Abstract
Recent advances in our understanding of the sophistication of the cellular microenvironment and the dynamics of tissue remodeling during development, disease, and regeneration have increased our appreciation of the current challenges facing tissue engineering. As this appreciation advances, we are better equipped to approach problems in the biology and therapeutics of even more complex fields, such as stem cells and cancer. To aid in these studies, as well as the established areas of tissue engineering, including cardiovascular, musculoskeletal, and neural applications, biomaterials scientists have developed an extensive array of materials with specifically designed chemical, mechanical, and biological properties. Herein, we highlight an important topic within this area of biomaterials research, protein–hydrogel interactions. Due to inherent advantages of hydrated scaffolds for soft tissue engineering as well as specialized bioactivity of proteins and peptides, this field is well-posed to tackle major needs within emerging areas of tissue engineering. We provide an overview of the major modes of interactions between hydrogels and proteins (e.g., weak forces, covalent binding, affinity binding), examples of applications within growth factor delivery and three-dimensional scaffolds, and finally future directions within the area of hydrogel–protein interactions that will advance our ability to control the cell–biomaterial interface.
Overview of Protein–Hydrogel Interaction Mechanisms
As we begin to understand more about the in vivo cellular milieu and strive to recapitulate more of its features, protein–hydrogel interactions are gaining increasing importance in tissue engineering. Protein-conjugated hydrogels are now routinely used as a simplified mimic of the extracellular matrix (ECM), where such cell populated constructs represent either platforms for basic discovery or fully functional tissue replacements. Whereas the value of hydrogel-based scaffolds has long been established for soft tissue engineering applications, the incorporation of various proteins in the scaffold structure has more recently been appreciated as beneficial toward eliciting a desired cell response, degradability, or manipulating the mechanical and physical properties of the scaffold itself. However, major concerns when incorporating proteins include the preservation of bioactivity as well as proper presentation for maximum desired effect.
This review provides an overview of strategies used to create protein–hydrogel conjugates, with emphasis on covalent and affinity interactions, but also touching upon weak interactions, such as adsorption. We focus on two applications of protein–hydrogel interactions in tissue engineering: immobilization of growth factors and conjugates for use as three-dimensional (3D) scaffolds for cell growth. Due to the nature of engineered tissues, which require a favorable cell environment, the emphasis will be on chemistries that are either nontoxic to cells or lead to nontoxic final products. In addition, because preservation of protein activity is essential, only water-based chemistries will be considered. The necessity to employ only water-based, nontoxic chemistries for the design of cell-compatible biomaterials, particularly in the context of 3D scaffold preparations, poses rather rigid constrains on materials processing. Here, the cell compatibility of the materials extends beyond chemical versatility of the hydrogels; it encompasses the design of physical and bioactive environments that are capable of eliciting specific interactions from biological systems. The authors recommend several comprehensive reviews1–3 stressing the importance and associated limitations of designing bioactive and biocompatible hydrogels.
Adsorption, electrostatic, and other weak interactions
Weak interactions, such as electrostatic binding or repulsion, hydrogen bonding, and van der Waals forces typically underline physisorption and chemisorption of biomolecules onto hydrogels. Adsorption has been extensively explored as means of incorporating biomolecules into scaffolds for release in tissue engineering applications. For example, a solution of vascular endothelial growth factor (VEGF) loaded onto freeze-dried collagen gels allowed the growth factor to adsorb onto the hydrogel during hydration and swelling. Adsorption had also been used for loading bone morphogenic protein-2 (BMP-2) into various particulate vehicles4,5 and basic fibroblast growth factor into hydrogel matrices.6 In some cases, such as the adsorption of human serum albumin onto chitosan/alginate systems, electrostatic and hydrophobic forces, as well as surface topography, were found to affect protein adsorption synergistically.7
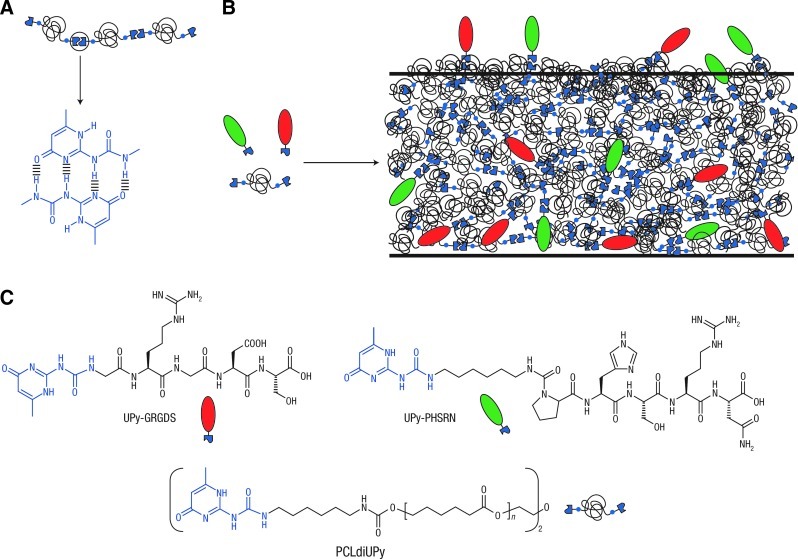
Hydrogen bonding is another weak interaction used in protein–polymer modifications, where plasma surface treatment can be used to introduce carboxylic functional groups to the polymer.8 Due to its reversible nature, hydrogen bonding is amenable to the creation of degradable polymer scaffolds. For example, the self-complimentary hydrogen-bonding moieties (ureido-pyrimidinone or UPy groups) were utilized as the basis to assemble polymers and biomolecules (Fig. 1).9 In gels with polar and charged functionality, hydrogen bonding, electrostatic interactions, and van der Waals forces can all influence protein–hydrogel interactions. For example, pH-sensitive insulin release was achieved with carboxylated chitosan-grafted nanoparticles.10
FIG. 1.
(A) The self-complementary hydrogen-bonding ureido-pyrimidinone (UPy) moiety in a supramolecular polymer. (B) The modular approach to constructing bioactive materials with various properties by mixing different UPy-functionalized biomolecules (green and red moieties) with UPy-polymers. (C) As building blocks for these materials, two UPy-functionalized peptides (UPy-GRGDS and UPy-PHSRN) were used in combination with an oligocaprolactone functionalized on both ends with UPy-units (PCLdiUPy). Image reproduced from Dankers et al.9 Color images available online at www.liebertpub.com/teb
Though adsorption may be sufficient for in vitro applications, this approach is associated with challenges in vivo due to desorption and exchange with physiological fluid. Further, inherently adsorptive scaffolds may cause a long-term inflammation or fibrosis. Conversely, nonionic hydrophilic polymers, such as poly(ethylene glycol) (PEG), poly(vinyl alcohol), and polyacrylamide, are considered inert to protein adsorption and other methods for efficient immobilization are generally required.
Affinity interactions
Inspired by the mechanism by which bioactive molecules are stored and presented in native ECM, interactions based on affinity between two biomolecules pose the advantage of tailoring the biomolecule release rate and mechanism. In hydrogels, affinity molecules or peptides are used to incorporate functionalized biomolecules, such as growth factors, into the gel structure.
Naturally derived affinity molecules
Sulfated glycosaminoglycans, such as heparin sulfate, are one class of ECM molecules that bind growth factors in vivo via electrostatic interactions. Due to their high content of functional groups available for modification with crosslinking sites, glycosaminoglycans have been utilized for several decades to modulate growth factor release in hydrogels. In 1991, Edelman et al. developed heparin-modified beads to achieve a controlled release of basic fibroblast growth factor by affinity binding.11 Since this work, heparin has been extensively explored in biomaterials for affinity interactions with a number of growth factors,12,13 including hepatocyte growth factor (HGF),14,15 neurotrophin-3,16 BMP-2,17 and VEGF.18 Other uses of heparin-based affinity include assembly of noncovalent 3D networks for enhancing cellular affinity19 or inducing stem cell differentiation.20 Another glycosaminoglycan, the negatively charged chondroitin sulfate, has demonstrated affinity for several positively charged growth factors, including nerve growth factor (NGF).21
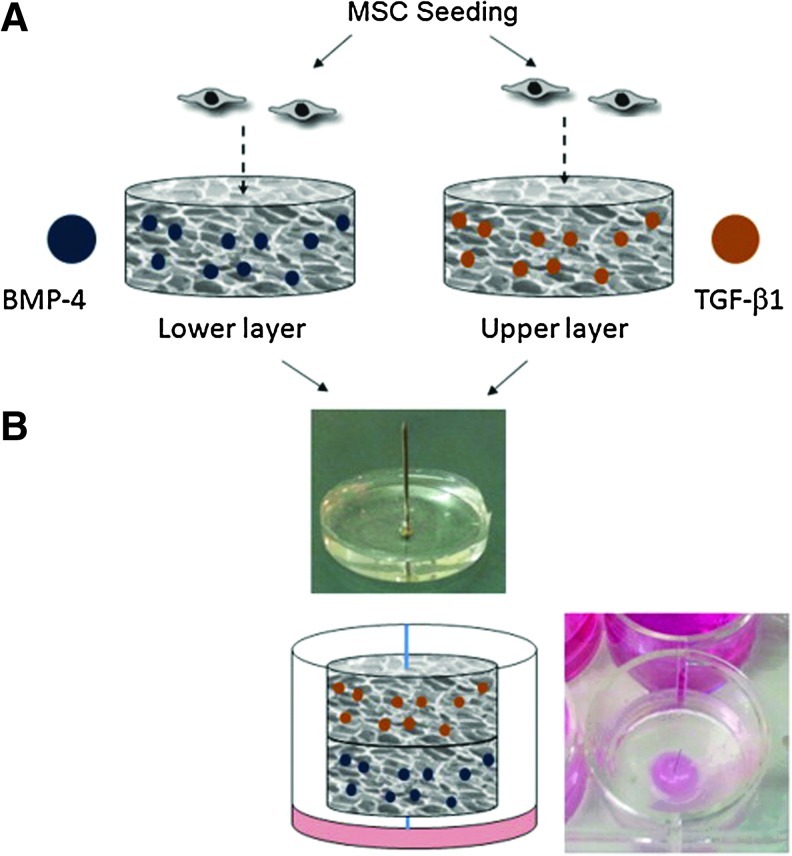
Hyaluronic acid (HA) and alginate are also naturally derived biomolecules and have structural similarities to heparin. Sulfated HA-alginate biomaterials have exhibited affinity binding to VEGF, platelet-derived growth factor-BB, and transforming growth factor-β1 (TGF-β1).22 Alginate hydrogels are simple injectable hydrogels, capable of controlled delivery of multiple proteins, while preserving the properties of the alginate.23 For example, alginate hydrogels were used to achieve dual delivery of insulin-like growth factor-1 and HGF to promote myocardial repair in a rat model of acute myocardial infarction,24 as well as for dual delivery of TGF-β1 and BMP-4 to induce articular cartilage regeneration in a rabbit model of subchondral defect (Fig. 2).25
FIG. 2.
