Abstract
Purpose
The aim of this report was to introduce a novel “core-membrane” microgel drug-delivery device for spontaneously pulsed release without any external trigger.
Methods
The microgel core was prepared with alginate and chitosan. The semipermeable membrane outside the microgel was made of polyelectrolytes including polycation poly(allylamine hydrochloride) and sodium polystyrene sulfonate. The drug release of this novel system was governed by the swelling pressure of the core and the rupture of the outer membrane.
Results
The size of the core-membrane microgel drug-delivery device was 452.90 ± 2.71 μm. The surface charge depended on the layer-by-layer coating of polyelectrolytes, with zeta potential of 38.6 ± 1.4 mV. The confocal microscope exhibited the layer-by-layer outer membrane and inner core. The in vitro release profile showed that the content release remained low during the first 2.67 hours. After this lag time, the cumulative release increased to 80% in the next 0.95 hours, which suggested a pulsed drug release. The in vivo drug release in mice showed that the outer membrane was ruptured at approximately 3 to 4 hours, as drug was explosively released.
Conclusion
These data suggest that the encapsulated substance in the core-membrane microgel delivery device can achieve a massive drug release after outer membrane rupture. This device was an effective system for pulsed drug delivery.
Keywords: polyelectrolyte, chitosan-alginate, microgels, layer-by-layer, pulsed drug delivery
Introduction
Many drug-delivery systems have focused on continuous drug release. However, some diseases, such as hyperpiesia, asthma, gastrelcosis, and angina pectoris, require precise drug release in a time-controlled manner. Pulsed drug delivery systems offer the advantage of releasing drugs at a specific time, to respond to pathological changes after a predetermined off-release period. For instance, a pulsed drug delivery system can release drug synchronizing with the body’s circadian rhythm or disease states to produce maximal therapeutic benefit and minimal side effects.1–10 At the same time, most pulsed drug-delivery systems need external triggers for drug release. These external triggers include changes in the physiological environment (eg, glucose levels, hydroxyl radicals, and hyaluronidase at inflammatory sites) or external stimuli (eg, magnetism, ultrasound, temperature changes, electrical effects, and irradiation).2
In this study, we reported a novel “core-membrane” microgel drug-delivery device for spontaneously pulsed release without any external trigger. It consisted of a chitosan–alginate microgel surrounded by a polyelectrolyte membrane formed by sodium polystyrene sulfonate (PSS) and poly(allylamine hydrochloride) (PAH). The polyelectrolyte membrane appeared to be impermeable to the microgel degradation products, which increased the inner pressure, but permeable to small molecules. When swelling pressure exceeded the tensile strength of the surrounding membrane, the membrane ruptured and the drug was promptly released. We applied a layer-by-layer (LBL) electrostatic self-assembly method for the polyelectrolyte coating of microgels.11
Polyelectrolyte multilayer films formed by consecutive adsorption of opposite electric charges attracted much interest in the 1990s. Various synthetic polyelectrolytes, biopolymers (proteins and nucleic acids), inorganic particles, dyes, drug crystals, and colloidal particles were applied LBL, producing multilayered films,12–14 which were used for adsorption, on macroscopic flat-charged substrates, in the field of polymer chemistry. Decher et al15 first reported the construction of ultrathin multicomponent films with an internal structure on the nanometer scale. Recently, polyelectrolyte multilayers have been used extensively to improve drug loading and release profiles, and for quantification of proteins.16,17 In the study by De Geest et al,11 layers were contributed to decrease burst release, resulting in massive drug release when the microcapsules ruptured. Although the study used dextran hydroxyethyl methacrylate (dex-HEMA) as a biodegradable core for time-controlled pulsed drug delivery, it has some limitations. For instance, the synthesis and purification procedures of dex-HEMA are time-consuming and complex,18 and the radical-polymerization reaction and size of the dex-HEMA microgels are difficult to control. Furthermore, dex-HEMA microgels are uncharged, which is unfavorable for coating by polyelectrolytes.
In our study we used chitosan and alginate to cope with those limitations. Chitosan and alginate have been used for many biomedical and pharmaceutical purposes, such as peptide and protein delivery,19–23 due to their low toxicity, low immunogenicity, and high biocompatibility and biodegradability.24 They can become gelatinized when interacting with calcium ions. In our study, chitosan made the surface of the microparticles positively charged, and these could then react with negatively charged molecules or biological membranes surfaces. Meanwhile, a portion of the pressure of the microgels was created as a result of pH sensitivity of chitosan–alginate microgels.25
The aim of this study was to formulate and evaluate the polyelectrolyte-coated chitosan–alginate microgel (PCAM) in vitro and in vivo. PCAM was not only able to explode under in vitro condition, but could also rupture under physiological conditions and release payload in a pulsed fashion.
Materials and methods
Materials
Chitosan (Mw 20, 100, and 200 kDa) with at least 85% deacetylation was obtained from Golden-Shell Biochemical Co. Ltd (Yuhuan, People’s Republic of China). Alginate sodium (1%, Pa S ≥ 0.02%) was obtained from Sinopharm Chemical Reagent Co. Ltd (Shanghai, People’s Republic of China). Fluorescein isothiocyanate dextrans (FITC-dextran) (Mw 40, 250 and 2000 kDa), Rhodamine B isothiocyanate (RhBITC), PAH (56 kDa), and PSS (70 kDa) were purchased from Sigma-Aldrich (St Louis, MO, USA). All other materials were analytical grade.
Kunming mice were obtained from Shanghai SLAC Laboratory Animal Co, Ltd (Shanghai, People’s Republic of China).
Preparation of chitosan–alginate microgels
Chitosan–alginate microgels were prepared with an electrostatic droplet generator (designed by the University of Shanghai for Science and Technology, Shanghai, People’s Republic of China).26 Briefly, chitosan and calcium chloride were dissolved in deionized water, which was adjusted to pH 1.2 with hydrochloric acid. Alginate was dissolved in deionized water and extruded through a 5 mL syringe, at a dropping rate of 30 mm/h, into the chitosan–calcium chloride solution under the high-voltage-field (at 3.5 KV) produced by the electrostatic droplet generator. Meanwhile, the mixture was left to gelate under gentle magnetic stirring, until all the alginate solution was added. Then, the beads were hardened, for another 15 minutes, in gelling medium with stirring. Finally, chitosan–alginate microgels were obtained through a grid after washing with deionized water three times. As a model drug in our study, FITC-dextran was loaded in the microgels by adding it to the alginate solution just before the gelatinization.
