Abstract
The foliar nematode Aphelenchoides besseyi causes white tip disease in rice (Oryza sativa L.) and floral malady in tuberose (Polianthes tuberosa L.). This nematode is widely distributed in the rice fields of many states of India, including West Bengal (WB), Andhra Pradesh (AP), Madhya Pradesh (MP) and Gujarat (GT). In order to generate information on intraspecific variations of A. besseyi as well as to confirm the identity of the nematode species infecting these important crops, morphological observation was undertaken of A. besseyi isolated from tuberose and rice from WB and rice from AP, MP and GT. The molecular study was only done for rice and tuberose populations from AP and WB. The variations were observed among the populations in the tail, esophageal and anterior regions, including the occurrence of four as well as six lateral lines in the lateral fields. The morphometrics of observed populations showed variations and those could be regarded as a consequence of host-induced or geographical variations. PCR amplification of the rDNA ITS 1 and 2 region of rice (AP) and tuberose (WB) populations of A. besseyi generated one fragment of approximately 830 bp, and the size of the ITS region was 788 bp and 791 bp for tuberose and rice population, respectively. Alignment of the two sequences showed almost 100% similarity. Blast analysis revealed a very high level of similarity of both the Indian strains to a Russian population. The Indian and Russian strains could be differentiated using restriction enzyme Bccl. Host tests revealed that rice (cv. IET 4094), oat (cv. OS-6) and teosinte (cv. TL-1) showed a typical distortion due to the infection of A. besseyi. Five germplasm lines of oat showed no infection of the nematode under field conditions. Local cultivars of onion, maize, chrysanthemum, gladiolus, and Sorghum halepense were also not infected by A. besseyi.
Keywords: Aphelenchoides besseyi, diagnosis, distribution, host, India, morphology, rice, tuberose
Amongst nematodes associated with rice (Oryza sativa L.) cultivation in West Bengal (WB), white tip nematode, Aphelenchoides besseyi is one of the most important due to its quarantine significance. Dastur (1936) reported its occurrence for the first time in rice from the former Central Provinces, now Chattisgarh region of Madhya Pradesh (MP), and later the nematode was reported widespread in India, particularly in the rice growing areas of Uttar Pradesh, MP, Tamil Nadu (Muniappan and Seshadri, 1964; Sivakumar, 1987), Gujarat (GT), Andhra Pradesh (AP) (Savitri et al., 1998) and Tripura (Nath et al., 1995). A serious outbreak of white tip disease was observed in 60% of the rice cultivars, with the most seriously affected cultivars being H.R.12 and Pankaj in AP (Jayaprakash and Joshi, 1979). The nematode causes yield losses of 10-15% in rice in GT (Thakar et al., 1987). The white tip disease of rice is severe in the southern and eastern states, where yield losses of 20% may occur (Prasad et al., 1986).
On tuberose (Polianthes tuberosa L.), the foliar nematode was first discovered to induce foliar disease in Hawaii (Holtzmann, 1968). Later, the occurrence of ‘floral malady’ caused by A. besseyi in tuberose was found in the Ranaghat areas of the Nadia district of WB (Chakraborti and Ghosh, 1993). Subsequently, A. besseyi was proven to be a pest of rice and tuberose in WB (Khan, 2001). The species was reported to cause a serious foliar disease of tuberose in the Mekong Delta of Vietnam, and based on morphological criteria and ribosomal RNA gene sequencing, the nematode was identified to be A. besseyi (Cuc and Pilon, 2007). Widespread occurrence of the white tip nematode infecting rice was reported from WB (Das and Khan, 2007, Khan and Das, 2009).