A scheme describing the construction of a bilayer affinity-binding alginate scaffold. Transforming growth factor-β (TGF-β1) and bone morphogenic protein-4 (BMP-4) were affinity-bound to alginate sulfate in macroporous alginate scaffolds. After cell seeding and a culture of 2 days (A), the seeded scaffolds were combined together by their assembly on a stainless steel pin placed perpendicular to a supporting poly(dimethyl siloxane) layer (B). Image reproduced from Re'em et al.25 Color images available online at www.liebertpub.com/teb
Protein components of the ECM also have affinity to growth factors and have been explored in affinity binding biomaterials. For example, collagens I, III, V, and VI have affinity to HGF26 and fibronectin and vitronectin domains can bind several growth factors, including HGF.27
Affinity peptides and other synthetic affinity molecules
A variety of affinity peptides have been identified which are derived from ligand–receptor binding motifs and ECM structural molecules. In comparison to large naturally derived biomolecules, short peptide sequences are generally easier to synthesize and incorporate into hydrogels, and offer more control over material properties. For example, Maynard and Hubbell identified a sulfated heparin-mimetic tetrapeptide that binds to VEGF28; Anseth and coworkers, identified affinity peptides that bind to TGF-β1,29 and Hudalla and Murphy used a peptide derived from the heparin-binding domain of FGF-2 to sequester heparin proteoglycans.30,31 The general approach to incorporate peptides into hydrogels is to utilize their various functional groups (i.e., amines, carboxyls, alcohols, thiols) to bind to the hydrogel structure (see also section Covalent interactions). For example, peptides containing Cys can react via Michael-type addition of thiol to vinyl sulfone or acrylate groups present in hydrogel polymers.32 Due to their versatility and relatively simple structure, the kinetics of a variety of peptide-based affinity interactions have been explored. Notably, Lin and Anseth determined the relationship between the peptide structure and affinity binding to the basic fibroblast growth factor within PEG hydrogels.33 Sakiyama-Elbert and coworkers identified peptides with affinity to NGF and also developed a mathematical model to describe the kinetics of the affinity binding reaction.34 Vulic and Shoichet uncovered the specific binding of the Src homology 3 domain expressed in growth factors to proline-rich peptides,35 and Wang et al. developed a new collagen mimetic peptide that binds to collagen type I via ionic interactions and shows specific affinity to VEGF.36
A variety of synthetic affinity molecules have been developed for affinity chromatography and are readily applied to biomaterial systems as well. For example, metal-ion chelation chemistry has been utilized to functionalize hydrogels with affinity motifs. Lin and Metters modified gels with iminodiacetic acid groups that chelate with hexa-histidine tagged protein.37 This approach may provide a specific advantage for controlled release as the growth factor can be dissociated and released on demand by the addition of metal ions. Further, multiple ligand–metal pairs may be implemented to provide cascades of multiple growth factors.38
Covalent interactions
Covalent tethering of peptides and proteins onto hydrogels is desirable in a variety of tissue engineering and controlled release applications and has been utilized with natural as well as synthetic hydrogels.39,40 Covalent attachment of biomolecules is generally more stable, provides greater control over biomolecule presentation and release, and is able to generate superior hydrogel mechanical properties as compared to physical entrapment, adsorption, or affinity interactions.41,42
For the covalent fuctionalization of hydrogels with proteins, one must consider reaction conditions that sustain the protein structure and activity. Protein immobilization reactions generally require aqueous conditions, since organic solvents may cause denaturation and degradation. Many of the widely used protein chemistries are nonspecific in that they target amines (N-terminus, Lys), carboxyls (C-terminus, Asp, Glu), and hydroxyls (Ser, Thr, Tyr). The presence of multiple available residues for modification makes these covalent interactions attractive for a wide range of applications, such as fluorescent labeling. However, in other applications, such as tethering of growth factors or adhesive motifs, random modifications can affect the proper biomolecule presentation by either altering protein conformation or by sterically blocking the protein-active site. Thus, random modification can lead to heterogeneous and poorly reproducible substrates. We highlight several immobilization strategies that have shown particular advantage for reaction specificity, patterning, or preserving bioactivity and biocompatibility. For an excellent resource on these and other covalent tethering chemistries, we recommend Hermanson's “Bioconjugate Techniques”.43
Carbodiimide and succinimide chemistries
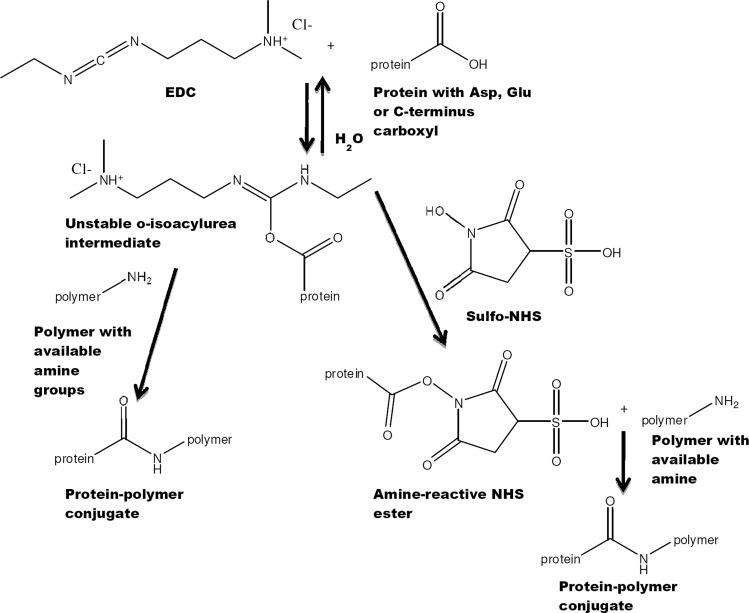
The carboxyl-activating agent 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) is a water soluble carbodiimide widely used for coupling to primary amines, yielding stable amide bonds (Fig. 3). Carbodiimide chemistry has been applied to modify amine-terminated polycaprolactone-PEG nanofibers with epidermal growth factor,44 to immobilize VEGF onto collagen scaffolds,45 and to immobilize NGF onto gelatin-tricalcium phosphate membranes.46 EDC reacts with a carboxyl to form an amine-reactive O-acylisourea intermediate. Since this intermediate rapidly hydrolyzes in an aqueous environment, EDC is often used in combination with N-hydroxysuccinimide (NHS) or sulfo-NHS to increase coupling efficiency by creating a more stable intermediate.43 Reaction conditions, such as pH, are key determinants of reaction outcome, with optimal pH from 4.7 to 6.0, even though reaction can occur at mildly alkaline conditions, but with a slower rate and lower yields. Potential side reactions to note when considering EDC/NHS chemistry with proteins include the reaction of EDC with Cys sulfhydryls and Tyr phenols as well as the reaction of sulfo-NHS with the imidazolyl group in His.43
FIG. 3.
Schematic representation of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)/sulfo-N-hydroxysuccinimide (NHS) chemistry. In this example, a stable amide bond is formed between the carboxylate group of a protein and the amine groups of a polymer, but due to the modular nature of the reaction, protein amines can also be reacted with polymer carboxylates. Sulfo-NHS is added to improve reaction yield by forming a semistable reaction intermediate.
