Abstract
Bacterial clearance is one of the most important beneficial consequences of the innate immune response. Chemokines are important mediators controlling leukocyte trafficking and activation, whereas reactive oxygen and nitrogen species are effectors in bacterial killing. In the present work, we used in vivo and in vitro models of infections to study the role of monocyte chemoattractant protein 1 (MCP-1)/CCL2 and nitric oxide (NO) in the bacterial clearance in sepsis. Our results show that MCP-1/CCL2 and NO levels are increased in the peritoneal cavity of mice 6 h after sepsis induced by cecal ligation and puncture. Pretreatment with anti–MCP-1/CCL2 monoclonal antibodies increased the number of colony-forming units (CFUs) recovered in the peritoneal lavage fluid. Moreover, CFU counts were increased in the peritoneal fluid of CCR2−/− mice subjected to cecal ligation and puncture. In vitro stimulation of peritoneal macrophages with recombinant MCP-1/CCL2 reduced CFU counts in the supernatant after challenge with Escherichia coli. Conversely, treatment with anti–MCP-1/CCL2 increased CFU counts under the same experimental condition. Stimulation of cultured macrophages with MCP-1/CCL2 and interferon had a synergistic effect on NO production. Macrophages from CCL2−/− mice showed a consistent decrease in NO production when compared with wild-type controls after stimulation with LPS + interferon. Finally, we showed incubation of macrophages with E. coli, and the ERK inhibitor U0126 increased CFU numbers and decreased intracellular levels of NO. In conclusion, we demonstrated for the first time that MCP-1/CCL2 has a crucial role in the clearance of bacteria by mechanisms involving increased expression of inducible NO synthase and production of NO by ERK signaling pathways.
Keywords: Chemokines, nitric oxide, bacterial infection, phagocytosis
INTRODUCTION
Sepsis is a major cause of death worldwide. Approximately 750,000 cases of sepsis occur annually in the United States, with a mortality rate of 28.6% (1, 2). Current evidence indicates that dysregulation of the host inflammatory response to the infectious agent is central to the mortality of patients with sepsis. Although regulated inflammation is important to control bacterial infection, an excessive systemic inflammatory response can lead to shock and early mortality (3). Factors related to the infectious agent that trigger the immune/inflammatory responses are amplified by molecules of the host origin including cytokines, chemokines, lipid mediators, and reactive oxygen species. These inflammatory mediators influence the recruitment and activation of leukocytes affecting pathogen clearance at a potential cost of promoting tissue damage (4).
Chemokines are critically involved in leukocyte migration but also affect the biology of leukocytes in several ways (5). Monocyte chemoattractant protein 1 (MCP-1)/CCL2 is the prototype of the CC chemokine subfamily and exhibits the most potent chemotactic activity for monocytes. Collectively, both experimental and clinical studies have clearly established a key role of MCP-1/CCL2 in the pathogenesis of macrophage-driven inflammatory diseases, such as atherosclerosis (6). In sepsis, studies suggested a protective role of MCP-1/CCL2, where it promotes the balance between anti-inflammatory and proinflammatory responses to infection. Treatment with recombinant MCP-1/CCL2 increased bacterial clearance and protected mice systemically infected with Pseudomonas aeruginosa or Salmonella typhimurium (7). Also, the pretreatment of mice with anti–MCP-1/CCL2 increased lethality and was associated with impaired bacterial clearance and reduced leukocyte recruitment in a model of peritoneal sepsis (8). In another study, administration of MCP-1/CCL2 24 h after the induction of sepsis promoted tissue repair by inducing phagocytosis of apoptotic neutrophils by macrophages (9).
We demonstrated that animals genetically deficient in the receptor for MCP-1/CCL2, CCR2, are more susceptible to polymicrobial sepsis induced by cecal ligation and puncture (CLP) (10). In another study, we observed similar results when we used the knockout mice for the MCP-1/CCL2 chemokine. These animals were more susceptible to systemic inflammatory response syndrome induced by LPS and to CLP model, and this susceptibility was associated with a poor balance between proinflammatory and anti-inflammatory factors. We identified MCP-1/CCL2 as a positive regulator of IL-10 and a negative regulator of the proinflammatory cytokine macrophage migration inhibitory factor, thus suggesting the mechanism for the increased lethality rate in the absence of MCP-1/CCL2 and the important immunomodulatory role for MCP-1/CCL2 in sepsis (11). Nevertheless, previous studies failed to address the impact of MCP-1/CCL2 signaling on the control of the infection and the involved mechanism.
In addition to MCP-1/CCL2, other mediators favor the elimination of bacteria by macrophages. It is well described in the literature that nitric oxide (NO) has an important role in host defense against infection and especially in bacterial elimination. The role of NO in sepsis has been controversial, since pharmacological manipulation of NO synthesis in septic patients was shown to be both deleterious and beneficial (12, 13). Increased NO synthesis following NO synthase (NOS) II induction plays a major part in the host defense against viral and bacterial pathogens and in the containment of tumor growth, yet elevated levels of NO can also exert deleterious effects in many acute inflammatory responses and chronic diseases (14). Endogenous NO production was associated to an increase in survival rate and to decreased numbers of bacteria in the lung and blood of animals inoculated with Klebsiella pneumoniae, indicating an important role of NO in the bacterial elimination by macrophages under septic conditions (15).
A connection between MCP-1/CCL2 and NO pathways has been established in models of tumoricidal activity (16), but this has not yet been studied in infectious conditions, nor a potential intracellular pathway been proposed in these circumstances.
In the present study, we investigate the role of MCP-1/CCL2 and NO in the clearance of bacteria after induction of polymicrobial sepsis, exploring the interrelationship between these mediators and the signaling pathways involved.
MATERIALS AND METHODS
Animals
Swiss and C57BL6 mice from the Oswaldo Cruz Foundation breeding unit weighing 20 to 25g were used for the studies. The animals were kept at a constant temperature (25°C) with free access to food and water in a room with a 12-h light-dark cycle. In this set of experiments, a C57BL/6 background was used with mice deficient in MCP-1/CCL2 chemokine (MCP-1/CCL2−/−), mice deficient in the MCP-1/CCL2 receptor CCR2 (CCR2−/−), or mice deficient in inducible NO synthase (iNOS−/−). The experiments in this study were approved by the Oswaldo Cruz Institute’s Animal Welfare Committee.
