Abstract
The WHO has developed new growth curves based on breast-fed infants. Recommendations for energy intake have been adopted based on measurements of total energy expenditure. Data on human milk (HM) intake are needed to estimate the energy intake from this food source. However, objective HM data from around the world have not been available, because these measurements are difficult to obtain. Stable isotope methods have been developed to provide objective measurements over a 14-d period. A pooled analysis of 1115 data points of HM intake, obtained using the dose to the mother deuterium oxide turnover method, was undertaken in infants aged 0–24 mo from 12 countries across 5 continents. A hierarchical model was needed to estimate mean HM intake and its variance within and between countries given the complexity of the data. The overall mean HM intake was 0.78 (95% CI = 0.72, 0.84) kg/d, and the age-specific estimates indicated that intake increased over the first 3–4 mo and remained above 0.80 kg/d until 6–7 mo. The variability of intake increased in late infancy. Boys consumed 0.05 kg/d more than girls (P < 0.01). HM intake was strongly, inversely associated with non-HM water intake [r = −0.448 (95% CI −0.511 to −0.385); P < 0.0001]. These objective isotope values of HM intake improve our understanding of the magnitude and variability of HM intake within and across populations and help to estimate nutrient intakes in breast-fed infants.
Introduction
Infant growth has been of major interest to health workers, researchers, and policy makers for several decades. A new set of growth curves has recently been introduced by the WHO based on a large multicenter study (1). These curves provide a normative reference for the growth of infants around the world. Requirements for energy intake have also recently been modified; where the 1985 WHO/FAO/UNU (2) requirements were based on measurements of energy intake, the new 2004 WHO/FAO/UNU (3) requirements are based on measurements of total energy expenditure with an added value for growth. For breast-fed infants to meet this recommendation, a certain amount of human milk (HM)9 intake of a particular energy density is required. However, there is uncertainty about the energy content of HM that is available for metabolic use by the infant and there is also uncertainty about the amount of HM breast-fed infants consume. Although 6 mo of age is now recommended as the optimal duration of exclusive breastfeeding (EBF), few data are available to allow assessment of HM consumption and its variability within and between settings (4). In the absence of such data, it is difficult to ascertain whether infants are receiving HM and associated nutrients in line with WHO recommendations.
Precise and accurate measurements of HM intake are difficult to obtain, because, unlike most food sources, the quantity ingested is not directly observable (4–6). Several methods for measuring HM intake in breast-fed infants have been applied, in particular infant or maternal test-weighing and maternal HM expression; however, both of these approaches risk generating data that do not reflect habitual infant intakes due to their interference with physiological or behavioral aspects of lactation. The introduction of isotope tracer methods to measure HM intakes was an important advance in the area. These techniques, pioneered by Coward et al. (7) in the late 1970s, represent a substantial improvement over test-weighing, which is difficult to apply in field conditions and unsuitable for large group studies.
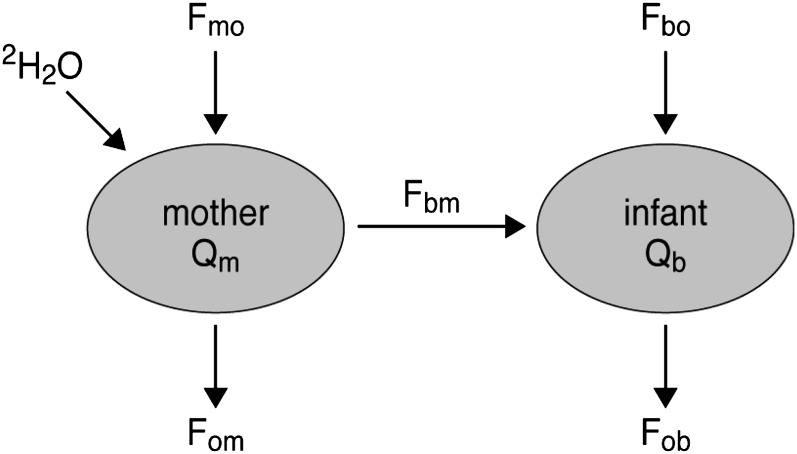
The isotope techniques use a nonradioactive tracer [deuterium-labeled water (2H2O)] to determine rates of water efflux from the mother and baby and the flux of water from the former to the latter. By considering the water balance, this can be related to intake in both (Fig. 1). The first measurements were performed by Coward et al. (7), who gave an oral 2H2O dose to the infant and collected urine samples for the following 2 wk. In this approach, it is assumed that the sole source of water to the infant is HM and therefore any other fluid intake results in an overestimation of HM consumed. This limitation can be overcome by incorporating the label in one of the water sources (either HM or a non-HM source) and in practice this is most easily achieved by administering the dose to the mother (8).
FIGURE 1.
