Abstract
Puerarin has multiple pharmacological effects and is widely prescribed for patients with cardiovascular diseases including hypertension, cerebral ischemia, myocardial ischemia, diabetes mellitus, and arteriosclerosis. We have successfully prepared puerarin-loaded solid lipid nanoparticles (Pue-SLNs) for oral administration. Pue-SLNs are prepared using monostearin, soya lecithin, and poloxamer 188. SLNs may alter the course of puerarin absorption predominantly to and through lymphatic routes and regions, presumably following a transcellular path of lipid absorption, especially by enterocytes and polar epithelial cells of the intestine. The alteration of absorption might influence the metabolic profile of puerarin when incorporated into SLNs. In the present study, we investigated the metabolic profile of puerarin in rat plasma and urine using rapid resolution liquid chromatography–tandem mass spectrometry after a single-dose intragastric administration of Pue-SLNs in comparison with puerarin suspension. Two glucuronidated metabolites of puerarin, puerarin-4′-O-glucuronide and puerarin-7-O-glucuronide, were detected in rat plasma and urine after intragastric administration of Pue-SLNs, with the latter acting as the major metabolite. Similar results were found in rat plasma and urine after intragastric administration of puerarin suspension. The results suggest that incorporation of puerarin into SLNs does not change either the position of glucuronidation or the metabolic pathway of puerarin in rats.
Keywords: puerarin, solid lipid nanoparticles, metabolic profiling
Introduction
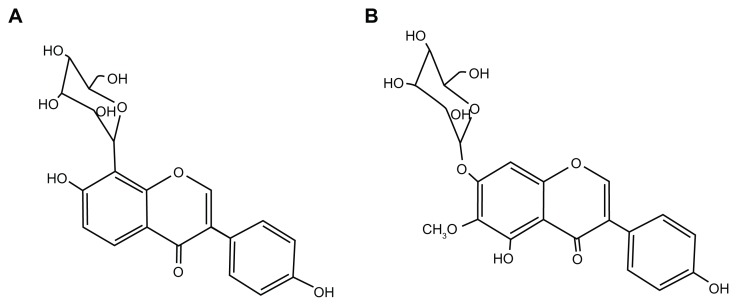
Puerarin (7,4′-dihydroxyisoflavone-8β-glucopyranoside; Figure 1A) is a major active ingredient in the Chinese medicine Puerariae Radix, which comes from the kudzu root (Pueraria lobota (Wild.) Howe). Puerarin is widely prescribed for patients with cardio-cerebrovascular diseases in China and it has been reported to have therapeutic effects on hypertension,1 cerebral ischemia,2 myocardial ischemia,3 diabetes mellitus,4 metabolic syndrome,5 and arteriosclerosis.6 The molecular mechanism underlying these pharmacological benefits is believed to involve puerarin’s ability to act as an antioxidant and a scavenger of reactive oxygen species.7 In diet-induced hypercholesterolemic rats, puerarin markedly reduces the total cholesterol by the promotion of cholesterol and bile acid excretion in the liver.6 Puerarin also improves endothelial function by inhibiting cellular factors, such as adhesive molecules8 and C-reactive protein,9 and stimulating endothelial nitric oxide synthase phosphorylation and nitric oxide production via activation of an estrogen receptor-mediated phosphatidylinositol 3-kinase/Akt- and calmodulin-dependent kinase II/AMP-activated protein kinase-dependent pathway.10 In addition, as a phytoestrogen, puerarin exhibits weak estrogenic activity in vivo.11
Figure 1.
Chemical structures of puerarin (A) and tectoridin (B).
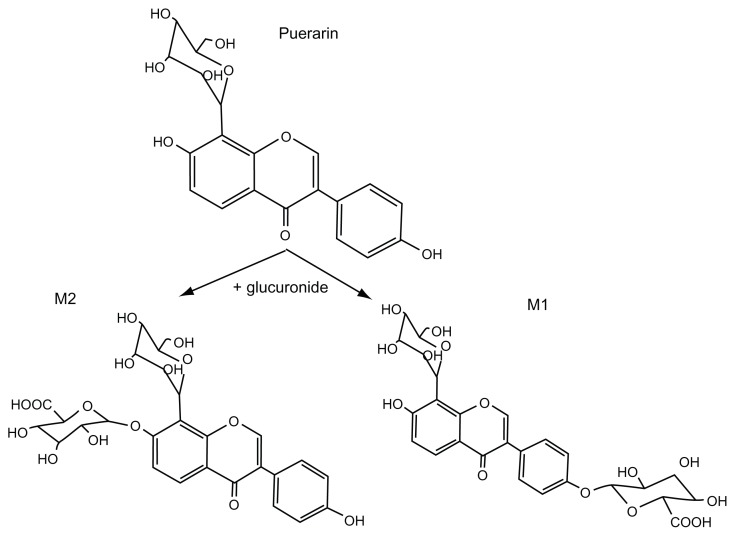
Understanding the metabolic pathway of puerarin will contribute to our understanding of its therapeutic as well as toxic effects. A few phase I functionalization reaction (oxidation and hydrolysis) and phase II conjugation reaction (glucuronidation and sulfation) metabolites have been found in biological samples after puerarin administration to both humans and rats.12–14 It has been reported that only two monoglucuronide conjugates of puerarin are detected in rat plasma, urine, or tissues.13,15,16 We have confirmed that the major metabolite (M2) is puerarin-7-O-glucuronide using rapid resolution liquid chromatography–tandem mass spectrometry (RRLC-MS/MS) and 1H and 13C NMR,15,16 and the minor metabolite (M1) is presumed to be puerarin-4′-O-glucuronide (Figure 2).