Materials
Murine recombinant MCP-1/CCL2 and murine recombinant interferon γ (IFN-γ) were from Peprotech (Rocky Hill, NJ). Lipopolysaccharide (LPS) from Escherichia coli 0111:B4, Griess reagent, and l-NAME (l-NG-nitroarginine methyl ester) were from Sigma-Aldrich (St. Louis, Mo). Thiopental (Thionembutal) was from Abbott Labs do Brasil, LTDA (São Paulo, Brazil), and ketamine was from Cristália (São Paulo, Brazil).
Cecal ligation and puncture
Mice were anesthetized with a mixture of thiopental (40 mg/kg) and ketamine (80 mg/kg) diluted in sterile saline and administered intraperitoneally (0.2 mL). Laparotomy was performed, and the cecum was exposed and ligated below the ileocecal junction with care to avoid bowel obstruction. The cecum was punctured once with an 18-gauge needle and was then gently squeezed to empty its contents through the puncture. The cecum was returned to the peritoneal cavity, and the abdominal muscle and skin incisions were closed in layers using a 3-0 nylon suture line. Immediately after the surgery, 0.5 mL of sterile saline was administered subcutaneously to the animals for volume resuscitation. Sham-operated mice were subjected to identical procedures except that ligation and puncture of the cecum were omitted.
Septic animals were rated as healthy (score of 0 points) or to have mild sepsis (score of 2 points), moderate sepsis (score of 3–4 points), or severe sepsis (score of 5–6 points) by a blinded observer using a severity score composed of the following parameters and respective awarded points: appearance (0 = normal, 1 = piloerection), activity (0 = active, 1 = lethargic), awareness (0 = alert, 1 = lethargic, 2 = moribund), breathing (0 = normal, 1 = amended), and appearance of eyes (0 = normal, 1 = with secretion).
Animals subjected to CLP developed early signs of sepsis, including lethargy, piloerection, and diarrhea. Survival of mice subjected to CLP or sham injury was determined daily for 7 days. The model performed as described above yielded a 7-day mortality rate of around 10% to 20% and a severity score of three points at 6 h (moderate sepsis) for C57BL6 wild-type (WT) animals and Swiss mice. CCR2−/− mice had a 7-day mortality rate of around 70% and a severity score of four to five points at 6 h (severe sepsis).
Bacteria inoculation
For these experiments, we used the E. coli ATCC25922 strain grown in LB medium from a single colony (10 g peptone, 5 g of yeast extract, and 10g NaCl, pH 7, sterilized by autoclaving at 120°C for 30 min). Mice were inoculated intraperitoneally with E. coli (5 × 105 bacteria/cavity). After 6 h, the peritoneal cavity was washed, and lavage fluid was collected to measure colony-forming units (CFUs) and NO levels.
Treatments
Treatment with l-NAME (10 mg/kg) or anti–MCP-1/CCL2 (250 µg/kg) was done 2 h after the CLP surgery by intraperitoneal injection. The ERK pathway inhibitor, U0126 (13 µM), was added to macrophage cultures 15 min before MCP-1/CCL2 and E. coli stimulation.
Cytokine measurements
The magnitude of the inflammatory response was evaluated by measuring the levels of MCP-1/CCL2 in the peritoneal fluid, using enzyme-linked immunosorbent assay technique with specific monoclonal antibodies, according to the manufacturer’s instructions (Duo Set Kit from R&D Systems, Minneapolis, Minn). Mice were killed in a CO2 chamber at designated time points, and the peritoneal cavity was opened and rinsed with Hanks balanced saline solution without calcium (Ca2+) or magnesium (Mg2+). The particulate matter was removed by centrifugation at 800g for 10 min, and the supernatant fractions were used for immunoassays.
Determination of CFUs
Twelve microliters of peritoneal lavage fluid from each mouse was placed on ice and was serially diluted with sterile saline. Twelve microliters of each dilution was placed on agar plates and incubated overnight at 37°C, after which the number of colonies was counted with the aid of a colony counter.
In vitro stimulation of peritoneal macrophages
Mice received an intraperitoneal dose of 3 mL of thioglycollate (3%; Sigma). Three days later, they were killed, and the peritoneal macrophages were obtained by washing the peritoneal cavity with 3 mL of saline. One million cells in RPMI media were distributed in 24-well culture plates and were placed into a 37°C CO2 incubator for 2 h. All nonadherent cells were subsequently removed, and the adherent cells were treated with MCP-1/CCL2 (100 ng/mL), anti–MCP-1/CCL2 (10 ng/mL), LPS from E. coli 0111: B4 (300 µg/mL), or IFN-γ (20 ng/mL) and after 1 h stimulated with live E. coli (105 bacteria/mL). The supernatants were removed 30 min after the stimulation and used for CFU counting and measurement of NO production. The cell viability was determined by trypan blue dye exclusion at the end of each experiment and was never lower than 90% for all experiments. To study the effect of ERK inhibitor, cells were pretreated with U0126 (13 µM) at 37°C for 15 min before treatment with MCP-1/CCL2 (100 ng/mL).
NO measurements
Mice were killed in a CO2 chamber at designated time points, and the peritoneal cavity was opened and rinsed with phosphate-buffered saline (PBS). The peritoneal fluid, or supernatant from peritoneal macrophages culture as described above, was collected and centrifuged for nitrite level determinations using Griess reagent, which detects NO2 −, a primary stable and nonvolatile breakdown product of NO. The Griess Reagent System is based on a chemical reaction that uses sulfanilamide and N-1-naphthylethylenediamine dihydrochloride under acidic (phosphoric acid) conditions.
Intracellular NO was detected in peritoneal macrophage cultures by incubating the cells with DAF-FM diacetate (d23844; Invitrogen, Chicago, Ill), followed by challenging them with E. coli (105 bacteria/mL). Fluorescence was detected by a fluorescence microplate reader (Molecular Devices, Eugene, Ore) and analyzed using a Softmax Pro program (Molecular Devices Corporation, Sunnyvale, Calif). The results were expressed as fluorescence intensity.