One challenge to EDC chemistry stems from the fact that the carbodiimide, due to its nonspecific nature, may cause inter- or intraprotein reactions and alter biomolecule activity or cause aggregation. To address this drawback, step immobilization can be used to first activate polymer carboxyls, and then react with protein amines; this approach was effective for modifying collagen gels to present uniform or gradient patterns of immobilized VEGF.47,48
Photo-immobilization
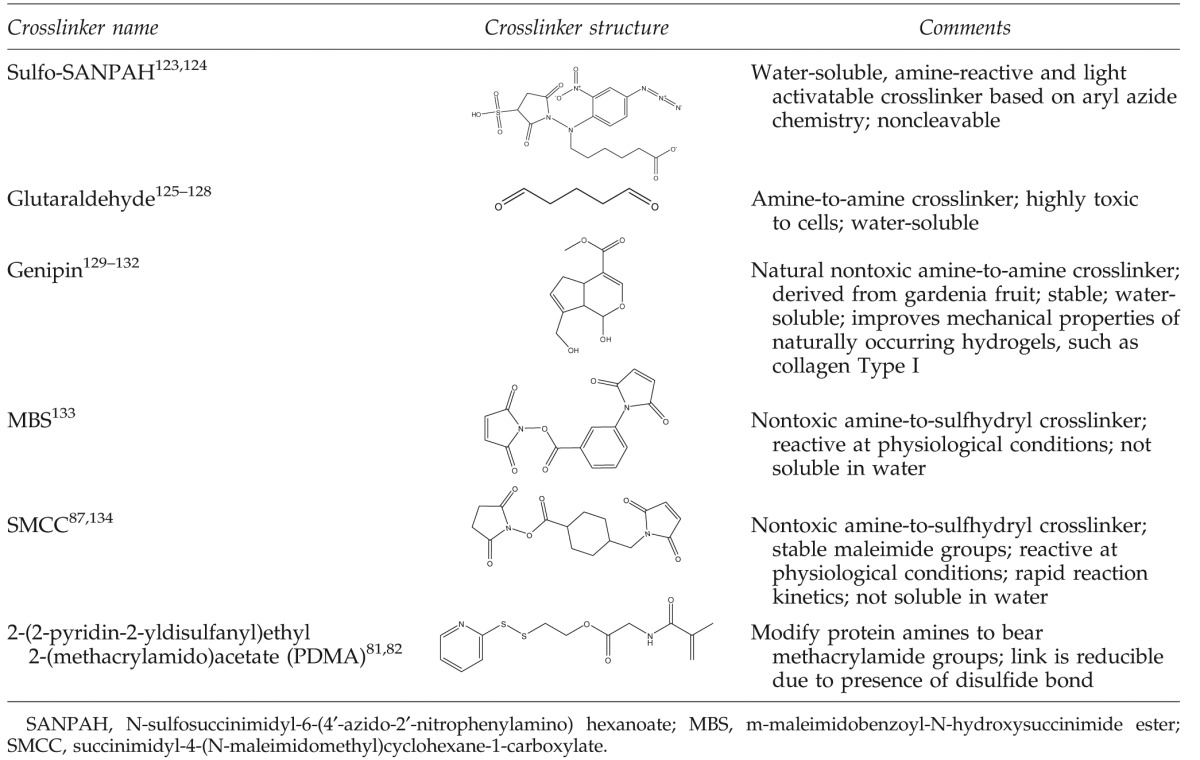
In many cases, it is beneficial to use photoreactive heterobifunctional crosslinkers, where one terminus is tailored to react with the desired functional groups (usually amines on proteins) and the other terminus is photoreactive (e.g., phenyl azide, benzophenone or diazo compound). These linkers react nonspecifically with ultraviolet (UV) light by creating singlet nitrenes that undergo insertion into C–H, N–H, and other bonds. Such crosslinkers are commercially available and their chemistry is simple, effective, and inexpensive. N-sulfosuccinimidyl-6-(4′-azido-2′-nitrophenylamino) hexanoate (sulfo-SANPAH, Table 1), which contains a photoactivatable phenyl azide and an amine-reactive NHS is one example of this class of crosslinkers. Sulfo-SANPAH has been widely used to conjugate biomolecules onto polyacrylamide gels for studying cellular response to substrate stiffness.49–51
Table 1.
Crosslinkers Used for Protein–Hydrogel Conjugation
 |
Alternatively, photochemistry can be combined with EDC chemistry, as in the case with the azidophenyl-derivatized polyallylamine method developed by Matsuda and Sugawara52 and modified by Ito et al.,53,54 where the protein is rendered photoreactive by modifying carboxyls with polyallylamine using EDC chemistry. This chemistry has been used for the immobilization of growth factors onto poly(dimethyl siloxane)55 and glass.56 Another type of photoreactive heterobifunctional crosslinkers contains an NHS or a succinimidyl carbonate group for conjugation to protein amines and a photoreactive acrylate or methacrylate group. Acrylates undergo free radical polymerization in the presence of a photoinitiator and UV irradiation. This chemistry is widely applied for growth factor immobilization, where the crosslinkers may contain a spacer arm to improve growth factor accessibility to the cells.57–60 Similar conjugations have been used to create protein–polymer and peptide–polymer conjugates to yield photopolymerizable biomimetic hydrogels.61–63
Photoimmobilization of proteins is an attractive strategy with many advantages, such as facile spatial and temporal control over reaction kinetics as well as rapid reaction conditions. However, to avoid the concern of generating active radical species during UV irradiation, which may affect protein bioactivity and cell viability, reaction conditions, such as UV exposure time and photoinitiator type and concentration must be optimized.64
Click chemistry
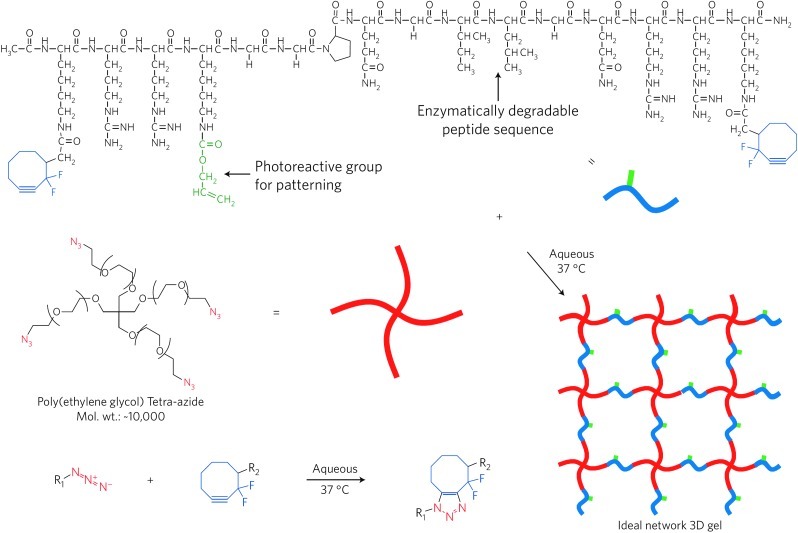
Click chemistry is a general concept that describes reactions that are high yielding, stereospecific, highly versatile, simple to perform and purify, and occur in benign solvents.65 Originally, click chemistry was introduced to accelerate drug discovery and testing, but it is increasingly becoming utilized in tissue engineering. To address a major drawback of conventional click chemistries, namely, the use of cytotoxic copper or ruthenium catalysts, copper-free click chemistry has been developed.66,67 For example, Nimmo et al. utilized click chemistry to design a one-step, aqueous-based method to crosslink HA hydrogels for use in tissue engineering.68 Anseth and coworkers adapted a click chemistry to perform spatial and temporal patterning of polypeptides onto PEG hydrogels to pattern 3D cell microenvironments (Fig. 4).69,70
FIG. 4.
Click-functionalized macromolecular precursors undergo the Cu-free [3+2] Huisgen cycloaddition to form a 3D ideal network hydrogel through a step-growth polymerization mechanism. Here, two orthogonal click chemistries are used: one for hydrogel formation and another for biochemical patterning within the preformed material. The modular aspect of these reactions allows for independent control of the network structure and chemistry, and facile incorporation of biological molecules. Image reproduced from DeForest et al.70 Color images available online at www.liebertpub.com/teb
Other chemistries
There are a large number of other chemistries used to create protein–hydrogel conjugates of which we will only attempt to describe a few. One such reaction is the Michael-type addition pioneered for use in tissue engineering by Elbert and Hubbell71 and Lutolf and Hubbell.72 In this reaction, unsaturated double bonds of chemical moieties, such as vinyl sulfone, acrylate, or methacrylate react under basic conditions with a thiol typically provided by Cys. For peptides, the reactivity of the thiol can be modulated by the presence of charged amino acids in the direct vicinity of the Cys.73 This chemistry is biocompatible and selective, and therefore ideal for protein conjugation and has been successfully used for conjugation of polymers to Cys-terminated peptides,74,75 genetically engineered growth factors with an added Cys,76,77 and other proteins.78,79
Homo- and hetero-bifunctional crosslinkers, despite their inherent nonspecific reactivity, are very useful for the crosslinking of polymers and proteins. Thus, in addition to linkers that have been applied for decades, such as glutaraldehyde, new crosslinkers (Table 1) are being developed with further improvements over existing technologies. Main advantages of the novel crosslinkers include lack of toxicity to cells, as well as improved versatility by incorporation of specific moieties, such as acrylates or disulfides, that permit controlled protein release, gelation strategies and kinetics, or gel degradation. For example, genipin, a naturally occurring crosslinker, has toxicity levels 10,000-fold lower than glutaraldehyde.80 Further, the acrylate functionality, which is responsible for photopolymerization of PEG-diacrylate gels, is utilized for several protein–hydrogel crosslinking reactions, including cleavable protein hydrogel crosslinkers (2-(2-pyridin-2-yldisulfanyl)ethyl 2-(methacrylamido)acetate in Table 1)81,82 and degradable hydrogels, where redox polymerization strategies allows for in-situ cell encapsulation.83 HA has been similarly modified with methacrylate to obtain gels that encompass the range of physiologically relevant mechanical properties, while maintaining HA cell receptors.84,85
Tissue Engineering Applications of Protein–Hydrogel Interactions
Protein–hydrogel interactions are desirable in a variety of tissue engineering applications, including sustained, localized, or targeted protein delivery, induction of specific cell signaling, and control of stem cell fate. We focus here on the two most common applications: immobilization of growth factors and polymer–protein conjugates for use as 3D scaffolds for cell growth, where the protein presents cell binding domains or degradation sites.
