Abstract
The vasculature, an organ that penetrates every other organ, is ideally poised to be the site where pools of stem cells are placed, to be deployed and committed in response to feedback regulation, and to respond to demands for new vascular structures. These pools of multipotent cells are often under the regulation of various members of the transforming growth factor-β superfamily, including the bone morphogenetic proteins and their antagonists. Regulation of stem cell populations affects their recruitment, differentiation, spatial organization, and their coordination with host tissue. Loss and dysregulation of feedback control cause a variety of diseases that involve ectopic tissue formation, including atherosclerotic lesion formation and calcification, diabetic vasculopathies, and arteriovenous malformations.
Keywords: Vascular stem cells, Endothelial cells, Bone morphogenetic proteins, Vascular disease
Introduction
The vasculature is a constantly adapting tissue that penetrates every other organ in the body except cornea and cartilage. Consequently, vascular disease has the potential to affect all organs and tissues. Tissue repair and regeneration will also have to involve the vasculature, where it may even be initiated or triggered. These features make the vasculature itself an ideal site to place stem cells strategically in pools that can be expanded rapidly to respond to injury and disease. This hypothesis suggests an interesting interface, where stem cell biology could greatly contribute to our understanding of the role of stem cells in vascular disease.
Common vascular disorders like atherosclerosis and diabetic vasculopathy feature ectopic tissue formation, including the formation of ectopic bone, fat, cartilage, and bone marrow-like elements in the vascular wall [1]. Indeed, it has long been known that the diseased arterial wall is the most common site for ectopic tissue formation [2]. This implies the existence and involvement of multipotent cells in the vasculature that respond to triggers of differentiation, which can occur functionally during development or dysfunctionally in disease. Many investigators have reported evidence supporting the existence of such cells, both as distinct cell populations and as cells that can potentially take on stem cell characteristics [1, 3]. Such “emergence of stemness,” defined as emergent properties of cell lineages under feedback control [4], has the potential of putting all vascular cells into play as latent stem cells.
Feedback regulation of stem cell populations, potential reversal of cell differentiation, and spatial organization and coordination of the vasculature with tissue-specific elements are all aspects of stem cell biology that are highly relevant to the function and regeneration of vascular structures. The members of the transforming growth factor (TGF)-β superfamily of growth factors have been implicated in several of these regulatory functions. One subgroup in particular, the bone morphogenetic proteins (BMPs), are surfacing as important regulators in vascular disease. The BMPs are known to support stem cell renewal [5, 6], promote vascular cell lineage differentiation, and affect the layout of the vasculature [7–9]. Dysregulation of vascular BMP activity can have striking effects on the vascular bed, which differ depending on location, as exemplified in the matrix Gla protein (MGP) knockout mouse, which lacks MGP, a BMP antagonist. The MGP knockout mouse suffers simultaneously from extensive calcification in the systemic arteries and widespread arteriovenous malformations (AVMs) in lungs and kidney [10, 11]. Dysregulated BMP signaling also contributes to the development of atherosclerosis, vascular calcification, hereditary hemorrhagic telangiectasia (HHT), and pulmonary arterial hypertension [12–15].
BMP-directed regulation of cell lineages may even extend to reversal of differentiation. Reports have suggested that fully differentiated endothelial (ECs) and smooth muscle cells (SMCs) can dedifferentiate and be recommitted to a different lineage such as osteochondrogenic lineages, in the context of enhanced BMP signaling in vivo and in vitro [16–18]. Thus, the BMPs would seem to be important regulators of stem cell function in vascular development and in disease.
As a general observation, it is clear that the vasculature and tissue-specific elements must be spatially and temporally coordinated for optimal organ function. The proliferation and differentiation of two or more distinct cell types (of which one is vascular) as well as their spatial patterning and coordination may be governed by principles that are applicable to all vascular beds with appropriate modification. Dysregulation of this control is likely to results in loss of functionality and disease.
Thus, the vasculature has to attain the appropriate morphology to achieve optimal function in each vascular bed. In this article, we discuss how vascular biology could gain insights from interfacing with stem cell research.
Feedback Regulation of Stem Cell Pools in the Vasculature
Vascular structures are unique. They are expected to take up little space and at the same time perfuse and deliver nutrients to all locations in the body. Thus, they will have to show inventiveness in penetrating organs and adapting to the spatial structure of each organ. Considering the stringent space restrictions, it is an interesting problem how stem cells are provided locally for repair or for the development of new vessels. Strategies will be needed to keep the number of progenitor cells low, yet keep them available for rapid mobilization and expansion. As has been proposed for other tissue types by Lander et al. [19, 20], stem cell pools may be one way that the vasculature responds to these requirements.