Immunolocalization
Leukocytes obtained from the peritoneal fluid were cytospun onto glass slides and fixed with 3.7% formaldehyde in PBS (pH 7.4) for 10 min and then were permeabilized with 0.2% Triton X-100 for 10 min. After cell fixation and permeabilization, macrophages were blocked with 2% normal donkey serum PBS for 15 min. After washing, cytospin preparations were incubated for 1 h at room temperature with goat polyclonal serum anti-iNOS, anti-pERK, or nonimmune goat immunoglobulin G (IgG) diluted in PBS. After washes in PBS, the preparations were incubated with biotin-conjugated rabbit anti–goat IgG (Sigma-Aldrich). The immunoreactivity was shown using an ABC Vectastatin glucose-oxidase kit according to the manufacturer’s instructions (Vector Laboratories, Burlingame, Calif) and then observed by light microscopy. Slides were then washed with PBS, and an aqueous mounting medium (Polysciences, Warrington, Pa) was applied to each slide before coverslip attachment. Slides were observed by phase-contrast and fluorescent microscopy. Digital photographs were taken with a Cool Snap camera (Roper Scientific, GmbH, Ottobrunn, Germany) and processed using Image Pro Express (Media Cybernetics, Silver Spring, Md).
ERK immunoblotting detection
Phosphorylated ERK and total ERK were detected in cell lysates of cultured macrophages treated with MPC-1/CCL2 and stimulated with E. coli as described above. Cell lysates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis in 10% acrylamide gradient gels and transferred onto nitrocellulose membranes. The membranes were then blocked with a solution of Tris-buffered saline Tween with 5% nonfat milk (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% Tween 20). The membranes were incubated with antibodies against phosphorylated ERK or ERK p42/44 (Cell Signaling Technology, Danvers, Mass), followed by incubation with horseradish peroxide–conjugated secondary anti–mouse antibody (Vector Laboratories). The signal was detected with Supersignal Chemiluminescence Pico (Pierce) by exposing the membrane to an autoradiography film (GE Healthcare, Rockford, Ill).
Statistical analysis
All other data are expressed as mean ± SEM and compared using a two-tailed Student t test. Data were considered statistically significant if P < 0.05.
RESULTS
MCP-1/CCL2 has a key role in bacterial clearance in models of sepsis
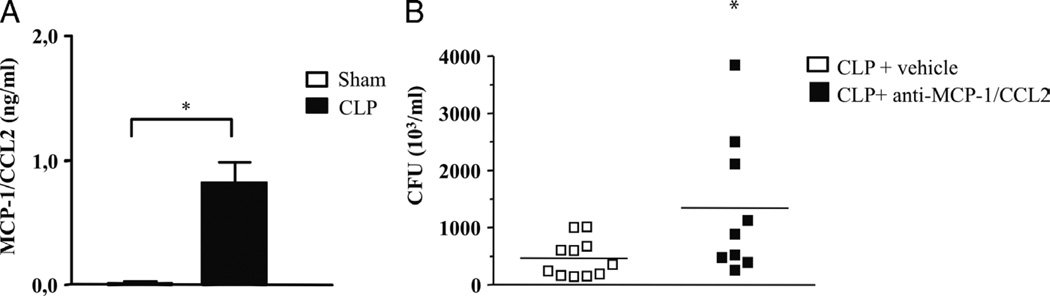
Monocyte chemoattractant protein 1/CCL2 levels increase in the plasma of patients with sepsis and septic shock (17). Therefore, to investigate if MCP-1/CCL2 levels increase in mice subjected to CLP, the animals were killed, and peritoneal fluid was collected 6 h after the surgery. As shown in Figure 1A, we observed a significant rise in MCP-1/CCL2 levels at 6 h after CLP (Fig. 1A).
Fig. 1. Monocyte chemoattractant protein 1/CCL2 levels are increased and are important for the control of bacterial burden in vivo.
A, Swiss mice were submitted to the CLP procedure, and 6 h later, the peritoneal lavage fluid was obtained and used for MCP-1/CCL2 determination by enzyme-linked immunosorbent assay. A group of sham-operated animals was used as the control. Each bar is the mean ± SEM from six animals. B, Swiss mice were treated with anti–MCP-1/CCL2 monoclonal antibody (10 ng/kg) 1 h after undergoing CLP. Six hours later, the peritoneal fluid was obtained, plated on tryptic soy agar medium (TSA) plates, and incubated overnight at 37°C for CFU counting. *Statistically significant differences when compared with sham-operated group.
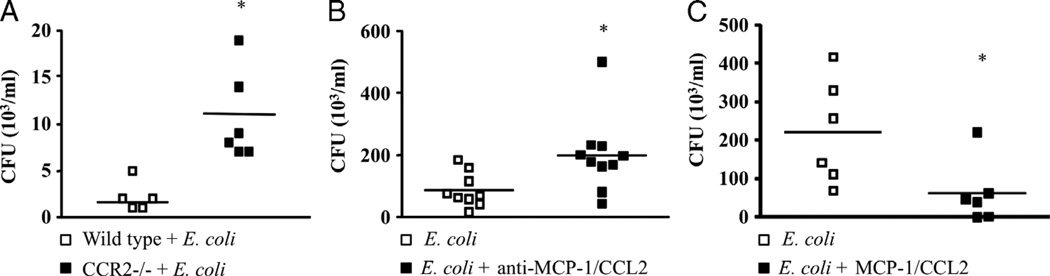
Nakano et al. (7) demonstrated that treatment with CCL2 increased bacterial clearance and protected the animals from mortality induced by Pseudomonas aeruginosa. To further investigate the role of MCP-1/CCL2 in bacterial clearance, the animals were treated with anti–MCP-1/CCL2, 1 h before CLP, and the peritoneal fluid was collected 6 h later and plated in agar plates for CFU determination. As shown in Figure 1B, CFU numbers were significantly increased in anti–MCP-1/CCL2–treated animals when compared with the control group. This result is additional evidence for the involvement of MCP-1/CCL2 in controlling bacterial clearance using the well-established and clinically relevant polymicrobial model of sepsis induced by CLP. Similar results were obtained when we used CCR2−/− mice in another model of bacterial sepsis. Challenge of CCR2−/− animals with E. coli (5 × 104/animal, i.p.; Fig. 2A) showed an increase in CFU numbers recovered from the peritoneal fluid as compared with WT animals, indicating that negative modulation of MCP-1/CCL2 signaling has a detrimental effect on bacterial clearance in different models of sepsis.
Fig. 2. Monocyte chemoattractant protein 1/CCL2 is important for the control of bacterial burden in in vivo and in vitro models of gram-negative infection.
