Abstract
Populations of Mesocriconema curvatum, M. kirjanovae, M. onoense, M. ornatum, M. sphaerocephala, M. surinamense, M. vadense, M. xenoplax, and Criconemoides informis from different geographical areas in the continental United States were characterized morphologically and molecularly. A new ring nematode from Washington County, Arkansas, is also described and named Mesocriconema ozarkiense n. sp., This new species is characterized by females with small flattened submedian lobes, lower than or at the same level as the labial disc, vagina straight, very well developed spermatheca without sperm, no more than one anastomoses, L=379-512 μm, V=89-93, stylet length = 49-61 μm, R=107-119, annuli with slightly crenate margins on tail portion and a simple anterior vulval lip. The molecular characterization of M. ozarkiense n. sp. using the ITS rRNA gene sequence and the phylogenesis relationship of this new species with the ring nematodes included in this study are provided.
Keywords: Criconematidae, Criconemoides, Criconemoides informis, internal transcribed spacer 1, Mesocriconema, Mesocriconema ozarkiense n. sp. M. crenatum, M. curvatum, M. kirjanovae, M. onoense, M. ornatum, M. sphaerocephala, M. surinamense, M. vadense, M. xenoplax, molecular biology, morphology, phylogenesis, taxon
Ring nematodes of the genera Criconemoides Taylor 1936 and Mesocriconema Andrassy, 1965 are damaging root ectoparasites of many economical important crops. Proper identification of these nematodes is critical for their management and development of germplasm resistant to these pests.
The taxonomic status of the genera Criconemoides Tylor 1936 and Mesocriconema Andrassy, 1965 is controversial and taxonomists have not reached a consensus of opinion about the validity and species composition of these genera. Many taxonomists including Brezski et al. (2002 a,b) consider these two genera valid, however, others, such as Siddiqi (2000), list Mesoscriconema as a junior synonym of Macroposthonia de Man, 1880. In a recent classification of plant parasitic nematodes by Decraemer and Hunt (2006) the genus Mesocriconema is synonymized with Criconemoides. In this paper, we follow the classification proposed by Brzeski et al. (2002 a,b). According to these authors the species of the genus Criconemoides are characterized morphologically by annuli more or less retrorse, first and second annuli separated from succeeding annuli, presence of six pseudolips on the first annulus, consisting of two lateral ones reduced to a connection with the four more developed and pronounced submedian lips; a closed vulva with a non-ornamented anterior lip; postvulval body short, conoid with a terminus rounded, conoid or acute. The species of the genus Mesocriconema are characterized by a cuticle with retrorse annuli with margin smooth or crenate; first annulus seldom separated; the four submedian lips are reduced and showing each a prominent outgrowth or true submedian lobes; an open vulva with often ornamented anterior lip; postvulval body short with terminus round or truncate.
Morphological studies concerning Criconemoides and Mesocriconema species are numerous in the literature, but data on the molecular characterization of these ring nematodes is insufficient and necessary in order to validate their taxonomic status and infer phylognetic relationships among the species of these genera. Molecular information derived from the high variable, D2-D3 expansion segment of the 28s rRNA gene of representatives of Criconematina was recently provided by Subbotin et al. (2005) based on the classification of Siddiqi (2000). The results of their phylogenetic analysis based on D2-D3 domain indicated monophyly among Mesocriconema, Hemicriconemoides, and Criconema and showed that a representative of the genus Criconemoides clustered together with Mesocriconema species. The nuclear rDNA internal transcriber regions (ITS) have been used as markers because its low intraspecific variation for species identification in several nematodes, representing useful information in order to develop tools for diagnostic purposes based on PCR reactions (Gasser, 2001). In a recent study by Powers et al. 2010, sequences of the nuclear ribosomal ITS1 were obtained for M. curvatum (Raski, 1952) Loof & De Grisse, 1989, M. rusticum (Micoletzky, 1915) Loof & De Grisse, 1989 and M. xenoplax (Raski, 1952) Loof & de Grisse, 1989.
The major objectives of this study were: i) to integrate the morphological and morphometrical characterization of populations of known Mesocriconema and Criconemoides species in the continental United States and describe a new species namely, Mesocriconema ozarkiense n. sp.: ii) to characterize molecularly M. ozarkiense and other ring nematodes included in this study using ITS1 rRNA gene; and iii) reconstruct the phylogenetic position of these species in the Criconematinae using the analysis of this gene. This is the first part of four intended to clarify and identify species of the superfamily Criconematoidea following the classification of Brzeski et al. (2002 a,b) and Raski and Luc (1987). The second part will provide the taxonomical and molecular identification of Bakernema, Criconema, Hemicriconemoides,Ogma, Xenocriconemella (subfamily Criconematinae), the third part Caloosia and Hemicycliophora (subfamily Hemicycliophorinae), Gracilacus and Paratylenchus (Family Tylenchulidae) and a final study about the phylogenesis relationships of Criconematoidea species.
Materials and Methods
Nematodes were collected from undisturbed natural locations in Arkansas, USA from 2008 to 2011 and a handheld global positional system device (GPS) (Etrex Garmin, Olathe, KS) was used to identify the location. Additional populations of nematodes were obtained from California, Kansas, Missouri, North Carolina and Tennessee. Nematodes from others States were received fixed in 3% formaldehyde for morphological purposes or they were preserved in a 1 M NaCl solution or 95% ethanol for molecular characterization. Nematodes collected in Arkansas were extracted from soil using Cobb sieving and flotation-centrifugation methods (Jenkins, 1964). Nematodes were killed and fixed in hot 3%formaldehyde, and subsequently infiltrated with glycerin using Seinhorst’s modified slow method (Seinhorst, 1959; Seinhorst, 1962) and mounted on slides for observation and preservation. Measurements of specimens were made with an ocular micrometer and drawings with a camera lucida. Abbreviations used are defined by Siddiqi, 2000. Photographs were taken with Canon EOS Rebel T3i digital camera mounted on a Nikon Optophot-2 compound microscope. Nematodes were fixed and gold coated before examination using a FEI Nano lab 200 Workstation scanning electron microscope at the Institute for Nanoscience and Engineering at University of Arkansas in Fayetteville, Arkansas.
Specimens of all populations of this study are deposited in the USDA Nematode Collection, Beltsville, MD. Morphometrics of related species to those identified in this work are included using data reported by Brzeski et al., 2002a,b.
Female specimens of each population were grouped and visibly checked for identification to select nematodes for morphological and molecular taxonomy characterization. Adult female nematodes for molecular analyses were crushed individually in 5μl of molecular grade (BDH Chemicals, Chester, PA) water and storage at -80°C until use.
PCR: Polymerase chain reaction (PCR) of the ITS1region was performed using 5 μl of the DNA extraction in a 50-μl PCR reaction mixture. Primers used to perform PCR reaction were rDNA2 (5’-TTGATTACGTCCCTGCCCTTT- 3’) (Vrain et al., 1992) and rDNA1.58s (5’-GCCACCTAGTGAGCCGAGCA- 3’) (Cherry et al., 1997). This PCR primer pair ampliflied the 3’ end of the 18S rDNA gene, the entire ITS1 region and the 5’ end of the 5.8S rDNA gene. The PCR mixture contained 4 μl of dNTP-mixture (0.2mM each) (Qiagen, Valencia, CA), 1 μl of each primer (0.4 μM), 0.4 μl (2 units) Taq DNA polymerase (New England Biolabs, Ipswich, MA) and 5 μl 10 X ThermoPol reaction buffer (New England Biolabs, Ipswich, MA). PCR was conducted using a Hybaid Express thermal cycler [Thermo Hybaid, Middlesex, UK] with the follow parameters: denaturation at 94 °C for 2 minutes, then 40 cycles of denaturation at 94 °C for 45 seconds, annealing at 52 or 56 °C for 45 seconds and extension at 72 °C for 60 seconds. A final extension for 5 minutes at 72 °C was performed. Visualization of PCR product was performed using a 5 μl of PCR product and 100 bp DNA ladder (Promega, Madison, WI) subjected to electrophoresis on a 1% agarose gel stained with ethidium bromide. A UV transluminator (BioDoc-it ™ system, UVP, Upland, CA) was used to visualize PCR products.
Sequencing: PCR products were purified using Nanosep centrifugal tubes 100k (Pall, Port Washington, NY) in a refrigerated centrifuge at 15°C for 20 minutes at 13,000 rev. Samples were sequenced in both directions using an Applied Biosystems Model 3100 genetic analyzer by the DNA sequencing core facility at the University of Arkansas Medical School, Little Rock, AR. Alignment of sequences was performance with CLUSTAL W (Thompson et al., 1994) and consensus sequences were obtained using BioEdit (Hall 1999) sequence alignment software.