Figure 2.
Puerarin and its metabolites detected in rat plasma and urine.
Notes: M1: puerarin-4′-O-glucuronide, M2: puerarin-7-O-glucuronide.
Although puerarin has protective effects on cardio-cerebrovascular diseases, the rapid clearance rate of puerarin in humans suggests frequent intravenous administration of high doses may be needed, possibly leading to severe and acute adverse effects including intravenous hemolysis. Oral administration is the preferred route for drug delivery, especially for the treatment of chronic diseases, but puerarin is not very water-soluble, and its absorption in vivo is very poor after oral administration,17 which diminishes its therapeutic effects.
Solid lipid nanoparticles (SLNs), a submicron nanoparticle drug delivery system, provide an attractive alternative for drug delivery. Previous studies indicate that the oral bioavailability of poorly hydrophilic drugs can be enhanced by incorporation into SLNs. We have successfully prepared puerarin-filled solid lipid nanoparticles (Pue-SLNs) for oral administration.18 The pharmacokinetic study in rats indicated that orally administered Pue-SLNs were rapidly absorbed and the bioavailability of puerarin was improved more than three-fold when incorporated into SLNs. In addition, puerarin concentrations in the tissues of interest were significantly increased after a single-dose oral administration of Pue-SLNs, especially in its target organs, the heart and brain.18
Several mechanisms might enhance the oral absorption and bioavailability of pharmacological ingredients when encapsulated in SLNs. These mechanisms include the general adhesiveness of nanoparticles and the absorption-enhancing effect of lipids.19 Paliwal et al reported that SLNs-based drug delivery showed a many-fold increase in methotrexate concentration in the lymphatic region compared with standard methotrexate solution administration,20 which indicates improved intestinal lymphatic transportation of drugs incorporated into SLNs. The lectin in Pue-SLNs enhances lymph formation and thus increases the lymphatic flow rate. Lymphatic absorption avoids first-pass metabolism to a certain degree and can be used to increase the oral absorption.19 These mechanisms that lead to an absorption enhancement might also influence the metabolic profile of puerarin when incorporated in SLNs. The present study focuses on investigating the metabolic profile of puerarin in rat plasma and urine using RRLC-MS/MS after a single intragastric administration of Pue-SLNs and comparing Pue-SLNs administration plasma kinetics with plasma kinetics for standard puerarin suspension administration.
Materials and methods
Chemicals and reagents
Puerarin was purchased from Guangdong Greatsun Biochemical Pharmaceutical Co, Ltd (Guangzhou, People’s Republic of China). Lyophilized Pue-SLNs powder was provided by the Drug Research Center of Guangzhou Medical University (Guangzhou, People’s Republic of China). Tectoridin (internal standard; Figure 1B) was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, People’s Republic of China). Methanol and acetonitrile (Merck KGaA, Darmstadt, Germany) were of HPLC grade. Formic acid and acetic acid were purchased from Acros Organics (Geel, Belgium). Drug-free rat plasma and urine were collected from healthy Sprague-Dawley rats of both sexes and stored at −20°C. Ultrapure water was prepared using a Millipore water-purification system (EMD Millipore, Billerica, MA, USA).
Animals
Specific-pathogen-free Sprague-Dawley rats, weighing 180–220 g, were obtained from Guangdong Medical Laboratory Animal Center (Guangzhou, People’s Republic of China). The rats were kept in an environmentally controlled room (temperature 25°C ± 2°C, humidity 60% ± 5%, 12/12-hour dark/light cycle) for 1 week prior to the experiments. They were fed a soy-free custom diet (Guangdong Medical Laboratory Animal Center) and water ad libitum. All rats were fasted overnight before the experiments, and all animal handling and treatments followed the Guide for the Care and Use of Laboratory Animals. The animal use and care protocol was reviewed and approved by the ethics committee of Guangzhou Medical University.