The widespread detection of white tip disease to rice in the southern and eastern states of India was reported and the foliar nematode problem is restricted to tuberose in WB and Odisha. Earlier studies were mostly on the occurrence of the problems and a detailed morphological and molecular evaluation of this nematode was thought to be essential for a better understanding of the species. Our preliminary light microscopic studies on the nematodes from these populations showed considerable variations in the body and stylet length including in the shape of tail, tail terminus and number of lateral lines. The objectives of this study were to evaluate populations of A. besseyi obtained from tuberose in WB and from rice in AP, MP, GT, and WB, using light and scanning electron microscopy (SEM) observations and molecular analysis and to assess the diagnostic value of morphological and molecular characters among the populations and to confirm the species infecting both rice and tuberose in India. Detailed morphometric and molecular observations of the nematode populations on rice and tuberose are presented herein.
Materials and Methods
Five populations of A. besseyi collected from rice (Oryza sativa L.) grains from Hyderabad, Andhra Pradesh (AP), Jabalpur, Madhya Pradesh (MP), Gujarat (GT), and West Bengal (WB) where A. besseyi populations infect both rice and tuberose, were studied for morphometric variations. Nematodes were extracted from rice grains after pounding with a mortar and pestle. The crushed grains were placed on single-ply tissues fitted over wire screens, which were then placed in Petri dishes containing tap water. The water level in the Petri dish was maintained to touch the bottom of the screen and tissue paper so that the nematodes could easily move down to the water below. Another Petri dish was placed on top of the screen and tissue paper to retard the evaporation of water. The entire assembly was kept for 15 to 20 hours at room temperature (30 to 35°C). Aphelenchoides besseyi from fresh, infected tuberose (Polianthes tuberosa L.) flower stalks obtained from foliar nematode-infested fields were extracted following the method of Khan and Pal (2001). Nematode specimens were killed in a hot-water bath, fixed in 3% formaldehyde and subsequently processed by the Seinhorst method (Seinhorst, 1959). The processed specimens were then mounted in anhydrous glycerine on glass slides. Photomicrographs of males, females and juveniles were taken with a Color Digital Camera (Retiga EXi, Q-Imaging, Austin, TX) attached to a compound microscope (a Leica Wild MPS48 Leitz DMRB, Leica Microsystems, Wetzlar, Germany). Altogether 24 parameters from at least 30 female of A. besseyi from each location and host in India were studied, as well as 30 males from WB on tuberose. The de-Man ratios and other values were determined from measurements taken with the help of an ocular micrometer (Hooper, 1986). For SEM, living specimens were fixed in 3% glutaraldehyde buffered with 0.05 M phosphate (pH 6.8), dehydrated in a graded series of ethanol, critical-point dried from liquid CO2, and sputter coated with a 20 to 30-nm layer of gold-palladium.
Collection of nematode populations for DNA extraction: DNA was extracted from the populations of A. besseyi infesting tuberose in WB and rice in AP. Tuberose flower stalks showing distortions caused by foliar nematodes were cut and placed in sterile spring water in a sterile Petri plate (5 cm diameter). After about an hour, nematodes that had migrated to the water were transferred into 1.5 ml microcentrifuge tubes, pelleted and used for DNA extraction. Similarly, rice seeds collected from nematode-infested rice fields were placed in water and dissected to allow the nematodes to move into the water.
Extraction of nematode DNA: DNA was extracted from the above two populations separately according to the method described by Subbotin et al., (2000). Ten to fifteen μl of sterile distilled water was added into each tube containing nematodes, which were crushed with a microhomogenizer. About 50 μl of nematode lysis buffer (125 mM KCl, 25 mM Tris-HCl pH 8.3, 3.75 mM MgCl2, 2.5 DTT, 1.125 % Tween 20, 0.025% gelatin) and 2 μl of proteinase K (600 g/ml) were added. The tubes were incubated at 65 °C (1h) and 95 °C (10 min) consecutively. Tubes were centrifuged at 16,000 g for 1 min and the supernatant containing DNA was stored at -20 °C until further use.