Molecular phylogenetic study: The distance matrix option of PAUP* 4.010 (Swofford, 2002) was used to calculate genetic distances according to the Kimura 2-parameter model (Kimura, 1980) of sequence evolution. Maximum likelihood and unweighted maximum parsimony analysis on the alignments were performed using PAUP* 4.010 (Swofford, 2002). Gaps were treated as missing characters for all analyses and the reliability of the trees was tested by a bootstrap test (Felsenstein, 1985). Parsimony bootstrap analysis included 1,000 resamplings using the branch and bound algorithm of PAUP*. The maximum likelihood parameter (Yang, 1994), the default likelihood parameter settings of PAUP* were used (HKY85 6-parameter model of nucleotide substitution, empirical base frequencies, and transition/transversion ratio set to 2:1). These parameters were employed to perform a heuristic search using PAUP*, using either the single most parsimonius tree as the starting tree or step-wise addition. Sequences of Mesocriconema xenoplax HM116073 and HM116057; M. curvatum HM 116066 and Heterorhabditis indica JQ178381 were obtained from GenBank and used for the phylogenetic analysis.
Results and Discussion
SYSTEMATICS
Mesocriconema ozarkiense n. sp.
Table 1.
Measurements and ratios of Mesocriconema ozarkiense n.sp. Morphometrics of related species are presented for comparison. Mean, standard deviation and range in μm.

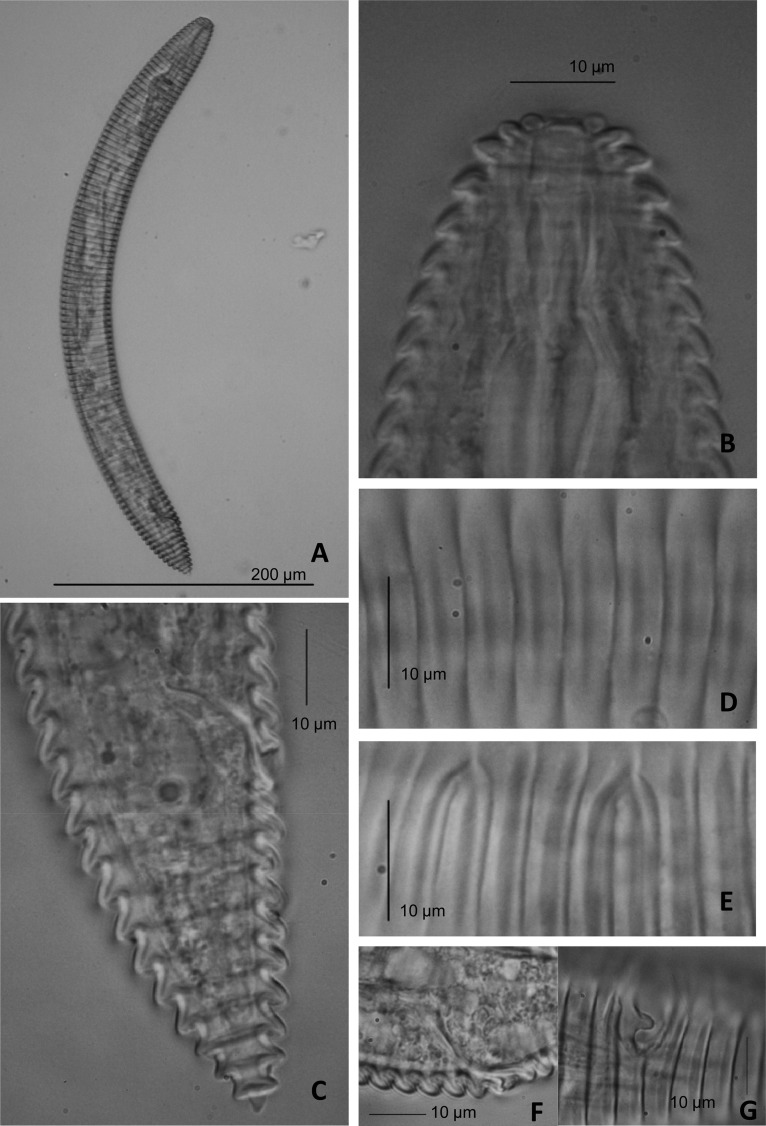
Fig. 1.
Light micrographs of Mesocriconema ozarkiense n. sp. A) Entire female. B, C, D) Anterior body portion showing lip region pattern and submedian lobes. E) Annuli margins. F, G, H) Posterior portion showing vulva, vagina and tail shape. Arrows showing crenate annuli margins in tail.
Fig. 2.
SEM micrograghs. A) Lateral view of lip region showing submedian lobes. B,C) Face view of lip region showing submedian lobes and labial plates. D) Posterior region. E) Detail of anterior vulval lip and anus. F) Tail end annuli.
Fig. 3.
Camera lucida drawings of Mesocriconema ozarkiense n. sp. A) Entire female. B) Anterior body portion. C, D) Posterior body portion showing vulva, vagina, tail shape and crenate annuli.
Description
Female nematodes ventrally arcuate. Annuli retrorse, smooth to irregular margins, crenate at the tail level. Not more than one anastomoses observed. Lip region not offset, tapering, slightly conical. First annulus with no constriction, retrorse. Labial plate minute and visible. Lip region with small submedian lobes, flattened and visible at same level or lower than labial plate. Stylet slender, robust, with knobs concave or anchor shaped. Typical criconematoid oesophagus. Excretory pore slightly anterior to or at the same level as the oesophagus basal gland, 27-34 annuli from the anterior end. Vulva open with anterior vulva lip simple. Vagina slightly curved or straight. Female genital tract monodelphic, prodelphic, outstretched, empty spermatheca, sometimes reaching more than ¾ of the nematode length (stylet knobs level). Tail uniformly conical decreasing to a pointed terminus of a single truncated annulus in most cases or small rounded end annulus, slightly dorsally arcuate.
Host and locality
Specimens were collected in August 2009 by M. Cordero in the Ozark National Forest at Illinois river in Washington County, Arkansas (GPS coordinates N 36° 09.979 min-W 094° 26.061 min) from the rhizosphere several Paspalum spp. (grasses).
Type specimens
Holotype (female): Specimen (T-656t) are deposited in U.S. Department of Agriculture Nematode Collection, Beltsville, Maryland.
Paratypes (females): Two paratypes are deposited in U.S. Department of Agriculture Nematode Collection, Beltsville, Maryland; and four paratypes are deposited as follows Department of Nematology, University of California, Riverside; CABI Bioscience, UK Centre, Surrey, UK; Department of Nematology, Agricultural University, Wageningen, The Netherlands and Nematode collection of the Royal Belgian Institute of Natural Sciences, Brussels, Belgium.
Diagnosis
Mesocriconema ozarkiense n. sp is characterized by small, flattened submedian lobes, smooth to irregular annuli body margins, except for those of the tail which are slightly irregular to slightly crenate. Tail annuli with crenate margins were visible with the compound microscope but indistinct with the scanning electron microscope. The vulva is open with a simple anterior vulval lip, straight vagina, tail conical with last annulus truncated or with a very small rounded dorsally arcuate tip and a specific ITS1 sequence (JQ708122) has been submitted to GenBank.
Relationships
Mesocriconema ozarkiense is related to several species which have a conical tail shape, stylet length about 40- 65 μm, number of body annuli around 70 to 120, and the absence or presence of anastomoses. There are differences in annuli margin appearance, shape of submedian lobes, shape of the vagina, and type of anterior vulval lip. The closest related species to Mesocriconema ozarkiense n.sp. is M. kirjanovae (Andrássy, 1962) Loof & De Grisse, 1989 which are similar in labial region with small labial plates, similar body length (378-512 vs. 350-790 μm), bigger value of c (13-23 vs. 12-13) similar stylet length (49-61 vs. 51-54 μm) similar V value (89-93 % vs. 88-90 %) but has a different tail shape, conical tail vs. conical-acute tail, respectively. Main features to differentiate M. ozarkiense from M. kirjanovae are small flattened submedian lobules reaching the border of the labial disc or lower (flattened) vs. rounded and elevated submedian lobes, a higher number of annuli (107-119 μm vs. 79-89), an anterior vulval lip simple, lacking lobes vs. rounded or thorn like-projections (Andrássy, 1962). Annuli margins in M. ozarkiense are smooth or irregular vs. smooth to finely crenate in M. kirjanovae. Annuli from vulva to posterior end are crenate and the last annulus in the tail is truncated or with a delicate rounded annulus instead of an acute end.