Two compartment, steady-state model of water flows in a mother and her PBF infant. The subscripts b, m, and o refer to the infant, mother, and outside, the combined subscripts indicate directions of water flow (e.g. bm is to the infant from the mother; mo to the mother from the outside). Qm and Qb refer to the total 2H2O distribution space of mother and infant, respectively.
In this revised version of the method, an oral bolus of 2H2O is given to the mother and the disappearance of the isotope from her total body water (sampled from saliva, urine, or HM) is monitored over a 14-d period. At the same time, the appearance of the tracer in, and its subsequent disappearance from, the infant’s body water (sampled as saliva or urine) is also measured. A simple mathematical model is used to obtain a precise estimate of both HM and water intake from foods other than HM (non-milk oral water intake). The importance of this method is that these measurements are made without interference with the normal pattern of feeding. Over a 20-y period, Coward conducted, supervised, and assisted research studies in different populations in various parts of the world. The data obtained from these studies are combined in the analysis reported here with the objective to derive a rational mean value for HM intake obtained by the standardized dose-to-the-mother 2H2O turnover method at successive stages during infancy. We examined HM intake from infants 0–24 mo old. HM intakes from 0–12 mo are contrasted with the values for 0–12 mo based on test-weighing published by WHO (4) and used to estimate nutrient requirements and complementary feeding of infants (9).
Participants and Methods
The data for the analysis are drawn from 12 countries from across 5 continents (Table 1): Bangladesh (10), Brazil (11, 12), Chile (13), Kenya (14), Malawi (15), Mexico (16), Papua New Guinea (PNG) (17), Senegal (18), The Gambia (19), USA (20), UK (21–23), and Zambia (24). Initially, these data were located through a list of research groups that conducted deuterium oxide measurements of lactation kept in Coward’s records and completed with a literature search. One of the chief investigators from these groups was contacted. Efforts were made to obtain all possible datasets including those presented as theses or technical reports. One study of 12 mothers from Pakistan with poor control of predose measurements was excluded (25). The literature search was conducted in PUBMED (26) and BIREME (27), which includes MEDLINE and LILACS reference databases. The search terms were: “breast milk and deuterium oxide,” “human milk and deuterium oxide,” and “breastfeeding and stable-isotope.” Twenty published studies were detected using these terms up to the end of 2007. Eight of them were excluded, because they focused on different metabolic aspects (cholesterol, albumin, modeling), used the dose-to-the-infant method, or studied cattle, and 1 publication was a review. From the 12 studies left, 5 were associated with the samples and countries included in this study (Brazil, Mexico, and Chile). From these countries, only 2 studies’ data were not recovered for the analysis. These 2 studies included 22 participants from Chile and 10 participants from Mexico. Therefore, the dataset includes the vast majority of the data worldwide using the dose-to-the-mother deuterium-oxide method (8) and constitutes the most comprehensive analysis performed to date. Ethical approval was obtained for each separate study.
TABLE 1.
Countries and characteristics of study included in the Human Milk Intake Analysis
| Country | Reference | Type of study | Infants, n | Age range or age at follow-up, mo | Measurements, n |
| UK | Laskey et al., 1991 (22) | Cross-sectional | 30 | 1–2 | 30 |
| UK | Jones, 2003 (23) | Cross-sectional | 50 | 1–2 | 50 |
| UK | YWP study (M. A. Laskey, unpublished) | Cross-sectional | 33 | 1–2 | 33 |
| USA | Butte et al., 1988 (20) | Cross-sectional | 9 | 1–6 | 9 |
| Senegal | Cissé et al., 2002 (18) | Cross-sectional | 129 | 2–3 | 129 |
| Bangladesh | Moore et al., 2007 (10) | Cross-sectional | 94 | 2–3 | 94 |
| Kenya | Ettyang et al., 2005 (14) | Cross-sectional | 10 | 2–5 | 10 |
| Brazil | Haisma et al., 2003 (11) | Cross-sectional | 70 | 3–4 | 70 |
| Brazil | Haisma et al., 2006 (12) | Cross-sectional | 74 | 7–8 | 74 |
| Zambia | Owino e t al, 2007 (24) | Cross-sectional | 52 | 9 | 52 |
| Mexico | Balaños et al., 2000 (16) | Longitudinal | 52 | 0.5, 3 | 104 |
| UK | Goldberg et al., 1991 (21) | Longitudinal | 10 | 1,2,3 | 29 |
| Chile | Alvear et al., 2004 (13) | Longitudinal | 29 | 1,3,6 | 78 |
| Gambia | Jarjou et al., 2006 (19) | Longitudinal | 30 | 4, 12 | 60 |
| Malawi | Golpin et al., 2007 (15) | Longitudinal | 42 | 6–9 | 79 |
| PNG | Orr-Ewing et al., 1986 (17) | Longitudinal | 23 | 0.7, 2, 3, 5, 7, 9, 12, 14, 17, 20, 24 | 214 |
| Total | 737 | 1115 |
The dataset was complex, because each individual study had specific and diverse objectives and designs (Table 1). Infant age ranged from 0.4 to 24 mo, but the majority of infants sampled were within the age range of 2–4 mo (n = 447) (Bangladesh, Brazil, Chile, Kenya, Mexico, Senegal, PNG, UK). Data for ages >10 mo came only from PNG (11–12 mo and 12–24 mo) and Gambia (12 mo). The data were therefore merged for ages 12–24 mo, following a previous protocol (9).