A, CCR2−/− mice were injected intraperitoneally with E. coli (105 bacteria/animal), and 6 h later, the peritoneal fluid was obtained and plated on plates and incubated overnight at 37°C for CFU counting. B, Peritoneal macrophages (106/well) from Swiss mice were incubated with anti–MCP-1/CCL2 monoclonal antibody (10 ng/mL) and 1 h later challenged with E. coli (105 bacteria/mL). C, Peritoneal macrophages (106/well) from Swiss mice were incubated with recombinant MCP-1/CCL2 (100 ng/mL) and 1 h later challenged with E. coli (105 bacteria/mL). After 30 min, the culture supernatant was collected in B and C and plated in TSA plates for CFU counting. Each figure represents one experiment out of three repetitions with similar results. Each square represents one well, and the horizontal lines represent the mean. *Statistically significant difference, P ≤ 0.05 (Student t test).
We also investigated the role of MCP-1/CCL2 in bacterial clearance using an in vitro model of gram-negative (E. coli) infection. Peritoneal macrophages were collected, plated, and treated with recombinant MCP-1/CCL2 or anti–MCP-1/CCL2 before the incubation with E. coli. Colony-forming units were then determined in the supernatant 30 min later. Similar to our results in the in vivo CLP model, we found that treatment of macrophages with anti–MCP-1/CCL2 caused a significant increase in CFU numbers after incubation with E. coli (Fig. 2B). On the other hand, decreased CFU numbers were detected after treatment of macrophages with recombinant MCP-1/CCL2 before incubation with E. coli (Fig. 2C).
NO is required for elimination of bacteria in the CLP model
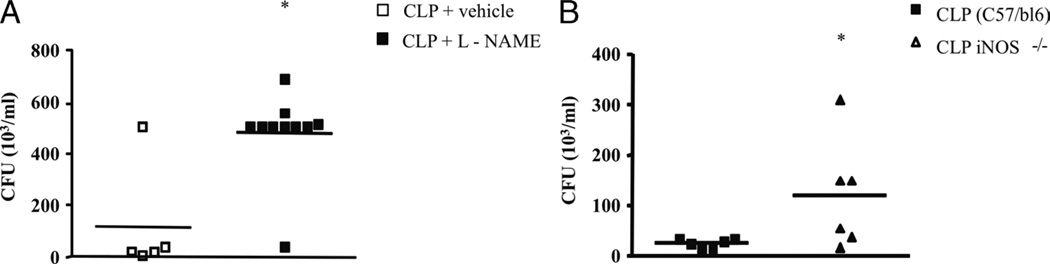
A variety of studies demonstrate that NO is an innate bactericidal effector molecule (18, 19). Enhanced NO production by macrophages and neutrophils is a critical defense mechanism in many fungal and bacterial infections (20). Initially, we measured the levels of NO in the peritoneal cavity after the CLP procedure. We observed an increase in NO levels 6 h after CLP when compared with the sham-operated control group (Fig. 3). Next, we used l-NAME (20 mg/kg) treatment to block NO synthesis and observed an increase in CFU counts in mice subjected to CLP (Fig. 4A). In addition, mice genetically deficient in the inducible isoform of NO synthase (iNOS−/−) were submitted to CLP, and 6 h after the procedure, the peritoneal fluid was collected and plated for CFU counts. As shown in Figure 4B, and in agreement with the pharmacological blockage of NO synthesis, CFU counts were markedly increased in animals genetically deficient of NOS. Together with these results, an important role for NO is suggested in the bacterial elimination in septic mice.
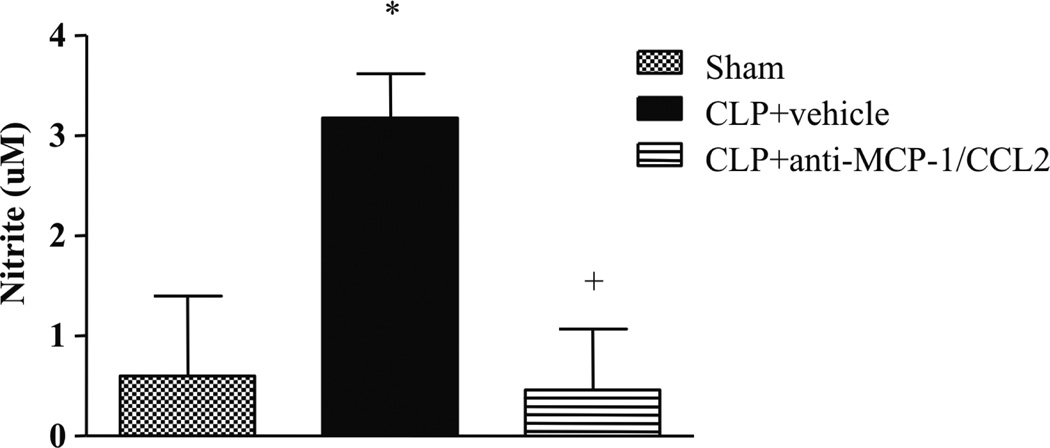
Fig. 3. Monocyte chemoattractant protein 1/CCL2 is involved in NO production after CLP-induced sepsis.
Nitrite levels in the peritoneal cavity 6 h after sham or CLP surgery in Swiss mice treated with anti–MCP-1/CCL2 monoclonal antibody (250 µg/kg) or vehicle. The peritoneal fluid was collected and used to measure nitrite levels by the Griess method. Each bar is the mean ± SEM from six animals in sham and nine animals in CLP + vehicle and CLP + anti–MCP-1/CCL2. +Statistically significant differences from CLP + vehicle group; *statistically significant differences between CLP + vehicle and sham-operated group, with a P ≤ 0.05 (Student t test).
Fig. 4. Nitric oxide is an effector molecule for bacterial killing in CLP-induced sepsis.
A, The Swiss mice were submitted to CLP and treated with l-NAME (20 mg/kg i.p.) 2 h later. After 6 h, the animals were killed, and the peritoneal fluid was collected, plated on TSA plates, and incubated at 37°C for CFU counting. B, Inducible NOS–deficient mice were submitted to CLP, and after 6 h, the peritoneal fluid was collected for CFU counting as described above. Each square represents one animal in the experimental group with the mean value represented by a horizontal line. The figures represent one experiment out of two repetitions with similar results. *Statistically significant difference, with a P ≤ 0.05 (Student t test).