Mesocriconema ozarkiense differs from others related species because: M. citricola (Siddiqui, 1965) Loof & De Grisse, 1989 has lower R= (107-119 vs. 73-78), no or at most one anastomose vs. few anastomoses, annuli margins smooth to irregular vs. crenate, vulval lip simple vs. vulval lip with lobes, both species have flat submedian lobes and a straight vagina; M. denoudeni (De Grisse, 1967) Loof & De Grisse, 1989 has no or at most one anastomose vs. 0-4 anastomoses, smooth to irregular annuli margins vs. smooth annuli margins, submedian lobes flattened vs. submedian lobes rounded, anterior vulval lip simple vs. anterior vulval lip with lobes, both species have a straight vagina; M. jessiense (Van der Berg, 1992) Van der Berg, 1994, has a smaller R (107-119 vs. 88-102), has no or at most one anastomose vs. few anastomoses, similar annuli margins which appear smooth to irregular, submedian lobes flattened vs. rounded, anterior vulval lip simple vs. anterior vulval lip flap, both species have a straight vagina; M. ornicauda (Vovlas, Inserra, & Esser, 1991) has no or at most one anastomose vs. few anastomoses, annuli margins smooth to irregular vs. annuli margins smooth, submedian lobes flattened vs. submedian lobes rounded, vagina straight vs. vagina sigmoidal and anterior vulval lip simple vs. anterior vulval lip with lobes; M. paradenoudeni (Rashid, Geraert, & Sharma, 1987) Loof & De Grisse, 1989 has higher R (107-119 vs. 102-130), lower RV (10-14 vs. 8-10), lower Ran (6-10 vs. 4-7), no or at most one anastomose vs. 0-5 anastomoses, annuli margins smooth to irregular vs. annuli margins smooth, submedian lobes flattened vs. submedian lobes rounded, both species have a straight vagina and a simple anterior vulval lip; M. parareedi (Ebsary, 1981) Loof & De Grisse, 1989, has no or at most one anastomose vs. no to few anastomoses, annuli margins smooth to irregular vs. annuli margins smooth, submedian lobes flattened vs. submedian lobes rounded, straight vagina vs. sigmoid vagina and anterior vulval lip simple vs. anterior vulval lip with lobes; M. reedi (Diab & Jenkins, 1966) Loof & De Grisse, 1989 has no or at most one anastomose vs. 0-5 anastomoses, annuli margins smooth to irregular vs. annuli margins smooth, submedian lobes flattened vs. submedian lobes rounded, straight vagina vs. sigmoid vagina, both species have a simple anterior vulval lip; M. sigillarium (Eroshenko & Volkova, 1997) has a shorter stylet length (49-61 vs. 46-51 μm), no or at most one anastomose vs. many anastomoses, annuli margins smooth to irregular vs. annuli margins crenate, submedian lobes flattened vs. submedian lobes rounded, both species have a straight vagina and a simple anterior vulval lip (Brzeski et al, 2002a; Diab and Jenkins, 1966).
Etymology
The species epithet is derived from the Ozark National Forest, the location where it was found in Arkansas, USA and the latin suffix ense, meaning belonging to or from.
Mesocriconema crenatum (Loof, 1964b) Andrássy, 1962.
Table 2.
Measurements and ratios of Mesocriconema curvatum and M. crenatum. Original morphomentrics of M. crenatum and species related are presented for comparison. Mean, standard deviation and range in μm.

Fig. 4.
Light micrographs of Mesocriconema crenatum. A) Entire female. B) Anterior body portion showing lip region pattern and submedian lobes. C) Posterior body portion showing open vulva and tail shape. D) Annuli margins crenate.
Description
Female nematodes ventrally arcuate. Annuli retrorse, crenate margins. Anastomoses not observed. Lip region not off set, submedian lobes small, rounded, visible. Labial plate minute. Stylet robust, knobs concave or anchor shaped. Typical criconematoid oesophagus. Excretory pore slightly posterior to oesophagus basal gland, 28-33 annuli from anterior end. Female genital tract monodelphic, prodelphic, outstretched, spermatheca not observed. Vulva open, simple without lobes. Tail conical, tip rounded.
Host and locality
Specimens were collected in August 2008 by K. Striegler from the rhizosphere of grape vines (Vitis vinifera) var. Chambourcin in Hermam, MO. No GPS coordinates provided.
Diagnosis
Mesocriconema crenatum has crenate body annuli, simple vulva without lobes, or spine like projections, or ornamentation. This population is in agreement with the original description (Loof, 1964b) and a specific ITS1 sequence (JQ708125) has been submitted to GenBank.
Relationships
This population of Mesocriconema crenatum is compared to populations of M. crenatum reported in Belgium (De Grisse, 1969) but has a longer stylet (71-83 μm vs. 38-51 μm), more body annuli (101-114 vs. 73-84), and a smaller c value (17-24. vs. 22-56). Populations from Romania were similar in c value (17-24 vs. 24-28), have a smaller stylet (71-83 μm vs. 38- 40 μm) and a smaller number of body annuli (101-114 vs. 80-81) (Popovici and Ciobanu, 2000). Mesocriconema crenatum is similar to M. ornatum but differs in having crenate annuli margins. However, differences in morphometrics of populations of M. crenatum described in Belgium and Romania suggested another species different from M. crenatum.
Mesocriconema curvatum (Raski, 1952) Loof & De Grisse, 1989.
Fig. 5.
Light micrographs of Mesocriconema curvatum. A) Entire female. B) Anterior body portion showing lip region pattern and submedian lobes. C) Posterior body portion showing open vulva and tail shape. D) Annuli margins.
Description
Female nematodes ventrally arcuate. Annuli retrorse, smooth margins. Anastomoses occasionally observed throughout the body. Lip region not offset, submedian lobes obvious, rounded. Labial plates minute or obvious. Stylet robust, knobs concave or anchor shaped. Typical criconematoid oesophagus. Excretory pore slightly posterior to oesophagus basal gland, 23-26 annuli from anterior end. Female genital tract monodelphic, prodelphic, outstretched, spermatheca rarely observed, if so empty of sperm. Vulva open, anterior lip with two round lobes variable in size. Tail conical, tip rounded.
Host and locality
Nematodes were collected in August 2008 by M. Cordero in the Ozark National Forest at Illinois river in Washington County, AR (GPS coordinates N 36° 09.979 min-W 094° 26.061 min and N 36° 05.900 min-W 094° 10.686 min.) from the rhizosphere of river cane (Arundinaria sp.), oak (Quercus robur) and turfgrass.
Diagnosis
Mesocriconema curvatum is characterized by body annuli with smooth margins, presence of anastomoses (1 to 3), rounded submedian lobes and anterior vulval lip with two rounded lobes. All the morphometric values of the specimens are in agreement with the ranges of the original description (Raski, 1952, Loof & De Grisse, 1989) and a specific ITS1 sequence (JQ708123) has been submitted to GenBank.
Relationships
Mesocriconema curvatum does not have either a high elevated or an emarginated first lip annule which is a main difference from M. xenoplax (Raski, 1952) Loof, 1989 and M. ornatum (Raski, 1958) Loof & De Grisse, 1989. It also has a straight vagina and smooth annuli margins. However, these three species have a labial disc that is somewhat elevated and obvious, with lateral submedian lobes. Mesocriconema curvatum and M. ornatum share a straight vagina and smooth annuli margins while M. xenoplax has a sigmoid vagina and smooth to irregular annuli margins (Raski, 1952; Brzeski et al., 2002a).
Mesocriconema kirjanovae (Andrássy, 1962) Loof & De Grisse, 1989.
Table 3.
Measurements and ratios Mesocriconema kirjanovae. Morphometric of species related are presented for comparison. Mean standard deviation and range in μm.

Fig. 6.
Light micrographs of Mesocriconema kirjanovae. A) Entire female. B) Anterior body portion showing lip region pattern and submedian lobes. C) Posterior body portion showing open vulva and tail shape. D) Annuli margins. E) Anastomoses. F) Vulva detail. G) Anterior vulva lip and lobe.
Description
Female nematodes ventrally arcuate. Annuli retrorse, smooth to slightly crenate margins. Anastomoses either absent or only one present. Lip region not off set, slightly conical. Submedian lobes small, rounded and visible. Labial plate minute. Stylet robust, knobs concave or anchor shaped. Typical criconematoid oesophagus. Excretory pore always posterior to the oesophagus basal gland, 26-31annuli from anterior end. Female genital tract monodelphic, prodelphic, outstretched, spermatheca full of sperm. Anterior vulval lip with two rounded projections of moderate size. Tail conical uniformly decreasing, tip acute.
Host and locality
Specimens were collected in May 2008 by R. T. Robbins and M. Cordero in the border of a swamp area near Pine Tree, AR (GPS coordinates N 35° 07.161 min-W 090o 66.581 min.) from the rhizosphere of young pine trees, hickory (Carya sp.) and grass (unidentified spp.). This is the first report of M. kirjanovae in the United States.