Classification of EBF and partial breastfeeding (PBF) was often not undertaken in the individual studies, so to avoid misclassification and reduction of sample size in the statistical analysis, we did not attempt to use this classification in our analyses. Earlier work by our group showed no difference in milk intake between those 2 categories in Brazilian infants at 3–4 mo of age (11) and the majority of infants can be considered as predominantly breast-fed.
The entire dataset was consistent in providing information for each dyad of mother-infant characteristics, such as infant’s age, weight, gender, and water flux from mother to infant. To progress from providing a separate analysis for each country and to give a broader estimate of the amount of HM infants consume, we therefore derived an analytical protocol to estimate HM intake by infant age, including all the data and splitting the variation into its relevant components. We achieved this by using a hierarchical model based on the assumptions that the data derive from a random selection of countries and that between-country correlation remains constant across the age range. We considered breastfeeding capacity to be the same among women in all the countries, because all the studies enrolled healthy pairs of mother-infant and previous studies have indicated no association between maternal BMI and infant HM intake across a wide range of maternal BMI (28).
Data organization and statistics.
All HM and non-milk water intakes were obtained by fitting the isotopic data to a model, which describes the transfer of HM from mother to the infant and water turnover in both mother and infant (7, 8). The organization of the data required recoding all variables to a common name, unit adjustment, and data merging for analysis. Recalculation of non-milk water intake was performed for the Chile, Brazil (8 mo), Kenya, and Gambia data to adjust for 2H fractionation. A critical review of values and calculation templates was performed. From a total of 1170 measurements, 55 cases (4.6%) were rejected because of apparent 2H enrichment of the infant’s predose body water compared with that of the mother (4 cases), non-milk water intake > 1.7 or < −1.7 kg/d (10 cases), or missing values for HM intake (41 cases).
HM intake data were analyzed using a hierarchical model that reflected the structure of the data in which measurements were nested within infants (repeat measurements per infant) and infants were nested within country, using Stata version 10 (StataCorp). This model allowed all the data to be used to obtain estimates of the parameters of interest. The model provided an overall pooled estimate of mean HM intake and estimates for the residual variance (σ12), between-infant, within-country variance (σ22), and between-country variance (σ32). The correlation among HM intakes within the same infant is (σ22+σ32)/(σ12+ σ22+σ32) and the correlation among HM intakes from different infants within the same country is (σ32)/(σ12+ σ22+σ32). Both non-milk water intake and age of the infant (categorized by month, with ages > 12 mo combined into a single group) were included as fixed effects in this model.