PCR amplification and cloning: Five μl of the DNA suspension was added to the PCR reaction mixture containing 0.2 mM dNTP′s, 0.2 μm of each primer (reverse and forward) and 2.5 μl 10 x Taq incubation buffer (10 mM Tris-HCl pH 8.3, 50 mM KCl, 1.5 mM MgCl2), 200 μM of each dNTP, 1 unit of Taq polymerase (Bangalore Genei, India), and double distilled water to a final volume of 25 μl. Primers (5′) TTGATTACGTCCCTGCCCTTT (3′) and (5′) TTCACTCGCCGTTACTAAGG (3′) as described by Joyce et al. (1994) for amplification of ITS 1 and 2 were used in the PCR reaction. Amplification reactions were performed in an Eppendorf Gradient thermal cycler. Each cycle had denaturation at 95 °C for 30 seconds, annealing at 55 °C for 30 seconds, and extension at 72 °C for 90 seconds; the cycle was repeated 44 times with a final extension of 72 °C for 5 min. Five-μl aliquots of amplification products were loaded on 1.2 % agarose gels prepared in 1X TAE (Tris-acetate-EDTA) buffer (pH 8.0), separated by electrophoresis (5 V/cm for 2.5 h) and stained with ethidium bromide. The gels were visualized using a gel documentation system (Vilber Lourmat, France). PCR products were purified with a PCR purification kit (Qiagen, Germany) as per manufacturer’s protocols, and 2 μl of purified product was used for TA cloning using pGEM T vector (Promega, Madison, USA). Recombinant colonies were identified using an ampicillin and the blue white colony selection method according to the manufacturer's instructions, and were confirmed by PCR using the same primers used for PCR amplification. Plasmids were prepared and the inserts were sequenced and analyzed. Multiple sequence alignment was done by MUSCLE, alignment curation by Gblock, phylogenetic analysis by PhyML and tree construction by TreeDyne (Dereeper et al., 2008).
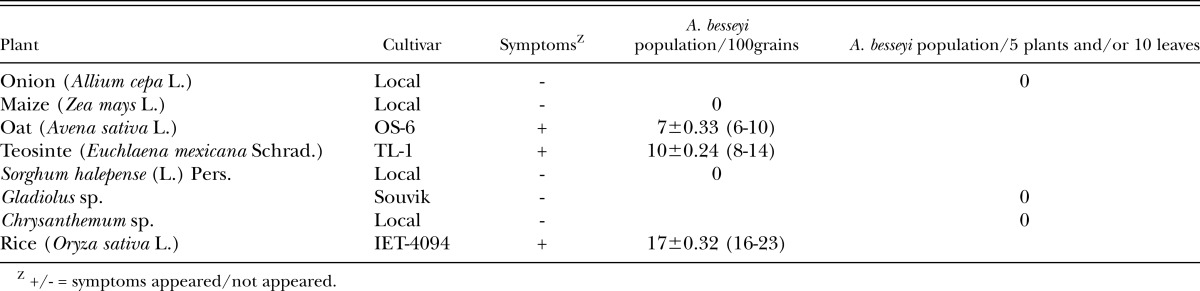
Host range tests: Onion (Allium cepa L. cv. Bombai red), chrysanthemum (Chrysanthemum sp.), gladiolus (Gladiolus sp. cv. Souvik), five germplasm of oat (Avena sativa L. cv. 0S-387, JO-03-309, JHO-2010-4, OL-1690, UPO-10-3), maize (Zea mays L. cv. Rajkumar), Sorghum halepense (L.) Pers.-Gramma-1, rice (Oryza sativa L. cv. IET 4094) and teosinte (Euchlaena mexicana Schrad. cv. TL-1) were grown in foliar nematode-infested plots at the Central Research Farm, Bidhan Chandra Krishi Viswavidyalaya, Gayespur, WB. The onion, oat, maize, Sorghum halepense, gladiolus and rice were grown in a plot of 10 m x 1 m inserted between the heavily infested plots of tuberose infected by A. besseyi. The crops were grown during 2009-2011 in a standard spacing of the crops during their growing seasons. The teosinte was sown in rows in a plot of 5 m × 2.5 m with spacing of 40 cm between plants and 60 cm between rows. The teosinte plants (one of the three rows) were inoculated with tuberose foliar nematodes extracted from tuberose at 30, 45 and 60 days after sowing at 500 nematodes/plant. After flowering, the plants were examined for infection by A. besseyi both in field and laboratory. Ten chrysanthemum plants were raised for two consecutive years (2009-2010) in pots and inoculated artificially by dispensing a nematode suspension of A.besseyi with a 5 ml dropper on the foliage and terminal points of 30 day-old cuttings at 2000 nematodes/plant. Infection by A. besseyi and development of symptoms on the plants were monitored regularly during the growing periods of the crops in both years. Five plant samples from ten sites within an experimental plot for each crop were collected and analysed in the laboratory for detection and estimation of nematode population. The observations on nematode population multiplication on the above crops were recorded from grains (100 grains) as well as from whole plants (at least five of onion and gladiolus) or plant parts (ten leaves including the growing points of chrysanthemum).