Diagnosis
Mesocriconema kirjanovae exhibited two projections from the anterior lip of the vulva, although they were sometimes difficult to observe. Crenate and smooth rings were observed in the margins of the annuli. This feature is highly variable among populations of this species (Brzeski, 1998; Castillo and Vovlas, 1992). Numbers of annuli from anterior end to the excretory pore, length of the sylet, ratios a, b and V are similar to the original population and those examined as M. annulatiformis (Andrássy, 1962). M. annulatiformis was later synonymized as the current species, even though the population from Arkansas was longer in body length and R (Andrássy, 1962; Brzeski, 1998; De Grisse and Loof, 1967). All morphometrics values of the specimens are in agreement with the original description (Andrássy, 1962) with the exceptions mentioned above and a specific ITS1 sequence (JQ708100) has been submitted to GenBank.
Relationships
This population of Mesocriconema kirjanovae has a slightly greater number of annuli in the body than the original description (98-115 vs. 71-105), similar stylet length (48-61 vs. 51-54 μm), Rex (26-31 vs. 26-27), RV (8-11 vs. 10-12), RVan (2 vs. 2-3), a (9-14 vs. 9-10), b (4-6 vs. 4-4-), m (69-78 vs. 74-75) and bigger value of c (12-26 vs. 12-13) compared with the original description. Mesocriconema kirjanovae has a conical-acute tail as M. bareilli (Misra & Edward, 1972); M. bilaspurense (Gupta & Gupta, 1981) Loof & De Grisse, 1989; M. calvatum (Eroshenko, 1981) Loof & De Grisse, 1989, M. reedi (Diab & Jenkins, 1966) Loof & De Grisse, 1989 and M. ripariensis (Eroshenko & Volkova, 1997) (Brzeski et al, 2002a). This species has lobes in its anterior vulval lip as M. calvatum and it is the only species of the above mentioned that has smooth to crenate annuli margins throughout the body. The remaining species vary from smooth margins in, M. bareilli, M. bilaspurense and M. reedi to crenate margins in M. calvatum and M. ripariensis.
Mesocriconema ornatum (Raski, 1958) Loof & De Grisse, 1989.
Table 4.
Measurements and ratios Mesocriconema ornatum and M. onoense. Morphometric of species related are presented for comparison. Mean, standard deviation and range in μm.

Fig. 7.
Light micrographs of Mesocriconema ornatum. A) Entire female. B) Anterior body portion showing lip region pattern and submedian lobes. C) Posterior body portion showing vulva, vagina, tail shape and folded annulus. D) Body annuli margins.
Description
Female nematodes ventrally arcuate. Annuli retrorse, smooth margins. Anastomoses present but no more than two randomly distributed in the body. Lip region not well off set, large submedian lobes, rounded and visible. Labial plate minute, slightly developed and, anteriorly projected. Stylet robust, knobs concave or anchor shaped. Typical criconematoid oesophagus. Excretory pore posterior to the oesophagus basal gland, 25-28 annuli from the anterior end. Female genital tract monodelphic, prodelphic, outstretched, empty spermatheca. Vulva open. Anterior vulval lip with two spicate projections of moderate size. Tail conical, tip rounded and somewhat truncated with last annulus folded.
Host and locality
Specimens were collected in 2009 by T. Todd, Kansas State University, from the rhizosphere of turfgrass. No Global positioned coordinates provided.
Diagnosis
This population of M. ornatum presented body annuli with smooth margins, one or two anastomoses along the body, lip region not so offset, submedian lobes prominent and rounded, labial plate slightly projected anteriorly and anterior vulval lip with two spicate projections. All the morphometric values of the specimens are in agreement with the ranges of the original description (Raski, 1952; Raski, 1958) and a specific ITS1 sequence (JQ708124) has been submitted to GenBank.
Relationships
The population of Mesocriconema ornatum reported here has a similar morphometrics compared with the original description as stylet length (52-59 vs. 43-46 μm), R (96-106 vs. 94-100), Rex (25-28 vs. 25-27), RV (9-12 vs. 7-9). Mesocriconema ornatum is very similar to M. crenatum (Loof, 1964) Andrássy, 1965 although margins of the annuli in M. ornatum are not crenate. Anastomoses, if present, no more than one in the entire body vs. M. ornatum does not show anastomoses at the posterior end of the body (Brzeski et al., 2002a). Previous descriptions of M. ornatum are similar to those reported from Argentina (Chaves, 1983) China (Ye, et al., 1997) Spain (Escuer and Bello, 1996) USA (Jaffe et al., 1987) and Venezuela (Loof 1964b; Crozzoli and Lamberti, 2001).
Mesocriconema onoense (Luc, 1959) Loof & De Grisse, 1989.
Fig. 8.
Light micrographs of Mesocriconema onoense. A) Entire female. B) Anterior body portion showing lip region pattern. Arrows showing submedian lobes. C) Posterior body portion showing vulva, vagina and tail shape. Arrows showing last annulus folded for the previous annulus
Description
Female nematodes ventrally arcuate. Annuli retrorse, smooth margins. Anastomoses occasionally observed in the body. Lip region not offset and tapering slightly anteriorly. Submedian lobes rounded, surrounded tightly by the first lip annulus, sometimes difficult to observe. Labial plate minute. Stylet robust, knobs concave or anchor shaped. Typical criconematoid oesophagus. Excretory pore slightly posterior to the oesophagus basal gland, 32-39 annuli from anterior end. Female genital tract monodelphic, prodelphic, outstretched, spermatheca present and full of sperm. Vulva open, simple with lobes. Tail rounded, tip rounded. Last annulus folded.
Host and locality
Specimens were collected in July 2008 from grass and maple (Acer saccharum) near Savoy, Washington County AR. by M. Cordero. GPS coordinates N 36° 06.246 min-W 094° 20.278 min.
Diagnosis
Mesocriconema onoense belongs to a group of species within the genus with a high number of annuli in the body, R= 106-143 similar to M. multiannulatum (Doucet, 1982) Loof & De Grisse, 1989, R= (143-150); M. oblongatum R= 134-148; M. onostre (Phukan & Sanwal, 1981) Loof & De Grisse, 1989 R= (133-147); and M. paranostre (Deswal & Bajaj, 1987) Loof & De Grisse, 1989 R= 117-150. Mesocriconema onoense has a very low lip region with small submedian lobes almost covered by the first lip annulus but visible. Spermatheca full of sperm in most specimens and a last tail annulus surrounded by the previous one. All the morphometric values of the specimens are in agreement with the ranges of the original description and redescription. (De Grisse and Loof, 1965; Luc, 1959; Loof and De Grisse, 1989) and a specific ITS1 sequence (JQ708120) has been submitted to GenBank.
Relationships
Mesocriconema onoense is similar to M. vadense (Loof, 1964) Loof & De Grisse, 1989 in the anterior portion, but submedian lobes in M. onoense are rounded while M. vadense has flattened submedian lobes. Last annulus is folded in M. onoense, a feature which is shared with M. ornatum (Raski, 1958) Loof & De Grisse, 1989; M. antipolitanum (De Guiran, 1963) Loof & De Grisse, 1989; and M. rusticum (Micoletzky, 1915) Loof & De Grisse, 1989.
Mesocriconema onoense is closely related also to M. onostre but can be differentiated by having small submedian lobes vs. large and obvious submedian lobes, RV = 9-11 vs. 7-9 for M. onostre, long conical rounded tail vs. a conical tail, anterior vulval lip simple with lobes vs. simple anterior vulval lip. In the description as M. onostris by (Phukan and Sanwal) 1980 it was mentioned that M. onoense has a broken first annulus that was considered to be a feature to differentiate between both species. However, after review of the original description of M. onoense (Luc, 1959) both species share an unbroken first lip annulus (Brzeski et al, 2002a).
Mesocriconema vadense (Loof, 1964b) Loof & De Grisse, 1989.
Table 5.
Measurements and ratios of Mesocriconema vadense. Morphometric of M. rusticum as related species is presented for comparison. Mean, standard deviation and range in μm.

Fig. 9.
Light micrographs of Mesocriconema vadense. A) Entire female. B, C, D) Anterior body portion showing lip region pattern, submedian lobes and labial plates. Arrows showing submedian lobes E, F, G, H, I) Posterior body portion showing vulva, vagina, tail shape. J) Margins annuli. K) Anastomoses.
Description
Female nematodes ventrally arcuate. Annuli retrorse, smooth margins. Anastomoses frequently observed throughout the body in groups of 3 or separately along with some interruptions in some annuli. Tapering slightly anteriorly, lip region not offset. Submedian lobes small, rounded and oriented in the same direction as the labial plate. Labial plate obvious. Stylet robust, knobs concave or anchor shape. Typical criconematoid oesophagus. Excretory pore frequently far posterior to the oesophagus basal gland, 30-45 annuli from anterior end. Female genital tract monodelphic, prodelphic, outstretched, spermatheca empty if observed. Vulva open with two small lobes and/or rounded spines in the anterior annulus. Tail conical, tip rounded without unfolded annuli.
