Abstract
Background: Adiponectin gene expression is modulated by peroxisome proliferator–activated receptor γ, which is a transcription factor activated by unsaturated fatty acids.
Objective: We investigated the effect of the interaction between variants at the ADIPOQ gene locus, age, sex, body mass index (BMI), ethnicity, and the replacement of dietary saturated fatty acids (SFAs) with monounsaturated fatty acids (MUFAs) or carbohydrates on serum adiponectin concentrations.
Design: The RISCK (Reading, Imperial, Surrey, Cambridge, and Kings) study is a parallel-design, randomized controlled trial. Serum adiponectin concentrations were measured after a 4-wk high-SFA (HS) diet and a 24-wk intervention with reference (HS), high-MUFA (HM), and low-fat (LF) diets. Single nucleotide polymorphisms at the ADIPOQ locus −11391 G/A (rs17300539), −10066 G/A (rs182052), −7734 A/C (rs16861209), and +276 G/T (rs1501299) were genotyped in 448 participants.
Results: In white Europeans, +276 T was associated with higher serum adiponectin concentrations (n = 340; P = 0.006) and −10066 A was associated with lower serum adiponectin concentrations (n = 360; P = 0.03), after adjustment for age, BMI, and sex. After the HM diet, −10066 G/G subjects showed a 3.8% increase (95% CI: −0.1%, 7.7%) and G/A+A/A subjects a 2.6% decrease (95% CI: −5.6%, 0.4%) in serum adiponectin (P = 0.006 for difference after adjustment for the change in BMI, age, and sex). In −10066 G/G homozygotes, serum adiponectin increased with age after the HM diet and decreased after the LF diet.
Conclusion: In white −10066 G/G homozygotes, an HM diet may help to increase adiponectin concentrations with advancing age. This trial was registered at clinicaltrials.gov as ISRCTN29111298.
INTRODUCTION
The metabolic syndrome is a complex disorder characterized by abdominal obesity, insulin resistance, hypertension, dyslipidemia, and inflammation (1). Observational evidence and intervention studies indicated that saturated fat worsens, whereas monounsaturated and polyunsaturated fats improve, insulin sensitivity (2). However, individuals show varying responses to dietary interventions, which suggests that a significant gene × dietary interaction occurs (3).
Adiponectin expressed in adipose tissue (4) is a potent insulin sensitizer that regulates energy homeostasis and glucose tolerance in the muscle and liver (5). Concentrations of circulating adiponectin are reduced in obese subjects (6), and hypoadiponectinemia appears to play an important causal role in insulin resistance, type 2 diabetes, and the metabolic syndrome (7–9). Adiponectin concentrations have a strong genetic component, with heritability estimated between 30% and 50% (10). Associations with the adiponectin concentration and/or the metabolic syndrome have been reported for genetic variants at the ADIPOQ gene locus in many studies (11–15). However, some of these associations could not be confirmed by others (16–18). These inconsistencies may relate to the interaction between the gene and environmental influences, such as dietary intake. Such an interaction may also underpin varied individual responses to diet in general.
Results from observational or weight-loss studies have suggested that diets low in carbohydrates (19) and high in unsaturated fat might increase adiponectin (20). Small intervention studies that investigated macronutrient effects independent of weight loss have been inconsistent (21–23). We aimed to discover whether reduction of saturated fat in a diet while maintaining a stable weight would increase serum adiponectin concentrations. We studied 448 participants at risk of developing the metabolic syndrome in the RISCK (Reading, Imperial, Surrey, Cambridge, and Kings) trial, which is a highly controlled dietary intervention that is based on the replacement of saturated fat with either carbohydrates or monounsaturated fat in isoenergetic diets (24). Unsaturated fatty acids are ligands for the transcription factor peroxisome proliferator–activated receptor γ (PPARγ) (25), which up-regulates ADIPOQ gene expression (26) and directly increases serum adiponectin concentrations (27). We hypothesized that variants in ADIPOQ could interact with the dietary intake of unsaturated fat to influence serum adiponectin in the absence of a significant change in fat mass. We examined 4 single nucleotide polymorphisms (SNPs) that we have previously shown to be significantly associated with fasting serum adiponectin in 2 independent samples: −11391 G/A (rs17300539) in the promoter, −100066 G/A (rs182052) and −7734 A/C (rs16861209) in intron 1, and +276 G/T (rs1501299) in intron 2 (28). The interaction of the SNP genotype with age and sex in the determination of serum adiponectin concentrations and measures of insulin sensitivity was assessed at baseline and after 24 wk of a dietary intervention in 366 white European subjects.
SUBJECTS AND METHODS
Subjects
Ethical approval for the RISCK study (ISRCTN29111298) was granted from the National Research Ethics Service, and written informed consent from participants was obtained, including subsequent genetic analyses. Men and women (age range: 30–70 y) recruited from the general population attended a clinic in a fasting state at participating centers (the University of Reading, Imperial College London, the University of Surrey, and the Medical Research Council Human Nutrition Research Centre, University of Cambridge and Kings College London). Eligibility for entry to the study was assessed by a point system and implementation of exclusion criteria described previously (24). A total of 47.5% of subjects had the metabolic syndrome according to the criteria of the International Diabetes Federation (29). A total of 549 subjects completed the RISCK study. The current study involved 448 subjects for whom DNA samples were available. Self-reported ethnicity was recorded as white, South Asian, black African, or other.