By including non-milk water intake as a fixed effect, it was possible to measure the association of complementary feeding with HM intake. An interaction test of infant gender and non-milk water intake was used to investigate whether the effects of non-milk water intake on HM intake depended on gender. Descriptive HM intake data are presented separately for each gender.
Results
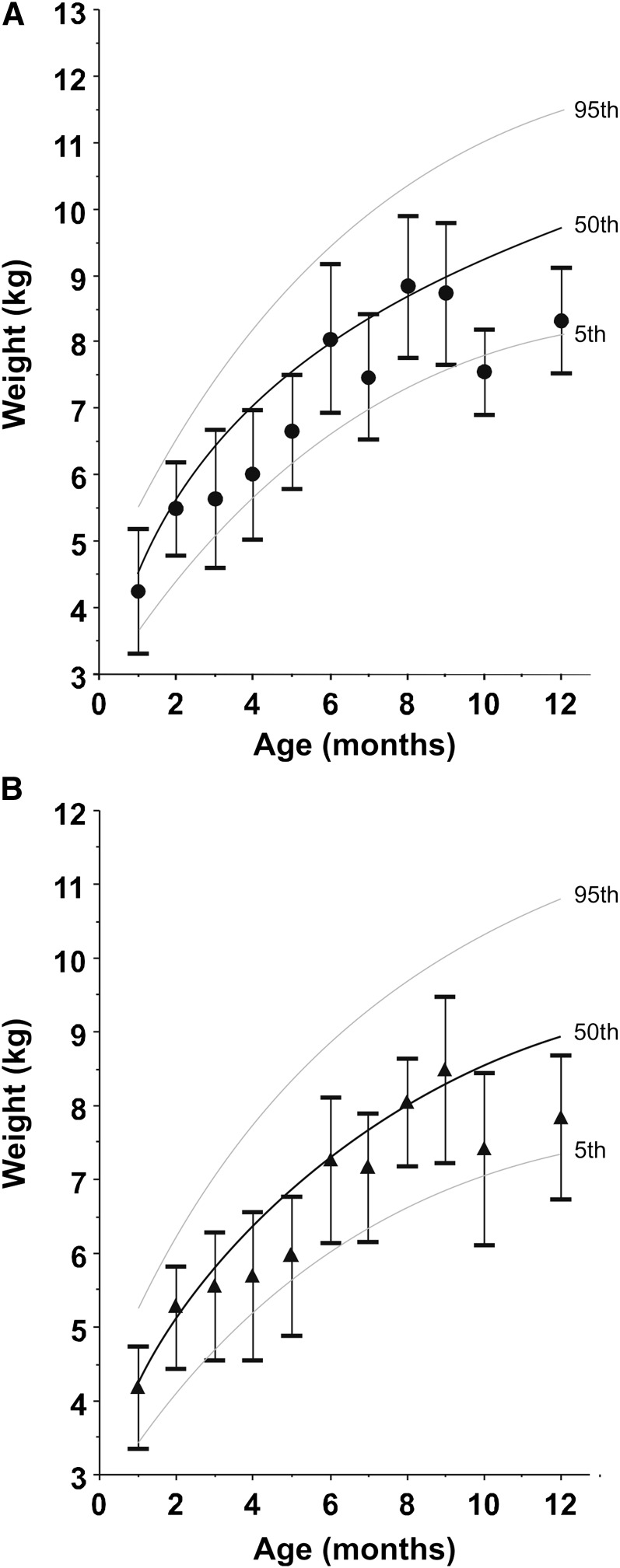
There were 6 longitudinal and 10 cross-sectional studies (Table 1). For the whole sample, the mean infant age was 5.2 mo (range 0.4–23 mo, n = 1115). Infant weight-for-age curves are shown in Figure 2 along with the WHO growth curve for boys (Fig. 2A) and girls (Fig. 2B) (1). In general, the infants showed a weight-for-age slightly below that of the WHO chart (except for 6–9 mo), with the shortfall in weight becoming more pronounced after 11 mo. In later infancy (>11 mo), the data came from PNG and Gambia only.
FIGURE 2.
Growth curve for weight-for-age for male (A) and female (B) infants included in the Human Milk Intake Analysis. Values are mean ± SD, n = 8–142 (A) or 12–124 (B). Lines are WHO standards for 5th, 50th, and 95th percentiles (1). There are no data available for 11-mo-old infants.
HM model excluding age effects.
The overall HM intake estimate from the model excluding age effects was 0.778 (95% CI = 0.717, 0.839) kg/d. The SD between countries was 0.102 kg/d, the SD between infants within countries was 0.116 kg/d, and the residual SD was 0.208 kg/d. The estimate of the correlation between different infants within a country was 0.155, and the correlation within infants was 0.355, implying that milk intake is associated more closely with the needs of the individual than with the feeding practices characterizing populations.
HM model including age group.
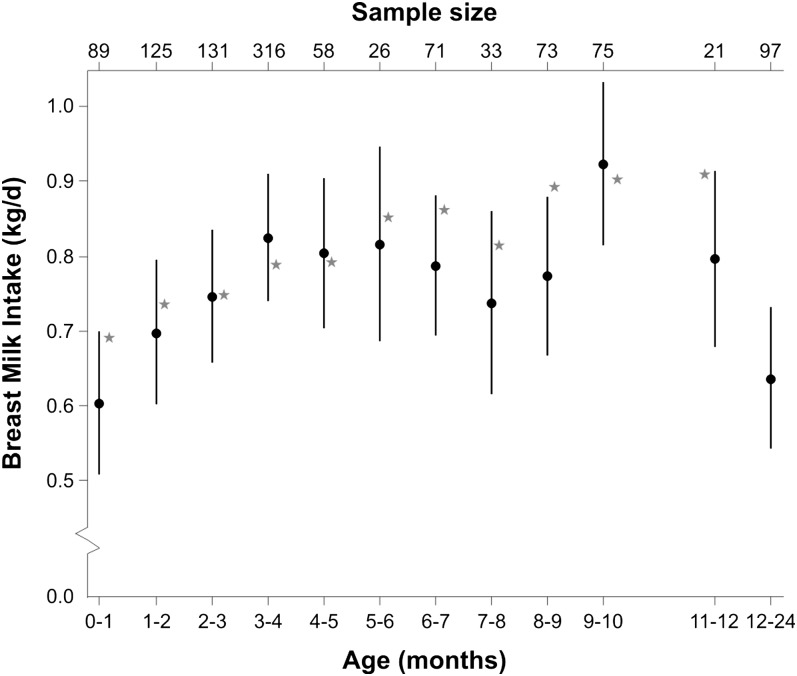
The expected HM intakes estimated from the model including age (categorical) are shown in Figure 3. The values are presented as adjusted mean and 95% CI. The general pattern indicated by our analysis was of a rapid rise from 0.6 kg/d (95% CI = 0.51–0.70) during the first month of life to 0.82 kg/d (95% CI = 0.74–0.91) at 3–4 mo and then very little decline until around 8–9 mo, by which time the variation was greater than at earlier ages and the number of countries included was reduced (9–10 mo: Malawi, PNG, and Zambia; 11–12 mo: PNG; 12–24 mo: PNG and Gambia). Mean HM intakes after 12 mo were derived only from data from PNG, which was the longest follow-up using isotope dilution ever conducted and hence data from other regions for comparison were lacking (Table 1). In the entire sample, the Wald test of whether every age parameter was zero was (P < 0.00001; chi-squared 118 on 11 d.f.), indicating an extremely strong relationship between age and HM intake.