Immobilization of growth factors
Growth factors, which are involved in cellular proliferation, differentiation, and tissue regeneration, can be supplied exogenously via solution injection or by immobilization onto scaffolds.40 The latter method is generally more efficient in promoting the desired cell outcome, while preserving bioactivity and stability, which in turn prolongs growth factor signaling and minimizes costs of growth factor therapeutics. Superior growth factor performance upon immobilization has been demonstrated by Chiu and Radisic, where VEGF tethered onto collagen scaffolds was associated with a higher endothelial cell proliferation versus treatment with VEGF in a solution.86 In addition, the immobilization method itself is important for growth factor performance, as demonstrated by Park et al., who determined that BMP-2 immobilized covalently onto chitosan nanofibers had higher bioactivity, promoted greater cell proliferation, alkaline phosphatase activity, and calcium deposition when compared with nanofibers with absorbed BMP-2.87
The concentration of the growth factor influences cell response, and when varying growth factor concentration is presented as a gradient, it has the potential to guide directional cell responses. For example, Davis et al. demonstrated that increased concentration of BMP-2 adsorbed onto polymer scaffolds enhanced the osteogenic response of human mesenchymal stem cells,4 while Odedra et al. found that VEGF gradients on collagen scaffolds promoted directional endothelial cell migration.48 Further, Moore et al. revealed that neurotrophin gradients on synthetic gels promoted guided neurite outgrowth from chick dorsal root ganglia neurons.88
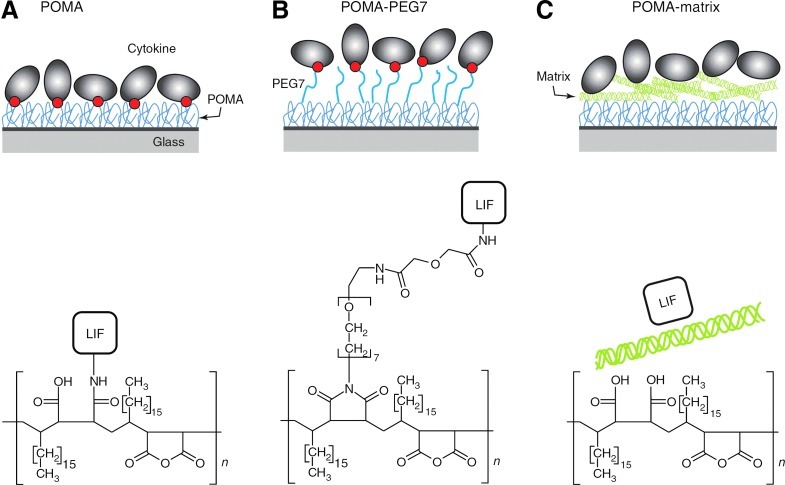
Growth factor tethering is especially favored for in-vivo scaffold applications, where it offers the additional advantage of localizing the effect of the growth factor within the scaffold itself rather than allow it to freely diffuse into the surrounding tissue. Further, immobilized growth factors have a physiological significance, as both bound and free growth factors are present in vivo. It is thought that cells first respond to the soluble growth factor, migrate toward its source, and then respond to the higher concentration of bound protein at the source. For example, Miyagi et al. covalently immobilized VEGF onto collagen scaffolds to promote neovascularization in vivo.89 The authors showed that scaffolds containing VEGF had greater blood vessel density than the control scaffolds with no VEGF, due to increased endothelial cell infiltration and proliferation.89 Further, Choi et al. demonstrated that the epithelial growth factor covalently immobilized onto polycaprolactone-PEG scaffolds promoted keratinocyte differentiation and resulted in superior wound healing in vivo than control groups with the soluble growth factor.44 Alberti and coworkers compared various methods of immobilizing the leukemia inhibitory factor onto hydrogels, including covalent attachment, covalent attachment via a flexible PEG spacer arm, and noncovalent binding (Fig. 5). They determined that the spacer arm increased growth factor accessibility and resulted in greater mouse embryonic stem cell pluripotency.90
FIG. 5.
(A–C) A cytokine, leukemia inhibitory factor (LIF), was immobilized by covalent attachment to a hydrogel composed of poly(octadecene-alt-maleic anhydride) (POMA) (A), covalent attachment to flexible poly(ethylene glycol) (PEG) spacer arms tethered to POMA (B) and noncovalent binding to extracellular matrix coating deposited on top of hydrolyzed POMA (C). Red circles indicate covalent bond. Note that POMA was covalently bound to amino-functionalized glass substrates and is the key component of the immobilization platform. POMA and POMA-PEG7 surfaces were coated with matrix for cell culture experiments (omitted from the figure for clarity). Only one covalent bond between the protein and the surface is shown, although more bonds are possible. Chemical structures depict the POMA layer and the immobilization mode of LIF. Image reproduced from Alberti et al.90 Color images available online at www.liebertpub.com/teb
Protein–hydrogel copolymers for use as 3D cell scaffolds
In tissue engineering applications, cell scaffolds ideally should mimic the natural structure of the tissue they are replacing, while providing a structural support for the cells. Polymer–protein conjugates are especially promising for use as tissue engineering scaffolds for a variety of reasons, including the addition of specific biological activity to already biocompatible substrates.
Conjugates of proteins and synthetic hydrogels
Synthetic biomaterials are widely used in tissue engineering because they offer precise control over a wide range of scaffold mechanical properties.85,91,92 Protein modification is especially advantageous for synthetic materials because proteins may provide intrinsic biological activity, such as cell adhesion or proteolytically degradable sites to typically inert polymers.
PEG, arguably the most widely used synthetic polymer in soft tissue engineering, has been conjugated to a variety of proteins to form biosynthetic hydrogel scaffolds. For example, Almany and Seliktar developed PEG-fibrinogen scaffolds as 3D environments for tissue regeneration therapies.78 The fibrinogen provided cell attachment motifs as well as proteolytic sensitivity for degradation, while PEG was responsible for the superior hydrogel elastic modulus.78 Comparing the properties of three different PEG-protein copolymers, Gonen-Wadmany et al. reported cell spreading and migration on PEG-fibrinogen and PEG-collagen gels, and not on PEG-bovine serum albumin gels due to lack of cell adhesion motifs on the bovine serum albumin backbone.93 Interestingly, the composite hydrogels also showed different elasticity, swelling, and degradability based on the incorporated protein, underscoring the importance of the molecular relationship between the polymer and the protein.93
Composites of collagen and polyanhydrides, such as polylactic acid, polyglycolic acid, and polycaprolactone, have been synthesized as nanofibrous matrices,94–96 grafted copolymers,97 and composite gels98–100 and are widely used in bone and cartilage tissue engineering, because of their similarity to natural bone or cartilage in main composition and hierarchical nanostructure. For example, these composite gels have been used in the form of a polylactic-glycolic acid sponge prepared with incorporated collagen microsponges in the pores, where the collagen facilitated homogeneous cell seeding and subsequent cell differentiation.101 Electrospun collagen/polycaprolactone materials have also been used as nerve guide conduits in vivo where they were able to support the nerve regeneration through an 8-mm sciatic nerve gap in adult rats, thus approaching the effectiveness of the nerve autograft.94 Collagen-poly(lactic acid-co-caprolactone) hybrid scaffolds have been used for bladder tissue regeneration, where the hybrid hydrogels exhibited a lower inflammatory reaction and degradation times than scaffolds without collagen.102
Peptides are commonly incorporated into hydrogels, including motifs derived from the ECM, such as the cell adhesive peptide RGD,83,103 as well as peptides susceptible to matrix metalloproteinase cleavage that allow proteolytic gel degradation.32,104 Methods to incorporate peptide ligands onto biomaterials have been reviewed extensively and, thus, can be found elsewhere.39,105
Conjugates of proteins and natural hydrogels
Because of their intrinsic biocompatibility, natural biomaterials are also very attractive for use in tissue engineering despite their relatively low mechanical strength. Usually, protein-hydrogel matrices are meant to either elicit a specific biological response from the cells (e.g., growth factor immobilization) or to improve the composite hydrogel mechanical, biochemical, or physical properties over the individual materials alone.106–108 The most common natural materials used in tissue engineering include HA, chitosan, collagen, fibrinogen, fibrin, and agarose.109
A variety of composites based on natural materials have been developed to address a major challenge in the construction of 3D scaffolds for tissue engineering; namely, the development of highly porous scaffolds with interconnected pores.110 Various collagen/HA/chitosan scaffolds with highly porous interconnected structures were manufactured by varying ratios between the constituents to find the optimal conditions for tissue engineering applications.111 Chitosan–HA-based hydrogels were created as injectable, biodegradable, and glucose-responsive hydrogels to deliver insulin in vitro.112 Highly porous collagen/HA composite hydrogels have also been developed as a model of the mammary gland,113 as a matrix for invertebral disk repair,114,115 and as a scaffold to study glioma cell invasion.116 Further, collagen/fibrin composite matrices were used as vascular constructs because of their superior mechanical strength in comparison to each of the components alone.117
Future Directions
While there is an array of well-established chemistries for physical, covalent, or affinity conjugation of proteins, one of the major areas in need of further development, especially in terms of growth factor conjugation, is the presentation of biomolecules. The majority of available conjugation chemistries are nonspecific, thus compromising molecule bioactivity by binding site unavailability due to orientation or steric hindrance. The nonspecific chemistries also raise issues of robustness, heterogeneities, as well as unwanted side interactions, such as competitive binding of media or serum proteins. These limitations are beginning to be addressed by adding tags to the biomolecule to ensure binding specificity or by adding spacer arms between the hydrogel and the protein to avoid steric hindrance. Examples of tags and linkers include Cys,118 Fc,119 polyhistidine,120,121 as well as de novo-designed coil-tagged chimeras.122 Overall, the development of more protein–hydrogel interactions, as well as the accumulation of knowledge about their effect on cell fate, will enable the design of engineered tissues that better mimic the in-vivo environment, as well as tissues that can be safely translated into the clinic.