Study design
A parallel 2 × 2 factorial design compared with a control intervention was used. Subjects were randomly assigned to treatments by using a computer-based minimization procedure to balance assignments by age, sex, waist, and HDL cholesterol as previously described (24). Power calculations were based on 113 subjects per group completing the study to give an 80% power to detect a difference in means of 1 (×10−4 mL ⋅ μU−1 ⋅ min−1) in the index of insulin sensitivity (Si) at P = 0.005. The final sample size, allowing for a dropout of 15%, was 130 per treatment group with equal numbers recruited at each of the 5 centers. Intervention diets were planned to provide similar intakes of dietary energy but to vary in the amount and type of fats and carbohydrates. All participants had a 4-wk run-in period during which they consumed a high–saturated fat reference diet before being randomly assigned to the reference diet or one of 4 isoenergetic dietary interventions designed to lower saturated fat. For the purposes of the current study the dietary intervention groups differing in carbohydrate quality were combined to focus the analyses on the manipulation of dietary fat, from which the effect on adiponectin concentrations and the homeostatic model assessment of insulin resistance was expected to be greater. The resulting 3 dietary groups were as follows: a high–saturated fat (HS) reference diet designed to reflect the saturated fat intake in a Western diet, a high–monounsaturated fatty acid (MUFA) diet (HM) in which saturated fatty acids (SFAs) were reduced and replaced with MUFAs, and a low-fat (LF) diet in which SFAs were reduced through the replacement of total fat with carbohydrates. The target intake for total fat was 38% of energy in the HS and HM diets and 28% of energy in the LF diet, with carbohydrate intakes of 45% and 55% of energy, respectively. The HM and LF diets were designed to reduce dietary SFAs to 10% of energy with a planned MUFA intake of 20% of energy in the HM diet and 12% of energy in the LF diet and HS reference diet. The dietary intervention was described in detail elsewhere (30). Measurements made after the run-in diet were referred to as baseline. All participants followed their randomly prescribed diets for 24 wk, after which an additional blood sample was collected and anthropometric variables were measured. Unweighed 4-d food diaries (3 weekdays and 1 weekend day) were collected to record dietary intakes at baseline and in the third and the final month of the intervention. Nutrient intakes were estimated by using the food-composition database software DINO (Medical Research Council Human Nutrition Research Unit, Elsie Widdowson Laboratory, Cambridge, United Kingdom) as previously described (24). The weight (in light clothing) and height (without shoes) of each subject were measured and an indwelling venous cannula was inserted into the forearm.
Blood analytic methods
Blood samples for analysis were drawn after a minimum of an 8-h overnight fast, and serum was stored at −45°C until analyzed. Insulin sensitivity as measured by the intravenous glucose tolerance test (IVGTT) and the measurement of fasting glucose and insulin were carried out as previously described (24). Glucose and insulin were measured at the Nutritional Biochemistry Laboratory, Medical Research Council Human Nutrition Research. Insulin resistance was indirectly assessed by estimated homeostatic model assessment of insulin resistance version 2 (HOMA2-IR) with software from the Diabetes Trials Unit, University of Oxford (http://www.dtu.ox.ac.uk/homa; Oxford, United Kingdom). Fasted plasma phospholipid fatty acids were measured by gas chromatography at the University of Reading as previously described (30). Serum adiponectin analysis was carried out at Unilever (Sharnbrook, United Kingdom) with the AutoDELFIA time-resolved fluorescence–based immunoassay (Perkin Elmer, Cambridge, United Kingdom). The immunoassay measured the total (low, middle, and high molecular weight) human adiponectin by using a mouse monoclonal anti-adiponectin antibody, mouse monoclonal adiponectin biotinylated detection antibody, and recombinant adiponectin (all from R&D systems, Abingdon, United Kingdom) and streptavidin Europium conjugate (Perkin Elmer). Tests of intra- and interassay CVs were performed in replicates of 25. Intra- and interassay percentage CVs, respectively, were as follows: 0.95 μg/mL: 7.2, 8.8; 2.88 μg/mL: 7.5, 8.2; and 8.60 μg/mL: 5.0, 1.4.
DNA extraction and SNP genotyping
Buffy coats removed from blood samples were stored in EDTA-coated tubes at −20°C. Genomic DNA was extracted from 200 μL buffy coat with an Illustra blood genomic prep mini spin kit (GE Healthcare, Amersham, United Kingdom) according to the manufacturer's instructions. Four SNPs at the ADIPOQ gene locus were genotyped [−11391 G/A (rs17300539), −10066 G/A (rs182052), −7734 C/A (rs16861209), and +276 G/T (rs1501299)]. Their relative positions with respect to the first coding base in exon 2 are indicated. Genotyping by pyrosequencing (Qiagen, Crawley, Surrey, United Kingdom) was performed in the 448 participants for whom DNA was available. Primers and PCR conditions for genotyping can be obtained upon request. We used internal controls, and accuracy as assessed by the inclusion of duplicates in the arrays was 98%. Genotyping success rates were 98.1%, 83.5%, 84.9%, and 92.1%, respectively.
Statistical analyses
All genotype distributions were tested for deviation from the Hardy-Weinberg equilibrium by a chi-square test with 1 df (P > 0.05). Statistical analyses were carried out with SPSS software (version 17.0 for Windows; SPSS Inc, Chicago, IL). When needed, variables were log transformed to obtain better approximations of the normal distribution before analysis. Data were analyzed by using analysis of covariance (ANCOVA), regressing follow-up measures against baseline measures with ethnicity, baseline age, sex, and diet as covariates. Outliers were excluded from the ANCOVA and were defined as points >2.5 times the interquartile range from the median on the transformed scale at baseline, follow-up, or the change from baseline. All data presented in the text and tables are expressed as means or geometric means ± SDs or 95% CIs. The unadjusted effect of each diet is expressed as the percentage change from the median value at baseline with 95% CIs. Correlations are presented as Spearman's r. Significance was accepted at P < 0.05.
RESULTS
Baseline characteristics of subjects
A total of 549 subjects completed the RISCK study. The ethnic mix of subjects was typical of England and predominantly white, with about one-fifth of subjects from ethnic minorities. On the basis of self-reported ethnicity, individuals of white (80%), South and Southeast Asian (9.5%), black African (8%), and other (2.5%) ancestry were distinguished. The 448 individuals for whom DNA samples were available were the subjects of this study, from which participants in the other ethnic subgroup were excluded. The characteristics of included subjects at baseline with respect to ethnicity, age, body mass index (BMI), and insulin homeostatic variables are presented in Table 1. After a 4-wk run-in period with the HS diet, there were significant differences between men and women in fasting glucose concentrations (higher in men) and insulin sensitivity (lower in men).
TABLE 1.