MCP-1/CCL2 is a trigger for NO production and bacterial clearance
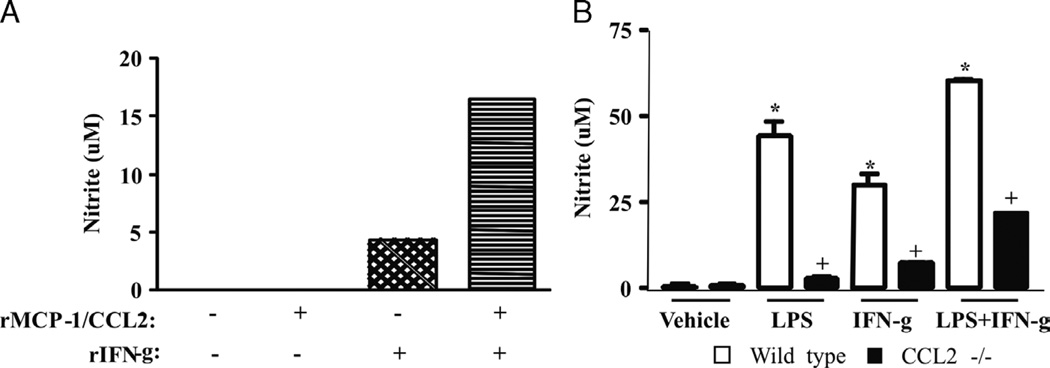
In view of the above results, we examined the possibility that there is a link between the production of MCP-1/CCL2 and NO in our models of infection. First, we observed that NO production after CLP was decreased by anti–MCP-1/CCL2 treatment (250 µg/kg) (Fig. 3). Similarly, NO levels were significantly decreased in CCR2−/− animals submitted to CLP as compared with the WT septic group (data not shown). We also treated peritoneal macrophages in vitro with recombinant MCP-1/CCL2 and IFN-γ and measured NO levels after 24 h. Figure 5A shows that cotreatment with MCP-1/CCL2 and IFN-γ increased the production of NO. Next, we treated peritoneal macrophages from MCP-1/CCL2−/− and WT mice with LPS, IFN-γ, or LPS + IFN-γ. As shown in Figure 5B, we observed a consistent decrease in NO production for all treatment groups in macrophages from MCP-1/CCL2−/− mice when compared with the macrophages from the WT control group.
Fig. 5. Monocyte chemoattractant protein 1/CCL2 and IFN-γ induce NO production in cultured peritoneal macrophages.
A, Peritoneal macrophages (106 cells/well) were incubated with rMCP-1/CCL2 (100 ng/mL), IFN-γ (10 ng/mL), or both. After 24 h, the culture supernatants were obtained for determination of nitrite levels by Griess. The figure represents one experiment out of three repetitions with similar results, and the bars are the mean of duplicate wells. B, Peritoneal macrophages (106 cells/well) from CCL2−/− mice were incubated with LPS (50 ng/mL), IFN-γ (10 ng/mL), or both. Each bar is the mean ± SEM of six wells. This is a representative experiment out of two repetitions with similar results. *Statistically significant difference when compared with macrophages incubated with vehicle (P ≤ 0.005). +Statistically significant difference when compared with stimulated macrophages obtained from WT animals (P ≤ 0.005).
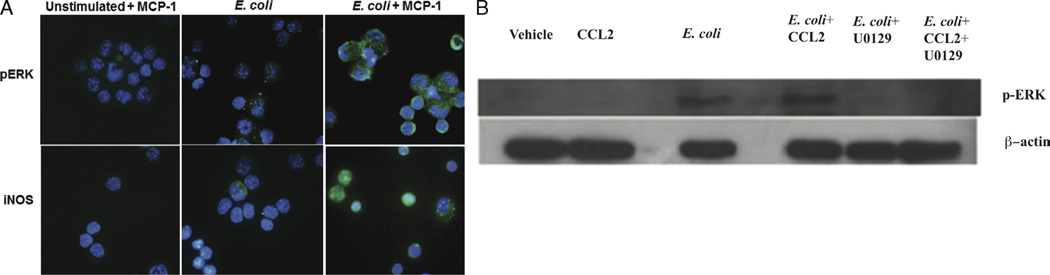
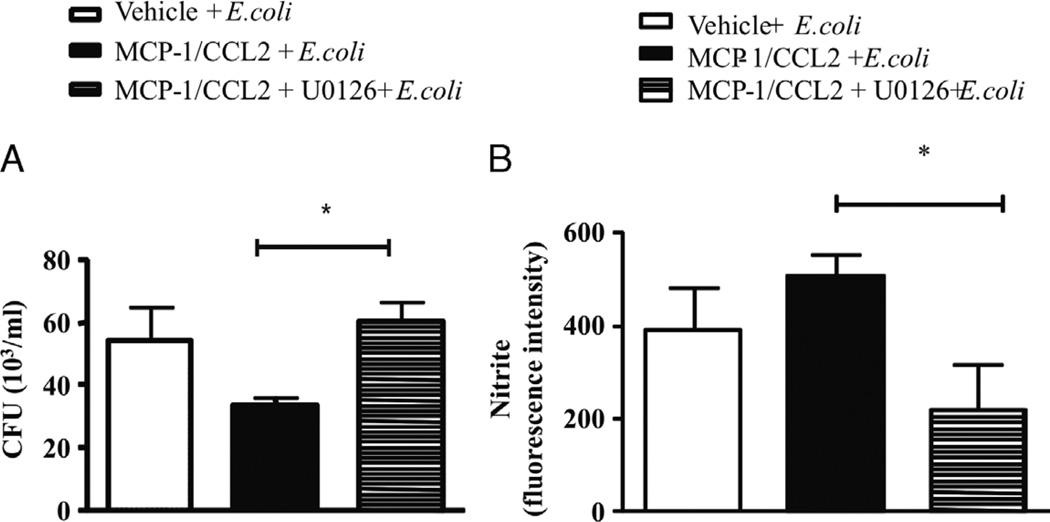
To investigate if NO production was related to the MCP-1/CCL2 signaling, we looked at iNOS expression by immunofluorescence in peritoneal macrophages after stimulation with E. coli and MCP-1/CCL2. As indicated in Figure 6A, stimulation of peritoneal macrophages from WT mice with MCP-1/CCL2, E. coli, or both increased the phosphorylation of pERK and increased the expression of iNOS when both stimuli were done together. The increase in pERK under the same conditions was confirmed by Western blotting (Fig. 6B). In addition, Figure 6 shows a decrease in pERK after treatment with ERK inhibitor U0126 (13 µM). Furthermore, incubation of macrophages with U0126 before stimulation with MCP-1/CCL2 and E. coli increased CFU numbers in the culture supernatant (Fig. 7A) and decreased intracellular levels of NO detected by the use of DAF probing (Fig. 7B). These results suggest that the mechanisms involved in NO production by murine peritoneal macrophages after MCP-1/CCL2 treatment are associated with the ERK1/2 signaling pathway and that NO is essential for the control of the bacterial load in this model of sepsis.