Preparation of Pue-SLNs
Pue-SLNs were prepared using the solvent injection method.18 Briefly, puerarin (20 mg), monostearin (150 mg), and soya lecithin (150 mg) were mixed in a 10 mL solvent consisting of methanol (1 mL) and ethanol (9 mL) and sonicated to form the organic phase. The aqueous phase, 0.5% poloxamer 188 (W/V) in ultrapure water, was heated to 75°C ± 2°C and then the organic phase was injected into the hot aqueous phase under mechanical agitation. The resulting solution was kept at the same temperature with the same agitation speed to remove the organic solvent. The condensed solvent (approximately 10 mL) was then injected into 0.5% poloxamer 188 (W/V) in ultrapure water at 0–2°C to form SLNs. The free drug in the Pue-SLNs suspension was separated from Pue-SLNs by ultrafiltration. To prepare the lyophilized Pue-SLNs powder, mannitol (final concentration was 10%, W/V) was added to the Pue-SLNs dispersion, and the solution was filtered through 0.45 μm polytetrafluoroethylene membranes (Jinteng, People’s Republic of China) and poured into sterilized glass vials. After freezing at −45°C for 8 hours, they were quickly moved to a freeze-dryer with a temperature of −50°C and a vacuum of 30 mmHg; the temperature was increased at a rate of 5°C/hour to −25°C and then maintained for 24 hours. Next, the temperature was increased at a rate of 5°C/hour to 30°C and then maintained for 24 hours, and the resulting Pue-SLNs lyophilized powder was collected. Before administration to rats, the Pue-SLNs lyophilized powder was re-suspended in ultrapure water at 1 mg/mL.
Drug administration and sampling
Twelve rats (six female and six male) were divided into two groups: Pue-SLNs and puerarin suspension. Animals in the first group received a single intragastric 20 mg/kg dose of lyophilized Pue-SLNs powder re-suspended in water at 1 mg/mL. Twenty mg/kg of puerarin suspended in 0.5% carboxymethyl cellulose sodium (1 mg/mL) was administered to the second group. For the Pue-SLNs group, blood samples (approximately 0.3 mL) were obtained from the jugular vein at 0, 10, 20, 30, and 40 minutes, and at 1, 2, 3, 4, 6, 8, 12, 18, and 24 hours post-dosing under anesthesia with sodium pentobarbital (30 mg/kg). In the puerarin suspension group, blood samples were obtained at 0, 20, and 40 minutes, and at 1, 1.5, 2, 3, 4, 6, 8, and 12 hours after dosing. For both groups, from the second hour post-dosing onwards, an equal volume of sterile physiological saline (0.3 mL) was injected into the animals after every blood sample withdrawal. The blood samples were centrifuged at 13,000 g for 3 minutes and the plasma was separated and collected. Urine was collected over 24 hours following the administration. All samples were stored at −20°C until analysis.
Plasma sample treatment
An aliquot of 100 μL of collected plasma was thawed on ice. Ten microliters of 35% methanol–water (V/V) solution and 10 μL tectoridin solution were added to the plasma and then 200 μL of a mixture of methanol and acetonitrile (90:10, V/V) was added to the mixture in order to precipitate the protein. The samples were vortexed for 5 minutes then centrifuged at 13,000 g for 10 minutes. The supernatant was collected and was centrifuged for 5 minutes at 13,000 g. The supernatant was analyzed by RRLC-MS/MS immediately after centrifugation.
Urine sample treatment
An aliquot of 100 μL of collected urine was thawed on ice, and 200 μL of a mixture of methanol and acetonitrile (90:10, V/V) was added. The mixture was vortexed for 3 minutes and centrifuged at 13,000 g for 10 minutes. The supernatant was then dried under nitrogen flow at 37°C. The residue was then dissolved in 200 μL of a mixture of acetonitrile and water (20:80, V/V) and was centrifuged at 13,000 g for 5 minutes after vortexing for 1 minute. The separated supernatant was analyzed immediately using RRLC-MS/MS.