Results
Morphometrics and Morphology: Morphometrics of A. besseyi females and males are given in Table 1. The nematode body length varied from 514 μm to 845 μm in females. The body length indicated that it was moderately to highly variable in all the studied A. besseyi populations, whereas the ratios a, b′, b, c, and c′ were highly variable in the tuberose population. Stylet length (10-11μm), head to median bulb distance (HMB 53-61μm), and V% (71-79) were moderately to least variable. The mean length of the post-uterine sac (PUS) in A. besseyi populations from WB- rice and Gujarat-rice was relatively larger (40μm) than that of other populations (34-35μm) in this study. The mean b-ratio for MP-rice, WB-rice and GT-rice was less (9) than that of WB-tuberose (12) and AP-rice (11).
Table 1.
Morphometrics of female and male of Aphelenchoides besseyi infesting rice and tuberose in IndiaX, mean ± SEY, Range, Measurements in μm.
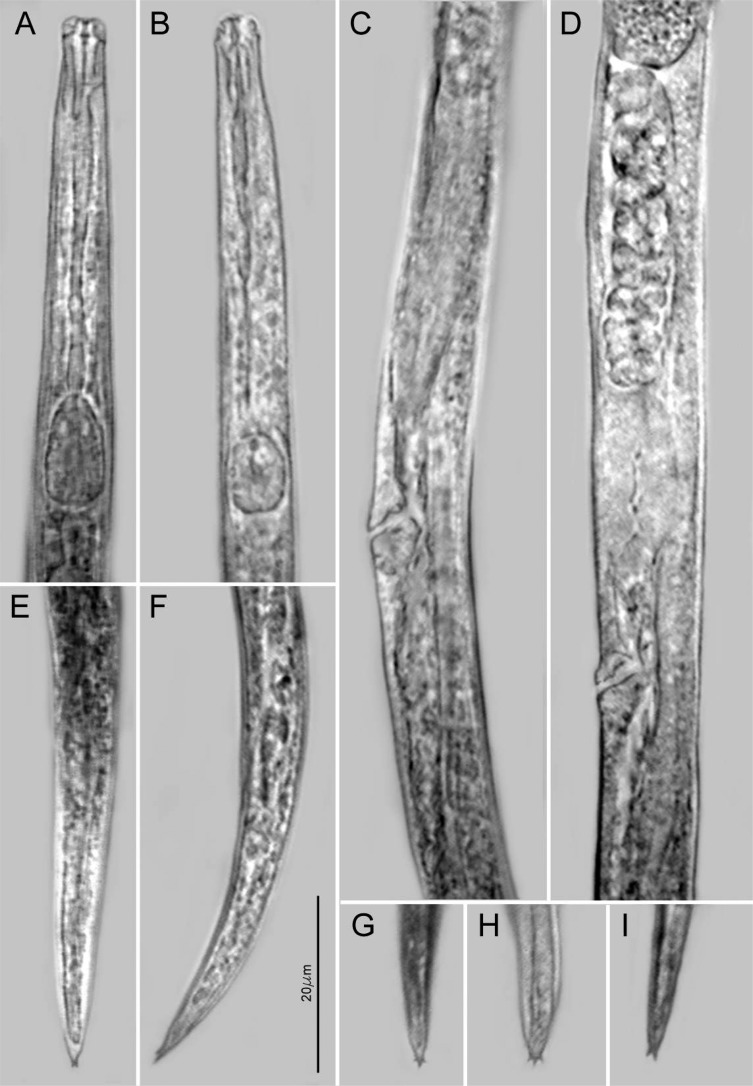
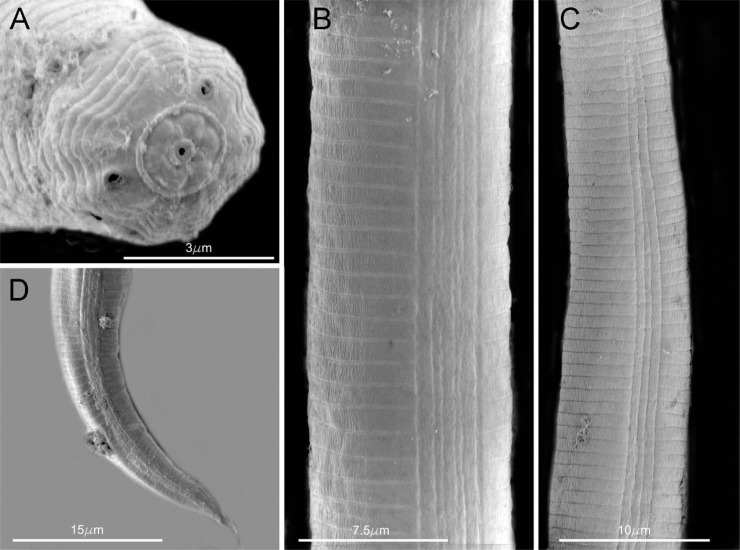
The A. besseyi populations studied in this investigation showed variations in the tail shape and terminal mucro and 2-3 processes, esophageal (shape of median bulb) and head end in the anterior regions, including the occurrence of four as well as six lateral lines in the lateral fields (Fig. 1-2).
Fig. 1.
Photomicrographs of Aphelenchoides besseyi female. A. Anterior end with esophagus; B-C: mid body region; E-F: Tail end; G-H-I: Tail tip, A-I: 20 μm scalebar.
Fig. 2.