There is dramatic evidence of multipotent cells in the vasculature, especially in vascular calcification and ectopic bone formation [1, 21]. Multiple studies have demonstrated that vascular mesenchymal cells, including microvascular pericytes, so-called calcifying vascular cells and SMCs, undergo osteochondrogenic lineage differentiation spontaneously or under special conditions such as hyperphosphatemia [1, 22]. These are readily expandable cell populations and may represent distinct types of local vascular stem cells, or they may represent specific, discernible stages in a spectrum of mesenchymal cell plasticity. In addition, circulating multipotent cells [23, 24] may provide additional access to a multipotent population.
Stem cells are often found near basement membrane, which has suggested the existence of so-called “niches” or specialized microenvironments for stem cells [25, 26]. The vascular basement membrane is located in proximity to the endothelium and is able to modulate the behavior of ECs through matrix components and growth factors. It may also assist in restricting the reach of growth factor, for example, to ECs, potentially promoting unequal segregation, cell polarity, and other characteristics important for cell fate determination [26]. A cell population in proximity to the basement membrane would be perfectly located to supply both EC and SMC progenitor cells, enabling quick regeneration. The origin of such progenitor cells would include resident vascular as well as circulating stem cells. Resident stem cells may be reserved and positioned during development [3], whereas circulating stem cells, mainly derived from the bone marrow [23, 24], may be rapidly mobilized from the circulation and modified by the vascular context. The recruited cells may be considered as a separate pool of cells or be added to already established pools. Differences in the recruiting factors or local contexts may determine which circulating stem cell pools are recruited.
Stem cell proliferation must be tightly controlled to balance progenitor cell versus mature populations in vessels whose dimensions are sometimes small. One strategy, in the mammalian olfactory epithelium, which has been explored with the help of mathematical models, is feedback inhibition of cell replication to regulate the output of cells at different stages of lineage differentiation [20]. Stem cells may pass through a number of intermediate steps before reaching a terminal differentiation stage. The characteristics of these stem cells in transit, and how they react to growth factors, may be a consequence of the feedback control or of the tissue context [19]. Systems where one feedback is aimed at the stem cell, and another is aimed at transiting cells, may respond with fast regeneration after a perturbation [20], maintaining a high ratio of differentiated to undifferentiated cells and minimizing the bulk of “unused” stem cells. If ECs and SMCs in the vasculature are maintained by specific feedback control, failure of these controls could result in an excess of poorly differentiated cells and the consequent development of vascular disease. Vascular calcification, atherosclerotic lesion formation, diabetic vascular calcification, and excessive neoangiogenesis are all pathologies that could involve rapidly expanding or inappropriately differentiating progenitor cell populations.
Negative feedback regulators of proliferation include many members of the TGF-β superfamily, for example, the growth and differentiation factor 11 in the olfactory epithelium [20]. Locally derived BMP and BMP antagonists are well-positioned to regulate local populations of progenitor pools. For example, BMP-4 is considered a renewal factor for stem cells [5, 6] but has also been shown to induce the activin-like kinase receptor (ALK) 1, which is stimulated by BMP-9 and is instrumental in the establishment of vasculature (Fig. 1A) [8, 27]. The importance of BMP signaling in vascular disease has been further emphasized by the work of several groups. Csiszar et al. and Sorescu et al. have shown that BMP-2 and BMP-4 are inflammatory mediators that can promote the early steps in the development of atherosclerosis [28, 29]. In addition, the induction of BMP antagonists such as follistatin, noggin, MGP, and crossveinless 2 (also referred to as BMPER) [12, 13, 28, 30] may be a key event in limiting vascular disease. Our work has shown that a decrease in BMP activity, as mediated by enhanced expression of MGP, an inhibitor of BMP-2, BMP-4, and BMP-7, limits atherosclerotic lesion development, lesion calcification, and diabetic medial calcification [12, 13]. Furthermore, mutations in the genes for the ALK1, a BMP type I, and the BMP type II receptors have been linked to the development of HHT, characterized by AVMs, and pulmonary arterial hypertension, respectively [8, 14, 15]. Thus, BMP signaling can direct proliferation and differentiation of vascular cells, and inadvertent activation can promote vascular disease.