RRLC-MS/MS analysis
RRLC-MS/MS analysis of rat plasma and urine samples was performed using an Agilent 1200 series RRLC and 6330 Ion Trap system consisting of a vacuum degasser, a binary pump, an autosampler, a column thermostat, and a 6330 Ion Trap XCT Ultra mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). Chromatography was carried out on a Zorbax SB C18 reversed-phase column (2.1 × 100 mm, 1.8 μm particle size; Agilent Technologies), preceded by a guard column filled with C18 (Zorbax SB, 1.8 μm particle size). The injection volume was 5 μL and the column temperature was set to 30°C. A gradient method was employed using 0.05% (V/V) formic acid–water solution as mobile phase A and acetonitrile as mobile phase B. For identification of metabolites of puerarin in rat plasma, the gradient started at 10% B and increased to 16% B over 6 minutes. For the kinetic study of metabolites, the gradient started at 8% B and increased to 20% B over 10 minutes, then solvent B was increased from 35% to 65% over the next 2 minutes, and finally isocratic conditions were held for 3 minutes. The initial conditions were held for 8 minutes prior to injection of the next sample. For the kinetic study of puerarin metabolites, a shorter Zorbax SB C18 reversed-phase column (2.1 × 50 mm, 1.8 μm particle size; Agilent Technologies) was used to achieve a lower column pressure compared with the 100 mm column For identification of metabolites of puerarin in rat urine, the mobile phase consisted of a mixture of 0.01% formic acid and acetonitrile (90:10, V/V) for the first 8 minutes. Then, a gradient method was employed with the gradient starting at 10% acetonitrile and increasing to 28% acetonitrile over 20 minutes. The flow rate was 0.2 mL/min for all samples.
The column effluent was then injected into the mass spectrometer, which was operated in electrospray ionization positive ionization mode. Nitrogen was used as the nebulizing and drying gas at 350°C, with a pressure of 40 psi and a flow rate of 10 L/min. In-source voltage was set to −3500V. The ion trap parameters were chosen using the smart parameter setting, and the number of ions stored in the ion trap was controlled, with a target number of 500,000 and a maximum accumulation time of 200 ms. The scanning mass-to-charge (m/z) range was from 250 to 800 with a scanning speed of 26,000 m/z per second. For identification of metabolites of puerarin, the autoMS, manual, or multiple reaction monitoring (MRM) mode was selected as required. For the kinetic study of metabolites, MRM analysis was conducted by monitoring the precursor ion to product ion transitions from m/z 593/417 (puerarin glucuronides), 417/399 (puerarin), or 463/301 (tectoridin, internal standard). The ratio of the peak area of the metabolite to that of the internal standard was used to determine the relative amount of the metabolite in each plasma sample and to obtain a time profile for the metabolite. The RRLC and the MS system were controlled by ChemStation version B.01.03 SR2 and Ion Trap software 6.1, respectively (Agilent Technologies).
Results
Characterization of Pue-SLNs
The average diameter and zeta potential of Pue-SLNs were measured via the laser-scattering method using a Zetasizer Nano ZS90 particle size analyzer (Malvern Instruments, Malvern, UK). The mean particle size of Pue-SLNs was 160 nm with a zeta potential of −35.43 ± 5.19 mV. There was no significant change in mean particle size or zeta potential after lyophilization of Pue-SLNs.