SEM photomicrographs of Aphelenchoides besseyi female. A. Head end; B-C: Lateral fields; D: Tail end.
PCR amplification of the rDNA ITS 1 and 2 region of each of the two studied populations of A. besseyi generated one fragment of approximately 830 bp. However, removal of primer sequences in the flanking 18S and 28S gave 788 and 791 bp ITS 1 and 2 sequences for rice and tuberose strains, respectively (Fig. 3). Further there was a thymine (T) instead of a guanine (G) at position 169 in case of the rice strain. Sequences of both populations have been deposited in GenBank under the accession numbers JF826519 (rice population) and JF826517 (tuberose population). The Indian populations are highly similar to a Russian population of A. besseyi (GenBank EU186069.1 790 bp). Multiple sequence alignment by Clustalw (Larkin et al., 2007) indicated a gap at position 176 in the sequence of Russian as in case of the tuberose strain. Interestingly, the thymine (T) at position 498 was replaced by cytosine (C) in the Russian population compared to both the sequences of the Indian strains. This sequence variation gave a restriction site for the enzyme Bccl only in the Russian population that could be used for differentiating it from the two Indian strains. The phylogenetic tree indicated that the two Indian populations and one Russian of A. besseyi were similar and distant from one population of A. ritzamabosi (Fig. 4).
Fig. 3.
Comparison of ITS 1 and 2 sequences of Aphelenchoides besseyi from India and Russia. Tuberose strain (T): rice strain (R) and Russian strain (Russian).
Fig. 4.