Fig. 6. Monocyte chemoattractant protein 1/CCL2 primes macrophages for enhanced expression of iNOS and phosphorylation of ERK.
A, Peritoneal macrophages were obtained and plated in 24-well culture plates (106 cells/well). Cells were then incubated with MCP-1/CCL2 (100 ng/mL) or vehicle and after 30 min challenged with E. coli (105 bacteria/mL) or vehicle. After 1 h, the cells were recovered and cytospun onto glass slides, fixed with 3.7% formaldehyde and permeabilized with 0.2% Triton X-100. Immunostaining with specific antibodies was performed as described in Materials and Methods, and the slides were observed under phase-contrast and fluorescent microscopy. B, Western blot analysis of pERK in macrophages stimulated with MCP-1/CCL2 and E. coli. Thioglycollate peritoneal macrophages were obtained and plated in 24-well culture plates (106 cells/well). Cells were then incubated with U0126 (13 µM) for 15 min followed by incubation with MCP-1/CCL2 (100 ng/mL) or vehicle for 30 min before they were challenged with E. coli (105 bacteria/mL) or vehicle. After 1 h, the cells were recovered and lysed in lysis buffer. The lysate was blotted in a sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel. Each experiment shown is representative of two repetitions with similar results.
Fig. 7. The pERK signaling pathway mediates MCP-1/CCL2–induced NO production and enhanced bacterial clearance.
Peritoneal macrophages (106 cells/well) from Swiss mice were incubated with vehicle or U0126 (13 µM) 15 min before the addition of rMCP-1/CCL2 (100 ng/mL). Thirty minutes later, E. coli (105 bacteria/mL) was added to the well, and after 1 h, the supernatant was recovered and plated to TSA plates for CFU determinations (A), while the cells were used for intracellular determinations of NO production using DAF probing (B). Each bar is the mean ± SEM of peritoneal macrophages from six different animals plated in separated wells in duplicate. *Statistically significant differences (P ≤ 0.005).
DISCUSSION
Early studies suggested that the mortality and organ injury associated with severe sepsis are primarily due to an exaggerated inflammatory response. Nevertheless, despite the association of inflammation with mortality, anti-inflammatory therapies have, for the most part, failed to significantly improve clinical outcomes (21). This may suggest that the balance between beneficial and detrimental effects of innate immune responses, that is, inflammation, is far more intricate than anticipated.
The control of bacterial burden is a key event in the management of septic patients. Antibacterial therapy is therefore required early after diagnosis, and its delay has a clear impact on mortality (22, 23). It has been long recognized that the most striking beneficial effect of the inflammatory response to an invading organism is the control of the spreading of the infection and the tissue colonization by the pathogenic organism. In this respect, phagocytosis by competent cells and the production of oxygen and nitrogen reactive species occupy a central role in the innate immune response dealing with control and/or elimination of the invading microorganism. Chemokines are known to control leukocyte influx to tissues under inflammatory conditions (24). Monocyte chemoattractant protein 1/CCL2 is a key chemokine that can recruit monocytes, macrophages, and lymphocytes by a mechanism dependent on its binding to the CCR2 receptor, but other activities such as an indirect control of neutrophil migration under infectious conditions have also been described (25). Our findings in this work, together with that in a previous publication (11), indicate that endogenous MCP-1/CCL2 protects mice from sepsis by regulating proinflammatory and anti-inflammatory cytokine production. We observed that, after CLP, the levels of MCP-1/CCL2 were increased and that blocking of this chemokine using anti–MCP-1/CCL2 antibodies caused an increase in CFU counts. This result suggested that the protective role of MCP-1/CCL2 may be related to its ability to limit bacterial load. Similarly, the increased bactericidal response to listeriosis after sepsis is related to CCR2-dependent recruitment of myeloid cells to the spleen and liver (26), adding support to the idea that MCP-1/CCL2 has important actions in the control of bacterial elimination in different preclinical models of infection. More importantly, we could mimic the effects of MCP-1/CCL2 in the control of bacterial clearance using an in vitro system of cultured macrophages challenged with E. coli as an infectious agent. In this system, stimulation of macrophages with recombinant MCP-1/CCL2 increased bacterial clearance, whereas incubation with anti–MCP-1/CCL2 monoclonal antibodies increased the numbers of CFUs in the culture supernatant. This important observation confirmed our current and previous observations in animal models and opened the possibility for mechanistic studies dissecting the molecular pathways involved in this effect.
The production of NO is critical for killing bacteria in different infectious conditions (27). Nitric oxide is produced by three different forms of NOS, namely, neuronal NOS (or NOS1), endothelial NOS (or NOS3), and iNOS (or NOS2). Because of the huge difference in the amount of NO produced, it has been generally assumed that neuronal NOS and endothelial NOS are critical for a normal physiology, whereas iNOS is associated more with injury (28). Inducible NOS–derived NO influences the physiological functions of most leukocytes and is implicated in phagocytic, antimicrobial, and tumoricidal activities (29, 30). However, the excessive production of NO by immune cells and nonimmune cells such as endothelial, epithelial, and smooth muscle cells can also lead to tissue damage, as demonstrated by the protective effects of NOS inhibitors in many inflammatory and autoimmune disease models (14). We confirmed previous studies showing that NO production is significantly increased after CLP or other septic conditions (31). Our results point to NO as an effector molecule in bacterial clearance in our polymicrobial model of sepsis. The role of NO in sepsis has been hotly contested, because pharmacological manipulation of NO synthesis in septic patients has been shown to be both deleterious and beneficial (12, 13, 32). The deleterious effects of NOS inhibition are related in part to the inability of NOS substrate inhibitors to discriminate between the constitutive and inducible isoforms of NOS, whereas the beneficial effects of these compounds have been attributed to the ability of NOS inhibitors to restore normal cardiovascular function in septic patients (32). Nitric oxide production in the early phases of sepsis is likely to contribute, together with respiratory burst, to efficient microbial clearance by phagocytic cells, especially because respiratory burst and NO-derived mediators have well-known antimicrobial activities (33). This effect is likely to explain why a nonspecific inhibitor of iNOS was found to be detrimental in a clinical trial (34) and as illustrated by our experiments, where the bacterial burden was increased either after treatment of animals with l-NAME, an inhibitor of iNOS (12), or the induction of sepsis in iNOS−/− mice.