Metabolites of puerarin in plasma after oral administration of Pue-SLNs and puerarin suspension
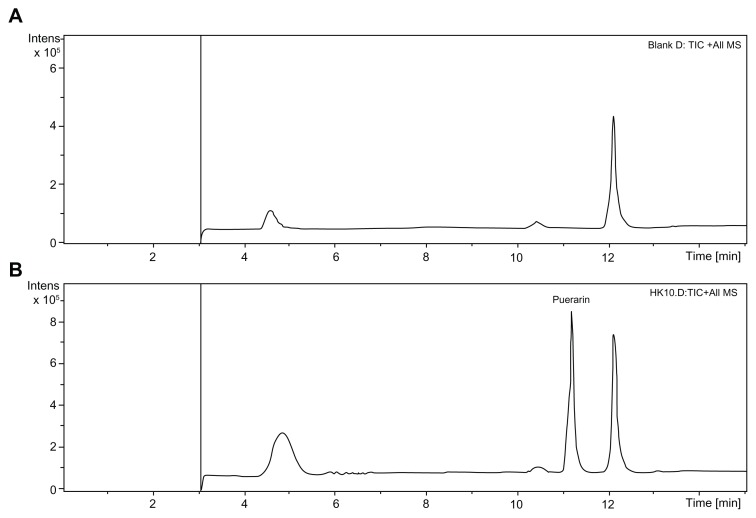
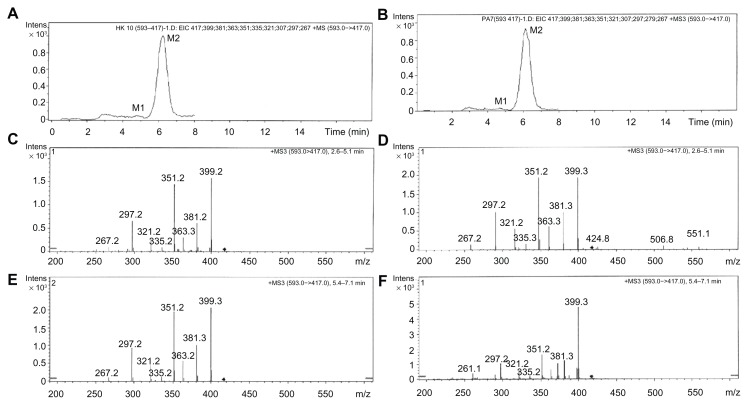
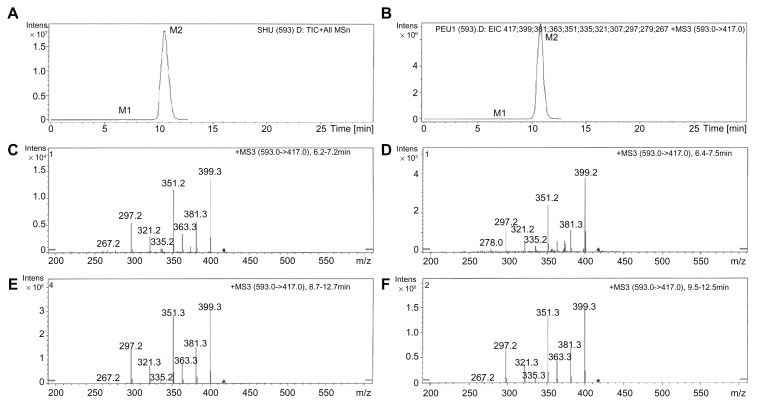
Total ion chromatography (TIC) was used to detect the metabolites of puerarin in the collected plasma after oral administration of Pue-SLNs (Figure 3) compared to the metabolites found in blank rat plasma (Figure 3A). However, no obvious additional peak was found in the TIC of the plasma of Pue-SLNs-treated rats, with the exception of the puerarin peak (Figure 3B), although we have previously reported that two glucuronidated metabolites of puerarin were detected in rat plasma and urine after intravenous administration of puerarin.15,16 To identify the glucuronidated metabolites of puerarin, the manual mode (m/z 593 → m/z 417) was selected in the analysis software, and two peaks were detected, named M1 and M2 in the order of elution (Figure 4A). Although the peak of M1 was much smaller than that of M2, the MS3 (m/z 593 → m/z 417) of M1 and M2 yielded product ions at m/z 399, 381, 363, 351, 335, 321, 297, and 267 (Figure 4C and E). The MS3 spectra and chromatographic action of M1 and M2 were similar to that of the two metabolites detected in rat plasma after intravenous administration of puerarin. Based on results from our previous work,15,16 M1 and M2 are puerarin-4′-O-glucuronide and puerarin-7-O-glucuronide, respectively, and M2 is the major metabolite. Similar results were found after oral administration of puerarin suspension (Figure 4B, D, and F).
Figure 3.
TIC of blank rat plasma and plasma after Pue-SLNs administration. Compared to that of blank rat plasma (A), no obviously additional peak was found in the TIC of plasma of Pue-SLNs-treated rats, with the exception of the puerarin peak (B).
Abbreviations: TIC, total ion chromatography; Pue-SLNs, puerarin-loaded solid lipid nanoparticles; MS, mass spectrometry.
Figure 4.
Metabolites of puerarin in rat plasma. Two additional peaks (M1 and M2) were present in the extracted ion chromatography of rat plasma after intragastric administration of Pue-SLNs (equivalent to 20 mg/kg of puerarin) (A) or puerarin suspension (20 mg/kg) (B). MS3 spectra of M1 (puerarin-4′-O-glucuronide) (m/z 593 → m/z 417) (C and D). MS3 spectra of M2 (puerarin-7-O-glucuronide) (m/z 593 → m/z 417) after intragastric administration of Pue-SLNs or puerarin suspension (E and F).
Abbreviations: Pue-SLNs, puerarin-loaded solid lipid nanoparticles; MS, mass spectrometry.
Metabolites of puerarin in rat urine after oral administration of Pue-SLNs and puerarin suspension
The two glucuronidated metabolites, puerarin-4′-O-glucuronide and puerarin-7-O-glucuronide, were also detected in rat urine after oral administration of Pue-SLNs (Figure 5A, C, and E), and the metabolic profile was similar to the profile measured after administration of puerarin suspension (Figure 5B, D, and F).
Figure 5.
Metabolites of puerarin in urine. Two additional peaks (M1 and M2) were present in the extracted ion chromatography of rat urine after intragastric administration of Pue-SLNs (equivalent to 20 mg/kg of puerarin) (A) or puerarin suspension (20 mg/kg) (B). MS3 spectra of M1 (puerarin-4′-O-glucuronide) (m/z 593 → m/z 417) (C and D). MS3 spectra of M2 (puerarin-7-O-glucuronide) (m/z 593 → m/z 417) after intragastric administration of Pue-SLNs or puerarin suspension (E and F).