Host and locality
Arkansas populations were collected in August 2008 by M. Cordero and R. T. Robbins in pine in Pine Tree, AR Saint Francis County, and Fayetteville, Washington County, AR. from grass at coordinates N 35° 07.004 min-W 090° 58.370 min and N 36° 05.918 min-W 094° 10.708 min., respectively.
Diagnostic
Mesocriconema vadense is characterized by having body annuli with smooth margins, frequent anastomoses throughout the body which tapers anteriorly with lip region not offset and anterior vulva lip with small lobes or rounded spines. All the morphometrics values of the specimens are in agreement with the original description and redescription (Loof, 1964b) Loof & De Grisse, 1989. However, a population of the species in Belgium (De Grisse, 1969) sometimes showed lobes at the anterior annuli of the vulva whereas others did not. The features at the cephalic portion, labial plate and the shape and orientation of the submedian lobes are typical for the species and specific ITS1 sequences (JQ708102 and JQ708121) have been submitted to GenBank.
Relationships
Mesocriconema vadense and M. curvatum are similar and difficult to separate morphologically. Shape and length of the tail and shape and orientation of the submedian lobes are the main features used to separate them. Tail shape in M. curvatum is rounded vs. a conical tail in M. vadense. Anastomoses are common in M. vadense, with 3 or 4 in the body vs. one in M. curvatum. Submedian lobes of M. vadense are small and rounded, similar to those observed on M. rusticum (Micoletzky, 1915) Loof & De Grisse, 1989. The cephalic portion of Mesocriconema curvatum appears flattened whereas the cephalic portion in M. vadense is not flattened (Ivanova, 1976; Brzeski et al, 2002a).
Mesocriconema sphaerocephala (Taylor, 1936) Loof, 1989.
Table 6.
Measurements and ratios of Mesocriconema sphaerocephala. morphometrics of M. sphaerocephaloides as related species is presented for comparison. Mean, standard deviation and range in μm.

Fig. 10.
Light micrographs of Mesocriconema sphaerocephala A) Entire female. B) Anterior body portion lip region pattern and showing submedian lobes. C) Anastomoses. D) Posterior body portion showing vulva and tail shape E) anastomoses in tail.
Description
Female nematodes small, ventrally arcuate. Annuli retrorse, margins finely crenate. Numerous anastomoses present throughout the body forming a zig-zag pattern. Lip region not off set, slightly tapering anteriorly. Submedian lobes small, rounded, barely visible. Labial plate not visible. Stylet robust, knobs concave or anchor shape. Typical criconematoid oesophagus. Excretory pore in most occasions anterior to the posterior end of the oesophagus basal gland, 15-25 annule from anterior end. Female genital tract monodelphic, prodelphic, outstretched, spermatheca empty. Vulva open and simple. Tail conical, tip rounded without unfolded annuli.
Host and locality
Specimens were collected in July 2008 from three different locations from the rhizosphere of turfgrass and daylily (Hemerocallis sp.) in Johnston, Sampsom, and Beaufort Counties in North Carolina by W. Ye. No GPS coordinates were provided.
Diagnostics
Mesocriconema sphaerocephala is characterized by small body size (294-406 μm) with body annuli margins finely crenate, tapering anteriorly, minute submedian lobes and numerosus anastomoses in the body. All the morphometric values of the specimens are in agreement with the ranges of the original description and redescription (De Grisse, 1967; Loof, 1989; Raski and Golden, 1965) and a specific ITS1 sequence (JQ708103) has been submitted to GenBank
Relationships
Mesocriconema sphaerocephala is characterized by the presence of high numbers of anastomoses throughout the body, annuli crenate and a conical-rounded tail. Presence of such numbers of anastomoses is present in M. brevistylus (Singh & Khera,1976) Loof & De Grisse, 1989; M. caelatum (Raski & Golden, 1966) Loof & De Grisse, 1989; M. paronostre (Deswal & Bajal, 1987) Loof & De Grisse, 1989; M. pseudosolivagum (De Grisse, 1964b) Andrássy, 1965; M. raskiensis (De Grisse, 1964) Andrássy, 1965; M. sigillarium (Eroshenko & Volkova, 1997), M. sphaerocephala (Taylor, 1967) Loof, 1989 and M. thabaum Van den Berg, 1996. However, the closest species related with M. sphaerocephala is M. sphaerocephaloides (De Grisse, 1967) Loof &De Grisse, 1989, showing variations in small submedian lobes vs. large and obvious submedian lobes, conical-rounded tail vs. rounded blunt tail, Sty%L (13-18 vs. 22), Rex (15-25 vs. 27), RV (4-7 vs.7), R (61-71 vs. 82) and annuli smooth to crenate vs. smooth to irregular (Brzeski et al., 2002a; De Grisse, 1967).
Mesocriconema surinamense (De Grisse & Maas, 1970) Loof & De Grisse, 1989.
Table 7.
Measurements and ratios of Mesocriconema surinamense. Morphometric of M. yossifovichi as related species is presented for comparison. Mean, standard deviation and range in μm.

Fig. 11.
Light micrographs of Mesocriconema surinamense. A) Entire female. B, C, D, E,) Anterior body portion showing lip region pattern and submedian lobes and labial plates. F, G, H) Posterior body portion showing vulva, vagina, tail shape.
Description
Female nematodes ventrally arcuate. Annuli retrorse, smooth margins. Anastomoses rare, no more than one in the body and sometimes present in the tail region. Lip region not offset, tapering and flattened anteriorly. Submedian lobes large and flattened. Labial plate visible. Stylet robust, knobs concave or anchor shape. Typical criconematoid oesophagus. Excretory pore at the posterior end of the oesophagus basal gland, 24-29 annuli from anterior end. Female genital tract monodelphic prodelphic, outstretched, spermatheca empty of sperm. Vulva open and simple with two small lobes sometimes difficult to observe in lateral view. Tail conical, tip rounded without unfolded annuli, unilobed.
Host and locality
Specimens were collected in August 2008 by M. Cordero in the Ozark National Forest and Savoy, Washington County, AR. from the rhizosphere of grass and maple (Acer saccharum) at GPS coordinates N 36° 09.969 min-W 094° 26.061 min and N 36° 06.246 min-W 094° 20.278 min., respectively.
Diagnostics
Mesocriconema surinamense is characterized by having a large, obvious and flattened submedian lobes, anastomoses rare or no more than one, annuli margins smooth, anterior vulva lip with two small lobes and last annulus unfolded. All the morphometric values of the specimens are in agreement with the ranges of the original description and redescription (De Grisse and Maas, 1970; Loof, and DeGrisse, 1967; Loof and DeGrisse, 1989) and a specific ITS1 sequence (JQ708101) has been submitted to GenBank.
Relationships
Mesocriconema surinamense belongs to a group of Mesocriconema that have flattened submedian lobes of different size: M. antipolitanum (De Guiran, 1963) Loof & De Grisse, 1989; M. caballeroi (Cid del Prado, 1978) Luc & Raski, 1981 synonym of M. surinamenese; M. vadense (Loof, 1964b) Loof & De Grisse, 1989; M. rusticum (Micoletzky, 1915) Loof & De Grisse, 1989 and M. yossifovich (Krnjaic, 1968) Luc & Raski, 1981). Mesocriconema surinamense is very similar to M. yossifovich, but the submedian lobes are not fused as in M. yossifovich where they form a plate with four lobes that surround the oral opening (Vovlas, 1984). In lateral view of M. surinamense a separation is observed between the two submedian lobes and the labial disc whereas M. yossifovich in lateral view shows a flat anterior end (Brzeski et al, 2002a; Cid del Prado, 1979; De Grisse & Maas, 1970).
Mesocriconema xenoplax (Raski, 1952) Loof, 1989.
Table 8.
Measurements and ratios of Mesocriconema xenoplax. Mean, standard deviation and range in μm.


Fig. 12.
Light micrographs of Mesocriconema xenoplax. A) Entire female. B) Anterior portion. C, F, G, H,J) Anterior body portion showing lip region pattern and submedian lobes, first lip annulus and labial plates. D,E,) Posterior body portion showing vulva, vagina and tail shape. I) Margins annuli and anastomoses.
Description
Female nematodes slightly ventral arcuate. Annuli retrorse, smooth to irregular margins. Labial disc elevated surrounding the oral opening. Anastomoses rare, no more than one in the body. Lip region not off set, large, rounded, conspicuous submedian lobes, equidistant of labial disc, anteriorly projected. First annulus indented. Stylet robust, knobs concave or anchor shape. Typical criconematoid oesophagus. Excretory pore at the posterior end of the oesophagus, 22-28 annuli from anterior end. Female genital tract monodelphic, prodelphic, outstretched, spermatheca empty of sperm. Vulva open, two sharp projections in anterior anule. Vagina sigmoid. Tail conical, tip rounded and unilobed.