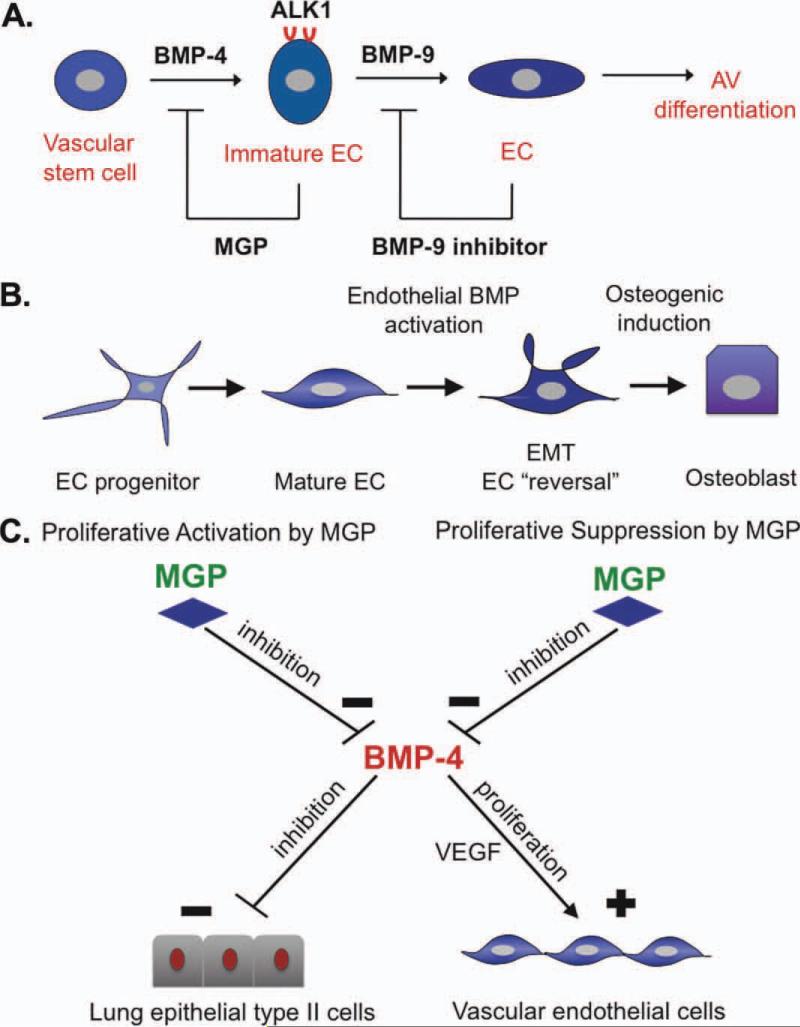
Figure 1.
Schematic diagrams of BMP involvement in vascular processes. (A): A two-step model of the proposed roles of BMP-4 and BMP-9 in EC differentiation. BMP-4 stimulates expression of the ALK1 receptor in immature ECs, which then become responsive to BMP-9, which allows for further proliferation and AV differentiation [8, 33, 34]. At each step, a BMP antagonist may be induced to limit the BMP stimulation. (B): Potential reversal of cell differentiation in ECs in the setting of increased BMP activity in vascular disease such as atherosclerosis (hyperlipidemia) or diabetic vasculopathy (hyperglycemia), with osteogenic induction by BMP-2 or other factors [12, 13, 16]. (C): Reciprocal regulation of proliferation in epithelial type II lung cells and vascular ECs. MGP promotes proliferation of lung cells but limits that of ECs. BMP-4 has the opposite effect. Thus, an increase in MGP will favor lung cell proliferation, whereas a decrease in MGP will favor EC proliferation [11]. Abbreviations: ALK1, activin-like kinase receptor 1; AV, arteriovenous; BMP, bone morphogenetic protein; EC, endothelial cell; EMT, endothelial-mesenchymal transition; MGP, matrix Gla protein; VEGF, vascular endothelial growth factor.
The stem cell field could advance vascular health in many ways by helping us understand and define intermediate steps in vascular cell lineages, identify mediators in the feedback regulation of proliferation, and define the context dependence of stemness in progenitor cells.
Reversal of Fortune: Is Vascular Differentiation Reversible?