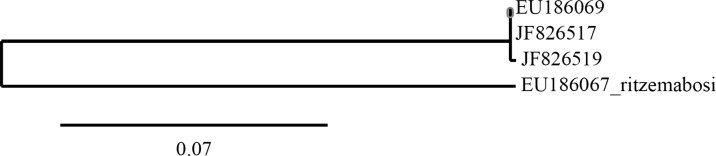
Phylogentic tree among the three populations of Aphelenchoides besseyi and Aphelenchoides ritzemabosi.
Studies on crop hosts of A. besseyi (Table 2) revealed that the rice (cv. IET 4094), oat (cv. OS-6) and teosinte showed a typical distortion due to the infection of A. besseyi. Although, five germplasm lines of oat procured from the All India Coordinated Research Project on Forage Crops (Kalyani Centre, West Bengal) showed no infection of the nematode under field conditions. No infection of A. besseyi on local cultivars of onion, maize, chrysanthemum, gladiolus and S. halepense were recorded even on the few plants that showed some minor distortion like symptoms.
Table 2.
Host tests with an Aphelenchoides besseyi population from tuberose.
Discussion
In this study, morphometrics of A. besseyi populations from AP-rice, MP-rice, WB-rice and WB-tuberose, GT-rice conform with the measurements from the type host and locality (Christie, 1942), rice population from India (Dastur, 1936), Senegal (Fortuner, 1970), Stylosanthes hamata Taubert population (cultured on Alternaria alternata) from Queensland of Australia (Gokte et al., 1992), onion population from Sri Lanka (Lamberti et al., 1996) and rice population from Egypt (Amin, 2002). There were considerable variations in measurements among the populations from India, Senegal and Sri Lanka due to genetic variation and or host-induced variability. B'Chir (1977) observed that the population reared on Impatiens balsamina was longer than the ones reared on Alternaria citri. Rajan and Mathur (1990) proposed two groups of A. besseyi from India and Philippines isolates based on morphometry and culturability on fungal hosts and considered Cuttack (eastern India) and Pune (western India) populations belonged to one and Hyderabad (southern India) and Philippines isolates (Philippines) to another group. However, in the present study, no appreciable variation in morphometry of rice and tuberose populations of A. besseyi from India was noticed. The morphology of A. besseyi populations (rice and tuberose) showed limited variation in the tail shape, medial bulb of esophagus and head end of anterior regions. Furthermore, the occurrence of four as well as six lateral lines in the lateral fields of the rice population was observed in SEM as well as light microscope (LM) observations. Viewing the number of lateral lines with LM can be difficult in a nematode genus like Aphelenchoides. Hooper and Ibrahim (1994) also described A. nechaleos, a very closely related species of A. besseyi and viewed four lines with LM and six lines with SEM. Aphelenchoides nechaleos was also differentiated from 14 species of Aphelenchoides but the number of lateral lines was not considered as differentiating character.
Host tests indicated that the A. besseyi population from tuberose induces symptoms in oat (cv. OS-6), rice (cv. IET-4094), and teosinte (cv. TL-1) but not onion, maize, S. halepense, gladiolus and chrysanthemum. Lamberti et al., (1996) found A. besseyi inducing a distortion and twisting of stems and discoloration of the leaf apical portion on onion in Sri Lanka, and proved the casual relationship of the nematode population on stem tissues and disease symptoms and bulb yield loss. Over 67 different crops including maize, chrysanthemum and oat are attacked by A. besseyi, among them rice, strawberry and tuberose are the principal hosts in India (Khan, 2010). The A. besseyi population from tuberose was proven earlier to be the same population causing white tip disease of rice (Khan, 2001). However, in our study, maize, chrysanthemum and gladiolus were not infected, probably because of differential preference of the nematode population for specific hosts.