Acknowledgments
We acknowledge NIH-NINDS R01NS065205 and funds from the Intramural Research Program of The Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH for supporting this work.
Disclosure Statement
No competing financial interests exist.
References
- 1.Lutolf M.P. Gilbert P.M. Blau H.M. Designing materials to direct stem-cell fate. Nature. 2009;462:433. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutolf M.P. Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 3.Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336:1124. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 4.Davis H.E. Case E.M. Miller S.L. Genetos D.C. Leach J.K. Osteogenic response to BMP-2 of hMSCs grown on apatite-coated scaffolds. Biotechnol Bioeng. 2011;108:2727. doi: 10.1002/bit.23227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie G. Sun J. Zhong G. Liu C. Wei J. Hydroxyapatite nanoparticles as a controlled-release carrier of BMP-2: absorption and release kinetics in vitro. J Mater Sci Mater Med. 2010;21:1875. doi: 10.1007/s10856-010-4038-0. [DOI] [PubMed] [Google Scholar]
- 6.Lefler A. Ghanem A. Development of bFGF-chitosan matrices and their interactions with human dermal fibroblast cells. J Biomater Sci Polym Ed. 2009;20:1335. doi: 10.1163/092050609X12457417534295. [DOI] [PubMed] [Google Scholar]
- 7.Martins G.V. Merino E.G. Mano J.F. Alves N.M. Crosslink effect and albumin adsorption onto chitosan/alginate multilayered systems: an in situ QCM-D study. Macromol Biosci. 2010;10:1444. doi: 10.1002/mabi.201000193. [DOI] [PubMed] [Google Scholar]
- 8.Wang S. Zhang Y. Wang H. Dong Z. Preparation, characterization and biocompatibility of electrospinning heparin-modified silk fibroin nanofibers. Int J Biol Macromol. 2011;48:345. doi: 10.1016/j.ijbiomac.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Dankers P.Y. Harmsen M.C. Brouwer L.A. van Luyn M.J. Meijer E.W. A modular and supramolecular approach to bioactive scaffolds for tissue engineering. Nat Mater. 2005;4:568. doi: 10.1038/nmat1418. [DOI] [PubMed] [Google Scholar]
- 10.Cui F. Qian F. Zhao Z. Yin L. Tang C. Yin C. Preparation, characterization, and oral delivery of insulin loaded carboxylated chitosan grafted poly(methyl methacrylate) nanoparticles. Biomacromolecules. 2009;10:1253. doi: 10.1021/bm900035u. [DOI] [PubMed] [Google Scholar]
- 11.Edelman E.R. Mathiowitz E. Langer R. Klagsbrun M. Controlled and modulated release of basic fibroblast growth factor. Biomaterials. 1991;12:619. doi: 10.1016/0142-9612(91)90107-l. [DOI] [PubMed] [Google Scholar]
- 12.Oh S.H. Kim T.H. Lee J.H. Creating growth factor gradients in three dimensional porous matrix by centrifugation and surface immobilization. Biomaterials. 2011;32:8254. doi: 10.1016/j.biomaterials.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Nie T. Baldwin A. Yamaguchi N. Kiick K.L. Production of heparin-functionalized hydrogels for the development of responsive and controlled growth factor delivery systems. J Control Release. 2007;122:287. doi: 10.1016/j.jconrel.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim M. Lee J.Y. Jones C.N. Revzin A. Tae G. Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials. 2010;31:3596. doi: 10.1016/j.biomaterials.2010.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruvinov E. Leor J. Cohen S. The effects of controlled HGF delivery from an affinity-binding alginate biomaterial on angiogenesis and blood perfusion in a hindlimb ischemia model. Biomaterials. 2010;31:4573. doi: 10.1016/j.biomaterials.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Taylor S.J. McDonald J.W. Sakiyama-Elbert S.E. Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury. J Control Release. 2004;98:281. doi: 10.1016/j.jconrel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Jeon O. Powell C. Solorio L.D. Krebs M.D. Alsberg E. Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J Control Release. 2011;154:258. doi: 10.1016/j.jconrel.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tae G. Scatena M. Stayton P.S. Hoffman A.S. PEG-cross-linked heparin is an affinity hydrogel for sustained release of vascular endothelial growth factor. J Biomater Sci Polym Ed. 2006;17:187. doi: 10.1163/156856206774879090. [DOI] [PubMed] [Google Scholar]
- 19.Lee J. Yoo J.J. Atala A. Lee S.J. Controlled heparin conjugation on electrospun poly(epsilon-caprolactone)/gelatin fibers for morphology-dependent protein delivery and enhanced cellular affinity. Acta Biomater. 2012;8:2549. doi: 10.1016/j.actbio.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 20.Tan H. Zhou Q. Qi H. Zhu D. Ma X. Xiong D. Heparin interacting protein mediated assembly of nano-fibrous hydrogel scaffolds for guided stem cell differentiation. Macromol Biosci. 2012;12:621. doi: 10.1002/mabi.201100502. [DOI] [PubMed] [Google Scholar]
- 21.Conovaloff A. Panitch A. Characterization of a chondroitin sulfate hydrogel for nerve root regeneration. J Neural Eng. 2011;8:056003. doi: 10.1088/1741-2560/8/5/056003. [DOI] [PubMed] [Google Scholar]
- 22.Freeman I. Cohen S. The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization. Biomaterials. 2009;30:2122. doi: 10.1016/j.biomaterials.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 23.Re'em T. Kaminer-Israeli Y. Ruvinov E. Cohen S. Chondrogenesis of hMSC in affinity-bound TGF-beta scaffolds. Biomaterials. 2012;33:751. doi: 10.1016/j.biomaterials.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Ruvinov E. Leor J. Cohen S. The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials. 2011;32:565. doi: 10.1016/j.biomaterials.2010.08.097. [DOI] [PubMed] [Google Scholar]
- 25.Re'em T. Witte F. Willbold E. Ruvinov E. Cohen S. Simultaneous regeneration of articular cartilage and subchondral bone induced by spatially presented TGF-beta and BMP-4 in a bilayer affinity binding system. Acta Biomater. 2012;8:3283. doi: 10.1016/j.actbio.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Schuppan D. Schmid M. Somasundaram R. Ackermann R. Ruehl M. Nakamura T. Riecken E.O. Collagens in the liver extracellular matrix bind hepatocyte growth factor. Gastroenterology. 1998;114:139. doi: 10.1016/s0016-5085(98)70642-0. [DOI] [PubMed] [Google Scholar]
- 27.Rahman S. Patel Y. Murray J. Patel K.V. Sumathipala R. Sobel M. Wijelath E.S. Novel hepatocyte growth factor (HGF) binding domains on fibronectin and vitronectin coordinate a distinct and amplified Met-integrin induced signalling pathway in endothelial cells. BMC Cell Biol. 2005;6:8. doi: 10.1186/1471-2121-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maynard H.D. Hubbell J.A. Discovery of a sulfated tetrapeptide that binds to vascular endothelial growth factor. Acta Biomater. 2005;1:451. doi: 10.1016/j.actbio.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 29.McCall J.D. Lin C.C. Anseth K.S. Affinity peptides protect transforming growth factor beta during encapsulation in poly(ethylene glycol) hydrogels. Biomacromolecules. 2011;12:1051. doi: 10.1021/bm101379v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hudalla G.A. Murphy W.L. Immobilization of peptides with distinct biological activities onto stem cell culture substrates using orthogonal chemistries. Langmuir. 2010;26:6449. doi: 10.1021/la1008208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudalla G.A. Murphy W.L. Biomaterials that regulate growth factor activity via bioinspired interactions. Adv Funct Mater. 2011;21:1754. doi: 10.1002/adfm.201002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lutolf M.P. Lauer-Fields J.L. Schmoekel H.G. Metters A.T. Weber F.E. Fields G.B. Hubbell J.A. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci U S A. 2003;100:5413. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin C.C. Anseth K.S. Controlling affinity binding with peptide-functionalized poly(ethylene glycol) hydrogels. Adv Funct Mater. 2009;19:2325. doi: 10.1002/adfm.200900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willerth S.M. Johnson P.J. Maxwell D.J. Parsons S.R. Doukas M.E. Sakiyama-Elbert S.E. Rationally designed peptides for controlled release of nerve growth factor from fibrin matrices. J Biomed Mater Res A. 2007;80:13. doi: 10.1002/jbm.a.30844. [DOI] [PubMed] [Google Scholar]
