérrez C. Screening Spanish isolates of steinernematid nematodes for use as biological control agents through laboratory and greenhouse microcosm studies. J Invertebr Pathol. 2009;100(2):100–105. doi: 10.1016/j.jip.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Chilton NB, Huby-Chilton F, Gasser RB, Beveridge I. The evolutionary origins of nematodes within the order Strongylida are related to predilection sites within hosts. Mol Phylogenet Evol. 2006;40(1):118–128. doi: 10.1016/j.ympev.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Chun J, Lee JH, Jung Y, Kim M, Kim S, Kim BK, Lim YW. EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol. 2007;57:2259–2261. doi: 10.1099/ijs.0.64915-0. [DOI] [PubMed] [Google Scholar]
- Dillman AR, Chaston JM, Adams BJ, Ciche TA, Goodrich-Blair H, Stock SP, Sternberg PW. An entomopathogenic nematode by any other name. PLoS Pathog. 2012;8(3):e1002527. doi: 10.1371/journal.ppat.1002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty EC, Nigon V. A new species of the free-living nematode genus Rhabditis of interest in comparative physiology and genetics. J Parasit. 1949;35(suppl.):11. [Google Scholar]
- Dyed W. A taxonomic study of the genus Erwinia I. The ‘amylovora’ group. NCW Zealand Journal of Science. 1968;11:590–607. [Google Scholar]
- Edgington S, Buddie AG, Moore D, France A, Merino L, Hunt DJ. Heterorhabditis atacamensis n. sp. (Nematoda: Heterorhabditidae), a new entomopathogenic nematode from the Atacama Desert, Chile. J Helminthol. 2010;19:1–14. doi: 10.1017/S0022149X10000702. [DOI] [PubMed] [Google Scholar]
- Ehlers RU. Current and future use of nematodes in biocontrol: practice and commercial aspects with regard to regulatory policy issues. Biocontr Sci Technol. 1996;6:303–317. [Google Scholar]
- Félix MA. 2006. Oscheius tipulae. In: The C. elegans Research Community, ed. WormBook: The Online Review of C. elegans Biology [Internet]. Pasadena (CA): WormBook. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Fitch DH, Bugaj-Gaweda B, Emmons SW. 18S ribosomal RNA gene phylogeny for some Rhabditidae related to Caenorhabditis. Mol Biol Evol. 1995;12(2):346–358. doi: 10.1093/oxfordjournals.molbev.a040207. [DOI] [PubMed] [Google Scholar]
- Gaugler R, Kaya HK. 1990. Entomopathogenic nematodes in biological control. Boca Raton, FL:CRC Press. [Google Scholar]
- Gaugler R. 2002 Entomopathogenic Nematology. New York: CABI Pub. [Google Scholar]
- Hwang UW, Choi EH, Kim DS, Decraemer W, Chang CY. Monophyly of the family Desmoscolecidae (Nematoda, Demoscolecida) and its phylogenetic position inferred from 18S rDNA sequences. Mol Cells. 2009;27(5):515–23. doi: 10.1007/s10059-009-0070-7. [DOI] [PubMed] [Google Scholar]
- Kanga FN, Waeyenberge L, Hauser S, Moens M. Distribution of entomopathogenic nematodes in Southern Cameroon. Journal of Invertebrate Pathology. 2012;109(1):41–51. doi: 10.1016/j.jip.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Kaya HK, Gaugler R. Entomopathogenic nematodes. Annual Review of Entomology. 1993;38:181–206. [Google Scholar]
- Kaya HK, Stock SP. 1997. Techniques in insect nematology. Pp. 281–324 in L.A. Lacey, ed. Manual of Techniques in Insect Pathology. San Diego: Academic Press. [Google Scholar]
- Kaya HK, Aguillera MM, Alumai A, Choo HY, de la Torre M, Fodor A, Ganguly S, Hazır S, Lakatos T, Pye A, Wilson M, Yamanaka S, Yang H, Ehlers RU. Status of entomopathogenic nematodes and their symbiotic bacteria from selected countries or regions of the world. Biological Control. 2006;38(1):134–155. [Google Scholar]