Stem cells are characterized both by multipotency and by their ability to maintain their own number through self-replication; ability to transdifferentiate into other lineages alone does not characterize a cell as a stem cell. However, it has increasingly been argued that the definition of stem cells should be one of context and condition, not of specific cell type [4]. The concept of “stemness,” loosely defined as the essential characteristics that distinguish stem cells from ordinary cells, may also be created as cell lineage properties that emerge under the control of growth factors and their inhibitors. Thus, the existence of stem cells may be context-dependent, which could explain the spectrum of multipotent cells reported in the vasculature [1, 3].
This raises the possibility that vascular lineages such as ECs and SMCs are capable of “reversing” their differentiation. Increasing evidence supports a great plasticity in vascular cells, and it is highly plausible that fully differentiated vascular cells may reverse their differentiation in response to special cues. Such emergence of stemness [4], as dictated by the vascular context, could be a real force driving vascular pathology. For example, phenotypic modulation causes SMCs to take on a less-differentiated, proliferative phenotype, add to the bulk of the atherosclerotic lesion mass, and show increased susceptibility to BMP-2 and other osteogenic inducers [33]. Indeed, Speer et al. [18] showed that frank transition or “lineage reprogramming” occurs in the aortic SMCs in mice lacking MGP. This mechanism is likely to play an important role also in more common types of vascular calcification, due to the increased BMP activity in both atherosclerotic lesions and diabetic vascular calcification [12, 13, 28, 30].
Transdifferentiation of ECs has also been proposed as a mechanism contributing osteogenic cells to the ectopic soft tissue calcification in fibrodysplasia ossificans progressive (FOP) [14]. FOP is caused by mutations in the gene for the ALK2, a BMP type 1 receptor, resulting in constitutive activation of the receptor. This BMP activation in ECs causes an apparent transition to osteogenic lineages and contributes directly to the calcified soft tissue lesions, implying that trans-differentiating ECs are a source of multipotent cells (Fig. 1B). Such a mechanism is plausible also in vascular calcification and would directly link factors like hyperlipidemia, hyperglycemia, and hyperphosphatemia to the induction of osteogenic differentiation in the vascular wall, through effects on BMP signaling [12, 13, 22].
If stemness emerges in vascular cells, it also opens the door to selective regulation of stemness, so that intermediate stages in differentiation might exist instead of distinct cell populations. Such intermediate stages might be necessary to help generate the layout of tissues and the accompanying vasculature during development. Spatial pattern formation may depend on the regulation of developmental stage, in that certain structures may need to form before progenitor cells reach terminal differentiation. For example, common stem cells could give rise to both ECs and surrounding pericytes or SMCs in a carefully orchestrated sequence that supports the proliferation, network formation, and terminal differentiation of the respective cells to create a functional vessel. Alternatively, there is the possibility that ECs transdifferentiate into pericytes or SMCs, or that pericytes act as a progenitor cells to SMCs [36, 37]. Thus, pericytes may take on different roles, likely due to the heterogeneity and relatively unclear origin of this cell population, which has mainly been defined anatomically as periendothelial cells surrounding capillaries and microvessels [36, 37].
Multipotent mesenchymal cells from the vasculature have also been shown to exhibit spatial pattern formation in vitro. Such stem cell-like cells generate patterns in the form of ridges and nodules [38, 39], in which bone formation subsequently occurs. Since BMPs and their inhibitors are concentrated by the pattern formation process, the pattern formation can contribute to differentiation by concentrating the factors that “flip the differentiation switch” in the differentiating cells. Proteins that could affect both cellular patterns and “the differentiation switch” would be excellent candidates to trigger vascular regeneration.
Thus, the stem cell biologist could greatly support vascular biology with knowledge of how to reverse terminal differentiation toward more stem cell-like characteristics, and the relation between stages of differentiation and the patterning of tissues, all central in vascular regeneration and disease.
How Does the Vasculature Coordinate with Organ-Specific Elements?
The vasculature clearly has to coordinate its growth with organ-specific elements to carry out organ function. Thus, it must be part of the pattern of the respective tissue, and vascular regeneration must take tissue pattern into account. The basic principles by which this coordination is carried out are likely to be applicable to all vascular beds. Since vascular stem cells may be exposed to stimuli targeting other, nearby, cell lineages, both stimuli and cells will need to be coordinated with each other at specific stages in development and regeneration. These processes are incompletely understood.