- 35.Vulic K. Shoichet M.S. Tunable growth factor delivery from injectable hydrogels for tissue engineering. J Am Chem Soc. 2012;134:882. doi: 10.1021/ja210638x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang A.Y. Leong S. Liang Y.C. Huang R.C. Chen C.S. Yu S.M. Immobilization of growth factors on collagen scaffolds mediated by polyanionic collagen mimetic peptides and its effect on endothelial cell morphogenesis. Biomacromolecules. 2008;9:2929. doi: 10.1021/bm800727z. [DOI] [PubMed] [Google Scholar]
- 37.Lin C.C. Metters A.T. Metal-chelating affinity hydrogels for sustained protein release. J Biomed Mater Res A. 2007;83:954. doi: 10.1002/jbm.a.31282. [DOI] [PubMed] [Google Scholar]
- 38.Lin C.C. Metters A.T. Bifunctional monolithic affinity hydrogels for dual-protein delivery. Biomacromolecules. 2008;9:789. doi: 10.1021/bm700940w. [DOI] [PubMed] [Google Scholar]
- 39.Hersel U. Dahmen C. Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 40.Masters K.S. Covalent growth factor immobilization strategies for tissue repair and regeneration. Macromol Biosci. 2011;11:1149. doi: 10.1002/mabi.201000505. [DOI] [PubMed] [Google Scholar]
- 41.Giraudier S. Hellio D. Djabourov M. Larreta-Garde V. Influence of weak and covalent bonds on formation and hydrolysis of gelatin networks. Biomacromolecules. 2004;5:1662. doi: 10.1021/bm049670d. [DOI] [PubMed] [Google Scholar]
- 42.Jeong K.J. Panitch A. Interplay between covalent and physical interactions within environment sensitive hydrogels. Biomacromolecules. 2009;10:1090. doi: 10.1021/bm801270k. [DOI] [PubMed] [Google Scholar]
- 43.Hermanson G.T. Bioconjugate Techniques. 2nd. San Diego, CA: Academic Press; 2008. [Google Scholar]
- 44.Choi J.S. Leong K.W. Yoo H.S. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF) Biomaterials. 2008;29:587. doi: 10.1016/j.biomaterials.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Shen Y.H. Shoichet M.S. Radisic M. Vascular endothelial growth factor immobilized in collagen scaffold promotes penetration and proliferation of endothelial cells. Acta Biomater. 2008;4:477. doi: 10.1016/j.actbio.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Chen P.R. Chen M.H. Lin F.H. Su W.Y. Release characteristics and bioactivity of gelatin-tricalcium phosphate membranes covalently immobilized with nerve growth factors. Biomaterials. 2005;26:6579. doi: 10.1016/j.biomaterials.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 47.Chiu L.L. Weisel R.D. Li R.K. Radisic M. Defining conditions for covalent immobilization of angiogenic growth factors onto scaffolds for tissue engineering. J Tissue Eng Regen Med. 2011;5:69. doi: 10.1002/term.292. [DOI] [PubMed] [Google Scholar]
- 48.Odedra D. Chiu L.L. Shoichet M. Radisic M. Endothelial cells guided by immobilized gradients of vascular endothelial growth factor on porous collagen scaffolds. Acta Biomater. 2011;7:3027. doi: 10.1016/j.actbio.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Kandow C.E. Georges P.C. Janmey P.A. Beningo K.A. Polyacrylamide hydrogels for cell mechanics: steps toward optimization and alternative uses. Methods Cell Biol. 2007;83:29. doi: 10.1016/S0091-679X(07)83002-0. [DOI] [PubMed] [Google Scholar]
- 50.Mih J.D. Sharif A.S. Liu F. Marinkovic A. Symer M.M. Tschumperlin D.J. A multiwell platform for studying stiffness-dependent cell biology. PLoS One. 2011;6:e19929. doi: 10.1371/journal.pone.0019929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacot J.G. McCulloch A.D. Omens J.H. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J. 2008;95:3479. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuda T. Sugawara T. Development of surface photochemical modification method for micropatterning of cultured cells. J Biomed Mater Res. 1995;29:749. doi: 10.1002/jbm.820290611. [DOI] [PubMed] [Google Scholar]
- 53.Ito Y. Chen G. Imanishi Y. Micropatterned immobilization of epidermal growth factor to regulate cell function. Bioconjug Chem. 1998;9:277. doi: 10.1021/bc970190b. [DOI] [PubMed] [Google Scholar]
- 54.Ito Y. Chen G. Imanishi Y. Morooka T. Nishida E. Okabayashi Y. Kasuga M. Differential control of cellular gene expression by diffusible and non-diffusible EGF. J Biochem. 2001;129:733. doi: 10.1093/oxfordjournals.jbchem.a002913. [DOI] [PubMed] [Google Scholar]
- 55.Gomez N. Lu Y. Chen S. Schmidt C.E. Immobilized nerve growth factor and microtopography have distinct effects on polarization versus axon elongation in hippocampal cells in culture. Biomaterials. 2007;28:271. doi: 10.1016/j.biomaterials.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 56.Ogiwara K. Nagaoka M. Cho C.S. Akaike T. Effect of photo-immobilization of epidermal growth factor on the cellular behaviors. Biochem Biophys Res Commun. 2006;345:255. doi: 10.1016/j.bbrc.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Porter A.M. Klinge C.M. Gobin A.S. Covalently grafted VEGF165 in hydrogel models up-regulates the cellular pathways associated with angiogenesis. Am J Physiol Cell Physiol. 2011;301:C1086. doi: 10.1152/ajpcell.00090.2011. [DOI] [PubMed] [Google Scholar]
- 58.Mann B.K. Schmedlen R.H. West J.L. Tethered-TGF-beta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22:4390. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 59.DeLong S.A. Moon J.J. West J.L. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26:3227. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 60.Leslie-Barbick J.E. Moon J.J. West J.L. Covalently-immobilized vascular endothelial growth factor promotes endothelial cell tubulogenesis in poly(ethylene glycol) diacrylate hydrogels. J Biomater Sci Polym Ed. 2009;20:1763. doi: 10.1163/156856208X386381. [DOI] [PubMed] [Google Scholar]
- 61.Hern D.L. Hubbell J.A. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 62.Scott R. Marquardt L. Willits R.K. Characterization of poly(ethylene glycol) gels with added collagen for neural tissue engineering. J Biomed Mater Res A. 2010;93:817. doi: 10.1002/jbm.a.32775. [DOI] [PubMed] [Google Scholar]
- 63.Dikovsky D. Bianco-Peled H. Seliktar D. The effect of structural alterations of PEG-fibrinogen hydrogel scaffolds on 3-D cellular morphology and cellular migration. Biomaterials. 2006;27:1496. doi: 10.1016/j.biomaterials.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 64.Lin C.C. Sawicki S.M. Metters A.T. Free-radical-mediated protein inactivation and recovery during protein photoencapsulation. Biomacromolecules. 2008;9:75. doi: 10.1021/bm700782c. [DOI] [PubMed] [Google Scholar]
- 65.Kolb H.C. Finn M.G. Sharpless K.B. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed Engl. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 66.Baskin J.M. Prescher J.A. Laughlin S.T. Agard N.J. Chang P.V. Miller I.A. Lo A. Codelli J.A. Bertozzi C.R. Copper-free click chemistry for dynamic in vivo imaging. Proc Natl Acad Sci U S A. 2007;104:16793. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laughlin S.T. Baskin J.M. Amacher S.L. Bertozzi C.R. In vivo imaging of membrane-associated glycans in developing zebrafish. Science. 2008;320:664. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nimmo C.M. Owen S.C. Shoichet M.S. Diels-Alder Click cross-linked hyaluronic acid hydrogels for tissue engineering. Biomacromolecules. 2011;12:824. doi: 10.1021/bm101446k. [DOI] [PubMed] [Google Scholar]
- 69.Polizzotti B.D. Fairbanks B.D. Anseth K.S. Three-dimensional biochemical patterning of click-based composite hydrogels via thiolene photopolymerization. Biomacromolecules. 2008;9:1084. doi: 10.1021/bm7012636. [DOI] [PubMed] [Google Scholar]
- 70.DeForest C.A. Polizzotti B.D. Anseth K.S. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat Mater. 2009;8:659. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elbert D.L. Hubbell J.A. Conjugate addition reactions combined with free-radical cross-linking for the design of materials for tissue engineering. Biomacromolecules. 2001;2:430. doi: 10.1021/bm0056299. [DOI] [PubMed] [Google Scholar]