- Kiontke K, Barriere A, Kolotuev I, Podbilewicz B, Sommer R, Fitch DH, Felix MA. Trends, stasis, and drift in the evolution of nematode vulva development. Curr Biol. 2007;17(22):1925–1937. doi: 10.1016/j.cub.2007.10.061. [DOI] [PubMed] [Google Scholar]
- Li WJ, Xu P, Schumann P, Zhang YQ, Pukall R, Xu LH, Stackebrandt E, Jiang CL. Georgenia ruanii sp. nov., a novel actinobacterium isolated from forest soil in Yunnan (China) and emended description of the genus Georgenia. Int J Syst Evol Microbiol. 2007;57:1424–1428. doi: 10.1099/ijs.0.64749-0. [DOI] [PubMed] [Google Scholar]
- Liu J, Berry RE, Moldenke AF. Phylogenetic relationships of entomopathogenic nematodes (Heterorhabditidae and Steinernematidae) inferred from partial 18S rRNA gene sequences. J Invertebr Pathol. 1997;69:246–252. doi: 10.1006/jipa.1997.4657. [DOI] [PubMed] [Google Scholar]
- Liu QZ, Mrácěk Z, Zhang LJ, Puzǎ V, Dong LM. Re-description of Oscheius chongmingensis (Zhang et al., 2008) (Nematoda: Rhabditidae) and its entomopathogenicity. Nematology. 2012;14(2):139–149. [Google Scholar]
- Malan AP, Knoetze R, Moore SD. Isolation and identification of entomopathogenic nematodes from citrus orchards in South Africa and their biocontrol potential against false codling moth. J Invertebr Pathol. 2011;108(2):115–25. doi: 10.1016/j.jip.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Mannion CM, Winkler HE, Shapiro DI, Gibb T. Interactions between halofenozide and the entomopathogenic nematode Heterorhabditis marelatus for control of Japanese beetle (Coleoptera: Scarabaeidae) larvae. J Econ Entomol. 2000;93:48–53. doi: 10.1603/0022-0493-93.1.48. [DOI] [PubMed] [Google Scholar]
- Marmur J. A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- Nguyen, K.B., and Smart Jr., G.C. 1995. Scanning electron microscope studies of Steinernema glaseri (Nematoda: Steinernematidae). Nematologica 41:183–190. [Google Scholar]
- Nguyen KB, Duncan LW. Steinernema diaprepesi n. sp. (Rhabditida: Steinenematidae)a parasite of the citrus root weevil Diaprepes abbreviatus(L) (Coleoptera:Curculionidae) J Nematol. 2002;34:159–170. [PMC free article] [PubMed] [Google Scholar]
- Nguyen KB, Půza V, Mrácek Z. Steinernema cholashanense n. sp. (Rhabditida, Steinernematidae) a new species of entomopathogenic nematode from the province of Sichuan, Chola Shan Mountains, China. J Invertebr Pathol. 2008;97(3):251–64. doi: 10.1016/j.jip.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Poinar GO. Rhabditis myriophila sp. n. (Rhabditidae: Rhabditida), associated with the millipede, Oxidis gracilis (Polydesmida: Diplopoda) Proc Helminthol Soc Wash. 1986;53:232–236. [Google Scholar]
- Qiu L, Hu X, Zhou Y, Mei S, Nguyen KB, Pang Y. Steinernema akhursti sp. nov. (Nematoda : Steinernematidae) from Yunan, China. J Invertebr Pathol. 2005;90(3):151–160. doi: 10.1016/j.jip.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Seinhorst JW. A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica. 1959;4:67–69. [Google Scholar]