Polyelectrolyes layer-by-layer coating of chitosan–alginate microgels
Chitosan–alginate microgels were coated with polyelectrolytes through adsorption, alternately, of oppositely charged polyelectrolytes. Microgels were first dipped into a four- to fivefold volumetric PSS solution (1 mg/mL, containing 0.5 M NaCl), under ultrasonication (80 W for 15 minutes) at room temperature. Then, microgels were collected with a grid and washed three times with deionized water to remove superfluous PSS. This process was repeated for coating the PAH and other PSS layers until predetermined multilayers were obtained.
Encapsulation efficiency of FITC-dextran
The polyelectrolyte coating was degradable at pH 13, while FITC-dextran was still stable. So PCAM was added to NaOH solution to destroy the polyelecrolyte membrane and extract FITC-dextran. After vortexing and centrifugation, the supernatant was measured using a fluorescent spectrophotometer.
| (1) |
Characterization of PCAM
Particle size and zeta potential
Microgels and polyelectrolyte-coated microgels were dispersed in deionized water with an 80 rpm stirring rate, and the average size was measured by a dynamic light-scattering detector (Mastersizer 2000; Malvern Instruments, Malvern, UK). Zeta potential was analyzed using a dynamic light-scattering detector (Zetasizer ZS90; Malvern Instruments). All the measurements were performed three times for average value and standard deviation (SD).
Scanning electron microscopy (SEM)
Because of the liquid environment inside the chitosan–alginate microgels, we removed only the water on the surface of the microparticles (with filter paper), to keep the spherical shape after preparation and collection. To avoid liquid loss from the microgels, the image of uncoated microgels and polyelectrolyte-coated microgels were obtained by SEM (TS5136MM; Tescan, Brno, Czech Republic), under low vacuum, although the details of surface morphology of the microgels were analyzed under high vacuum and water-free conditions, for clear images. The microgels were dehydrated by transferring them successively to a series of increasing ethanol/water mixtures ranging from 30% to 100%. The microgels were left for 10 minutes in each solution, and dried at room temperature.27
Confocal laser scanning microscopy (CLSM)
FITC-dextran-loaded chitosan–alginate microgels were coated with PSS and with PAH labeled with RhBITC (PAH-RhBITC). Uncoated and coated microgels were each dispersed in deionized water. A drop of the microgel dispersion was placed on a microscope slide and observed by CLSM (SP5 CLSM; Leica Microsystems, Wetzlar, Germany). Meanwhile, fluorescence intensity and multisection observations were analyzed to confirm the structure and to model drug distribution of the microgels.
Permeability of the polyelectrolytes membrane
The uncoated microgels and microgels with different layers were incubated in 1 mg/mL FITC-dextran solution for 30 minutes under continuous shaking at 100 rpm (MAXQ2000; Thermo Fisher Scientific, Waltham, MA, USA), and the suspension observed by CLSM.
Swelling degree of chitosan–alginate microgels in vitro
The original sizes of the chitosan–alginate microgels were measured by laser diffraction (Mastersizer 2000) immediately after preparation. Then, 1.5 g microgel was incubated in 20 mL phosphate-buffered solution (PBS) (pH 7.4) at 37°C, concurrently shaken in an SHZ-88 water-bath oscillator (Taicang Laboratorial Equipment Factory, Taicang, People’s Republic of China). Microgels were collected with a grid again at 1, 2, and 3 hours, and the size was measured immediately. All experiments were carried out in triplicate. The swelling degree (%SW) for different time periods was determined using Equation 2:
| (2) |
where L0 was the original particle diameter of microgels and Ln was the particle diameter after incubation in PBS for a predetermined time.
In vitro experiments of FITC-dextran loaded PCAM
The morphologic change of PCAM in vitro
The in vitro degradation behavior of FITC-dextran-loaded PCAM was followed with CLSM. To accelerate in vitro release of the PCAM and observe conveniently, the in vitro release of PCAM was conducted in PBS adjusted to pH 11 with NaOH, as the PCAM ruptured easily and quickly at the higher pH value. PCAM was added to a 35 mm-diameter culture dish fixed on a thermostatic airbath at 37°C. The morphologic changes of PCAM during the degradation were monitored under CLSM.
In vitro release of FITC-dextran loaded PCAM
The 800 mg FITC-dextran-loaded PCAM was dispersed in a 50 mL centrifuge tube containing 7 mL of PBS (pH 7.4) with 0.1% (w/v) NaN3 as a preservative. The PCAM was dispersed in PBS (pH 7.4) and shaken in a water-bath oscillator (SHZ-88) at 37°C. At predetermined time points, 0.7 mL medium was taken out and centrifuged. The supernatant was measured by a fluorospectrophotometer (F-7000; Hitachi, Tokyo, Japan) and then another 0.7 mL fresh medium was added to the centrifuge tube. All experiments were carried out in triplicate. A systematic study was performed to gain insight into the effect on FITC-dextran release of different factors, including polyelectrolyte layers, ratio of polyelectrolyte to NaCl, concentration of alginate, concentration of chitosan, molecular weight of chitosan, and concentration of calcium chloride.
The morphologic change of PCAM in vivo
The protocol for the animal experimental procedures was approved by the Animal Experimentation Ethics Committee of Second Military Medical University, Shanghai, People’s Republic of China.
Male Kunming mice of 6–8 weeks were anesthetized with chloral hydrate (400 mg/kg body weight, intraparenterally) and injected subcutaneously with 0.2 mL of PCAM suspension in PBS (pH 7.4). All procedures were carried out under sterile conditions. Mice were sacrificed at 1, 2, 3, 4, and 6 hours after injection. The PCAM and surrounding tissues were carefully taken out and frozen, and then 8 μm sections were cut with a cryo-ultramicrotome (CM1850; Leica Microsystems). All sections were examined by CLSM.