A connection between MCP-1/CCL2 signaling and NO production has been established by previous observations. Murine peritoneal macrophages, upon activation with MCP-1/CCL2, show a dose- and time-dependent production of NO together with increased tumoricidal activity. l-NMMA (N-monomethyl-l-arginine), a specific inhibitor of NO production, inhibits the MCP-1/CCL2–induced NO secretion and macrophage-mediated tumoricidal activity (16). Consistent with this finding in anti-tumor assays, we observed by immunostaining that stimulation of macrophages with MCP-1/CCL2 and E. coli is able to induce an increase in iNOS expression. In contrast, stimulation of CCR2−/− macrophages with LPS and IFN-γ failed to increase iNOS expression. Taken together, these findings demonstrate that MCP-1/CCL2 signaling is central to NO production by macrophages challenged by infectious stimuli to increase the expression of the inducible isoform of NOS.
The production of NO after stimulation of peritoneal macrophages with MCP-1/CCL2 was shown to occur via signal transduction cascades involving the participation of protein kinases, phosphatases, and the secondary messenger calcium (16). Our results extend this conclusion because we localized by immunofluorescence phosphorylated ERK and iNOS in macrophages stimulated with MCP-1/CCL2 in the presence of E. coli. Furthermore, we established functional relevance for ERK phosphorylation in this system; the ERK inhibitor U0126 decreased the intracellular production of NO while increasing the CFU counts in the supernatants of MCP-1/CCL2–stimulated cultured macrophages challenged with E. coli. These results indicate that the mechanism involved in MCP-1/CCL2 bacterial clearance is closely associated with the increased local release of NO, which is an important mediator for the death of microorganisms, and the signaling pathway involved in this mechanism is dependent on MAP kinases and, more specifically, an ERK signaling pathway. Although the protective effect of MCP-1/CCL2 has been established before (11, 25), this is an important new contribution to the understanding of the mechanism by which MCP-1/CCL2 exerts a protective role in septic syndromes.
The clinical importance of MCP-1/CCL2 in sepsis is still a matter of interest and debate. In previous studies, we demonstrated that MCP-1/CCL2 plays an important role in the survival of septic animals, by a mechanism based on the balance of inflammatory mediators. Several reports in the literature have shown that this balance is essential for the homeostasis to be restored after noxious stimulation, such as bacterial infection in septic syndromes. Clinical data obtained in septic patients by our group demonstrated a role for MCP-1/CCL2 as a biomarker to predict early mortality (<48 h). Monocyte chemoattractant protein 1/CCL2 levels showed the best accuracy for predicting 28-day mortality and were also associated with prognosis (35). With this new study, we are further reinforcing the importance of MCP-1/CCL2 in the pathophysiology of sepsis pointing to its central role in the elimination of microorganisms.
In summary, we demonstrated a crucial role of MCP-1/CCL2 in the clearance of bacteria by a mechanism that involves increased expression of iNOS and production of NO by ERK signaling pathways. The data further support the postulate that maintenance of a balance between proinflammatory and anti-inflammatory factors during sepsis is necessary for limiting tissue damage and maximizing beneficial infection control effects of inflammation in models of sepsis. This has also provided a novel mechanism through which MCP-1/CCL2 and NO participate in bacterial clearance in septic responses.
ACKNOWLEDGMENTS
The authors thank Dr. Claudia Benjamin and Dr. Willian A. Kuziel for CCR2 knockout animals, and Dr. Craig Gerard from the Children’s Hospital (Harvard Medical School) for kindly providing MCP-1/CCL2 knockout mice and their backcrossed controls. They also thank Dr. Alan de Brito Carneiro for support in Western blot analysis and Dr. Adriana Vieira de Abreu for support in the immunolocalization protocol.
This work was supported by PAPES-FIOCRUZ, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), and Fundaçã o de Amparo à Pesquisa do Rio de Janeiro (FAPERJ, Brazil). The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Dr. Zimmerman’s research activities are in part supported by National Institutes of Health/National Heart, Lung, and Blood Institute award SR37WLO44525.
ABBREVIATIONS
- CLP
cecal ligation and puncture
- NOS
nitric oxide synthase
- iNOS
induced nitric oxide synthase
- l-NAME
l-NG-nitroarginine methyl ester
Footnotes
The authors have declared that no competing interests exist.