FIGURE 3.
Hierarchical model for HM intake obtained with the dose-to-the-mother deuterium oxide method according to infant age group. This model estimates an overall HM intake with random effect terms for between-country SD and between-individual, within-country SD. The values are mean HM estimates and 95% CI from the hierarchical model with categorical age. There are no available data for 10- to 11-mo-old infants and data for infants ≥12 mo old were grouped. The sample size is included on the top axis. The star plotted in the graph indicates the intake of HM reported by WHO (4) for exclusively HM-fed infants from 1 to 11 mo of age from developed countries.
From this hierarchical model, the SD between countries was 0.136 kg/d, the SD between individuals within country was 0.138 kg/d, and the residual SD was 0.186 kg/d. The correlation between infants within the same country was 0.256 and the correlation within infants was 0.520 (an indication of consistency). In this model, therefore, consistency within infants was again substantially greater than that within countries.
HM model with non-milk water intake as a fixed effect.
The model with non-milk water intake as a fixed effect gave qualitatively similar results but with fewer observations from 11 countries (no data for USA): 694 infants and 1002 measurements in total. The association between non-milk water intake and HM intake was significant (P < 0.0001) and the correlation coefficient was −0.448 (95% CI = −0.511 to −0.385), which is interpreted as a decrease of 45 g/d HM intake for each additional 100 g/d of non-milk water intake. We assume that the cause of this was complementary feeding.
HM intake by gender.
Median HM intake of male (n = 555) infants was higher than girls (n = 551), with both presenting a symmetrical distribution. The sample number was marginally reduced, because there were missing data for infant gender (n = 9). Median HM intake was 0.828 kg/d for male infants and 0.772 kg/d for females, with the male value 5% higher and significantly different (P < 0.01). Lower (× 0.25.) and higher (× 0.75) quartiles were 0.676 and 0.972 kg/d for male and 0.640 and 0.920 kg/d for female infants, respectively. The lowest and highest nonoutlier observations were very close for both sexes as were the number of outliers.
Despite this clear association of gender with HM consumption, gender was not associated with the relative quantity of non-milk water received (and therefore the magnitude of complementary food intake).
Discussion
We report isotopically determined estimates of HM and non-milk water intake at each month of life from 0.4 to 12 mo and a net value for 13–24 mo of age, based on a pooling analysis of 1115 measurements. This pooling analysis gains efficiency compared with looking at each study separately, because variance parameters are shared. It also allows the estimation of between-country correlations and thus the quantification of these differences between populations. We assumed that the country means are themselves normally distributed and thus the results can be expressed in terms of a typical country, allowing the expected mean HM intake to be calculated. Additionally, we reported information of non-milk water intake, which was not always available in previous publications (14, 15, 21, 22, 24). Although there is a paucity of adequate data in the second 6 mo of infancy, our analysis has a number of important findings concerning the developmental pattern of breastfeeding in humans that are relevant to the current debates about optimal infant nutrition.
The inability to assess HM intake directly has long been a challenge for nutrition researchers. Estimated nutrient requirements are based on HM intakes combined with HM composition (6, 29), yet objective data on actual HM intake remain sparse. The majority of infant HM intake data have been collected using test weighing (4,28). This technique is prone to incorrect estimation of intake: first, corrections must be made for insensible water loss during the period of each feeding; second, many infants take numerous, small feedings interspersed with non-nutritive suckling, making it difficult to identify genuine periods of intake; and third, the technique intrudes on the normal behavior of both mother (who is confined near the weighing equipment) and infant (who experiences abnormal amounts of handling before and after every feed). Nighttime feedings are particularly difficult to incorporate in the study protocol; hence, some studies test-weigh during the day only (4). The isotope methodology resolves these issues while also providing an estimate of HM intake averaged over 14 d, thereby providing more representative data for each infant.