Characteristics of subjects in the RISCK (Reading, Imperial, Surrey, Cambridge, and Kings) study at baseline1
| Characteristic | All (n = 448) | Men (n = 264) | Women (n = 184) | P (men vs women) |
| Ethnicity [n (%)]2 | — | |||
| South Asian | 44 (9.8) | 25 (9.4) | 19 (10.3) | |
| Black African | 38 (8.5) | 28 (10.6) | 10 (5.4) | |
| White European | 366 (81.7) | 211 (80.0) | 155 (84.3) | |
| Age (y)3 | 52.5 ± 9.9 | 53.6 ± 10.2 | 51.8 ± 9.5 | 0.05 |
| BMI (kg/m2)3 | 28.8 ± 4.7 | 28.5 ± 3.9 | 28.9 ± 5.2 | 0.43 |
| Fasting insulin (pmol/L)4 | 60.4 ± 52.1 | 63.4 ± 40.8 | 58.2 ± 58.7 | 0.10 |
| Fasting glucose (mmol/L)3 | 5.7 ± 0.8 | 5.9 ± 0.9 | 5.5 ± 0.6 | <0.001 |
| Si (IVGTT) (mU ⋅ Lminus1 ⋅ minminus1)4 | 2.7 ± 2.7 | 2.4 ± 1.9 | 2.9 ± 3.1 | 0.001 |
| HOMA2-IR4 | 1.3 ± 0.7 | 1.3 ± 0.8 | 1.3 ± 0.7 | 0.54 |
Si (IVGTT), insulin sensitivity as measured by the intravenous glucose-tolerance test; HOMA2-IR, homeostatic model assessment of insulin resistance version 2. Data are presented for all subjects who completed the study and for whom DNA was available (n = 448). All variables were measured at baseline after a 4-wk run-in period on a reference high–saturated fat diet. The significance of differences between men and women was determined by Student's t test.
Self-reported.
Values are means ± SDs.
Values are geometric means ± SDs.
Serum adiponectin with respect to age, BMI, and ethnicity
Regression analysis showed that adiponectin was positively correlated with age (β = 0.217, P < 0.001) and was negatively correlated with BMI (β = −0.161, P < 0.001). As shown in Table 2, there was a significant increase in the geometric mean serum adiponectin concentration with age after adjustment for BMI, sex, and ethnicity. The serum adiponectin concentration decreased with increasing BMI after adjustment for age, sex, and ethnicity. There were highly significant differences with respect to ethnicity after adjustment for BMI, age, and sex. Adiponectin concentrations were significantly higher in white Europeans than in South Asians (P = 0.001) and black Africans (P = 0.001). Serum adiponectin concentrations were significantly higher in women (11.1± 6.2 μg/mL) than in men (8.5 ± 4.1 μg/mL) (P < 0.001). However, there were no significant interactions between sex × age (P = 0.697), sex × BMI (P = 0.139), or sex × ethnicity (P = 0.15) in the determination of serum adiponectin concentrations.
TABLE 2.
Serum adiponectin concentrations at baseline stratified by age, BMI, and ethnicity1
| Age |
BMI |
Ethnicity2 |
|||
| Age group | Adiponectin | BMI group | Adiponectin | Ethnic group | Adiponectin |
| μg/mL | kg/m2 | μg/mL | μg/mL | ||
| <40 y [63] | 8.5 (7.5, 9.7)3 | <25 [88] | 11.4 (10.2, 12.6) | South Asian [44] | 7.5 (6.5, 8.7) |
| 41–50 y [129] | 8.4 (7.7, 9.2) | 25–29 [210] | 9.6 (8.9, 10.2) | Black [38] | 6.8 (5.8, 8.0) |
| 51–60 y [148] | 10.1 (9.3, 11.0) | 30–35 [107] | 8.7 (7.9, 9.5) | White [366] | 10.2 (9.7, 10.8) |
| 61–70 y [108] | 11.2 (10.2, 12.4) | >35 [43] | 8.9 (7.6, 10.3) | — | — |
| P value | <0.001 | — | 0.002 | — | <0.001 |
| Adjusted P value | <0.0014 | — | <0.0015 | — | <0.0016 |
Data are presented for subjects for whom DNA samples and serum adiponectin measurements were available (n = 448). n in brackets. All variables were measured at baseline after 4-wk run-in period on a reference high–saturated fat diet. The significance of differences between age, BMI, and ethnic groups was determined by one-factor ANOVA (P values without adjustments) or ANCOVA (P values with adjustments).
Self-reported.
Geometric mean; 95% CI in parentheses (all such values).
Adjusted for BMI, sex, and ethnicity.
Adjusted for age, sex, and ethnicity.
Adjusted for age, BMI, and sex.
Correlation of serum adiponectin with insulin sensitivity measures
To establish any relation between serum adiponectin concentrations and insulin resistance in RISCK subjects, we tested correlation between geometric mean adiponectin and insulin homeostatic variables in all subjects at baseline. Serum adiponectin was negatively correlated with HOMA2-IR (r = −0.38; P < 0.001) and positively correlated with Si as measured by IVGTT (r = 0.37; P < 0.001). We also determined the partial correlation coefficient with adjustment for BMI, age, sex, and ethnicity. The correlation was decreased but remained significant [partial r for HOMA2-IR = −0.279 (P < 0.001); partial r for Si = 0.323 (P < 0.001)].
SNP minor allele and genotype frequencies
We determined the minor allele frequencies (MAFs) and genotype frequencies of the 4 SNPs typed at the ADIPOQ locus in 448 subjects stratified by ethnic group (see supplemental Table 1 under “Supplemental data” in the online issue). Genotype distributions for each SNP did not deviate from Hardy-Weinberg expectations. Allele frequencies were compared with those listed on the National Center for Biotechnology Information SNP database (31). MAFs in white Europeans were as follows: −11391 G/A, 0.09; −10066 G/A, 0.38; −7734 C/A, 0.02; and +276 G/T, 0.24. The −11391 G/A (MAF: 0.02) was not expected to be present in blacks, −10066 G/A (MAF: 0.32) was not as frequent as expected in blacks [0.40 HapMap-Yoruba (YRI)], and the −7734 C/A frequency was lower than expected in Europeans (MAF: 0.02) and blacks (MAF: 0.05) [0.09 HapMap-Western European (CEU) and 0.13 HapMap-YRI, respectively]. The +276 G/T MAFs were as expected. There were no comparable data available for South Asians.