No genetic difference was found between the rice and tuberose strains of A. besseyi. Absence of the restriction site for Bccl in the ITS sequence of Indian strains could be used to differentiate the Indian strains and the Russian strains. Cuc and Pilon (2007) identified A. besseyi infection in tuberose from the Mekong Delta of Vietnam using morphological characters and partial sequences of two rRNA genes (small subunit 18S and large sub unit 28S). Their BLAST search of the GenBank database also showed homologous sequences of the 18S rRNA sequence with those of Aphelenchoides ritzemabosi (GenBank DQ901554) and Aphelenchoides besseyi isolate 98 from North Florida, USA (GenBank AY508035), with 602/628 (95%) and 595/643 (92%) nucleotide identities in homologous regions, respectively. Therefore, the present work provides both morphological and molecular sequence variations found in populations of A. besseyi infecting rice and tuberose that will enable researchers to identify the species infecting both rice and tuberose and to select DNA-cutting enzymes for use in molecular identifications tests that will be more rapid and accurate than conventional methods based on LM study. In addition, this study provides fundamental information that is needed for morphological and molecular diagnosis of this species to provide continued protection for the rice and tuberose industry in India and throughout the world.
Literature Cited
- Amin WA. Aphelenchoides besseyi (Christie, 1942) on rice: A new record in Egypt. Pakistan Journal of Biological Sciences. 2002;5:297–298. [Google Scholar]
- B'Chir MM. Biometric variations of some species of the genus Aphelenchoides according to the properties of the host. Mededelingen van de Faculteit Landbouwwetenschappen Rijksuniversiteit Gent. 1977;42:1497–1512. [Google Scholar]
- Chakraborti HS, Ghosh SC. Studies on the floral malady of Polianthes tuberosa L. Journal of Mycopathological Research. 1993;33:25–28. [Google Scholar]
- Christie JR. A description of Aphelenchoides besseyi n.sp., the summer dwarf nematode of strawberries, with comments on the identity of Aphelenchoides subtenuis (Cobb, 1929) and Aphelenchoides hodsoni Goodey, 1935. Proceedings of the Helminthological Society of Washington. 1942;9:82–84. [Google Scholar]
- Cuc NTT, Pilon M. An Aphelenchoides sp. nematode parasitic of Polianthes tuberosa in the Mekong Delta. Journal of Nematology. 2007;39:248–257. [PMC free article] [PubMed] [Google Scholar]
- Das TK, Khan MR. Occurrence and distribution of white tip nematode, Aphelenchoides besseyi in West Bengal, India. Indian Journal of Nematology. 2007;27:94–97. [Google Scholar]
- Dastur JF. A nematode disease of rice in the Central Provinces. Proceedings of Indian Academy of Science. 1936;4:108–122. [Google Scholar]
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O. 2008 doi: 10.1093/nar/gkn180. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Research. 2008 Jul 1;36 (Web Server issue): W465-9. Epub 2008 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuner R. On the morphology of Aphelenchoides besseyi Christie, 1942 and A.siddiqi n.sp. (Nematoda, Aphelenchoidea) Journal of Helminthology. 1970;44:141–152. [Google Scholar]
- Gokte N, Mathur VK, Lal A, Rajan On the occurrence of Aphelenchoides besseyi and some free living nematode species in Stylosanthes hamata seeds. Nematologia Mediterranea. 1992;20:63. [Google Scholar]
- Holtzmann OV. A foliar disease of tuberose caused by Aphelenchoides besseyi. Plant Disease Reporter. 1968;52:56. [Google Scholar]
- Hooper DJ. 1986. Drawing and measuring nematodes. Pp. 87–106 in J. F. Southey, J.F. ed. Laboratory methods for work with plant and soil nematodes. Ministry of Agriculture, Fishery and Food, London. [Google Scholar]