Abbreviations: Pue-SLNs, puerarin-loaded solid lipid nanoparticles; MS, mass spectrometry; m/z, mass-to-charge.
Kinetics of puerarin-7-O-glucuronide in rat plasma after oral administration of Pue-SLNs
Prepared rat plasma samples were analyzed by RRLC-MS/MS. M1, M2, puerarin, and tectoridin (the internal standard) were well separated under the gradient mobile phase, with retention times of 3.9, 5.2, 9.7, and 11.9 minutes, respectively. Because the peak area of M1 was much smaller than that of M2, the kinetics of M1 was not studied.
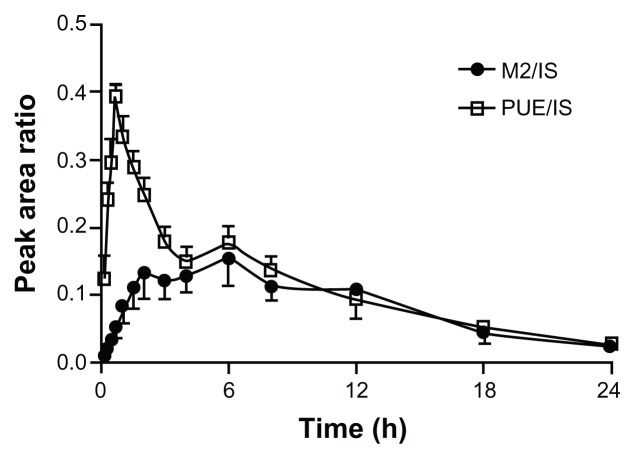
To understand the kinetics of M2 in plasma after oral administration of Pue-SLNs, the ratio of M2 peak area to the internal standard (tectoridin) peak area was plotted against time. The kinetics of M2 and puerarin in rat plasma are shown in Figure 6. The profile of puerarin is similar to a profile on which we have previously reported:18 that profile of puerarin was outlined in the plasma concentration of puerarin after oral administration of Pue-SLNs. In the present work, M2 was present in rat plasma 10 minutes after intragastric administration of Pue-SLNs, and the kinetic curve of M2 in plasma was a two-peak type, with the first peak appearing 2 hours post-dosing, the level of M2 increasing again, and the second peak appearing at 6 hours, at the same time the second peak of puerain was obtained. Subsequently, the level of M2 in plasma descended slowly.
Figure 6.
Kinetics of M2 in plasma after a single dose intragastric administration of Pue-SLNs (20 mg/kg, n = 6).
Note: M2: metabolite of puerarin (puerarin-7-O-glucuronide).
Abbreviations: Pue-SLNs, puerarin-loaded solid lipid nanoparticles; PUE, puerarin; IS, internal standard.
Discussion
Our previous study demonstrated that the incorporation of puerarin into SLNs enhanced the absorption of puerarin after oral administration. Oral absorption of Pue-SLNs is increased due to several mechanisms, such as nanoparticle adhesion to the gastrointestinal tract wall, intestinal lymphatic transportation, and the role of lectin in Pue-SLNs formulation.18 At present, it is uncertain whether the metabolic profile of administered active pharmacological ingredients could be influenced by the incorporation of those ingredients into SLNs. In the present study, two glucuronide metabolites, puerarin-4′-O-glucuronide and puerarin-7-O-glucuronide, were detected in rat plasma and urine after intragastric administration of Pue-SLNs, with the latter acting as the major metabolite. However, similar metabolic profiles were found in rat plasma and urine after intragastric administration of puerarin suspension. These results indicate that incorporation of puerarin into SLNs did not change the position of glucuronidation or the metabolic pathway of puerarin.
Glucuronidation has been considered to represent a metabolic pathway performed mainly by the liver. However, UDP-glucuronosyltransferase (UGT) activity is resident in human extrahepatic tissues, such as the intestine, kidney, and colon.21 Xenobiotic material first contacts the gastrointestinal tract after oral administration. Thus, intestinal disposition is important for drug metabolism. It has been reported that the intestine is the main organ for genistein glucuronide formation and excretion in rats and may serve as the main organ of first-pass metabolism.22 The metabolic profiles of puerarin were similar in the rat liver and intestine investigated by in situ liver and intestine perfusion, indicating that no metabolic regioselectivity of puerarin occurs in the liver or intestine.16 We have also confirmed that UGT1A1 is the principal enzyme responsible for puerarin metabolism in human liver microsomes.15 UGT1A1 mRNA is expressed in several tissues including the liver and intestine.21 Although lymphatic absorption avoids liver disposition to a certain degree and other mechanisms changed or enhanced the absorption of puerarin when incorporated into SLNs, the metabolic profile of puerarin was not changed; it is similar to that achieved by administration of puerarin suspension. Certainly, adhesion of nanoparticles to the gut enhanced the intestinal disposition of puerarin, which may provide another reason that a similar metabolic profile was found.