REFERENCES
- 1.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 2.Marshall JC. Such stuff as dreams are made on: mediator-directed therapy in sepsis. Nat Rev Drug Discov. 2003;2:391–405. doi: 10.1038/nrd1084. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 4.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 5.Rot A, von Andrian UH. chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 6.Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 7.Nakano Y, Kasahara T, Mukaida N, Ko YC, Nakano M, Matsushima K. Protection against lethal bacterial infection in mice by monocyte-chemotactic and -activating factor. Infect Immun. 1994;62:377–383. doi: 10.1128/iai.62.2.377-383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Strieter RM, Kunkel SL. Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: cross-talk between MCP-1 and leukotriene B4. J Immunol. 1999;163:6148–6154. [PubMed] [Google Scholar]
- 9.Amano H, Morimoto K, Senba M, Wang H, Ishida Y, Kumatori A, Yoshimine H, Oishi K, Mukaida N, Nagatake T. Essential contribution of monocyte chemoattractant protein-1/C-C chemokine ligand-2 to resolution and repair processes in acute bacterial pneumonia. J Immunol. 2004;172:398–409. doi: 10.4049/jimmunol.172.1.398. [DOI] [PubMed] [Google Scholar]
- 10.Gomes RN, Bozza FA, Amancio RT, Japiassu AM, Vianna RC, Larangeira AP, Gouvea JM, Bastos MS, Zimmerman GA, Stafforini DM, et al. Exogenous platelet-activating factor acetylhydrolase reduces mortality in mice with systemic inflammatory response syndrome and sepsis. Shock. 2006;26:41–49. doi: 10.1097/01.shk.0000209562.00070.1a. [DOI] [PubMed] [Google Scholar]
- 11.Gomes RN, Figueiredo RT, Bozza FA, Pacheco P, Amancio RT, Laranjeira AP, Castro-Faria-Neto HC, Bozza PT, Bozza MT. Increased susceptibility to septic and endotoxic shock in monocyte chemoattractant protein 1/cc chemokine ligand 2–deficient mice correlates with reduced interleukin 10 and enhanced macrophage migration inhibitory factor production. Shock. 2006;26:457–463. doi: 10.1097/01.shk.0000228801.56223.92. [DOI] [PubMed] [Google Scholar]
- 12.Petros A, Lamb G, Leone A, Moncada S, Bennett D, Vallance P. Effects of a nitric oxide synthase inhibitor in humans with septic shock. Cardiovasc Res. 1994;28:34–39. doi: 10.1093/cvr/28.1.34. [DOI] [PubMed] [Google Scholar]
- 13.Spain DA, Wilson MA, Bar-Natan MF, Garrison RN. Nitric oxide synthase inhibition aggravates intestinal microvascular vasoconstriction and hypoperfusion of bacteremia. J Trauma. 1994;36:720–725. doi: 10.1097/00005373-199405000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Moilanen E, Vapaatalo H. Nitric oxide in inflammation and immune response. Ann Med. 1995;27:359–367. doi: 10.3109/07853899509002589. [DOI] [PubMed] [Google Scholar]
- 15.Tsai WC, Strieter RM, Zisman DA, Wilkowski JM, Bucknell KA, Chen GH, Standiford TJ. Nitric oxide is required for effective innate immunity against Klebsiella pneumoniae. Infect Immun. 1997;65:1870–1875. doi: 10.1128/iai.65.5.1870-1875.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas SK, Sodhi A, Paul S. Regulation of nitric oxide production by murine peritoneal macrophages treated in vitro with chemokine monocyte chemo-attractant protein 1. Nitric Oxide. 2001;5:566–579. doi: 10.1006/niox.2001.0370. [DOI] [PubMed] [Google Scholar]
- 17.Bossink AW, Paemen L, Jansen PM, Hack CE, Thijs LG, Van Damme J. Plasma levels of the chemokines monocyte chemotactic proteins-1 and -2 are elevated in human sepsis. Blood. 1995;86:3841–3847. [PubMed] [Google Scholar]
- 18.Forslund T, Sundqvist T. Nitric oxide– releasing particles inhibit phagocytosis in human neutrophils. Biochem Biophys Res Commun. 1997;233:492–495. doi: 10.1006/bbrc.1997.6490. [DOI] [PubMed] [Google Scholar]
- 19.Franchini A, Conte A, Ottaviani E. Nitric oxide: an ancestral immunocyte effector molecule. Adv Neuroimmunol. 1995;5:463–478. doi: 10.1016/0960-5428(95)00029-1. [DOI] [PubMed] [Google Scholar]
- 20.Moncada S. The 1991 Ulf von Euler Lecture. The l-arginine: nitric oxide pathway. Acta Physiol Scand. 1992;145:201–227. doi: 10.1111/j.1748-1716.1992.tb09359.x. [DOI] [PubMed] [Google Scholar]
- 21.Cross AS, Opal SM. A new paradigm for the treatment of sepsis: is it time to consider combination therapy? Ann Intern Med. 2003;138:502–505. doi: 10.7326/0003-4819-138-6-200303180-00016. [DOI] [PubMed] [Google Scholar]
- 22.Gaieski DF, Mikkelsen ME, Band RA, Pines JM, Massone R, Furia FF, Shofer FS, Goyal M. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2012;38:1045–1053. doi: 10.1097/CCM.0b013e3181cc4824. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui S, Salahuddin N, Raza A, Razzak J. How early do antibiotics have to be to impact mortality in severe sepsis? A prospective, observational study from an emergency department. J Ayub Med Coll Abbottabad. 2009;21:106–110. [PubMed] [Google Scholar]
- 24.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 25.Balamayooran G, Batra S, Balamayooran T, Cai S, Jeyaseelan S. Monocyte chemoattractant protein 1 regulates pulmonary host defense via neutrophil recruitment during Escherichia coli infection. Infect Immun. 2011;79:2567–2577. doi: 10.1128/IAI.00067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia T, Serbina NV, Brandl K, Zhong MX, Leiner IM, Charo IF, Pamer EG. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol. 2008;180:6846–6853. doi: 10.4049/jimmunol.180.10.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hauser B, Radermacher P, Thiemermann C, Matejovic M. Nitric oxide, bacteria, and host defense in sepsis: who needs what? Shock. 2004;22:588–590. doi: 10.1097/00024382-200412000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Beck PL, Xavier R, Wong J, Ezedi I, Mashimo H, Mizoguchi A, Mizoguchi E, Bhan AK, Podolsky DK. Paradoxical roles of different nitric oxide synthase isoforms in colonic injury. Am J Physiol Gastrointest Liver Physiol. 2004;286:G137–G147. doi: 10.1152/ajpgi.00309.2003. [DOI] [PubMed] [Google Scholar]
- 29.Liew FY. Nitric oxide in infectious and autoimmune diseases. Ciba Found Symp. 1995;195:234–239. discussion 239–244. [PubMed] [Google Scholar]
- 30.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 31.Vromen A, Arkovitz MS, Zingarelli B, Salzman AL, Garcia VF, Szabo C. Low-level expression and limited role for the inducible isoform of nitric oxide synthase in the vascular hyporeactivity and mortality associated with cecal ligation and puncture in the rat. Shock. 1996;6:248–253. doi: 10.1097/00024382-199610000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Kilbourn RG, Szabo C, Traber DL. Beneficial versus detrimental effects of nitric oxide synthase inhibitors in circulatory shock: lessons learned from experimental and clinical studies. Shock. 1997;7:235–246. doi: 10.1097/00024382-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Linares E, Giorgio S, Mortara RA, Santos CX, Yamada AT, Augusto O. Role of peroxynitrite in macrophage microbicidal mechanisms in vivo revealed by protein nitration and hydroxylation. Free Radic Biol Med. 2001;30:1234–1242. doi: 10.1016/s0891-5849(01)00516-0. [DOI] [PubMed] [Google Scholar]
- 34.Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L, Breen D, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 35.Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC, Bozza PT/ Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11:R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]