Our experiments have suggested a potential mechanism for this coordination. In the MGP-deficient mice, we found that ECs and type II lung epithelial cells have opposite responses to the same stimulus [11]. EC proliferation was stimulated by BMP-4, as mediated by the vascular endothelial growth factor (VEGF), whereas lung cells were inhibited by BMP-4. Lung cell proliferation, however, was stimulated when BMP-4 activity was suppressed [11]. This principle of “reciprocal regulation” (Fig. 1C) was reflected in vivo in the balance between vasculature and airways, where airways dominated the lung space in MGP transgenic mice and vasculature dominated the lung space in MGP-deficient mice [9, 11]. Thus, optimal gas exchange in the lungs can actually be directly linked to the proliferative response of two different cell types. This basic principle of reciprocity between two neighboring cell types could be applied anywhere coordination is needed. However, relating the overall pattern of the tissue, such as branching, to cell differentiation still needs further study.
A less-discussed abnormality in MGP-deficient mice is the lack of hypertrophic chondrocytes and a loss of the columnar organization in the bone growth plate associated with these cells [10]. Formation of vascular channels and vascular invasion are necessary for the architecture, affects longitudinal bone growth, and are dependent on appropriate expression of VEGF-A [38, 39]. The lack of MGP leads to premature and poorly patterned mineralization and an overall picture of osteopenia, suggesting that the uncontrolled BMP activity in these mice also affects the coordination between vascular elements and differentiating chondrocytes, likely through VEGF expression.
Adipose tissue provides another interesting case of cellular cooperation. Adipose tissue is able to expand and shrink as needed throughout life, and the vasculature adapts and responds to cues indicating whether fat needs to be stored or mobilized. Adipogenesis and angiogenesis are coupled during development, and several studies suggest that vascular growth may be decisive in the growth of adipose tissue, and that obesity can be limited by inhibiting angiogenesis [42]. In addition, the adipose microvascular environment changes with the state of the adipocytes and the development of obesity and disease, such as diabetes, thereby affecting vascular growth [43, 44]. For example, the adipose tissue of obese, insulin-resistant subjects was found to have fewer capillaries and more large vessels compared with lean subjects [43].
Studies have suggested a common progenitor cell for adipocytes and adipose ECs [45, 46], which would simplify keeping a balance between fat and vasculature, but the regulation of this “fork” in differentiation is not clear although the microenvironment would be expected to have a strong effect. Multipotent so-called dedifferentiated cells derived from white mature adipocytes may represent cardiovascular precursor cells that are able to differentiate into ECs or adipocytes depending on the immediate need [47, 48]. Other studies have proposed that adipocytes can be derived from cells residing in the mural cell compartment of the adipose vasculature in close relationship to ECs [46]. Thus, an understanding of the cellular choices in fat tissue would provide us with tools to modify the fat in various locations.
Finally, it is well-known that the vasculature shows coalignment with nerves; vessels track the nerves and vice versa. This so-called neurovascular link may be explained by the ECs and neurons responding to the same molecular cues or a coregulation of cell migration [49]. Important vascular signals such as VEGF and Notch have been shown to be central in cell fate in the nervous system as well as in the vascular system [49–52]. Overall, current knowledge about the spatial and temporal development of the nervous system suggests that the vasculature plays a role in coordinating neurogenesis. The maintenance of this link is likely central in preserving normal neurological function.
Thus, coordination between the vasculature and organ-specific elements is an everyday but poorly understood process that is critical for optimal regulation of organ function, which requires cooperation and the sharing of space and resources between tissues. Disturbances of this coordination form the bases of regeneration and in many cases, disease. Further studies will be required to elucidate how stem cells are correctly directed to the vasculature versus organ-specific elements. We need to know how the tissue context can be optimized to promote a specific stem cell stage or lineage, and the exact mechanisms of this process, to be truly successful in regenerating new tissues.
Conclusion
The vasculature is essential for normal organ function. Tissue repair and regeneration will necessarily involve the vasculature and may indeed be initiated from the vasculature. It would also be the ideal location for stem cell populations that could rapidly be expanded locally to respond to injury and disease.
However, in order to optimize the use of vasculature to support regeneration, provide stem cells, or coordinate tissue growth and differentiation, a greater understanding of the cells and their regulation in vascular disease and development has to be achieved. The development of a better interface between the vascular biology field, including the role of BMPs, and the stem cell field holds great promise for reaching this goal.
Acknowledgments
This work was supported in part by NIH Grants HL30568 and HL81397, NSF Grant BECS 1025073, and the American Heart Association.