Our isotope data can be compared with both the WHO report (4), which is based on highly selected EBF infants from developed countries using mainly test-weighing data, and also the broader literature reporting numerous studies from both developed and developing countries and incorporating both EBF and PBF infants (4,6, 9, 29). Our dataset does not support the high intakes considered in the WHO report (4) over 6 mo. Compared with these, our values are 0.1 kg/d (14%) lower in the first month but converge by mo 4. From 4 mo, our data did not show any increase, which results in the WHO values being 0.1 kg/d (11%) higher by 7 mo. From 9 mo onward, our data suggest extreme heterogeneity, with data from PNG consistent with high WHO values of 0.9 kg/d at 9 mo. Our mean value at 3–4 mo is very close to the average 0.796 kg/d calculated in a systematic review of 33 studies of EBF infants of this age in developed countries (29), although our data do not replicate the 0.1-kg increase between 4 and 6 mo described in that review, based on longitudinal measurements in 72 infants. If our data are compared with averaged values for EBF infants from developing countries (4), we find our values typically 0.05–0.08 kg/d higher, although there is convergence at 6 mo. From 2–3 mo to 5–6 mo, our values are 0.18–0.21 kg/d higher than previously reported for predominantly breast-fed babies from developed countries. This supports the underestimation of HM intake previously performed with test-weighing in developing countries. Our values are also higher than previously reported from a 1985 WHO report (30) and higher than the values published in a recent review of dietary reference intakes for the United States and Canada (6).
On this basis, our data indicate HM intakes generally higher than reported elsewhere in developing countries (4,29) and are consistent with those previously published for developed countries (29), but lower at almost all time points than the WHO reported intake values in EBF infants from developed countries (4). These results therefore contribute to the discussion on the optimal nutrient requirements of infants. Currently, the WHO protein requirements for infants (31) are based on HM intake data from the WHO report (4), whereas WHO energy requirements (3) for infants are not based on the observed intakes but on a factorial approach considering total energy expenditure and the energy needs for growth. Thus, there is a discrepancy between the HM intakes considered ideal for protein compared with energy, and our data provide an indication of typical HM intakes. This information is important for nutritionists, because the infant’s exposure to nutrients or compounds that are transferred from mothers to infants through HM can be better estimated on a 100-g HM/d basis and their variance and range determined. This information can help nutritionists calculate recommendations for infant complementary food intakes (32).
Male infants had HM intakes 5% greater than those of female infants. This is likely due both to biological factors, with male infants having greater lean mass than females throughout infancy, and behavioral factors, because mothers may consider male infants as having higher energy requirements and hence feed them differently. Our data are consistent with an earlier study demonstrating increased energy intakes by mothers of male compared with female fetuses and suggest that there is a cumulative increased maternal cost of nourishing males (33). Unsurprisingly, our data reveal a strong correlation between the intake of HM and the intake on non-HM foods, represented in our model by non-HM water source. The distribution of non-milk water intake is very skewed, with most of the values close to zero, which cannot be analyzed using the same multilevel model as applied to the HM intake data. Some of the difference between our values and those reported in the WHO report (4) is likely due to the mothers in our sample introducing non-milk foods earlier than the WHO sample.
A limitation of our analysis is that we were unable to separate between EBF infants and predominantly breast-fed infants, because this information is obtained by maternal verbal information and not objectively verified due to lack of a gold standard. Accurate questionnaires and awareness for EBF is recent and this limitation underlies previous reports (4,6, 29, 30). However, Haisma et al. (11), using the same deuterium oxide-method, found no difference in HM intake between EBF and PBF infants. A second limitation is the paucity of data for later infancy and the fact that all data from 12 mo onward came from a single population, PNG. However, there are several strengths of our study, which is unique in the literature to date: it is based on an objective technique and it has a broad inclusion of countries, gender separation, and a large age range. Our analysis emphasizes the lack of existing data on HM intake in very early and later infancy and the urgent need for new data, especially on EBF infants.
Our study, offers an overview of HM intake using an objective stable isotope method. Our analysis of a large sample of mother-infant dyads from 12 countries showed a steady increase in HM intake from mo 1 to 4 of life, followed by a plateau beyond 6 mo, during which mean HM intake remained above 0.8 kg/d. Our values are similar to those reported by others for developed countries and higher than those for developing countries. We suggest that HM intake may have been underestimated by test-weighing in developing countries. These data provide a novel and valuable perspective on typical HM intakes for use with energy and nutrient density estimation in breast-fed infants. HM intake significantly declines as the level of non-milk water intake increases.
Acknowledgments
The work reported here is dedicated to the memory of Andy Coward, for pioneering the dose to the mother method. All authors contributed to the conceptualization of the paper. T.H.M.C., L.B., J.W., and H.H. contacted researchers to obtain data; T.H.M.C. undertook the data organization; A.M. performed statistical analysis and participated in data organization; T.H.M.C. prepared the drafts. All authors read and approved the final manuscript.
Footnotes
T.H.M.C. is supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Ministry of Education, Brazil, and DPP “Decanato de Pesquisa e Pós-graduação”, University of Brasilia, Brazil.