ADIPOQ SNP genotype associations with serum adiponectin and insulin homeostatic variables at baseline
In view of the small sample size of the south Asian and black subgroups, we chose to focus our genetic investigation on the white subjects (n = 366). Measurements of serum adiponectin, Si measured by IVGTT, and HOMA2-IR at baseline (after the 4-wk run-in period with the HS diet) with respect to −11391 G/A, −10066 G/A, −7734 C/A, and +276 G/T genotype groups are shown in Table 3. Analysis of variance was used to test the association between genotypes and phenotypic values adjusted for BMI, age, and sex on the basis of a dominant model. Carriers of the +276 T allele had significantly higher mean serum adiponectin concentrations at baseline than did noncarriers (P = 0.006), and carriers of the −10066 A allele had significantly lower serum adiponectin concentrations than did noncarriers (P = 0.03) before correction for multiple testing. After correction using 4-factor analysis of variance, the association of serum adiponectin with the −10066 G/A genotype remained significant (P = 0.03) but not with the +276 G/T genotype (P > 0.05). The +276 G/T and −10066 G/A genotypes did not significantly interact with age in the determination of serum adiponectin concentrations at baseline (P > 0.05). No phenotypes were significantly associated with −11391 G/A or −7734 A/C SNP genotypes at baseline (P > 0.05).
TABLE 3.
Serum adiponectin concentrations and insulin homeostatic variables by ADIPOQ single nucleotide polymorphism genotype in white European subjects at baseline1
| −11391 G/A |
−10066 G/A |
−7734 C/A |
+276 G/T |
|||||||||
| Phenotype | G/G | G/A+A/A | P | G/G | G/A+A/A | P | C/C | C/A+A/A | P | G/G | G/T+T/T | P |
| M/F (n) | 125/141 | 17/41 | 57/75 | 95/133 | 128/152 | 3/9 | 87/109 | 55/89 | ||||
| Adiponectin (μg/mL) | 9.9 (9.4, 10.5)2 | 10.7 (9.6, 11.9) | 0.24 | 10.9 (10.2, 11.8) | 9.9 (9.3, 10.4) | 0.0334 | 10.4 (9.9, 10.9) | 10.8 (8.5, 13.8) | 0.73 | 9.6 (9.1, 10.2) | 11.0 (10.2, 11.8) | 0.0063 |
| Si (mU ⋅ Lminus1 ⋅ minminus1) | 2.7 (2.5, 2.9) | 2.5 (2.1, 2.9) | 0.28 | 2.8 (2.5, 3.2) | 2.6 (2.4, 2.9) | 0.28 | 2.7 (2.5, 2.9) | 2.4 (1.7, 3.5) | 0.56 | 2.6 (2.4, 2.9) | 2.8 (2.5, 3.1) | 0.37 |
| HOMA2-IR | 2.2 (2.0, 2.3) | 2.1 (1.8, 2.4) | 0.72 | 2.0 (1.8, 2.2) | 2.2 (2.1, 2.4) | 0.11 | 1.3 (1.2, 1.4) | 1.1 (0.9, 1.4) | 0.28 | 1.3 (1.3, 1.4) | 1.2 (1.1, 1.3) | 0.07 |
Si, insulin sensitivity; HOMA2-IR, homeostatic model assessment of insulin resistance version 2. Data are presented for white European subjects for whom DNA samples and serum adiponectin measurements were available (n = 366). All variables were measured at baseline after 4-wk run-in period on a reference high–saturated fat diet. Associations were tested by univariate ANOVA on the basis of a dominant model. P values were derived from geometric mean adiponectin concentrations, Si as measured by the intravenous glucose tolerance test, and HOMA2-IR values. Unless otherwise indicated, P values were not significant (P > 0.05) after adjustment for multiple testing.
Mean; 95% CI in parentheses (all such values).
P value adjusted for BMI, age, and sex was nominally significant (P < 0.05) when unadjusted for multiple comparisons.
P value remained significant after correction for multiple testing by 4-factor ANOVA (P = 0.03).
Changes in measured variables after dietary intervention
The RISCK study was designed to address the effects of diets with different macronutrient compositions but similar energy intakes to measure changes in the absence of a significant alteration in weight. Subjects were randomly assigned to continuation on the HS reference diet, or 24 wk on an isoenergetic diet in which saturated fats were replaced with either MUFAs (HM diet) or carbohydrates (LF diet). The diet during the run-in was monitored by a weighed intake. There was no significant difference in intakes of saturated fat between subjects who continued on the HS reference diet or were later assigned to the HM and LF diets (30). Body weights were relatively stable. Additional information is provided elsewhere (24, 30). Changes in percentages of SFA and MUFAs differed between diets over the 24 wk of intervention. The HM group had a significantly lower plasma phospholipid percentage of SFA than did the LF group and a higher percentage of MUFAs, but other fatty acid classes [(n−3) polyunsaturated fatty acids (PUFAs), (n−6) PUFAs, and trans fatty acids] were not affected (30). The mean serum adiponectin concentration was not significantly different between diet groups [after the reference HS diet (n = 85), the mean concentration was 9.2 μg/mL (95% CI: 8.2, 10.3 μg/mL); after the HM diet (n = 227), the mean concentration was 9.3 μg/mL (95% CI: 8.7, 10.0 μg/mL), and after the LF diet (n = 235), the mean concentration was 9.6 μg/mL (95% CI: 9.0, 10.2 μg/mL), with P = 0.44 after adjustment for BMI, age, sex, and ethnicity]. Dietary interventions had no significant effect on Si, which was unaltered after adjustment for changes in weight (24).
Changes in adiponectin concentrations after dietary intervention with respect to ADIPOQ SNP genotype
The −10066 G/A and +276 G/T genotypes were significantly associated with serum adiponectin concentrations at baseline in white subjects (Table 3). We investigated the significance of any changes in adiponectin concentrations after the HM and LF diets with respect to −10066 G/A and +276 G/T genotypes. There was no significant difference in the change after HM or LF diets with respect to the +276 G/T genotype or after the LF diet with respect to the −10066 G/A genotype. However, after the HM diet, there was a significant difference in the change in serum adiponectin concentrations between −10066 G/A genotype groups. The geometric mean concentration (95% CI) at baseline was 10.4 μg/mL (9.3, 11.6 μg/mL) in G/G subjects (n = 57) and 9.6 μg/mL (8.8, 10.4 μg/mL) in G/A+A/A subjects (n = 94). The geometric mean adiponectin concentration (95% CI) at follow-up was 10.8 μg/mL (9.7, 12.1 μg/mL) in G/G subjects and 9.3μg/mL (8.6, 10.2 μg/mL) in G/A+A/A subjects. The −10066 G/G subjects showed an increase of 3.8% (−0.1%, 7.7%), and G/A+A/A subjects showed a decrease of −2.6% (−5.6%, 0.4%) after the HM diet. The difference in the percentage change between G/G homozygotes and carriers of the A allele was significant (P = 0.006) after adjustment for changes in BMI, age, and sex. However, the gene × diet interaction in the determination of serum adiponectin concentrations was not significant (P = 0.12) after adjustment for changes in BMI, age, and sex.