Results and discussion
Preparation of PCAM
Alginate, composed of linear chains of guluronic and mannuronic acids, is a natural biopolymer extracted from brown algae. The high number of carboxyl groups contained in the alginate make it negatively charged. It forms hydrogels in the presence of divalent cations like Ca2+. Chitosan, which has positive charge, is used both for the complexation and coating of alginate beads (through electrostatic attraction), in order to alter the diffusion rate of the encapsulated drug, and as an additive for the bulk swelling of the hydrogel structure.28 We obtained uniform-size microgels by using an electrostatic droplet generator. The chitosan–alginate microgels had a particle size of 409.18 ± 6.47 μm (n = 3). We did not observe any significant difference among microgels with various ratios of materials or reaction conditions (P > 0.05, Student’s t-test). The size uniformity was due to the electrostatic droplet generator, which could strongly control particle diameter through its high tension electricity. The low variability of microgel size would contribute to simultaneous rupture of polyelectrolyte coating.
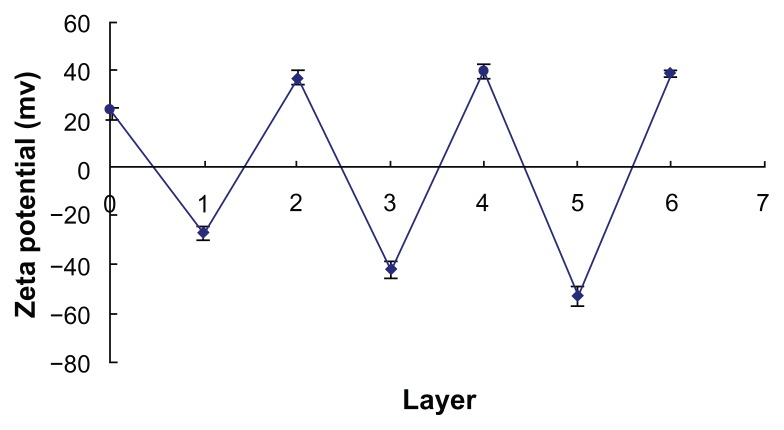
Multilayer microgels were formed by alternate deposition of PSS and PAH through electrostatic interactions. Since the polyelectrolyte coating was a thick film, the size of coated microgels increased during the LBL self-assembly, the size of coated microgels increased during the LBL self-assembly. When three PSS/PAH polyelectrolyte layers were deposited on the chitosan–alginate microgels, the average size of microgels increased to 452.90 ± 2.71 μm (n = 3), 10.68% larger than the uncoated microgels. The negatively charged PSS and the positively charged PAH contributed to changes of zeta potential of the microgels. The changes of zeta potential of the coated microgels during LBL coating are shown in Figure 1. Chitosan, which was outside the chitosan–alginate microgels, was positively charged in order to make the zeta potential of microgels 23.4 ± 0.90 mV (n = 3) before LBL coating. The zeta potentials of microgels coated with PSS, PSS/PAH, PSS2/PAH, PSS2/PAH2, PSS3/PAH2, and PSS3/PAH3 were −27.2 ± 2.9 mV, 36.6 ± 3.3 mV, −42.3 ± 3.6 mV, 40.0 ± 2.6 mV, −52.7 ± 3.7 mV, and 38.6 ± 1.4 mV, respectively. These changes demonstrated that PSS and PAH had been successively deposited on the surface of microgels during the LBL coating process.
Figure 1.
Changes in zeta potential with different numbers of layers during layer-by- layer coating.
The number of coating layers could influence FITC-dextran encapsulation efficiency. The encapsulation efficiency of microgels coated with (PSS/PAH)0, (PSS/PAH)2, (PSS/PAH)4, and (PSS/PAH)6 were 70.31% ± 5.54%, 57.87% ± 3.45%, 45.87% ± 3.98%, and 25.20% ± 5.36%, respectively. The encapsulation efficiency decreased when the layer number increased (P < 0.05, Student’s t-test), which might be explained by the diffusion of drug molecules into solution under vigorous ultrasound during polyelectrolyte coating.
Microscopy characterization of PCAM
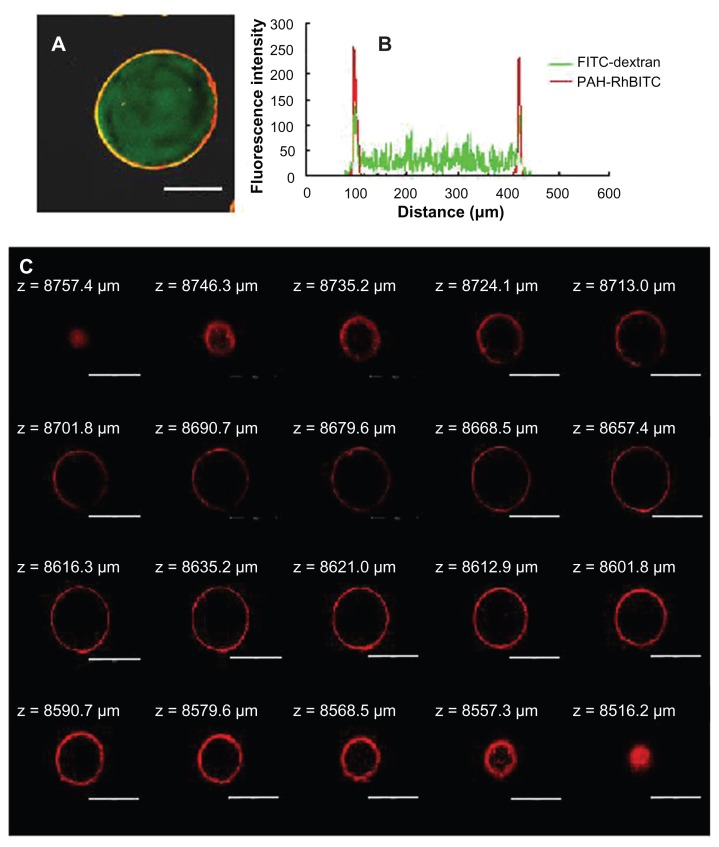
PCAM was coated with PAH-RhBITC for observation of the polyelectrolyte membrane (Figure 2A). A homogeneous distribution of FITC-dextran is shown in the red ring, which stands for the polyelectrolyte membrane. The fluorescence intensity (Figure 2B) also shows the distribution of the encapsulated drug and the coating film. As shown in multisection images (Figure 2C), a distinct red ring surrounding the microgel can also be observed. The results above confirmed the successful LBL coating on the surface of the chitosan– alginate microgels.