- 72.Lutolf M.P. Hubbell J.A. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules. 2003;4:713. doi: 10.1021/bm025744e. [DOI] [PubMed] [Google Scholar]
- 73.Lutolf M.P. Tirelli N. Cerritelli S. Cavalli L. Hubbell J.A. Systematic modulation of Michael-type reactivity of thiols through the use of charged amino acids. Bioconjug Chem. 2001;12:1051. doi: 10.1021/bc015519e. [DOI] [PubMed] [Google Scholar]
- 74.Zustiak S.P. Leach J.B. Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules. 2010;11:1348. doi: 10.1021/bm100137q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zustiak S.P. Durbal R. Leach J.B. Influence of cell-adhesive peptide ligands on poly(ethylene glycol) hydrogel physical, mechanical and transport properties. Acta Biomater. 2010;6:3404. doi: 10.1016/j.actbio.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leipzig N.D. Wylie R.G. Kim H. Shoichet M.S. Differentiation of neural stem cells in three-dimensional growth factor-immobilized chitosan hydrogel scaffolds. Biomaterials. 2011;32:57. doi: 10.1016/j.biomaterials.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 77.Zisch A.H. Lutolf M.P. Ehrbar M. Raeber G.P. Rizzi S.C. Davies N. Schmokel H. Bezuidenhout D. Djonov V. Zilla P. Hubbell J.A. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17:2260. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 78.Almany L. Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005;26:2467. doi: 10.1016/j.biomaterials.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 79.Oss-Ronen L. Seliktar D. Polymer-conjugated albumin and fibrinogen composite hydrogels as cell scaffolds designed for affinity-based drug delivery. Acta Biomater. 2011;7:163. doi: 10.1016/j.actbio.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 80.Sung H.W. Chang W.H. Ma C.Y. Lee M.H. Crosslinking of biological tissues using genipin and/or carbodiimide. J Biomed Mater Res A. 2003;64:427. doi: 10.1002/jbm.a.10346. [DOI] [PubMed] [Google Scholar]
- 81.Verheyen E. Delain-Bioton L. der Wal S. El Morabit N. Hennink W.E. van Nostrum C.F. Protein macromonomers for covalent immobilization and subsequent triggered release from hydrogels. J Control Release. 2010;148:e18. doi: 10.1016/j.jconrel.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 82.Verheyen E. Delain-Bioton L. van der Wal S. el Morabit N. Barendregt A. Hennink W.E. van Nostrum C.F. Conjugation of methacrylamide groups to a model protein via a reducible linker for immobilization and subsequent triggered release from hydrogels. Macromol Biosci. 2010;10:1517. doi: 10.1002/mabi.201000168. [DOI] [PubMed] [Google Scholar]
- 83.Chien H.W. Tsai W.B. Jiang S. Direct cell encapsulation in biodegradable and functionalizable carboxybetaine hydrogels. Biomaterials. 2012;33:5706. doi: 10.1016/j.biomaterials.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 84.Hachet E. Van Den Berghe H. Bayma E. Block M.R. Auzely-Velty R. Design of biomimetic cell-interactive substrates using hyaluronic acid hydrogels with tunable mechanical properties. Biomacromolecules. 2012;13:1818. doi: 10.1021/bm300324m. [DOI] [PubMed] [Google Scholar]
- 85.Leach J.B. Bivens K.A. Collins C.N. Schmidt C.E. Development of photocrosslinkable hyaluronic acid-polyethylene glycol-peptide composite hydrogels for soft tissue engineering. J Biomed Mater Res A. 2004;70:74. doi: 10.1002/jbm.a.30063. [DOI] [PubMed] [Google Scholar]
- 86.Chiu L.L. Radisic M. Scaffolds with covalently immobilized VEGF and angiopoietin-1 for vascularization of engineered tissues. Biomaterials. 2010;31:226. doi: 10.1016/j.biomaterials.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 87.Park Y.J. Kim K.H. Lee J.Y. Ku Y. Lee S.J. Min B.M. Chung C.P. Immobilization of bone morphogenetic protein-2 on a nanofibrous chitosan membrane for enhanced guided bone regeneration. Biotechnol Appl Biochem. 2006;43:17. doi: 10.1042/BA20050075. [DOI] [PubMed] [Google Scholar]
- 88.Moore K. MacSween M. Shoichet M. Immobilized concentration gradients of neurotrophic factors guide neurite outgrowth of primary neurons in macroporous scaffolds. Tissue Eng. 2006;12:267. doi: 10.1089/ten.2006.12.267. [DOI] [PubMed] [Google Scholar]
- 89.Miyagi Y. Chiu L.L. Cimini M. Weisel R.D. Radisic M. Li R.K. Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials. 2011;32:1280. doi: 10.1016/j.biomaterials.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 90.Alberti K. Davey R.E. Onishi K. George S. Salchert K. Seib F.P. Bornhauser M. Pompe T. Nagy A. Werner C. Zandstra P.W. Functional immobilization of signaling proteins enables control of stem cell fate. Nat Methods. 2008;5:645. doi: 10.1038/nmeth.1222. [DOI] [PubMed] [Google Scholar]
- 91.Hammouche S. Hammouche D. McNicholas M. Biodegradable bone regeneration synthetic scaffolds: in tissue engineering. Curr Stem Cell Res Ther. 2012;7:134. doi: 10.2174/157488812799219018. [DOI] [PubMed] [Google Scholar]
- 92.Fernandes S. Kuklok S. McGonigle J. Reinecke H. Murry C.E. Synthetic matrices to serve as niches for muscle cell transplantation. Cells Tissues Organs. 2012;195:48. doi: 10.1159/000331414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gonen-Wadmany M. Oss-Ronen L. Seliktar D. Protein-polymer conjugates for forming photopolymerizable biomimetic hydrogels for tissue engineering. Biomaterials. 2007;28:3876. doi: 10.1016/j.biomaterials.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 94.Yu W. Zhao W. Zhu C. Zhang X. Ye D. Zhang W. Zhou Y. Jiang X. Zhang Z. Sciatic nerve regeneration in rats by a promising electrospun collagen/poly(epsilon-caprolactone) nerve conduit with tailored degradation rate. BMC Neurosci. 2011;12:68. doi: 10.1186/1471-2202-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ngiam M. Liao S. Patil A.J. Cheng Z. Yang F. Gubler M.J. Ramakrishna S. Chan C.K. Fabrication of mineralized polymeric nanofibrous composites for bone graft materials. Tissue Eng Part A. 2009;15:535. doi: 10.1089/ten.tea.2008.0011. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y.Z. Venugopal J. Huang Z.M. Lim C.T. Ramakrishna S. Characterization of the surface biocompatibility of the electrospun PCL-collagen nanofibers using fibroblasts. Biomacromolecules. 2005;6:2583. doi: 10.1021/bm050314k. [DOI] [PubMed] [Google Scholar]
- 97.Chen J.P. Li S.F. Chiang Y.P. Bioactive collagen-grafted poly-L-lactic acid nanofibrous membrane for cartilage tissue engineering. J Nanosci Nanotechnol. 2010;10:5393. doi: 10.1166/jnn.2010.1945. [DOI] [PubMed] [Google Scholar]
- 98.Liao S.S. Cui F.Z. Zhang W. Feng Q.L. Hierarchically biomimetic bone scaffold materials: nano-HA/collagen/PLA composite. J Biomed Mater Res B Appl Biomater. 2004;69:158. doi: 10.1002/jbm.b.20035. [DOI] [PubMed] [Google Scholar]
- 99.Liao S.S. Cui F.Z. Zhu Y. Osteoblasts adherence and migration through three-dimensional porous mineralized collagen based composite: nHAC/PLA. J Bioact Compat Polym. 2004;19:117. [Google Scholar]
- 100.Liao S.S. Cui F.Z. In vitro and in vivo degradation of mineralized collagen-based composite scaffold: nanohydroxyapatite/collagen/poly(L-lactide) Tissue Eng. 2004;10:73. doi: 10.1089/107632704322791718. [DOI] [PubMed] [Google Scholar]
- 101.Chen G. Sato T. Ushida T. Ochiai N. Tateishi T. Tissue engineering of cartilage using a hybrid scaffold of synthetic polymer and collagen. Tissue Eng. 2004;10:323. doi: 10.1089/107632704323061681. [DOI] [PubMed] [Google Scholar]