Changes in adiponectin concentrations after dietary intervention with respect to age and minus10066 G/A genotype
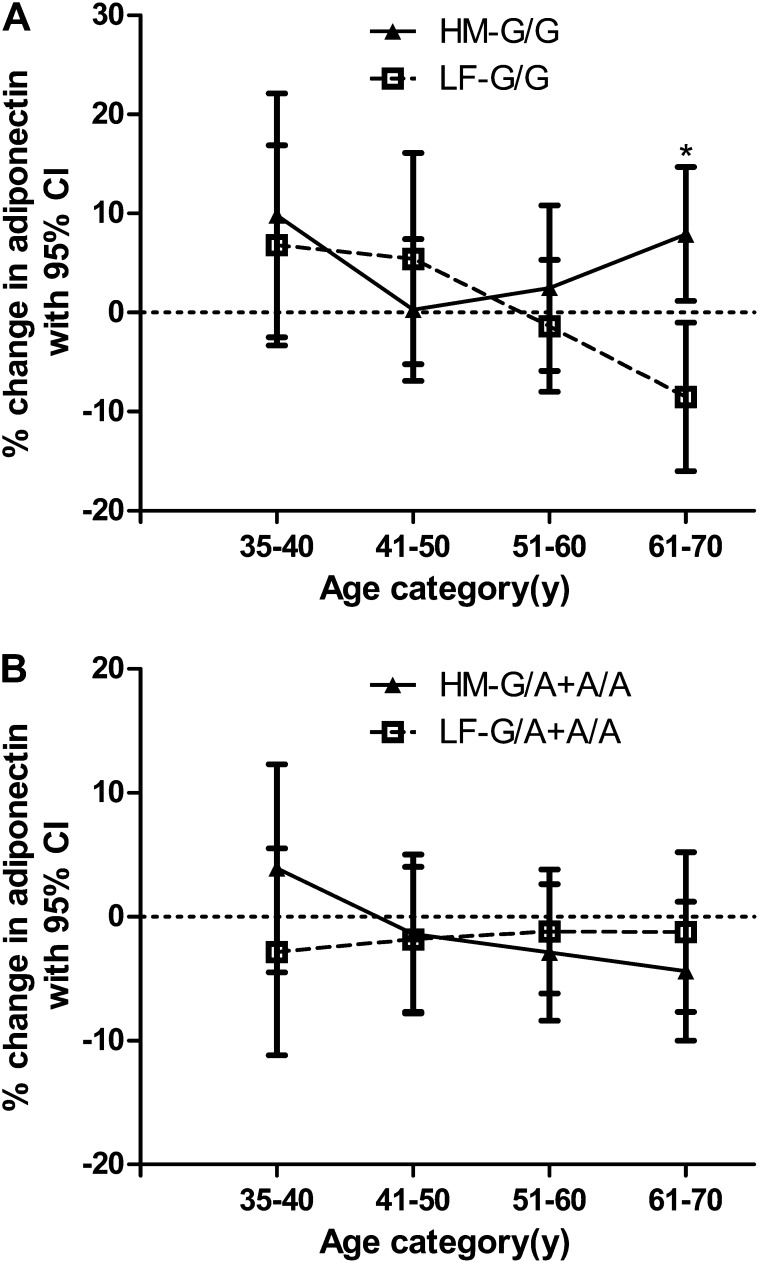
We were interested to determine whether the significant increase in serum adiponectin concentrations with age that we observed at baseline (Table 2) was modified by dietary interventions. After dietary treatments, the interaction between diet × age was not significant in the determination of the change in concentrations (P = 0.83) adjusted for changes in BMI and sex. We proceeded to determine whether age and the −10066 G/A genotype interacted to influence changes in adiponectin concentrations after dietary interventions. Effects of HM and LF diets on the percentage change in serum adiponectin concentrations in white −10066 G/G homozygotes and A-allele carriers Figure 1. Although there were inconsistent effects in the smallest number of subjects in the group aged 35–40 y (n = 41), general trends were evident across 10-y categories from age 41–70 y. In G/G homozygotes >40 y of age, adiponectin concentrations increased progressively after the HM diet and decreased after the LF diet (Figure 1A). The difference in the percentage change in serum adiponectin between G/G subjects on HM and LF diets in the oldest age group of 61–70 y was significant (P = 0.003). In A-allele carriers there was little change in serum adiponectin concentrations compared with at baseline with increasing age after HM or LF diets (Figure 1B). The interaction between gene × age × diet in the determination of change in serum adiponectin concentration approached significance after adjustment for sex and change in BMI (n = 303; P = 0.07) and remained insignificant after correction for multiple testing. However, the interaction between gene × age × diet × sex was not significant (P = 0.67) after adjustment for change in BMI.
FIGURE 1.
Effect of high–monounsaturated fatty acid (HM) and low-fat (LF) diets on adiponectin concentrations with respect to −10066 G/A genotype and age in white subjects. Percentage changes (95% CI) in geometric mean adiponectin concentration adjusted for change in BMI and sex are shown in each age group after subjects consumed an HM (n = 151) or LF (n = 152) diet. A: −10066 G/G (HM-GG and LF-GG) subjects (n = 111). B: −10066 G/A+A/A (HM-G/A+A/A and LF-G/A+A/A) subjects (n = 192). (See supplemental Table 2 under “Supplemental data” in the online issue for the numbers of subjects in each mean.) The interaction between gene × age × diet in the determination of changes in serum adiponectin concentrations as determined by ANCOVA was not significant after adjustment for changes in BMI (n = 303; P = 0.07). *Significant difference in percentage change in serum adiponectin between G/G subjects on HM and LF diets, P = 0.003.