Figure 2.
CLSM images of (A) 2000 kDa FITC-dextran (green color) loaded microgels coated with (PSS/PAH-RhBITC)2; (B) the fluorescence intensity of FITC-dextran and PAH-RhBITC in PSS/PAH-coated microgels; and (C) multisection images of coated microgels with(PSS/PAH-RhBITC)2. The PAH was fluorescently labeled with RhBITC (red color). The scale bar represents (A) 150 μm and (C) 250 μm.
Abbreviations: CLSM, confocal laser scanning microscopy; FITC, fluorescein isothiocyanate dextran; PSS, sodium poly(styrenesulfonate); PAH, poly(allylamine hydrochloride); RhBITC, Rhodamine B isothiocyanate.
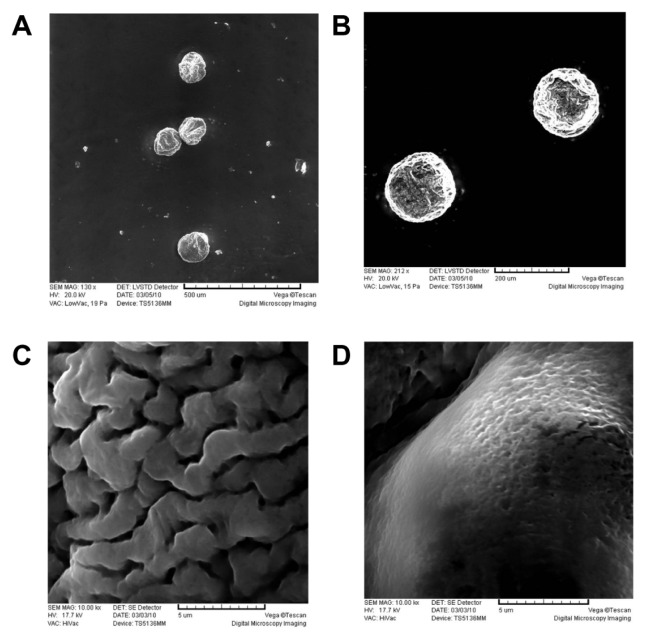
Chitosan–alginate microgels tended to shrink without aqueous solutions, because the microgel was not a kind of infarctate structure, but a capsule with solution inside. The polymer network of alginate–calcium ion–chitosan allowed the entrapment of a wide range of bioactive substances, cells, and drug molecules, but with minor interactions between these and the biopolymer.29 Under the vacuum condition of SEM, a portion of water inside the microgels was removed, which created some wrinkles on the surface of the microgels, caused by partial collapsing of the polymer network (Figure 3A). However, the polyelectrolyte-coating on the microgels caused significant improvement on the maintenance of the spherical shape, due to the mechanical strength of polyelectrolyte coating (Figure 3B). Because of the special network of chitosan–alginate microgels, the microgels were not suitable for lyophilization or long-term storage in a dry environment. To get a detailed examination of the microgel surface, uncoated microgels and LBL-coated microgels were observed with SEM under high-vacuum conditions (water was removed before SEM observation). It was found that the surface of the microgel became smoother after LBL self-assembly (Figure 3C and D).
Figure 3.
SEM micrographs of (A) chitosan–alginate microgels and (B) (PSS/PAH)3 coated microgels. (C) and (D) are the surface of uncoated and coated microgels. The scale is (A) 500 μm; (B) 200 μm; (C) 5 μm; and (D) 5 μm.
Abbreviations: SEM, scanning electron microscope; PSS, sodium poly(styrenesulfonate); PAH, poly(allylamine hydrochloride).
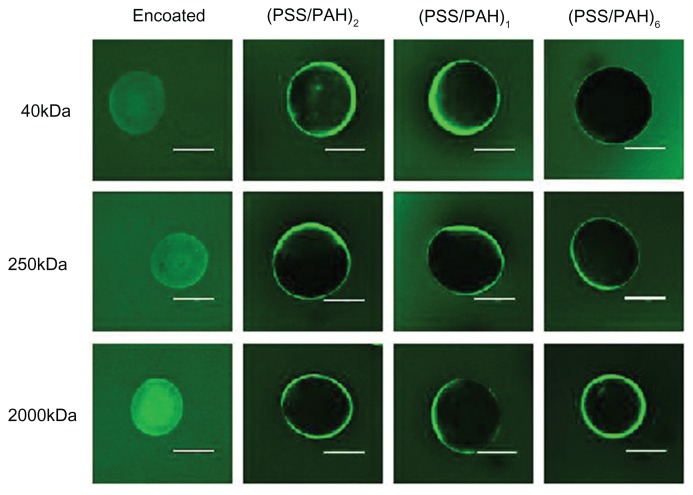
Permeability of the PSS/PAH coating
The content of the microgels would not diffuse before the rupture of polyelectrolyte membrane. The permeability of the semipermeable membrane was investigated with the goal of preventing drug escape from the microgels. The uncoated and coated microgels were incubated in different solutions of FITC-dextran with molecular weights of 40 kDa, 250 kDa, and 2000 kDa, respectively. Results obtained from the CLSM images are shown in Figure 4. After 30 minutes of incubation, uncoated microgels were filled with one of the FITC-dextran solutions. However, it was clear that the permeability of the bilayers decreased when the number of layers increased. In the case of the same number of PSS/PAH layers, 40 kDa FITC-dextran entered into microgels more easily compared with molecular weight of 250 kDa and 2000 kDa; that is to say, the drug with low molecular weight diffused through the polyelectrolyte membrane more easily. When the number of polyelectrolyte bilayers increased to six, it was observed that even 40 kDa FITC-dextran could not diffuse through the membrane.
Figure 4.
Permeability of the LBL coating to different molecular-weight FITC-dextran.
Note: The scale bar represents 250 μm.
Abbreviations: LBL, layer-by-layer; FITC, fluorescein isothiocyanate dextran; PAH, poly(allylamine hydrochloride); PSS, sodium polystyrene sulfonate.