- 102.Engelhardt E.M. Micol L.A. Houis S. Wurm F.M. Hilborn J. Hubbell J.A. Frey P. A collagen-poly(lactic acid-co-varepsilon-caprolactone) hybrid scaffold for bladder tissue regeneration. Biomaterials. 2011;32:3969. doi: 10.1016/j.biomaterials.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 103.Zhu J. He P. Lin L. Jones D.R. Marchant R.E. Biomimetic poly(ethylene glycol)-based hydrogels as scaffolds for inducing endothelial adhesion and capillary-like network formation. Biomacromolecules. 2012;13:706. doi: 10.1021/bm201596w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bahney C.S. Hsu C.W. Yoo J.U. West J.L. Johnstone B. A bioresponsive hydrogel tuned to chondrogenesis of human mesenchymal stem cells. FASEB J. 2011;25:1486. doi: 10.1096/fj.10-165514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shin H. Jo S. Mikos A.G. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 106.Lai V.K. Lake S.P. Frey C.R. Tranquillo R.T. Barocas V.H. Mechanical behavior of collagen-fibrin co-gels reflects transition from series to parallel interactions with increasing collagen content. J Biomech Eng. 2012;134:011004. doi: 10.1115/1.4005544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang X. Sang L. Luo D. Li X. From collagen-chitosan blends to three-dimensional scaffolds: the influences of chitosan on collagen nanofibrillar structure and mechanical property. Colloids Surf B Biointerfaces. 2011;82:233. doi: 10.1016/j.colsurfb.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 108.Rowe S.L. Stegemann J.P. Microstructure and mechanics of collagen-fibrin matrices polymerized using ancrod snake venom enzyme. J Biomech Eng. 2009;131:061012. doi: 10.1115/1.3128673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Silva S.S. Mano J.F. Reis R.L. Potential applications of natural origin polymer-based systems in soft tissue regeneration. Crit Rev Biotechnol. 2010;30:200. doi: 10.3109/07388551.2010.505561. [DOI] [PubMed] [Google Scholar]
- 110.Yang S. Leong K.F. Du Z. Chua C.K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001;7:679. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 111.Lin Y.C. Tan F.J. Marra K.G. Jan S.S. Liu D.C. Synthesis and characterization of collagen/hyaluronan/chitosan composite sponges for potential biomedical applications. Acta Biomater. 2009;5:2591. doi: 10.1016/j.actbio.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 112.Tan H. Rubin J.P. Marra K.G. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for adipose tissue regeneration. Organogenesis. 2010;6:173. doi: 10.4161/org.6.3.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Davidenko N. Campbell J.J. Thian E.S. Watson C.J. Cameron R.E. Collagen-hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater. 2010;6:3957. doi: 10.1016/j.actbio.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 114.Alini M. Li W. Markovic P. Aebi M. Spiro R.C. Roughley P.J. The potential and limitations of a cell-seeded collagen/hyaluronan scaffold to engineer an intervertebral disc-like matrix. Spine. 2003;28:446. doi: 10.1097/01.BRS.0000048672.34459.31. discussion 453. [DOI] [PubMed] [Google Scholar]
- 115.Calderon L. Collin E. Velasco-Bayon D. Murphy M. O'Halloran D. Pandit A. Type II collagen-hyaluronan hydrogel—a step towards a scaffold for intervertebral disc tissue engineering. Eur Cell Mater. 2010;20:134. doi: 10.22203/ecm.v020a12. [DOI] [PubMed] [Google Scholar]
- 116.Yang Y.L. Sun C. Wilhelm M.E. Fox L.J. Zhu J. Kaufman L.J. Influence of chondroitin sulfate and hyaluronic acid on structure, mechanical properties, and glioma invasion of collagen I gels. Biomaterials. 2011;32:7932. doi: 10.1016/j.biomaterials.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cummings C.L. Gawlitta D. Nerem R.M. Stegemann J.P. Properties of engineered vascular constructs made from collagen, fibrin, and collagen-fibrin mixtures. Biomaterials. 2004;25:3699. doi: 10.1016/j.biomaterials.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 118.Backer M.V. Patel V. Jehning B.T. Claffey K.P. Backer J.M. Surface immobilization of active vascular endothelial growth factor via a cysteine-containing tag. Biomaterials. 2006;27:5452. doi: 10.1016/j.biomaterials.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 119.Ogiwara K. Nagaoka M. Cho C.S. Akaike T. Construction of a novel extracellular matrix using a new genetically engineered epidermal growth factor fused to IgG-Fc. Biotechnol Lett. 2005;27:1633. doi: 10.1007/s10529-005-2605-0. [DOI] [PubMed] [Google Scholar]
- 120.Kato K. Sato H. Iwata H. Immobilization of histidine-tagged recombinant proteins onto micropatterned surfaces for cell-based functional assays. Langmuir. 2005;21:7071. doi: 10.1021/la050893e. [DOI] [PubMed] [Google Scholar]
- 121.Nakaji-Hirabayashi T. Kato K. Arima Y. Iwata H. Oriented immobilization of epidermal growth factor onto culture substrates for the selective expansion of neural stem cells. Biomaterials. 2007;28:3517. doi: 10.1016/j.biomaterials.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 122.Boucher C. St.-Laurent G. Loignon M. Jolicoeur M. De Crescenzo G. Durocher Y. The bioactivity and receptor affinity of recombinant tagged EGF designed for tissue engineering applications is defined by the nature and position of the tags. Tissue Eng Part A. 2008;14:2069. doi: 10.1089/ten.tea.2008.0037. [DOI] [PubMed] [Google Scholar]
- 123.Tilghman R.W. Cowan C.R. Mih J.D. Koryakina Y. Gioeli D. Slack-Davis J.K. Blackman B.R. Tschumperlin D.J. Parsons J.T. Matrix rigidity regulates cancer cell growth and cellular phenotype. PLoS One. 2010;5:e12905. doi: 10.1371/journal.pone.0012905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lin Y.C. Tambe D.T. Park C.Y. Wasserman M.R. Trepat X. Krishnan R. Lenormand G. Fredberg J.J. Butler J.P. Mechanosensing of substrate thickness. Phys Rev E Stat Nonlin Soft Matter Phys. 2010;82:041918. doi: 10.1103/PhysRevE.82.041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Annabi N. Fathi A. Mithieux S.M. Martens P. Weiss A.S. Dehghani F. The effect of elastin on chondrocyte adhesion and proliferation on poly (epsilon-caprolactone)/elastin composites. Biomaterials. 2011;32:1517. doi: 10.1016/j.biomaterials.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 126.Ji C. Annabi N. Khademhosseini A. Dehghani F. Fabrication of porous chitosan scaffolds for soft tissue engineering using dense gas CO2. Acta Biomater. 2011;7:1653. doi: 10.1016/j.actbio.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 127.Huang C. Chen R. Ke Q. Morsi Y. Zhang K. Mo X. Electrospun collagen-chitosan-TPU nanofibrous scaffolds for tissue engineered tubular grafts. Colloids Surf B Biointerfaces. 2011;82:307. doi: 10.1016/j.colsurfb.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 128.Miranda S.C. Silva G.A. Hell R.C. Martins M.D. Alves J.B. Goes A.M. Three-dimensional culture of rat BMMSCs in a porous chitosan-gelatin scaffold: a promising association for bone tissue engineering in oral reconstruction. Arch Oral Biol. 2011;56:1. doi: 10.1016/j.archoralbio.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 129.Tan H. DeFail A. Rubin J.P. Constance R.C. Marra K.G. Novel multiarm PEG-based hydrogels for tissue engineering. J Biomed Mater Res Part A. 2009;92A:979. doi: 10.1002/jbm.a.32438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schek R.M. Michalek A.J. Iatridis J.C. Genipin-crosslinked fibrin hydrogels as a potential adhesive to augment intervertebral disc annulus repair. Eur Cell Mater. 2011;21:373. doi: 10.22203/ecm.v021a28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bi L. Cao Z. Hu Y. Song Y. Yu L. Yang B. Mu J. Huang Z. Han Y. Effects of different cross-linking conditions on the properties of genipin-cross-linked chitosan/collagen scaffolds for cartilage tissue engineering. J Mater Sci Mater Med. 2011;22:51. doi: 10.1007/s10856-010-4177-3. [DOI] [PubMed] [Google Scholar]
- 132.Wang C. Lau T.T. Loh W.L. Su K. Wang D.A. Cytocompatibility study of a natural biomaterial crosslinker—genipin with therapeutic model cells. J Biomed Mater Res B Appl Biomater. 2011;97:58. doi: 10.1002/jbm.b.31786. [DOI] [PubMed] [Google Scholar]
- 133.Mochizuki M. Kadoya Y. Wakabayashi Y. Kato K. Okazaki I. Yamada M. Sato T. Sakairi N. Nishi N. Nomizu M. Laminin-1 peptide-conjugated chitosan membranes as a novel approach for cell engineering. FASEB J. 2003;17:875. doi: 10.1096/fj.02-0564fje. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Q. He Q.F. Zhang T.H. Yu X.L. Liu Q. Deng F.L. Improvement in the delivery system of bone morphogenetic protein-2: a new approach to promote bone formation. Biomed Mater. 2012;7:045002. doi: 10.1088/1748-6041/7/4/045002. [DOI] [PubMed] [Google Scholar]