DISCUSSION
In this cohort of mainly overweight men and women, we have shown significant effects of age, BMI, and ethnicity on serum adiponectin concentrations and a novel influence of the ADIPOQ −10066 G/A genotype dependent on age and diet. After the HM diet, G/G homozygotes showed a progressive increase in serum adiponectin concentrations between 41–70 y of age, whereas after the LF diet, there was a progressive decline in serum adiponectin concentrations.
The significant increase in serum adiponectin concentrations that we showed with age after adjustment for BMI was in agreement with previous reports (32). Because insulin sensitivity declines with age, this may reflect the development of a resistance or survival in individuals with higher adiponectin concentrations. The inverse relation that we showed with BMI is well known (33). Serum adiponectin was significantly higher in white Europeans than in South Asians and black Africans after adjustments. Inconsistent correlations of adiponectin with BMI and age in white and black women (34, 35) suggested that relations with obesity and insulin sensitivity may not be generalizable to all ethnic groups. We focused our genetic investigation on white Europeans.
We previously observed strong associations of the 4 SNPs with serum adiponectin concentrations in 2 much larger cohorts of healthy white women (28). At baseline in the current study, we showed higher mean serum adiponectin concentrations and lower fasting insulin concentrations and HOMA2-IR in carriers of the variant +276 T allele than in noncarriers. Significance disappeared after adjustment for multiple testing; however, association of elevated adiponectin with +276T has been reported in several larger studies (11, 12, 36). We showed that carriage of the variant −10066 A allele was associated with lower serum adiponectin concentrations at baseline than in noncarriers. This result remained significant after correction for multiple comparisons and was in agreement with our previous finding (28) and the only other report of –10066 G/A association (37). The remainder of our study centered on this SNP. Although the association of elevated adiponectin with the −11391 A allele was widely reported (13–15, 37), we observed no association in RISCK subjects.
Most intervention studies have shown that a higher intake of saturated fat was detrimental to maintaining insulin sensitivity whereas unsaturated fat was beneficial (38). Various studies have reported higher concentrations of adiponectin after diets rich in unsaturated fat than in diets rich in carbohydrates or protein, although these higher concentrations were not always accompanied by significant changes in insulin sensitivity (19–21, 39). The replacement of SFA by isoenergetic MUFA or carbohydrate diets for 24 wk did not significantly improve adiponectin concentrations. We previously reported no significant effect on insulin sensitivity after this dietary regimen (24). Small changes in adiponectin concentrations after the dietary intervention may not have been sufficient to affect insulin sensitivity, or the intervention period may not have been long enough to produce an effect. In a recent study, an interaction between the ADIPOQ rs266729 (−11377 C/G) genotype with SFAs, but not MUFAs or PUFA, significantly affected the homeostatic model assessment of insulin resistance. However, there were no significant effects on serum adiponectin concentrations, which suggested that insulin resistance did not reflect changes in the ADIPOQ gene expression elicited by SFAs (40).
We hypothesized that the stratification by genotype might have uncovered an influential interaction between dietary MUFAs and ADIPOQ variants. There were no significant differences in changes in serum adiponectin concentration after HM or LF diets with respect to the +276 G/T genotype and none after the LF diet with respect to −10066 G/A. However, after the HM diet, G/G subjects had an increase of 3.8%, and G/A+A/A subjects had a decrease of 2.6%. Although the difference in changes was highly significant, the gene × diet interaction was not a significant determinant of serum adiponectin concentrations. Activation of PPARγ by unsaturated fatty acids increases with the chain length and degree of unsaturation (41). The switch from SFAs to MUFAs could have led to the increased expression of the ADIPOQ gene and serum adiponectin concentrations through the increased availability of PPARγ-activating MUFA ligands. The peroxisome proliferator response element (PPRE) is 250 base pairs upstream of exon 1 (26) and lies in a linkage disequilibrium block of 1.3 kb in length, bounded 5′ by −11377 G/C in the promoter and 3′ by −10066 G/A in intron 1 (15). If the rare −10066 A allele was in linkage disequilibrium with a variant in the PPRE, which reduced the affinity for the receptor, this could have accounted for the higher serum adiponectin concentrations in response to MUFAs in G/G homozygotes and the lower concentrations in A allele carriers. The −11391 G/A SNP, which was not associated with adiponectin concentrations in RISCK subjects, lies upstream of the LD block that contains the PPRE (15).
We were interested to discover whether the strong relation between adiponectin concentrations and age seen at baseline was modified by diet. There was no significant diet × age interaction. We then looked at whether age × genotype interaction was influential after a dietary intervention. After the HM diet, −10066 G/G homozygotes showed a progressive increase in serum adiponectin between 41–70 y of age, which culminated in a highly significant difference in the oldest age group. After the LF diet, G/G homozygotes showed a progressive fall. Both diets produced little effect in carriers of the A allele. Serum adiponectin concentrations might be expected to be lower in G/G subjects after the LF diet, in which carbohydrates replaced PPARγ-activating fatty acids that were present in the HM diet. In carriers of the A allele, the substitution of carbohydrates for MUFAs would have had little effect if the reduced affinity of the PPRE, rather than ligand activation, was the rate-limiting step. This scenario is compatible with other reports of lower serum adiponectin concentrations after high-carbohydrate than after HM diets (19–21, 39). If aging is associated with the development of adiponectin resistance, the change in adiponectin amounts may reflect a capability to respond by increasing production after an HM diet but not after a LF diet.
Few additional studies have investigated an effect of the interaction between ADIPOQ SNP genotypes and dietary intakes of fat on serum adiponectin concentrations. Male −11377 C/C homozygotes had lower adiponectin concentrations than did C/C women and were less insulin resistant after MUFA-rich and LF diets than were carriers of the G allele (42). In the largest study to date, there was no interaction between dietary fat and the −11391 G/A genotype in the determination of serum adiponectin concentrations, but carriers of the A allele had lower BMI than noncarriers when MUFAs comprised >13% of the total energy intake (43).