The morphologic changes of PCAM in vitro
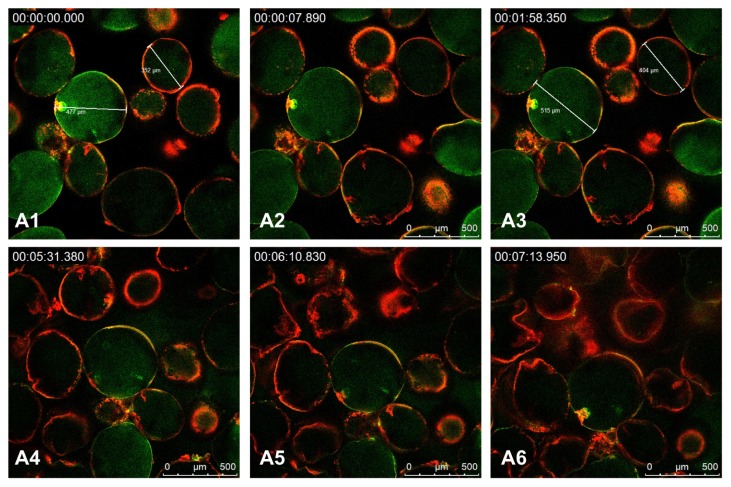
To visualize the rupture of the polyelectrolyte membrane, we observed the morphologic change of PCAM by CLSM. As a pH-sensitive microgel, PCAM ruptured faster in the solution at higher pH value. PSS and PAH were stable at a lower pH,1 2,30 so the solution at pH 11 was used as the release medium to accelerate swelling and rupture of PCAM. Figure 5A1–6 shows images of PCAM during the release process. With the swelling of PCAM, the size of microgels had evidently changed. As shown in Figure 5A1–3, the diameters of two microparticles selected randomly varied from 477 μm to 515 μm and from 352 μm to 404 μm. At a certain moment (Figure 5A4), most of them ruptured leading to the release of 2000 kDa FITC-dextran. The PCAM could not retain the spherical shape (Figure 5A6).
Figure 5.
CLSM images of (PSS/PAH-RhBITC)2-coated microgels at pH 11.
Note: The microgels were loaded with 2000 kDa FITC-dextran (green color) and the PAH was labeled with RhBITC (red color) (n = 3).
Abbreviations: CLSM, confocal laser scanning microscopy; PSS, sodium poly(styrenesulfonate); PAH, poly(allylamine hydrochloride); RhBITC, Rhodamine B isothiocyanate; FITC, fluorescein isothiocyanate dextran.
Swelling behavior and in vitro release of FITC-dextran
The explosion time of the PCAM was governed by the swelling pressure of the chitosan–alginate microgels. The swelling capability mainly came from the pH-sensitivity of the microgels and partly from the repulsion of the chitosan group (instability of the chitosan at pH 7.4, based on its low solubility). The slow degradation was perhaps one of the driving forces. Figure 6 shows the evolution of microgel diameters during the swelling of the microgel core in PBS (pH 7.4). As can be observed in Figure 6, the degree of swelling had an important influence on the lag time; a clear lag time can be seen during the initial phase of the FITC-dextran release. After this initial phase, the model drug massively released in a couple of hours, allowing us to conclude that PSS/PAH coating of the chitosan–alginate microgels causes the release of the 2000 kDa FITC-dextran to be much more pulsatile.
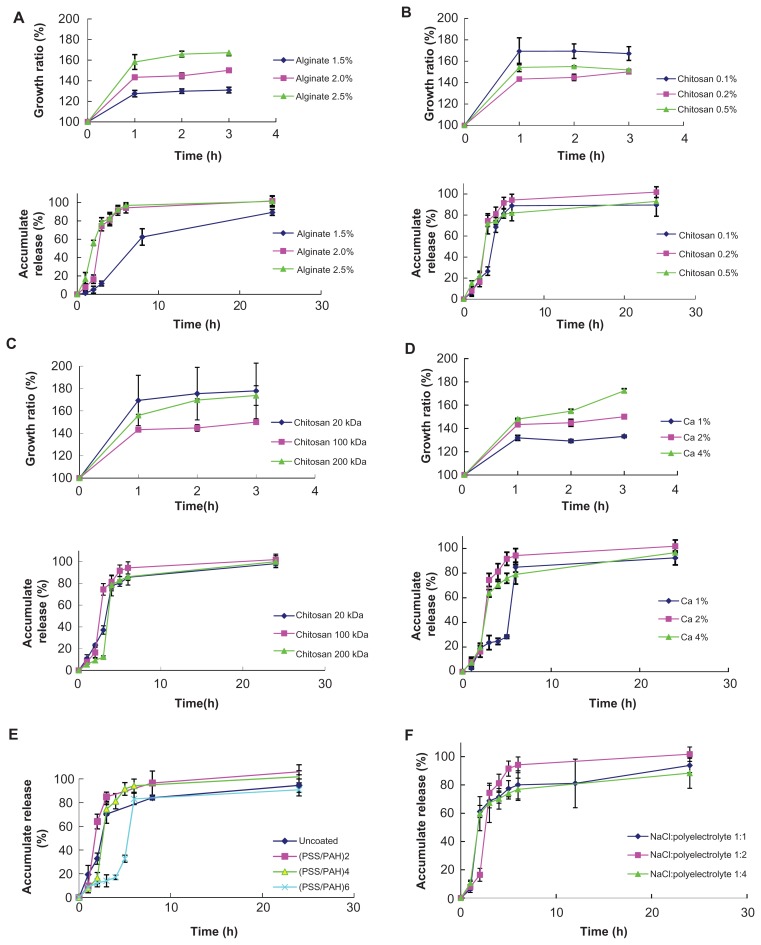
Figure 6.
Effect of various factors on swelling degree of uncoated microgels and release behavior of coated microgels. (A) Alginate concentration; (B) chitosan concentration; (C) chitosan molecular weight; (D) CaCl2 concentration; (E) polyelectrolyte layers; and (F) ratio of polyelectrolyte and NaCl (n = 3).
Abbreviations: PAH, poly(allylamine hydrochloride); PSS, sodium poly(styrene-sulfonate).