- Ross JL, Ivanova ES, Spiridonov SE, Waeyenberge L, Moens M, Nicol GW, Wilson MJ. Molecular phylogeny of slug-parasitic nematodes inferred from 18S rRNA gene sequences. Mol Phylogenet Evol. 2010;55(2):738–43. doi: 10.1016/j.ympev.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Shapiro-Ilan DI, Lewis EE, Campbell JF, Kim-Shapiro DB. Directional movement of entomopathogenic nematodes in response to electrical field: effects of species, magnitude of voltage, and infective juvenile age. J Invertebr Pathol. 2012;109(1):34–40. doi: 10.1016/j.jip.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Sicard M, Le Brun N, Pagès S, Godelle B, Boemare N, Moulia C. Effect of native Xenorhabdus on the fitness of their Steinernema hosts: contrasting types of interaction. Parasitol Res. 2003;91:520–524. doi: 10.1007/s00436-003-0998-z. [DOI] [PubMed] [Google Scholar]
- Sicard M, Ferdy J-B, Pagès S, Le Brun N, Godelle B, Boemare N, Moulia C. When mutualists are pathogens: an experimental study of the symbioses between Steinernema (entomopathogenic nematodes) and Xenorhabdus (bacteria) J Evol Biol. 2004;17:985–993. doi: 10.1111/j.1420-9101.2004.00748.x. [DOI] [PubMed] [Google Scholar]
- Stock SP, Pryor BM, Kaya HK. Distribution of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) in natural habitats in California, USA. Biodivers Conserv. 1999;8:535–549. [Google Scholar]
- Stock SP, Caicedo AM, Calatayud PA. Rhabditis (Oscheius) colombiana n. sp. (Nematoda: Rhabditida), a necromenic associate of the subterranean burrower bug Cyrtomenus bergi (Hemiptera: Cydnidae) from the Cauca Valley, Colombia. Nematology. 2005;7:363–373. [Google Scholar]
- Stock SP, Rivera-Orduño B, Flores-Lara Y. Heterorhabditis sonorensis n. sp. (Nematoda: Heterorhabditidae), anatural pathogen of the seasonal cicada Diceroprocta ornea (Walker) (Homoptera: Cicadidae) in the Sonoran desert. J Invertebr Pathol. 2009;100(3):175–184. doi: 10.1016/j.jip.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Sudhaus W, Hooper DJ. Rhabditis (Oscheius) guentheri sp. nov., an unusual species with reduced posterior ovary, with observations on the Dolichura and Insectivora groups (Nematoda: Rhabditidae) Nematologica. 1994;40:508–533. [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Barragan A, Suazo A, Buhler WG, Cardoza YJ. 2011 Studies on the entomopathogenicity and bacterial associates of the nematode Oscheius carolinensis. Biological Control 59:123–129. [Google Scholar]
- Vrain TC, Wakarchuk DA, Levesque AC, Hamilton RI. Intraspecific rDNA restriction fragment length polymorphisms in the Xiphinema americanum group. Fundam. Appl Nematol. 1992;15:563–574. [Google Scholar]
- White G. A method for obtaining infective nematode larvae from culture. Science. 1927;66:302–303. doi: 10.1126/science.66.1709.302-a. [DOI] [PubMed] [Google Scholar]
- Ye W, Torres-Barragan A, Cardoza YJ. Oscheius carolinensis n. sp. (Nematoda: Rhabditidae), a potential entomopathogenic nematode from vermicompost. Nematology. 2010;12:121–135. [Google Scholar]
- Zhang CX, Liu JR, Xu MX, Sun J, Yang SY, An XH, Gao GF, Lin MS, Lai R, He ZY, Wu YD, Zhang KY. Heterorhabditidoides chongmingensis gen. nov., sp. nov. (Rhabditida: Rhabditidae), a novel member of the entomopathogenic nematodes. J Invertebr Pathol. 2008;98:153–168. doi: 10.1016/j.jip.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Zhang CX, Yang SY, Xu MX, Sun J, Liu H, Liu JR, Liu H, Kan F, Sun J, Lai R, Zhang KY. A novel species of Serratia, family Enterobacteriaceae: Serratia nematodiphila sp.nov., symbiotically associated with entomopathogenic nematode Heterorhabditidoides chongmingensis (Rhabditida: Rhabditidae) Int J Syst Evol Microbiol. 2009;59:1603–1608. doi: 10.1099/ijs.0.65718-0. [DOI] [PubMed] [Google Scholar]