The strength of our study was in its design as a randomized, tightly controlled, feeding trial with high adherence and retention rates and diets with a practical relevance to the general population. Limitations included a relatively small sample size and changes in serum adiponectin concentrations associated with genotype, which were small compared with those with BMI, age, or sex. We measured total adiponectin rather than the most bioactive high molecular weight form, but a strong correlation has been shown between the 2 measures regardless of obesity status or the dietary period (39). A wide interindividual variation at baseline and in response to diets could have limited the significance of some outcomes. Multiple testing remains a controversial issue in the interpretation of association studies. Standard multiple-correction methods are appropriate when several SNP and phenotype associations are analyzed simultaneously, but replication in other cohorts is the most reliable method to distinguish true from false-positive associations. If substantiated in a larger sample, a recommendation to −10066 G/G homozygotes to substitute SFAs with MUFAs to maintain adiponectin concentrations with advancing years would be justified. Elucidation of the effects of common SNPs in modifying the outcome of dietary-intervention studies should help in the identification of individuals at risk of complex disease who would benefit from personalized dietary recommendations.
Supplementary Material
Acknowledgments
We acknowledge the contributions of the additional RISCK Study Group members as follows—University of Reading: Hannah Farrant (local coordinator), Claire Lawrence, Edel Magee, and Kit Tsoi (research assistants), Darren Cole (database manager), Steve Austin, Hanneke Mfuni, and Kate Guberg (sample analyses of glucose and insulin), Anna Gent, Celia Greenberg, and Caroline Stokes (coding and analyses of dietary data), and Mario Siervo and Rosemary Hall (clinicians); Imperial College London: Louise Goff (local coordinator), Claire Howard, Namrata Dhopatkar and Bushra Siddiqui (research assistants), and Anne Dornhurst (clinician); Kings College London: Fiona Lewis (local coordinator), Samantha Bowen, Lan Chen, and Robert Gray (research assistants), Roy Sherwood (sample analyses of clinical biochemistry), and Anthony Leeds, A Shah, G Saran, J Niehuser-Saran, and JA Cockburn (clinicians); University of Reading: Rachel Gitau (local coordinator) and Katie Newens and Sean Lovegrove (research assistants); University of Reading and University of Surrey: John Wright (clinician); and University of Surrey: Margaret Griffin (local coordinator). We thank Duncan Talbot at Unilever Colworth Laboratory, Sharnbrook, United Kingdom, for quantitation of plasma adiponectin.
The authors’ responsibilities were as follows—SDO and TABS: conceived the current research project, developed the overall research plan, and had primary responsibility for the final content of the manuscript; AA: extracted DNA and performed the genotyping; GSF, BAG, JAL, SAJ, and TABS: were primary investigators at the 5 RISCK study centers and provided access to databases; AA and TABS: analyzed data and performed statistical analyses; and SDO: wrote the manuscript. The authors and their research groups have a number of links with the food industry. In a personal capacity, GSF is a consultant to Coca-Cola, Premier Foods, and Unilever; and TABS has acted as a consultant to Seven Seas, is a member of the Scientific Advisory Committee for the Global Dairy Platform and external scientific review committee of the Malaysian Palm Oil Board, and chairs Cadbury's Global Nutrition Advisory Panel. TABS, BAG, JAL, SAJ, and GSF have received ad hoc honoraria for lectures or writing articles. In a nonpersonal capacity, BAG was formerly a member of an expert group known as the Fat Panel, which was supported by Dairy Crest, Kerry Gold, and Unilever; SAJ is a member of Scientific Advisory Boards for Coca-Cola, Heinz, PepsiCo, Nestlé, and Kellogg's. SAJ sits on government advisory boards that also include food-industry members. All research groups received products from a range of food companies gratis for research purposes, including Archer Daniel Mills, Croda, Matthews Foods, Nestle, PepsiCo, Jordan, GlaxoSmithKline, and Unilever. AA and SDO reported no conflicts of interest.
REFERENCES
- 1.Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med 2005;56:45–62 [DOI] [PubMed] [Google Scholar]
- 2.Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res 2009;48:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corella D, Ordovas JM. Single nucleotide polymorphisms that influence lipid metabolism: Interaction with dietary factors. Annu Rev Nutr 2005;25:341–90 [DOI] [PubMed] [Google Scholar]
- 4.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002;8:1288–95 [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001;7:941–6 [DOI] [PubMed] [Google Scholar]
- 6.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999;257:79–83 [DOI] [PubMed] [Google Scholar]
- 7.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 2001;86:1930–5 [DOI] [PubMed] [Google Scholar]
- 8.Duncan BB, Schmidt MI, Pankow JS, et al. Adiponectin and the development of type 2 diabetes: the Atherosclerosis Risk in Communities study. Diabetes 2004;53:2473–8 [DOI] [PubMed] [Google Scholar]
- 9.Ryo M, Nakamura T, Kihara S, et al. Adiponectin as a biomarker of the metabolic syndrome. Circ J 2004;68:975–81 [DOI] [PubMed] [Google Scholar]
- 10.Comuzzie AG, Funahashi T, Sonnenberg G, et al. The genetic basis of plasma variation in adiponectin, a global endophenotype for obesity and the metabolic syndrome. J Clin Endocrinol Metab 2001;86:4321–5 [DOI] [PubMed] [Google Scholar]
- 11.Hara K, Boutin P, Mori Y, et al. Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes 2002;51:536–40 [DOI] [PubMed] [Google Scholar]
- 12.Menzaghi C, Ercolino T, Di Paola R, et al. A haplotype at the adiponectin locus is associated with obesity and other features of the insulin resistance syndrome. Diabetes 2002;51:2306–12 [DOI] [PubMed] [Google Scholar]
- 13.Vasseur F, Helbecque N, Dina C, et al. Single-nucleotide polymorphism haplotypes in the both proximal promoter and exon 3 of the APM1 gene modulate adipocyte-secreted adiponectin hormone concentrations and contribute to the genetic risk for type 2 diabetes in French Caucasians. Hum Mol Genet 2002;11:2607–14 [DOI] [PubMed] [Google Scholar]