Most of the absorbed flavonoids are present in the blood as conjugates (eg, glucuronide conjugates and sulfate conjugates).23 The fact that flavonoids exert biological effects in vivo in human intervention studies suggests that flavonoid conjugates may retain some biologically active properties. Indeed, some glucuronide conjugates have been shown to be active. For example, quercetin 3-O-β-D-glucuronide can inhibit cell migration and proliferation of vascular smooth muscle cells.24 In spontaneously hypertensive rats, quercetin and quercetin-3-glucuronide progressively reduced mean blood pressure.25 However, the hypotensive effect of quercetin-3-glucuronide was abolished when rats were treated with a specific inhibitor of β-glucuronidase. This result indicates that quercetin-3-glucuronide provides plasmatic transport of quercetin to the target tissues, where quercetin is released and exerts a hypotensive effect.25 In the present work, the content of M2 or puerarin was expressed by the ratio of the peak area of M2 or puerarin to that of internal standard, respectively. The profile of puerarin is similar to a previously reported profile.18 In that previous work, the puerarin profile followed the plasma concentration of puerarin after oral administration of Pue-SLNs, suggesting that the ratio of peak area of M2 to that of the internal standard represents the fluctuating M2 plasma concentration in the present work. However, the plasma level of M2 was steady between 1.5 hours and 8 hours after intragastric administration of Pue-SLNs, suggesting further investigation is needed to study its pharmacological effect.
Conclusion
Similar metabolic profiles were found in rat plasma and urine for intragastric administration of Pue-SLNs and puerarin suspension. Incorporation of puerarin into SLNs did not change the position of glucuronidation or the metabolic pathway.
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (“863” Program; grant number 2007AA022002), the Research Fund for the Doctoral Program of Higher Education of China (grant number 20114423120004), and the Guangdong Natural Science Foundation (grant number S2011040003552).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Song XP, Chen PP, Chai XS. Effects of puerarin on blood pressure and plasma renin activity in spontaneously hypertensive rats. Zhongguo Yao Li Xue Bao. 1988;9(1):55–58. Chinese. [PubMed] [Google Scholar]
- 2.Gao L, Ji X, Song J, et al. Puerarin protects against ischemic brain injury in a rat model of transient focal ischemia. Neurol Res. 2009;31(4):402–406. doi: 10.1179/174313209X444017. [DOI] [PubMed] [Google Scholar]
- 3.Zhang S, Chen S, Shen Y, et al. Puerarin induces angiogenesis in myocardium of rat with myocardial infarction. Biol Pharm Bull. 2006;29(5):945–950. doi: 10.1248/bpb.29.945. [DOI] [PubMed] [Google Scholar]
- 4.Hsu FL, Liu IM, Kuo DH, Chen WC, Su HC, Cheng JT. Antihyperglycemic effect of puerarin in streptozotocin-induced diabetic rats. J Nat Prod. 2003;66(6):788–792. doi: 10.1021/np0203887. [DOI] [PubMed] [Google Scholar]
- 5.Xu ME, Xiao SZ, Sun YH, Zheng XX, Ou-Yang Y, Guan C. The study of anti-metabolic syndrome effect of puerarin in vitro. Life Sci. 2005;77(25):3183–3196. doi: 10.1016/j.lfs.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 6.Yan LP, Chan SW, Chan AS, Chen SL, Ma XJ, Xu HX. Puerarin decreases serum total cholesterol and enhances thoracic aorta endothelial nitric oxide synthase expression in diet-induced hypercholesterolemic rats. Life Sci. 2006;79(4):324–330. doi: 10.1016/j.lfs.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Han RM, Tian YX, Becker EM, Andersen ML, Zhang JP, Skibsted LH. Puerarin and conjugate bases as radical scavengers and antioxidants: Molecular mechanism and synergism with beta-carotene. J Agric Food Chem. 2007;55(6):2384–2391. doi: 10.1021/jf062796c. [DOI] [PubMed] [Google Scholar]
- 8.Hu W, Zhang Q, Yang X, Wang Y, Sun L. Puerarin inhibits adhesion molecule expression in TNF-alpha-stimulated human endothelial cells via modulation of the nuclear factor kappaB pathway. Pharmacology. 2010;85(1):27–35. doi: 10.1159/000264938. [DOI] [PubMed] [Google Scholar]