Host and locality
Specimens were collected in June – August 2008 by M. Cordero at various locations in Washington county, AR including: Farmington ( GPS coordinates N 36° 01.530 min-W 094° 19.274 min); Fayetteville (N 36° 10.223 min-W 094° 16.444 min and N 36° 05.918 min-W 094° 10.708 min.), near Savoy N (36° 06.246 min-W 094° 20.278 min), from the rhizosphere of oak (Quercus robur), pine (Pinus sp.), elm (Ulmus sp.) river cane (Arundinaria sp.), and grass. Nematodes from North Carolina were associated with the rhizosphere of bermuda grass (Cynodon dactylon), peach (Prunus persica) and turfgrass. Populations from California were sent by Dr. Howard Ferris - University of California at Davis and were collected in the rhizosphere of grapes vines (Vitis vinifera) at Ripon, Parlier, Los Alamos, Russian River, Mendocino, Fresno and Livingston. No global positioned coordinates were provided for California and North Carolina populations.
Diagnosis
Mesocriconema xenoplax is the type species of the genus characterized by body annuli margins smooth to irregular, submedian lobes large and anteriorly projected, first cephalic annulus elevated and indented and vulva sigmoid. All the morphometric values of the specimens are in agreement with the ranges of the original description (Raski, 1952) and redescription (Loof and DeGrisse, 1989) and specific ITS1 sequences (JQ708104 to JQ708117 and JQ708119) have been submitted to GenBank.
Relationships
Mesocriconema xenoplax is different from other species in its elevated labial disc and first cephalic annulus are indented or projected anteriorly. Mesocriconema xenoplax is closer to M. rusticum and M. ornatum. Mesocriconema xenoplax has a stylet longer (65 -80 μm vs. 50-60 μm) than for M. rusticum (Micoletzky, 1915) Loof & De Grisse, 1989 and M. ornatum (Raski, 1958) Loof & De Grisse, 1989 (65-80 μm vs. 44-56 μm). Submedian lobes in M. xenoplax and M. ornatum are rounded while M. rusticum has flattened submedian lobes and a tapering anterior end. The tail in M. xenoplax is rounded and conical while M. rusticum has a rounded tail terminus. M. ornatum has a smaller body length (324-736 vs. 330-520 μm), lower labial plate, annuli margins that are smooth, anterior annulus of the vulva with lobes, straight vs. sigmoid vagina and a similar conoid-rounded tail shape in comparison with M. xenoplax. According to Brzeski et al (2002a) these three species are frequently misidentified. Population from Russian River, Ca. showed variations in submedian lobes which appeared in some cases flattened along with a lower labial disc and a longer and conical tail, as compared with the others 6 populations studied. See tables 4 and 5 to compare with morphometrics of M. ornatum and M. rusticum.
Criconemoides informis (Micoletzky, 1922) Taylor, 1936.
Table 9.
Measurements and ratios of Criconemoides informis. Morphometrics of C. mongolensis and C. morgensis as related species is presented for comparison. Mean, standard deviation and range in μm.

Fig. 13.
Light micrographs of Criconemoides informis. A) Entire female. B, C, D, E) Anterior body portion showing first annulus and oral opening. F, G) Posterior body portion showing vulva, vagina and tail shape.
Description
Female nematodes straight or dorsally arcuate. Annuli retrorse, smooth to irregular margins. Anastomoses absence. Lip region not offset, without submedian lobes. Labial disc elevated surrounding the oral opening. First lip annulus sometimes anteriorly projected, smaller than the second one. Second lip annulus smaller than rest of body annuli. Stylet robust, knobs concave or anchor shaped. Typical criconematoid oesophagus. Excretory pore posterior to the oesophagus basal gland, 19-22 annules from anterior end. Female genital tract monodelphic, prodelphic, outstretched, spermatheca full of sperm. Vulva closed as a simple narrow slit located at 2–4 annuli from the posterior end. Tail conical, tip rounded and unilobed
Host and locality
Specimens were collected in June 2010 by E. Bernard in the Smoky Mountains from the rhizosphere of tulip poplar (Liriodendron tulipifera) in Tennessee. No global coordinates provided.
Diagnostic
Criconemoides informis has a lip region no off set without submedian lobes, first annulus elevated and anteriorly projected and vulva close as a simple narrow slit. All the morphometric values of the specimens are in agreement with the ranges of the original description (De Grisse, 1969) and a specific ITS1 sequence (JQ708118) have been submitted to GenBank.
Relationships
Criconemoides informis has a conical tail shape as C. mongolensis Andrássy, 1964 and C. morgensis (Hofmänner in Hofmänner & Menzel, 1914) Taylor, 1936. Both species have an elevated labial plate that surrounds the oral opening. The submedian lobes are either absent or are not developed in both species. Criconemoides informis has a shorter stylet than C. morgensis (45- 50 vs. 68-108 μm). The stylet in C. informis is robust when compared with the stylet of C. mongolensis Andrássy, 1964 which is slender and delicate (Choi et al., 2000). Recently, populations of C. informis found in Iran exhibited a longer stylet (45- 50 vs. 64-87 μm), a similar position of the excretory pore Rex (15-22 vs. 18-25), a longer tail (8-16 vs. 16-31 μm), and a similar body length (415-506 vs. 440-600 μm). This last population was divided in females with or without sperm in the spermatheca but the purpose for this division wasn’t mentioned and no significant measurement differences were found (Brzeski et al., 2002a; Eskandari, 2010).
Molecular phylogenetic analysis
For the species of Mesocriconema and Criconemoides studied the length of the PCR product ranged between 560 bp to 680 bp. The portion of internal transcribed spacer 1 length used for phylogenetic analysis was 387 bp with 7 characters constant (7%) and 332 characters parsimony-informative (85%). The population group have an average nucleotide composition of 24.1% (A), 25.2% (C), 26.8 (G) and 23.8 (T). The nucleotide composition of the ITS1 region for each species showing similarities and differences as percentages of bases among them is shown in Table 10.
Table 10.
Nucleotides composition of the nuclear ITS1 ribosomal region (387 bp) of the populations of Mesocriconema and Criconemoides obtained in this study and those sequences obtained from GenBank.

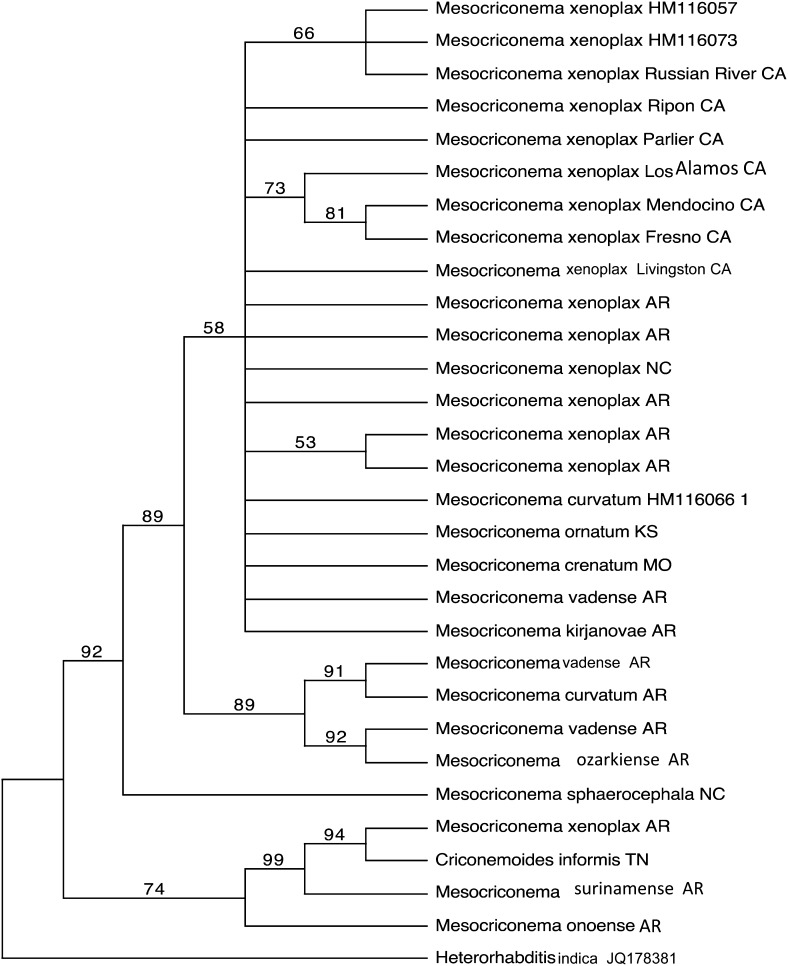
Only one most parsimonious tree was obtained from Mesocriconema and Criconemoides data (Fig. 14). (Length = 1396; C.I =0.58). Two clades were originated. The first clade has two clusters that are mainly conformed by populations of M. xenoplax, M. curvatum, M. ornatum, M. crenatum, M. kirjanovae, M. vadense, M. ozarkiense n.sp. and M. sphaerocephala as sister species with a 92% bootstrap support. The second clade included 4 populations: M. onoense, M. surinamensis, M. xenoplax and Criconemoides informis with 74% bootstrap support. The maximum likelihood tree included the species in two clades as well (Fig. 15) (-Ln likelihood = 5362.01162), Topology of maximum parsimony and maximum likelihood trees kept the same clades among the species including the clade with 89% bootstrap support which clustered M. vadense, M. curvatum and M. ozarkiense n.sp. however, M. sphaerocephala was clustered as a species close related with M. ornatum in the maximum likelihood tree.