- 14.Bouatia-Naji N, Meyre D, Lobbens S, et al. ACDC/adiponectin polymorphisms are associated with severe childhood and adult obesity. Diabetes 2006;55:545–50 [DOI] [PubMed] [Google Scholar]
- 15.Heid IM, Wagner SA, Gohlke H, et al. Genetic architecture of the APM1 gene and its influence on adiponectin plasma concentrations and parameters of the metabolic syndrome in 1,727 healthy Caucasians. Diabetes 2006;55:375–84 [DOI] [PubMed] [Google Scholar]
- 16.Filippi E, Sentinelli F, Trischitta V, et al. Association of the human adiponectin gene and insulin resistance. Eur J Hum Genet 2004;12:199–205 [DOI] [PubMed] [Google Scholar]
- 17.Ohashi K, Ouchi N, Kihara S, et al. Adiponectin I164T mutation is associated with the metabolic syndrome and coronary artery disease. J Am Coll Cardiol 2004;43:1195–200 [DOI] [PubMed] [Google Scholar]
- 18.Vozarova de Courten B, Hanson RL, Funahashi T, et al. Common polymorphisms in the adiponectin gene ACDC are not associated with diabetes in Pima Indians. Diabetes 2005;54:284–9 [DOI] [PubMed] [Google Scholar]
- 19.Pischon T, Girman CJ, Rifai N, Hotamisligil GS, Rimm EB. Association between dietary factors and plasma adiponectin concentrations in men. Am J Clin Nutr 2005;81:780–6 [DOI] [PubMed] [Google Scholar]
- 20.Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA 2003;289:1799–804 [DOI] [PubMed] [Google Scholar]
- 21.Kasim-Karakas SE, Tsodikov A, Singh U, Jialal I. Responses of inflammatory markers to a low-fat, high-carbohydrate diet: effects of energy intake. Am J Clin Nutr 2006;83:774–9 [DOI] [PubMed] [Google Scholar]
- 22.Lithander FE, Keogh GF, Wang Y, et al. No evidence of an effect of alterations in dietary fatty acids on fasting adiponectin over 3 weeks. Obesity (Silver Spring) 2008;16:592–9 [DOI] [PubMed] [Google Scholar]
- 23.Paniagua JA, Gallego de la Sacristana A, Romero I, et al. Monounsaturated fat-rich diet prevents central body fat distribution and decreases postprandial adiponectin expression induced by a carbohydrate-rich diet in insulin-resistant subjects. Diabetes Care 2007;30:1717–23 [DOI] [PubMed] [Google Scholar]
- 24.Jebb SA, Lovegrove JA, Griffin BA, et al. Effect of changing the amount and type of fat and carbohydrate on insulin sensitivity and cardiovascular risk: the RISCK (Reading, Imperial, Surrey, Cambridge, and Kings) trial. Am J Clin Nutr 2010;92:748–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferré P. The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes 2004;53(suppl 1):S43–50 [DOI] [PubMed] [Google Scholar]
- 26.Iwaki M, Matsuda M, Maeda N, et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 2003;52:1655–63 [DOI] [PubMed] [Google Scholar]
- 27.Maeda N, Takahashi M, Funahashi T, et al. PPAR{gamma} ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 2001;50:2094–9 [DOI] [PubMed] [Google Scholar]
- 28.Kyriakou T, Collins LJ, Spencer-Jones NJ, et al. Adiponectin gene ADIPOQ SNP associations with serum adiponectin in two female populations and effects of SNPs on promoter activity. J Hum Genet 2008;53:718–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med 2006;23:469–80 [DOI] [PubMed] [Google Scholar]
- 30.Moore C, Gitau R, Goff L, et al. Successful manipulation of the quality and quantity of fat and carbohydrate consumed by free-living individuals using a food exchange model. J Nutr 2009;139:1534–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NCBI. Entrez SNP. Available from: http://www.ncbi.nlm.nih.gov/snp build 132 (cited 11 November 2010)
- 32.Adamczak M, Rzepka E, Chudek J, Wiecek A. Ageing and plasma adiponectin concentration in apparently healthy males and females. Clin Endocrinol (Oxf) 2005;62:114–8 [DOI] [PubMed] [Google Scholar]
- 33.Marques-Vidal P, Bochud M, Paccaud F, Mooser V, Waeber G, Vollenweider P. Distribution of plasma concentrations of adiponectin and leptin in an adult Caucasian population. Clin Endocrinol (Oxf) 2010;72:38–46 [DOI] [PubMed] [Google Scholar]
- 34.Cohen SS, Gammon MD, Signorello LB, et al. Serum adiponectin in relation to body mass index and other correlates in black and white women. Ann Epidemiol 2011;21:86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hulver MW, Saleh O, MacDonald KG, Pories WJ, Barakat HA. Ethnic differences in adiponectin concentrations. Metabolism 2004;53:1–3 [DOI] [PubMed] [Google Scholar]
- 36.Qi L, Li T, Rimm E, et al. The +276 polymorphism of the APMI gene, plasma adiponectin concentration, and cardiovascular risk in diabetic men. Diabetes 2005;54:1607–10 [DOI] [PubMed] [Google Scholar]
- 37.Woo JG, Dolan LM, Deka R, et al. Interactions between noncontiguous haplotypes in the adiponectin gene ACDC are associated with plasma adiponectin. Diabetes 2006;55:523–9 [DOI] [PubMed] [Google Scholar]
- 38.Hu FB, van Dam RM, Liu S. Diet and risk of Type II diabetes: the role of types of fat and carbohydrate. Diabetologia 2001;44:805–17 [DOI] [PubMed] [Google Scholar]
- 39.Yeung EH, Appel LJ, Miller ER, III, Kao WH. The effects of macronutrient intake on total and high-molecular weight adiponectin: results from the OMNI-Heart trial. Obesity (Silver Spring) 2010;18:1632–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson JF, Phillips CM, Tierney AC, et al. Gene-nutrient interactions in the metabolic syndrome: single nucleotide polymorphisms in ADIPOQ and ADIPOR1 interact with plasma saturated fatty acids to modulate insulin resistance. Am J Clin Nutr 2010;91:794–801 [DOI] [PubMed] [Google Scholar]
- 41.Sanderson LM, de Groot PJ, Hooiveld GJ, et al. Effect of synthetic dietary triglycerides: a novel research paradigm for nutrigenomics. PLoS One 2008;3:e1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pérez-Martínez P, López-Miranda J, Cruz-Teno C, et al. Adiponectin gene variants are associated with insulin sensitivity in response to dietary fat consumption in Caucasian men. J Nutr 2008;138:1609–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warodomwichit D, Shen J, Arnett DK, et al. ADIPOQ polymorphisms, monounsaturated fatty acids, and obesity risk: the GOLDN study. Obesity (Silver Spring) 2009;17:510–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.