Footnotes
Author contributions: K.I.B.: conception, financial support, and manuscript writing; A.G.: conception and manuscript writing; Y.Y. and M.J.: manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflict of interest.
References
- 1.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7:528–536. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huggins CB. The formation of bone under the influence of epithelium of the urinary tract. Arch Surg. 1931;22:377–408. [Google Scholar]
- 3.Torsney E, Xu Q. Resident vascular progenitor cells. J Mol Cell Cardiol. 2011;50:304–311. doi: 10.1016/j.yjmcc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Lander AD. The ‘stem cell’ concept: Is it holding us back? J Biol. 2009;8:70. doi: 10.1186/jbiol177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watabe T, Miyazono K. Roles of TGF-beta family signaling in stem cell renewal and differentiation. Cell Res. 2009;19:103–115. doi: 10.1038/cr.2008.323. [DOI] [PubMed] [Google Scholar]
- 6.Varga AC, Wrana JL. The disparate role of BMP in stem cell biology. Oncogene. 2005;24:5713–5721. doi: 10.1038/sj.onc.1208919. [DOI] [PubMed] [Google Scholar]
- 7.Lowery JW, de Caestecker MP. BMP signaling in vascular development and disease. Cytokine Growth Factor Rev. 2010;21:287–298. doi: 10.1016/j.cytogfr.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David L, Feige JJ, Bailly S. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev. 2009;20:203–212. doi: 10.1016/j.cytogfr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Yao Y, Nowak S, Yochelis A, et al. Matrix GLA protein, an inhibitory morphogen in pulmonary vascular development. J Biol Chem. 2007;282:30131–30142. doi: 10.1074/jbc.M704297200. [DOI] [PubMed] [Google Scholar]
- 10.Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 11.Yao Y, Jumabay M, Wang A, et al. Matrix Gla protein deficiency causes arteriovenous malformations in mice. J Clin Invest. 2011;121:2993–3004. doi: 10.1172/JCI57567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao Y, Bennett BJ, Wang X, et al. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 2010;107:485–494. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bostrom KI, Jumabay M, Matveyenko A, et al. Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ Res. 2010;108:446–457. doi: 10.1161/CIRCRESAHA.110.236596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shovlin CL. Hereditary haemorrhagic telangiectasia: Pathophysiology, diagnosis and treatment. Blood Rev. 2010;24:203–219. doi: 10.1016/j.blre.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Rudarakanchana N, Morrell NW. Primary pulmonary hypertension: Molecular basis and potential for therapy. Expert Rev Mol Med. 2004;6:1–15. doi: 10.1017/S1462399404007483. [DOI] [PubMed] [Google Scholar]
- 16.Medici D, Shore EM, Lounev VY, et al. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wylie-Sears J, Aikawa E, Levine RA, et al. Mitral valve endothelial cells with osteogenic differentiation potential. Arterioscler Thromb Vasc Biol. 2011;31:598–607. doi: 10.1161/ATVBAHA.110.216184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Speer MY, Yang HY, Brabb T, et al. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lander AD. Pattern, growth, and control. Cell. 2011;144:955–969. doi: 10.1016/j.cell.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lander AD, Gokoffski KK, Wan FY, et al. Cell lineages and the logic of proliferative control. PLoS Biol. 2009;7:e15. doi: 10.1371/journal.pbio.1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bostrom KI, Rajamannan NM, Towler DA. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ Res. 2011;109:564–577. doi: 10.1161/CIRCRESAHA.110.234278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shanahan CM, Crouthamel MH, Kapustin A, et al. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ Res. 2011;109:697–711. doi: 10.1161/CIRCRESAHA.110.234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dimmeler S. Regulation of bone marrow-derived vascular progenitor cell mobilization and maintenance. Arterioscler Thromb Vasc Biol. 2010;30:1088–1093. doi: 10.1161/ATVBAHA.109.191668. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka K, Sata M. Contribution of circulating vascular progenitors in lesion formation and vascular healing: Lessons from animal models. Curr Opin Lipidol. 2008;19:498–504. doi: 10.1097/MOL.0b013e32830dd566. [DOI] [PubMed] [Google Scholar]