Fig. 14.
Consensus tree from the maximum parsimony bootstrap analysis for ITS1-rDNA region of Mesocriconema and Criconemoides. The percentages of bootstrap replicates supporting the clades are indicated at the branch points.
Fig. 15.
Best maximum likelihood tree for ITS1-rDNA region of Mesocriconema and Criconemoides. Changes lengths are proportional to the number of inferred changes.
Genetic variation among M. xenoplax populations and M. curvatum, M. ornatum, M. crenatum, M. kirjanovae, M. vadense ranged from 0.7% to 33%. Genetic divergence between M. vadense and M. curvatum, two species difficult to separate morphologically, ranged between 27% to 32%. Maximum likelihood showed a close relationship of M. sphaerocephala with M. ornatum with 43% of genetic divergence. Morphologically, these two species have very low submedian lobes and a cylindrical body. Morphological differences of M. sphaerocephala with the rest of the species are evident having a small body length average (354 ± 29 μm), very small submedian lobes, labial plates no evident, numerosus anastomoses and vulva simple. These differences seen to agree with the genetic variation mentioned above. Mesocriconema ornatum do not have anastomoses, has a larger body length and it is morphologically most close to M. xenoplax. The genetic variation between M. ornatum and M. xenoplax population ranged from 5% to 15.1%, except for the population of M. xenoplax from North Carolina which showed a higher genetic variation of 58%. Mesocriconema ozarkiense showed a genetic divergence of 30% with M. vadense and one population of M. curvatum. The populations of M. xenoplax obtained and studied from California showed a genetic variation of 0.5% to 2.8%
Criconemoides informis showed the typical morphological differences that separate the genus from Mesocriconema species and a range of genetic divergence of 55-60% between both genera. Besides, ITS1 DNA sequences were able to show similarities with those species of Mesocriconema that have similar molecular structure but are different from the M. xenoplax group and to separate species with notorious morphological differences as M. onoense, M. sphaerocephala and M. surinamense with a range of genetic divergence with the others species of 59- 62%, 42%-54% and 52-55%, respectively.
The topology of maximum parsimony and maximum likelihood showed monophyletic and paraphyletic relationships with different rates of substitutions in the ITS1sequences and possibly different evolutionary histories.
A recent proposal to synonymize genera Criconemella, Macroposthonia and Mesocriconema as Criconemoides (Decraemer and Hunt, 2006) is not shared by the authors because the proposal did not take into consideration the clear differences mentioned early in this work regarding the presence of true submedian lobes and open vulva in Mesocriconema and the absence of true submedian lobes and closed vulva in Criconemoides, as important characters of diagnostic extensively studied by Brzeski et al (2002a,b) and before them by Loof and De Grisse (1967) . Therefore, Criconemoides and Mesocriconema are considered here as valid genera of the subfamily Criconematinae.
Genetic variation in the nuclear rDNA ITS1 region could be the results of different lineages or multiple substitutions because mutations events evolving at different rates within the group according with genetic variation percentages. These molecular differences among Mesocriconema spp. and Criconemoides sp. are important in order to determine barcodes for identification and diagnostic purposes for those species with many similarities and just a few differences even though, the known high variability of the internal transcribed spacer 1.
Accurate morphological and taxonomical identification is essential to avoid confusion and help to detect real relationships and possible lineages among species when molecular information is obtained. Ye et al. (2004) using ITS1 sequences reported genetic variation between Xiphinema chambersi and Longidorus crassus was 38.6%; X. diversicaudatum and X. bakeri 3.8%, X.chambersi and X. italiae 29.9%; L.crassus and L. grandis 8.9% and L. fragilis and L. diadecturus 32.4%. The genetic variation between different species of Punctoderinae and Heteroderinae ranged from 0.0 to 31.4% and 0.3 to 14.7% within each subfamily (Subbotin et al., 2001). The genetic variation of ITS1 sequences between Paratrichodorus macrostylus and Trichorus primitivus was 65% and 21.7% between P. macrostylus and P. pachydermus. (Boutsika et al., 2004). Useful information after characterization of the nuclear ITS1 ribosomal region using PCR-RFLP had been obtained. Variation within individuals and between isolates from US and India of Heterodera zeae and, between isolates of H. goettingiana from North Ireland and US (Szalanski et al., 1997); Presence of Heterodera avenae, H.glycines, H. hordecalis, H. latipons, H. schachtii, H. trifolii, H. elachista, H. turcomanica, H. mothi and Cactodera cacti were confirmed and identified from Iran (Tanha Maafi et al., 2003); populations of Naccobus aberrans from Peru were differentiated from those studied in Mexico and Argentina. Furthermore, two different populations from Argentina were detected and similarities between populations of the species from Peru and Bolivia were found (Reid et al., 2003) and presence of Globodera pallida in Idaho in 2007 was confirmed using ITS1 sequence (Skantar, et al, 2007) Recently, morphology studies and sequences of ITS1 of Discocriconemella inarata Hoffmann, 1974, M. curvatum, M. rusticum and M. xenoplax allowed to confirm that D. inarata was morphological different from the others Mesocriconema species however, molecular information showed a close relation with Mesocriconema species but distantly related to Discocriconemella species (Powers et al, 2010).
Authors are in agreement with the opinion of several researchers (Luc et al., 2010) that DNA sequence data from a study involving molecular diagnostics or molecular phylogenetics should be integrated with morphological identification in order to avoid confusion when morphology and biology relationships need to be studied. Further researches are needed in order to have a more clear idea about the relationships between taxonomic and molecular identification and the phylogeny of Criconematoidea.