Abbreviations used: HM, human milk; EBF, exclusive breastfed, PBF, partially breastfed; PNG, Papua New Guinea.
Literature Cited
- 1.WHO WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for height and body mass index-for-age: methods and development; 2006. [cited 2010 Mar 3]. Available from: http://www.who.int/bookorders/anglais/detart1.jsp?sesslan=1&codlan=1&codcol=15&codcch=660
- 2.WHO Energy and protein requirements: report of a joint FAO/WHO/UNU expert consultation. Geneva: WHO; 1985. WHO Technical Report Series No. 724 [PubMed] [Google Scholar]
- 3.WHO Human energy requirements. Report of a Joint FAO/WHO/UNU Expert Consultation. Rome: FAO; 2004. FAO Food and Nutrition Technical Report Series, No. 1 [Google Scholar]
- 4.WHO Nutrient adequacy of exclusive breastfeeding for the term infant during the first six months of life. Geneva: WHO; 2002. [cited 2010 Mar 3]. Available from: http://www.who.int/child-adolescent-health/publications/NUTRITION/Nutrient_Adequacy.htm [Google Scholar]
- 5.WHO The optimal duration of exclusive breastfeeding. A systematic review. WHO/NHD/01.08 or WHO/FCH/CAH/01.23. Geneva: WHO; 2002. [cited 2010 Mar 3]. Available from: http://www.who.int/child-adolescent-health/publications/NUTRITION/WHO_FCH_CAH_01.23.htm [Google Scholar]
- 6.Institute of Medicine Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington, DC: National Academies Press; 2002 [DOI] [PubMed] [Google Scholar]
- 7.Coward WA, Whitehead RG, Sawyer MB, Prentice AM. New method for measuring milk intakes in breast-fed babies. Lancet. 1979;2:13–4 [DOI] [PubMed] [Google Scholar]
- 8.Coward WA, Cole TJ, Sawyer MB, Prentice AM. Breast milk intake measurement in mixed-fed infants by administration of deuterium oxide to their mothers. Hum Nutr Clin Nutr. 1982;36:141–8 [PubMed] [Google Scholar]
- 9.WHO/UNICEF/Orstrom/University of California at Davis Complementary feeding of young children in developing countries: a review of current knowledge. WHO/NUT/98.1. Geneva: WHO; 1998. [cited 2010 Mar 3]. Available from:;http://www.who.int/child-adolescent-health/publications/NUTRITION/WHO_NUT_98.1.htm [Google Scholar]
- 10.Moore SE, Prentice AM, Coward WA, Wright A, Frongillo EA, Fulford AJC, Mander AP, Persson L, Arifeen SE, et al. Use of stable-isotope techniques to validate infant feeding practices reported by Bangladeshi women receiving breastfeeding counseling. Am J Clin Nutr. 2007;85:1075–82 [DOI] [PubMed] [Google Scholar]
- 11.Haisma H, Coward WA, Albernaz E, Visser GH, Wells JCK, Wright A, Victora CG. Breast milk and energy intake in exclusively, predominantly, and partially breast-fed infants. Eur J Clin Nutr. 2003;57:1633–42 [DOI] [PubMed] [Google Scholar]
- 12.Haisma H, Coward WA, Visser GH, Vonk R, Wells JCK, Wright A, Victora CG. Socio-economic and environmental factors influence energy utilization in Brazilian breast-fed infants. J Nutr. 2006;136:2945–51 [DOI] [PubMed] [Google Scholar]
- 13.Alvear J, Salazar G, Berlanga R, Anciani A, Pizzaro F. Breastfeeding and growth in a group of selected 0 to 24 months infants. In: International Atomic Energy Agency (IAEA). Coordinated research project on isotopic evaluation in infant growth monitoring: a collaboration with WHO (partly RCA). Vienna: INIS; 2004 [Google Scholar]
- 14.Ettyang GA, Lichtenbelt WDM, Esamai F, Saris WHM, Westerterp KR. Assessment of body composition and breast milk volume in lactating mothers in pastoral communities in Pokot, Kenya, using deuterium oxide. Ann Nutr Metab. 2005;49:110–7 [DOI] [PubMed] [Google Scholar]
- 15.Galpin L, Thakwalakwa C, Phuka J, Ashorn P, Maleta K, Wong WW, Manary M J. Breast milk intake is not reduced more by the introduction of energy dense complementary Food than by typical infant porridge. J Nutr. 2007;137:1828–33 [DOI] [PubMed] [Google Scholar]
- 16.Balaños AV, Caire G, Valencia ME, Casanueva E, Pérez R, Calderón de La Barca AM. Energy intake and growth of breastfed infants in two regions of Mexico. In: Koletzko B, editor. Short and long term effects of breast feeding on child health. New York: Kluwer Academic Plenum Publishers; 2000. P. 371–2 [Google Scholar]