Chitosan–alginate microgels showed a swelling degree that was dependent on the concentration of alginate. The in vitro FITC-dextran release from PCAM with different alginate concentrations is shown in Figure 6A. The PCAM with the higher content of alginate owned the higher degree of swelling. This contributed to the shorter lag time than in lower alginate-content-containing PCAM. The concentration and the molecular weight of chitosan had no significant effect on the swelling degree or release profile (Figure 6B and C). There were two reasons for this phenomenon: first, when gelatinated with alginate, chitosan with lower molecular weight easily overcame the stereospecific blockade among polymer chains to form a more compact structure with short chains. The compact network hindered microgel swelling, as did chitosan with high concentration. On the other hand, the swelling property of chitosan itself and its insoluble nature at pH 7.4 led to less stable beads and then to increased swelling. It has been demonstrated that the addition of chitosan to the beads can increase the swelling degree by about 30%.27 Either of the reasons analyzed above is a chief factor affecting swelling ability under various conditions. Consequently, it was difficult to investigate the regularity of the release profiles of microgels with different kinds of chitosan. The swelling degree of the chitosan–alginate microgels decreased and the lag time increased when the concentration of CaCl2 decreased (Figure 6D). Hydroxyapatite Ca10 (PO4)6(OH)2 is a kind of calcium phosphate salt, the formation of which is favored at neutral and basic pH. The calcium ions in the microgels tend to form calcium phosphate salts with phosphate radical in PBS. However, this needs further research in vivo where the environment is more complicated. The lag time could be extended by building up more bilayers, which had the mechanical strength (Figure 6E). The release profile was not affected by the addition of NaCl to the LBL-coated solution even though NaCl could make the membrane more permeable. It is possible the salt also changed the adsorbability of the polyelectrolytes on the substrate. In other words, the higher the NaCl content, the more polyelectrolytes were deposited (Figure 6F).
Finally, an orthogonal experiment was carried out to point out the significant reaction parameters of FITC-dextran release. The concentrations of alginate and calcium chloride and the number of the layers were the significant factors influencing lag phase during in vitro FITC-dextran release (F > F0.05 [2, 2]). Lower alginate or calcium chloride content gave rise to lower swelling degrees and thus, leading to the longer lag phase. The release profile consisted of two apparent phases: a little- or no-drug release period (lag-phase) and a majority-of-drug release after lag time (II-phase). Meanwhile, the concentration of alginate was also the significant factor influencing the II-phase (F > F0.05 [2, 2]). At the same time, neither the number of the polyelectrolyte layers nor the NaCl content could influence the II-phase. The result was ascribed to the distribution of the encapsulated substrates. As FITC-dextran was loaded into the chitosan–alginate microgels, the dissolution time of drug molecule from the polymer network was the rate-limiting factor of II-phase release, which was not influenced by the outside membrane. What is more, the neutral macromolecules FITC-dextran had no interactions with the charged polyelectrolytes. If the encapsulated molecule was charged, II-phase release profiles may be affected by the polyelectrolyte membrane.31
In our work, FITC-dextran was chosen as the model drug for investigation. In clinical work, the lag time and II-phase release profile should be adjusted according to therapeutic needs.
The morphologic change of PCAM in vivo
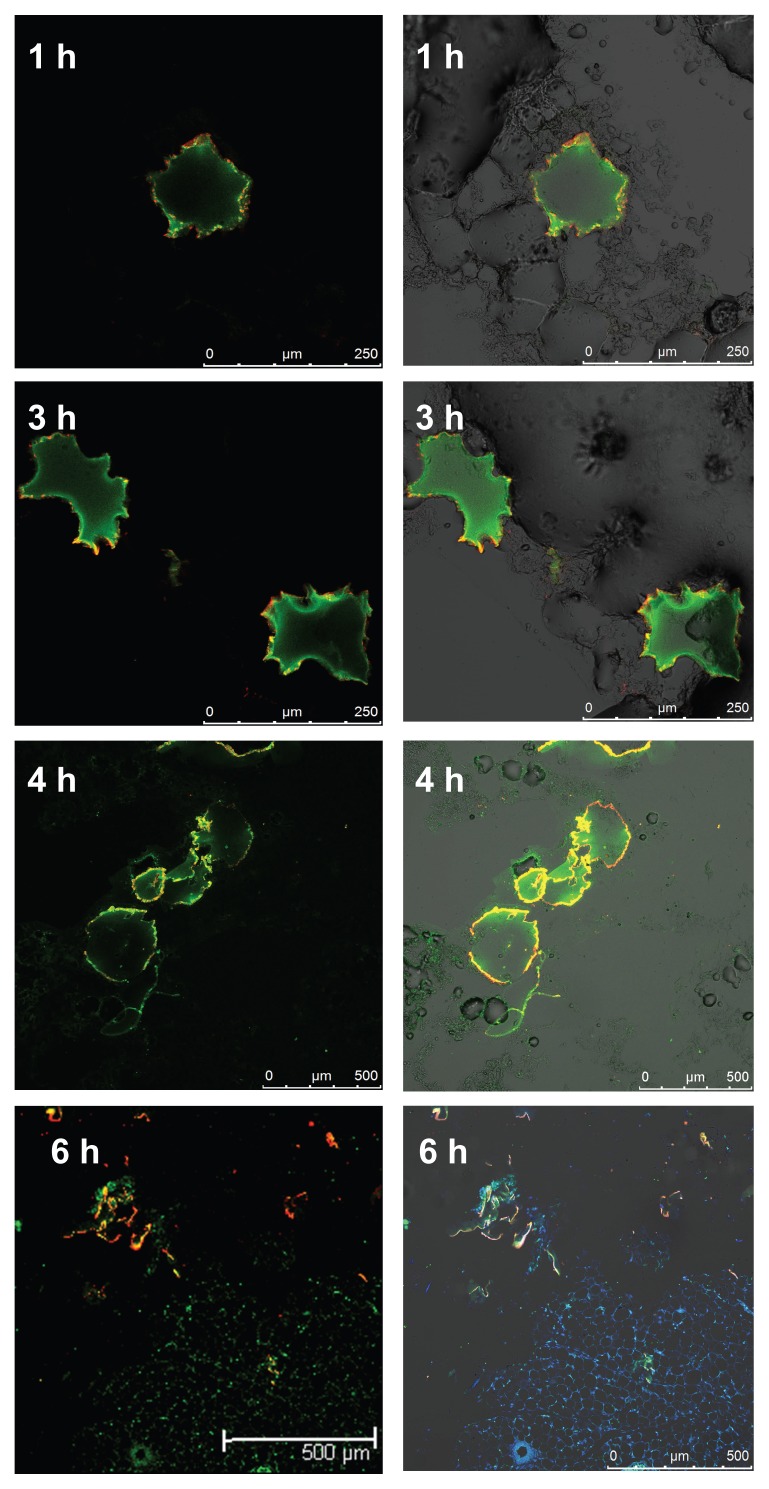
As can be seen in Figure 7, in the sections taken 1 hour after subcutaneous injection of the PCAM, the microgels appeared spherical in shape; after 3 hours, the microgels began to deform, but the membrane structure still remained intact, and drug was still trapped in the microgels; after 4 hours, PCAM began to rupture (suggesting that the lag time should be between 3 to 4 hours); 6 hours later, the polyelectrolyte layers had completely broken, and the structure of the drug device collapsed, with FITC-dextran diffusing into the subcutaneous tissue and cells. (The blue fluorescence is due to 4′,6-diamidino-2-phenylindole, which selectively stains the cell nuclei.)