- 9.Yang X, Hu W, Zhang Q, Wang Y, Sun L. Puerarin inhibits C-reactive protein expression via suppression of nuclear factor kappaB activation in lipopolysaccharide-induced peripheral blood mononuclear cells of patients with stable angina pectoris. Basic Clin Pharmacol Toxicol. 2010;107(2):637–642. doi: 10.1111/j.1742-7843.2010.00548.x. [DOI] [PubMed] [Google Scholar]
- 10.Hwang YP, Kim HG, Hien TT, Jeong MH, Jeong TC, Jeong HG. Puerarin activates endothelial nitric oxide synthase through estrogen receptor-dependent PI3-kinase and calcium-dependent AMP-activated protein kinase. Toxicol Appl Pharmacol. 2011;257(1):48–58. doi: 10.1016/j.taap.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Malaivijitnond S, Tungmunnithum D, Gittarasanee S, Kawin K, Limjunyawong N. Puerarin exhibits weak estrogenic activity in female rats. Fitoterapia. 2010;81(6):569–576. doi: 10.1016/j.fitote.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Prasain JK, Jones K, Brissie N, Moore R, Wyss JM, Barnes S. Identification of puerarin and its metabolites in rats by liquid chromatography-tandem mass spectrometry. J Agric Food Chem. 2004;52(12):3708–3712. doi: 10.1021/jf040037t. [DOI] [PubMed] [Google Scholar]
- 13.Prasain JK, Peng N, Moore R, Arabshahi A, Barnes S, Wyss JM. Tissue distribution of puerarin and its conjugated metabolites in rats assessed by liquid chromatography-tandem mass spectrometry. Phytomedicine. 2009;16(1):65–71. doi: 10.1016/j.phymed.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasuda T, Kano Y, Saito K, Ohsawa K. Urinary and biliary metabolites of puerarin in rats. Biol Pharm Bull. 1995;18(2):300–303. doi: 10.1248/bpb.18.300. [DOI] [PubMed] [Google Scholar]
- 15.Luo CF, Cai B, Hou N, et al. UDP-glucuronosyltransferase 1A1 is the principal enzyme responsible for puerarin metabolism in human liver microsomes. Arch Toxicol. 2012;86(11):1681–1690. doi: 10.1007/s00204-012-0874-7. [DOI] [PubMed] [Google Scholar]
- 16.Luo CF, Yuan M, Chen MS, Liu SM, Ji H. Metabolites of puerarin identified by liquid chromatography tandem mass spectrometry: similar metabolic profiles in liver and intestine of rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(3–4):363–370. doi: 10.1016/j.jchromb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Quan DQ, Xu GX, Wu XG. Studies on preparation and absolute bioavailability of a self-emulsifying system containing puerarin. Chem Pharm Bull (Tokyo) 2007;55(5):800–803. doi: 10.1248/cpb.55.800. [DOI] [PubMed] [Google Scholar]
- 18.Luo CF, Yuan M, Chen MS, et al. Pharmacokinetics, tissue distribution and relative bioavailability of puerarin solid lipid nanoparticles following oral administration. Int J Pharm. 2011;410(1–2):138–144. doi: 10.1016/j.ijpharm.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 19.Muchow M, Maincent P, Muller RH. Lipid nanoparticles with a solid matrix (SLN, NLC, LDC) for oral drug delivery. Drug Dev Ind Pharm. 2008;34(12):1394–1405. doi: 10.1080/03639040802130061. [DOI] [PubMed] [Google Scholar]
- 20.Paliwal R, Rai S, Vaidya B, et al. Effect of lipid core material on characteristics of solid lipid nanoparticles designed for oral lymphatic delivery. Nanomedicine. 2009;5(2):184–191. doi: 10.1016/j.nano.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581–616. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Wang S, Jia X, et al. Disposition of flavonoids via recycling: comparison of intestinal versus hepatic disposition. Drug Metab Dispos. 2005;33(12):1777–1784. doi: 10.1124/dmd.105.003673. [DOI] [PubMed] [Google Scholar]
- 23.Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342(8881):1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- 24.Ishizawa K, Izawa-Ishizawa Y, Ohnishi S, et al. Quercetin glucuronide inhibits cell migration and proliferation by platelet-derived growth factor in vascular smooth muscle cells. J Pharmacol Sci. 2009;109(2):257–264. doi: 10.1254/jphs.08236fp. [DOI] [PubMed] [Google Scholar]
- 25.Galindo P, Rodriguez-Gómez I, González-Manzano S, et al. Glucuronidated quercetin lowers blood pressure in spontaneously hypertensive rats via deconjugation. PLoS One. 2012;7(3):e32673. doi: 10.1371/journal.pone.0032673. [DOI] [PMC free article] [PubMed] [Google Scholar]