Literature Cited
- Andrássy I. Neu nematoden-Arten aus Ungarn. 1. Zehn neu Arten der Unterklasse Secernentea (Phasmidia) Acta Zoologica Academiae Scientiarum Hungaricae. 1962;8:1–23. [Google Scholar]
- Brzeski M. Morphological observations on three species of Mesocriconema Andrássy, 1962 and nomenclatorial note on M. goodeyi (Jairajpuri, 1963) Loof and De Grisse, 1989 (Nematoda:Criconematidae. Journal of Nematology, Morphology and Systematics. 1998;1:47–56. [Google Scholar]
- Brzeski M, Loof PAA, Choi YE. Compendium of the genus Criconemoides Taylor, 1936. Nematology. 2002a;4:325–339. [Google Scholar]
- Brzeski M, Loof PAA, Choi YE. Compendium of the genus Mesocriconema Andrássy, 1965. Nematology. 2002b;4:341–360. [Google Scholar]
- Boutsika K, Brown DJK, Phillips M, Blok V. Molecular characterization of the ribosomal DNA of Paratrichodorus macrostylus, P. pachydermus, Trichodorus primitivus and T. similis (Nematoda: Trichodoridae) Nematology. 2004;6:641–654. [Google Scholar]
- Castillo P, Vovlas N. Mesocriconema kirjanovae (Nematoda: Criconematidae) from southeastern Spain. Journal of Nematology. 1992;24:61–66. [PMC free article] [PubMed] [Google Scholar]
- Chaves E. Criconematoidea (Nematoda) from Argentina. Nematologica. 1983;29:404–424. [Google Scholar]
- Cherry T, Szalanski AT, Todd TC, Powers TO. The internal transcribed spacer region of Belonolaimus (Nemata: Belonolaimidae) Journal of Nematology. 1997;29:23–29. [PMC free article] [PubMed] [Google Scholar]
- Choi YE, Brzeski MW, Kim JI. Observations of some species of Criconemoides Taylor, 1936 (Nematoda; Criconematidae) with proposals of new synonyms. Nematology. 2000;2:273–284. [Google Scholar]
- Cid del Prado VI. Three new species of Macroposthonia (Nematoda: Criconematidae) from Mexico. Nematologica. 1979;24:29–36. [Google Scholar]
- Crozzoli R, Lamberti F. Known and new species of Mesocriconema Andrássy, 1965 (Nematoda: Criconematidae) from Venezuela. Russian Journal of Nematology. 2001;9:85–105. [Google Scholar]
- Decraemer W, Hunt D. 2006. Structure and classification. Pp. 3–32 in R.N. Perry and M. Moens eds. Plant Nematology. UK. CAB International. [Google Scholar]
- De Grisse AT, Loof PAA. Revision of the genus Criconemoides (Nematoda) Mededelingen Faculteit Landbouwhogeschool en Opzoekingsstations Gent. 1965;30:577–603. [Google Scholar]
- De Grisse AT, Loof PAA. Mesocriconema annulatiformis n. sp. (Criconematidae) Nematologica. 1967;13:459–465. [Google Scholar]
- De Grisse AT. Description of fourteen new species of Criconematidae with remarks on different species of this family. Biologisch Jaarboek Dodonaea. 1967;35:66–125. [Google Scholar]
- De Grisse AT. 1969. Contribution to the morphology and the systematic of the Criconematidae (Taylor, 1936) Thorne, 1949. PhD Thesis, University of Gent, Belgium. [Google Scholar]
- De Grisse AT, Maas PW. Macroposthonia longistyleta n.sp. and Discocriconemella surinamensis n.sp. from Surinan (nematode: Criconematidae. Nematologica. 1970;16:123–132. [Google Scholar]
- Diab KA, Jenkins WR. 1966. Three species of Criconemoides (Nematoda: Criconematidae) Proceedings of the Helminthological society 33:3–5. [Google Scholar]
- Escuer M, Bello A. Nematodos de la subfamilia Macroposthoniidae (Nematoda: Criconematidae) en la España peninsular. Orsis. 1996;1:59–92. [Google Scholar]
- Eskandari A, Karegar A, Pourjam E, van der Berg E, Tiedt L. Additional data on some poorly known species of Criconemoides Taylor, 1936 (Nematoda: Criconematidae) Nematology. 2010;12:505–518. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenics: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Gasser RB. 2001. Identification of parasitic nematodes and study of genetic variability using PCR approaches Pp 53–82 in Kennedy, M. and W. Harnett eds. Parasitic nematodes. Molecular biology, biochemistry and immunology. UK. CAB international. [Google Scholar]
- Hall AH. BioEdit: a user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Ivanova TS. 1976. Root parasitic nematodes. Family Criconematidae. Leningrad, Russia. [Google Scholar]
- Jaffe BA, Nyczepir AP, Golden M. Criconemella spp. In Pennsylvannia peach orchard with morphological observations of C. curvata and C. ornate. Journal of Nematology. 1987;19:420–423. [PMC free article] [PubMed] [Google Scholar]
- Jenkins WR. A rapid centrifugal-flotation technique for separating nematodes. Plant Disease Report. 1964;48:692. [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative study of nucleotide sequences. Journal of Molecular Evolution. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Loof PAA. Free-living and plant parasitic nematodes from Venezuela. Nematologica. 1964b;10:201–300. [Google Scholar]
- Loof PAA, De Grisse A. 1967 Re-establishment of genus Criconemoides Taylor, 1936 (Nematoda: Criconematidae) Mededelingen Faculteit Landbouwwetenschappen Rijksuniversiteit Gent. 32:466–475. [Google Scholar]
- Loof PAA, De Grisse A. Taxonomic and nomenclatorial observations on the genus Criconemella De Grisse & Loof, 1965. Sensu Luc & Raski, 1981. Mededelingen Faculteit Landbouwwetenschappen Rijksuniversiteit Gent. 1989;54:53–74. [Google Scholar]
- Luc M. Nouveaux Criconematidae de la zone intertropicale (Nematoda: Tylenchida) Nematologica. 1959;4:16–22. [Google Scholar]
- Luc M, Doucet M, Fortuner R, Castillo P, Decraemer W, Lax P. Usefulness of morphological data for the study of nematode biodiversity. Nematology. 2010;12:495–504. [Google Scholar]
- Phukan PN, Sanwal KC. Two new species of Macroposthonia De Man, 1880 (Criconematidae: Nematoda) from Assam. Indian Journal of Nematology. 1980;10:135–140. [Google Scholar]
- Popovici I, Ciobanu M. New records of some Criconematidae (Nematoda) from Rumania. Journal of Nematology, Morphology and Systematics. 2000;2:149–157. [Google Scholar]
- Powers TO, Harris T, Higgins R, Sutton L, Powers K. Morphological and molecular characterization of Discocriconemella inarata, an endemic nematode from North A merican native tallgrass prairies. Journal of Nematology. 2010;42:35–45. [PMC free article] [PubMed] [Google Scholar]
- Raski D. On the morphology of Criconemoides Taylor, 1936, with descriptions of six new species. Proceedings of the Helminthological society. 1952;19:85–99. [Google Scholar]
- Raski D. Nomenclatorial notes on the genus Criconemoides (Nematoda: Criconematidae) with a key to the species. Proceedings of the Helminthological society. 1958;25:139–142. [Google Scholar]
- Raski D, Golden AM. Studies on the genus Criconemoides Taylor, 1936 with descriptions of eleven new species and Bakernema variabile n.sp. (Criconematidae: Nematoda) Nematologica. 1965;11:501–565. [Google Scholar]
- Raski D, Luc M. A reappraisal of tylenchina (Nemata) 10. The superfamily Criconematoidea Taylor, 1936. Revue de Nématologie. 1987;10:409–444. [Google Scholar]
- Reid A, Manzanilla-Lopez R, Hunt D. Naccobus aberrans (Thorne, 1955) Thorne & Allen, 1944 (Nematoda: Pratylenchidae); a nascent species complex revealed by RFLP analysis and sequencing of the ITS-rDna region. Nematology. 2003;5:441–451. [Google Scholar]
- Seinhorst JW. A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica. 1959;4:67–69. [Google Scholar]
- Seinhorst JW. On the killing, fixation, and transferring to glycerin of nematodes. Nematologica. 1962;8:29–32. [Google Scholar]
- Siddiqui MR. 2000. Tylenchida parasites of plants and insects. St. Albans, UK. Commonwealth Agricultural Bureaux Publishing. [Google Scholar]
- Skantar MA, Handoo ZA, Carta LK, Chitwood DJ. Morphological and molecular identification of Globodera pallida associated with potato in Idaho. Journal of Nematology. 2007;39:133–144. [PMC free article] [PubMed] [Google Scholar]
- Subbotin S, Vierstraete A, De Ley P, Rowe J, Waeyenberge L, Moens M, Vanfleteren JR. Phylogenetics relationships within the cyst-forming nematodes (Nematoda, Heteroderidae) based on analysis of sequences from the ITS regions of ribosomal DNA. Molecular Phylogenetics and Evolution. 2001;21:1–16. doi: 10.1006/mpev.2001.0998. [DOI] [PubMed] [Google Scholar]
- Subbotin S, Vovlas N, Crozzoli R, Sturhan D, Lamberti F, Moens M, Baldwin J. Phylogeny of Criconematina Siddiqui, 1980 (Nematoda: Tylenchida) based on morphology and D2-D3 expansion segments of the 28S-rRNA gene sequences with application of a secondary structure model. Nematology. 2005;7:927–944. [Google Scholar]
- Swofford DL. 2002. PAUP: Phylogenetic analysis using parsimony (and other methods). Version 4. Sinauer Associated, Sunderland, Massachussets. [Google Scholar]
- Szalanski A, Sui D, Harris TS, Powers T. Identification of cyst nematodes of agronomic and regulatory concern with PCR-RFLP of ITS1. Journal of nematology. 1997;29:255–267. [PMC free article] [PubMed] [Google Scholar]
- Tanha Maafi Z, Subbotin S, Moens M. Molecular identification of cyst nematodes (Heteroderidae) from Iran and the phylogeny based on ITS1-rDNA sequences. Nematology. 2003;5:99–111. [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence aligments through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vovlas N. Morphological characterisitics of Criconemella yossifovich (Krnjaic, 1968) Luc et Raski from Italian vineyards. Nematologia Mediterranea. 1984;12:201–206. [Google Scholar]
- Vrain TC, Wakarchuk DA, Levesque AC, Hamilton RI. Intraspecific rDNA restriction fragment length (bp) polymorphism in the Xiphinema americanum group. Fundamental and Applied Nematology. 1992;15:563–573. [Google Scholar]
- Yang Z. Maximum likelihood phylogenetic estimation from DNA sequences with variable rates over sites: Approximate methods. Journal of Molecular Evolution. 1994;39:306–314. doi: 10.1007/BF00160154. [DOI] [PubMed] [Google Scholar]
- Ye W, Lin W, Cai W. Some criconematids from China. International Journal of Nematology. 1997;7:137–141. [Google Scholar]
- Ye W, Szalanski A, Robbins RT. Phylogenetics relationships and genetic variation in Longidorus and Xiphinema species (Nematoda: Longidoridae) using ITS1 sequences of nuclear ribosomal DNA. Journal of Nematology. 2004;36:14–19. [PMC free article] [PubMed] [Google Scholar]