Figure 7.
CLSM images of tissue sections taken at several time points after subcutaneous injection of PCAM.
Abbreviations: CLSM, confocal laser scanning microscopy; PCAM, polyelectrolyte-coated chitosan–alginate microgel.
Compared with the lag-phase of FITC-dextran release in vitro (2.67 hours ), there was a little delay of this in vivo (~3–4 hours ). This phenomenon may be due to the structure of the microgels, which is a kind of colloid with liquid inside. Initially, the microgels were forced to shrink by mechanical pressure from the surrounding tissue when injected subcutaneously, and the quantity of subcutaneous liquid was less than the medium in the centrifuge in vitro release. Initially, the microgels were forced to shrink by mechanical pressure from the surrounding tissue when injected subcutaneously, and the quantity of subcutaneous liquid was less than the medium in the centrifuge in vitro release. The drug release underwent three processes that created a longer lag time: 1) shrinkage of the beads that expel amounts of water; 2) swell; 3) rupture with drug molecules out of the beads. In addition, the polyelectrolyte multilayers may have degraded naturally in vivo and by enzymatic attack from living cells.32
Conclusion and outlook
In this study, we have shown that an exploding drug device can be prepared by LBL coating of pH-sensitive and biodegradable chitosan–alginate microgels, with polyelectrolyte PSS and PAH. Zeta potential measurements and CLSM images proved from the structure of the novel device that polyelectrolytes can be sequentially adsorbed onto the surface of microgels successfully. PCAM was not only able to explode under in vitro conditions, but could also rupture under physiological conditions and release payload in a pulsed fashion. The microcapsules have been implicated as high-potential systems for pharmaceutical use because they can tailor many different requirements and satisfy various clinical needs, so as to deliver drug at the right moment, in the right place, and at an adequate concentration in terms of therapeutic efficacy and compliance. The study may be a promising step toward a vaccine or other macromolecule. Further, it is possible to control the mechanical properties and the permeability of the polyelectrolyte shell by tuning the layer thickness and density on the nanometer scale in order to load more kinds of molecules, such as peptides, proteins, and oligonucleotides. What is more, development of new polymer, derivant, and coating material is also the research direction of novel pulsed drug delivery.
Various sizes of chitosan–alginate microgels can be obtained with an electrostatic droplet generator, and a particle diameter under 20 μ can be achieved with a spray dryer. A formulation consisting of different particle sizes of self-rupturing microcapsules, each population exploding at a different time after injection, would offer the possibility to generate multiple drug boosters after a single administration.
Research on this kind of drug device is, however, still in the conceptual phase, and most delivery systems are only for lab use. Their performance in vivo needs testing and investigation, and clinical results are lacking. It may also be that large-scale manufacturing is costly. But this system offers potential for chronopharmacology and these hurdles can be overcome in future by rapid increases in technique and science. Therefore, our further research will focus on the pharmacokinetics and pharmacodynamics in vivo.
Acknowledgments
We thank Mr Baoguo Li for the technical assistance with the electrostatic droplet generator (designed by the University of Shanghai for Science and Technology, Shanghai, People’s Republic of China). This work was supported in part by the National Natural Science Foundation of China (30801441, program projects).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Roy P, Shahiwala A. Multiparticulate formulation approach to pulsatile drug delivery: current perspectives. J Control Release. 2009;134(2):74–84. doi: 10.1016/j.jconrel.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Stubbe BG, De Smedt SC, Demeester J. “Programmed polymeric devices” for pulsed drug delivery. Pharm Res. 2004;21(10):1732–1740. doi: 10.1023/b:pham.0000045223.45400.01. [DOI] [PubMed] [Google Scholar]
- 3.Mastiholimath VS, Dandagi PM, Jain SS, Gadad AP, Kulkarni AR. Time and pH dependent colon specific, pulsatile delivery of theophylline for nocturnal asthma. Int J Pharm. 2007;328(1):49–56. doi: 10.1016/j.ijpharm.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 4.He HY, Guan J, Lee JL. An oral delivery device based on self-folding hydrogels. J Control Release. 2006;110(2):339–346. doi: 10.1016/j.jconrel.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 5.He H, Cao X, Lee LJ. Design of a novel hydrogel-based intelligent system for controlled drug release. J Control Release. 2004;95(3):391–402. doi: 10.1016/j.jconrel.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Mohamad A, Dashevsky A. Development of pulsatile multiparticulate drug delivery system coated with aqueous dispersion Aquacoat ECD. Int J Pharm. 2006;318(1–2):124–131. doi: 10.1016/j.ijpharm.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Gazzaniga A, Palugan L, Foppoli A, Sangalli ME. Oral pulsatile delivery systems based on swellable hydrophilic polymers. Eur J Pharm Biopharm. 2008;68(1):11–18. doi: 10.1016/j.ejpb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Intra J, Glasgow JM, Mai HQ, Salem AK. Pulsatile release of biomolecules from polydimethylsiloxane (PDMS) chips with hydrolytically degradable seals. J Control Release. 