- Hooper DJ, Ibrahim SK. Aphelenchoides nechaleos n.sp. and A. paraanechaleos n. sp. (Nematoda: Aphelenchoididae) from rice plants. Fundamental and Applied Nematology. 1994;17:153–160. [Google Scholar]
- Joyce SA, Reid A, Driver F, Curran J. 1994. Application of polymerase chain reaction (PCR) methods to the identification of entomopathogenic nematodes. Pp. 178–187 in A. M. Burnell, R. U. Ehlers, and J. P. Masson, eds. COST 812 Biotechnology: Genetics of entomopathogenic nematodes bacterium complexes. Proceedings of symposium and workshop, St Patrick’s College, Maynooth, County Kildare, Ireland. Luxembourg, European Commission, DGXII. [Google Scholar]
- Jayaprakash A, Joshi NC. A serious outbreak of white tip nematode disease (Aphelenchoides besseyi) in rice crops at Hyderabad. Indian Journal of Plant Protection. 1979;7:218–219. [Google Scholar]
- Khan MR. 2001. White-tip nematode, Aphelenchoides besseyi in rice-tuberose cropping system. Pp. 142–144 in S. K. Mukhopadhyay, D. C. Ghosh and G. C. De, eds. Proceedings of National seminar on frontiers of crop management. Visva-Bharati, Sriniketan, India. [Google Scholar]
- Khan MR, Pal AK. Plant parasitic nematodes associated with tuberose (Polianthes tuberosa L.) in West Bengal. Annals of Plant Protection Sciences. 2001;9:357–359. [Google Scholar]
- Khan MR, Das TK. 2009. White tip nematode, Aphelenchoides besseyi, a threat to rice in West Bengal, India. p. 53. in Proceedings of International conference on quality seed and food security at Bangladesh Agricultural University, Mymensingh, Bangladesh, 82p. [Google Scholar]
- Khan MR. 2010. White tip nematode infestation in rice. Pp 140–170 in M. R. Khan and M. S. Jairajpuri, eds. Nematode Infestation, Part-I: Food Crops. The National Academy of Sciences, India. [Google Scholar]
- Lamberti F, Ekanyake HMRK, Sasanelli N, Larizza A. Aphelenchoides besseyi on onion in Sri Lanka. Nematologia Mediterranea. 1996;24:63–71. [Google Scholar]
- Larkin M A, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007 doi: 10.1093/bioinformatics/btm404. Clustal W and Clustal X version 2.0. Bioinformation 23:2947–2948. [DOI] [PubMed] [Google Scholar]
- Muniappan R, Seshadri AR. On the occurrence of white tip nematode of rice in Madras State. Madras Agricultural Journal. 1964;51:510–511. [Google Scholar]
- Nath RC, Mukherjee B, Dasgupta MK, Siddiqi MR. Prevalence and distribution of plant-parasitic nematodes in rice fields of Tripura, India. Afro-Asian Journal of Nematology. 1995;4:147–150. [Google Scholar]
- Prasad JS, Panwar MS, Rao YS. Nematode problems of rice in India. Tropical Pest Management. 1986;32:127–136. [Google Scholar]
- Rajan, Lal A, Mathur VK. Host range and morphological studies on four isolates of Aphelenchoides besseyi Christie. Indian Journal of Nematology. 1990;20:177–183. [Google Scholar]
- Savitri H, Wahab Tayaba, Sattar MA, Reddy BM, Wahab T. Prevalence of white tip nematode (Aphelenchoides besseyi Christie) in rice samples of Andhra Pradesh. The Journal of Research, ANGRAU. 1998;26:74–76. [Google Scholar]
- Sivakumar CV. White tip nematode in Kanyakumari district, Tamil Nadu. Indian Journal of Nematology. 1987;17:72–75. [Google Scholar]
- Seinhorst JW. A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica. 1959;15:67–69. [Google Scholar]
- Subbotin SA, Waeyenberge L, Moens M. Identification of cyst forming nematodes of the genus Heterodera (Nematoda: Heteroderidae) based on the ribosomal DNA-RFLP. Nematology. 2000;2:153–164. [Google Scholar]
- Thakar NA, Patel BK, Patel HR, Patel CC. 1987. Occurrence of white tip disease of rice in Gujarat. Gujarat Agricultural University Research Journal 13:65. [Google Scholar]