- 17.Orr-Ewing AK, Heywood PF, Coward WA. Longitudinal measurements of breast milk output by a 2H2O tracer technique in rural Papua New Guinean women. Hum Nutr Clin Nutr. 1986;40:451–67 [PubMed] [Google Scholar]
- 18.Cissé AS, Dossou N, Ndiaye M, Guèye AL. Diop el HI, Diaham B, Guiro AT, Cissé D, Sarr CS, et al. Stable isotope aided evaluation of community nutrition program: effect of food supplementation schemes on maternal and infant nutritional status. Food Nutr Bull. 2002;23 Suppl 3:169–73 [PubMed] [Google Scholar]
- 19.Jarjou LM, Prentice A, Sawo Y, Laskey MA, Bennett J, Goldberg GR, Cole TJ. Randomized, placebo-controlled, calcium supplementation study in pregnant Gambian women: effects on breast-milk calcium concentrations and infant birth weight, growth, and bone mineral accretion in the first year of life. Am J Clin Nutr. 2006;83:657–66 [DOI] [PubMed] [Google Scholar]
- 20.Butte NF, Wong WW, Patterson BW, Garza C, Klein PD. Human-milk intake measured by administration of deuterium oxide to the mother: a comparison with the test-weighing technique. Am J Clin Nutr. 1988;47:815–21 [DOI] [PubMed] [Google Scholar]
- 21.Goldberg GR, Prentice AM, Coward WA, Davies HL, Murgatroyd PR, Sawyer MB, Ashford J, Black AE. Longitudinal assessment of the components of energy balance in well-nourished lactating women. Am J Clin Nutr. 1991;54:788–98 [DOI] [PubMed] [Google Scholar]
- 22.Laskey MA, Prentice A, Hanratty LA, Jarjou LM, Dibba B, Beavan SR, Cole TJ. Bone changes after 3 mo of lactation: influence of calcium intake, breast-milk output, and vitamin D–receptor genotype. Am J Clin Nutr. 1998;67:685–92 [DOI] [PubMed] [Google Scholar]
- 23.Jones D. Genetic and biochemical determinants of interindividual variability in the skeletal response to lactation [PhD thesis]. Cambridge: Darwin College, University of Cambridge; 2003 [Google Scholar]
- 24.Owino VO, Kasonka LM, Sinkala MM, Wells JK, Eaton S, Darch T, Coward A, Tomkins AM, Filteau SM. Fortified complementary foods with or without α-amylase treatment increase hemoglobin but do not reduce breast milk intake of 9-mo-old Zambian infants. Am J Clin Nutr. 2007;86:1094–103 [DOI] [PubMed] [Google Scholar]
- 25.Bhutta Z A, Abbass S, Wright A, Coward A. Isotopic evaluation of breast milk intake, energy metabolism, growth and body composition of exclusively breastfed infants in Pakistan. In: International Atomic Energy Agency (IAEA). Coordinated research project on isotopic evaluation in infant growth monitoring: a collaboration with WHO (partly RCA). Vienna: INIS; 2004 [Google Scholar]
- 26.PUBMED, US National Library of Medicine, NIH. [cited 2010 Mar 30]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/
- 27.BIREME. “Biblioteca Virtual em Saúde”, OPAS, WHO [cited 2010 Mar 30]. Available from: http://regional.bvsalud.org/php/index.php.
- 28.Prentice AM, Goldberg GR, Prentice A. Body mass index and lactation performance. Eur J Clin Nutr. 1994;48 Suppl 3:S78–86 [PubMed] [Google Scholar]
- 29.Reilly JJ, Ashworth S. Wells JCK. Metabolisable energy consumption in the exclusively breast-fed infant aged 3–6 months from the developed world: a systematic review. Br J Nutr. 2005;94:56–63 [DOI] [PubMed] [Google Scholar]
- 30.Organización Mundial de la Salud Cantidad y calidad de la leche maternal. Ginebra: Organización Mundial de la Salud; 1985 [Google Scholar]
- 31.WHO Protein and amino acid requirements in human nutrition. Report of a Joint WHO/FAO/UNU Expert Consultation. WHOTechnical Report Series, No. 935. Geneva: WHO; 2007 [Google Scholar]
- 32.WHO Complementary feeding: family foods for breastfed children. WHO/NHD/00.1, WHO/FCH/CAH/00.6. Geneva: WHO; 2000. [cited 2010 Mar 3]. Available from: http://www.who.int/nutrition/publications/infantfeeding/WHO_NHD_00.1/en/index.html [Google Scholar]
- 33.Tamimi RM, Lagiou P, Mucci LA, Hsieh CC, Adami HO, Trichopoulos D. Average energy intake among pregnant women carrying a boy compared with a girl. BMJ. 2003;326:1245–6 [DOI] [PMC free article] [PubMed] [Google Scholar]