- 25.Nikolova G, Strilic B, Lammert E. The vascular niche and its basement membrane. Trends Cell Biol. 2007;17:19–25. doi: 10.1016/j.tcb.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Watt FM, Hogan BL. Out of Eden: Stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 27.Yao Y, Zebboudj AF, Shao E, et al. Regulation of bone morphogenetic protein-4 by matrix GLA protein in vascular endothelial cells involves activin-like kinase receptor 1. J Biol Chem. 2006;281:33921–33930. doi: 10.1074/jbc.M604239200. [DOI] [PubMed] [Google Scholar]
- 28.Csiszar A, Lehoux S, Ungvari Z. Hemodynamic forces, vascular oxidative stress and regulation of BMP-2/4 expression. Antioxid Redox Signal. 2009;11:1683–1697. doi: 10.1089/ars.2008.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorescu GP, Song H, Tressel SL, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 30.Chang K, Weiss D, Suo J, et al. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: Role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation. 2007;116:1258–1266. doi: 10.1161/CIRCULATIONAHA.106.683227. [DOI] [PubMed] [Google Scholar]
- 31.Shao ES, Lin L, Yao Y, et al. Expression of vascular endothelial growth factor is coordinately regulated by the activin-like kinase receptors 1 and 5 in endothelial cells. Blood. 2009;114:2197–2206. doi: 10.1182/blood-2009-01-199166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki Y, Ohga N, Morishita Y, et al. BMP-9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. J Cell Sci. 2010;123:1684–1692. doi: 10.1242/jcs.061556. [DOI] [PubMed] [Google Scholar]
- 33.Iyemere VP, Proudfoot D, Weissberg PL, et al. Vascular smooth muscle cell phenotypic plasticity and the regulation of vascular calcification. J Intern Med. 2006;260:192–210. doi: 10.1111/j.1365-2796.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- 34.Corselli M, Chen CW, Crisan M, et al. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30:1104–1109. doi: 10.1161/ATVBAHA.109.191643. [DOI] [PubMed] [Google Scholar]
- 35.Diaz-Flores L, Gutierrez R, Madrid JF, et al. Pericytes morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 36.Garfinkel A, Tintut Y, Petrasek D, et al. Pattern formation by vascular mesenchymal cells. Proc Natl Acad Sci USA. 2004;101:9247–9250. doi: 10.1073/pnas.0308436101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zebboudj AF, Shin V, Bostrom K. Matrix GLA protein and BMP-2 regulate osteoinduction in calcifying vascular cells. J Cell Biochem. 2003;90:756–765. doi: 10.1002/jcb.10669. [DOI] [PubMed] [Google Scholar]
- 38.Ballock RT, O'Keefe RJ. Physiology and pathophysiology of the growth plate. Birth Defects Res Part C Embryo Today. 2003;69:123–143. doi: 10.1002/bdrc.10014. [DOI] [PubMed] [Google Scholar]
- 39.Zelzer E, Mamluk R, Ferrara N, et al. VEGFA is necessary for chondrocyte survival during bone development. Development. 2004;131:2161–2171. doi: 10.1242/dev.01053. [DOI] [PubMed] [Google Scholar]
- 40.Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9:107–115. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- 41.Spencer M, Unal R, Zhu B, et al. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab. 2011;96:E1990–E1998. doi: 10.1210/jc.2011-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee MJ, Wu Y, Fried SK. Adipose tissue remodeling in pathophysiology of obesity. Curr Opin Clin Nutr Metab Care. 2010;13:371–376. doi: 10.1097/MCO.0b013e32833aabef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 44.Tang W, Zeve D, Suh JM, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jumabay M, Zhang R, Yao Y, et al. Spontaneously beating cardiomyocytes derived from white mature adipocytes. Cardiovasc Res. 2010;85:17–27. doi: 10.1093/cvr/cvp267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen JF, Sugawara A, Yamashita J, et al. Dedifferentiated fat cells: An alternative source of adult multipotent cells from the adipose tissues. Int J Oral Sci. 2011;3:117–124. doi: 10.4248/IJOS11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quaegebeur A, Lange C, Carmeliet P. The neurovascular link in health and disease: Molecular mechanisms and therapeutic implications. Neuron. 2011;71:406–424. doi: 10.1016/j.neuron.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 48.Erskine L, Reijntjes S, Pratt T, et al. VEGF signaling through neuropilin 1 guides commissural axon crossing at the optic chiasm. Neuron. 2011;70:951–965. doi: 10.1016/j.neuron.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Licht T, Goshen I, Avital A, et al. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci USA. 2011;108:5081–5086. doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104:576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]