2008;127(3):280–287. doi: 10.1016/j.jconrel.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Lin CC, Metters AT. Hydrogels in controlled release formulations: network design and mathematical modeling. Adv Drug Deliv Rev. 2006;58(12–13):1379–1408. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Li B, Zhu J, Zheng C, Gong W. A novel system for three-pulse drug release based on “tablets in capsule” device. Int J Pharm. 2008;352(1–2):159–164. doi: 10.1016/j.ijpharm.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 11.De Geest BG, Déjugnat C, Verhoeven E, et al. Layer-by-layer coating of degradable microgels for pulsed drug delivery. J Control Release. 2006;116(2):159–169. doi: 10.1016/j.jconrel.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Fan YF, Wang YN, Fan YG, Ma JB. Preparation of insulin nano-particles and their encapsulation with biodegradable polyelectrolytes via the layer-by-layer adsorption. Int J Pharm. 2006;324(2):2158–2167. doi: 10.1016/j.ijpharm.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 13.Sun B, Lynn DM. Release of DNA from polyelectrolyte multilayers fabricated using ‘charge-shifting’ cationic polymers: Tunable temporal control and sequential, multi-agent release. J Control Release. 2010;148(1):91–100. doi: 10.1016/j.jconrel.2010.07.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radtchenko I, Sukhorukov G, Möhwald H. A novel method for encapsulation of poorly water-soluble drugs: precipitation in polyelectrolyte multilayer shells. Int J Pharm. 2002;242(1–2):219–223. doi: 10.1016/s0378-5173(02)00161-8. [DOI] [PubMed] [Google Scholar]
- 15.Decher G, Lehr B, Lowack K, Lvov Y, Schmitt J. New nanocomposite films for biosensors: layer-by-layer adsorbed films of polyelectrolytes, proteins or DNA. Biosens Bioelectron. 1994;9(9–10):677–684. [Google Scholar]
- 16.Derveaux S, Stubbe BG, Roelant C, et al. Layer-by-layer coated digitally encoded microcarriers for quantification of proteins in serum and plasma. Anal Chem. 2008;80(1):85–94. doi: 10.1021/ac071212i. [DOI] [PubMed] [Google Scholar]
- 17.Shao YY, Zhu BQ, Li J, Liu XR, Tan X, Yang XL. Novel chitosan microsphere-templated microcapsules suitable for spontaneous loading of heparin. Materials Sci Eng C. 2009;29(3):936–941. [Google Scholar]
- 18.van Dijk-Wolthuls WNE, Tsang SKY, Kettenes-van den Bosch JJ, Hennink WE. A new class of polymerizable dextrans with hydrolyzable groups: hydroxyethyl methacrylated dextran with and without oligolactate spacer. Polymer. 1997;38(25):6235–6242. [Google Scholar]
- 19.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 20.Park DJ, Choi BH, Zhu SJ, Huh JY, Kim BY, Lee SH. Injectable bone using chitosan-alginate gel/mesenchymal stem cells/BMP-2 composites. J Craniomaxillofac Surg. 2005;33(1):50–54. doi: 10.1016/j.jcms.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Zhan C, Fan L, Wang L, Zheng H. Preparation of dual crosslinked alginate-chitosan blend gel beads and in vitro controlled release in oral site-specific drug delivery system. Int J Pharm. 2007;336(2):329–337. doi: 10.1016/j.ijpharm.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 22.González-Rodríguez ML, Holgado MA, Sánchez-Lafuente C, Rabasco AM, Fini A. Alginate/chitosan particulate systems for sodium diclofenac release. Int J Pharm. 2002;232(1–2):225–234. doi: 10.1016/s0378-5173(01)00915-2. [DOI] [PubMed] [Google Scholar]
- 23.Taqieddin E, Amiji M. Enzyme immobilization in novel alginate-chitosan core-shell microcapsules. Biomaterials. 2004;25(10):1937–1945. doi: 10.1016/j.biomaterials.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 24.Dai C, Wang B, Zhao H. Microencapsulation peptide and protein drugs delivery system. Colloids Surf B Biointerfaces. 2005;41(2–3):117–120. doi: 10.1016/j.colsurfb.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 25.Hu B, Jin XH, Tan TW. Swelling characteristic and the application of compound sodium alginate gel-a pH sensitive chitosan. Chinese Journal of Clinical Rehabilitation. 2004;8(11):2162–2163. [Google Scholar]
- 26.Zhang WJ, Li BG, Zhang C, Xie XH, Tang TT. Biocompatibility and membrane strength of C3H10T1/2 cell-loaded alginate-based microcapsules. Cytotherapy. 2008;10(1):90–97. doi: 10.1080/14653240701762372. [DOI] [PubMed] [Google Scholar]
- 27.Pasparakis G, Bouropoulos N. Swelling studies and in vitro release of verapamil from calcium alginate and calcium alginate-chitosan beads. Int J Pharm. 2006;323(1–2):34–42. doi: 10.1016/j.ijpharm.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 28.Anal AK, Stevens WF. Chitosan-alginate multilayer beads for controlled release of ampicillin. Int J Pharm. 2005;290(1–2):45–54. doi: 10.1016/j.ijpharm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Griffith LG. Polymeric biomaterials. Acta Mater. 2000;48(1):263–277. [Google Scholar]
- 30.Déjugnat C, Sukhorukov GB. pH-responsive properties of hollow polyelectrolyte microcapsules templated on various cores. Langmuir. 2004;20(17):7265–7269. doi: 10.1021/la049706n. [DOI] [PubMed] [Google Scholar]
- 31.Van Tomme SR, De Geest BG, Braeckmans K, et al. Mobility of model proteins in hydrogels composed of oppositely charged dextran microspheres studied by protein release and fluorescence recovery after photobleaching. J Control Release. 2005;110(1):67–78. doi: 10.1016/j.jconrel.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 32.De Geest BG, De Koker S, Demeester J, De Smedt SC, Hennink WE. Pulsed in vitro release and in vivo behavior of exploding microcapsules. J Control Release. 2009;135(3):268–273. doi: 10.1016/j.jconrel.2009.01.017. [DOI] [PubMed] [Google Scholar]