Abstract
We evaluated a commercial point-of-care circulating cathodic antigen (POC-CCA) test for assessing Schistosoma mansoni infection prevalence in areas at risk. Overall, 4,405 school-age children in Cameroon, Côte d'Ivoire, Ethiopia, Kenya, and Uganda provided urine for POC-CCA testing and stool for Kato-Katz assays. By latent class analysis, one POC-CCA test was more sensitive (86% versus 62%) but less specific (72% versus ∼100%) than multiple Kato-Katz smears from one stool. However, only 1% of POC-CCA tests in a non-endemic area were false positives, suggesting the latent class analysis underestimated the POC-CCA specificity. Multivariable modeling estimated POC-CCA as significantly more sensitive than Kato-Katz at low infection intensities (< 100 eggs/gram stool). By linear regression, 72% prevalence among 9–12 year olds by POC-CCA corresponded to 50% prevalence by Kato-Katz, whereas 46% POC-CCA prevalence corresponded to 10% Kato-Katz prevalence. We conclude that one urine POC-CCA test can replace Kato-Katz testing for community-level S. mansoni prevalence mapping.
Introduction
As part of its strategy for assigning treatment coverage in mass drug administration plans, such as Preventive Chemotherapy (PCT) programs, for control of morbidity caused by Schistosoma mansoni infection the World Health Organization (WHO) recommends using duplicate Kato-Katz thick smear examination (two slides of a single stool specimen) from school-age children to estimate local infection prevalence and to map areas for intervention.1–3 Community-wide PCT is recommended for areas classified as having high prevalence (> 50%) by Kato-Katz in school-age children, whereas school-based PCT is recommended for communities having moderate prevalence according to the Kato-Katz technique (10–49%). It is recognized that the single stool Kato-Katz method is incompletely sensitive for detection of active S. mansoni infection in individual patients.4–6 However, barring laboratory error; it is considered essentially 100% specific. In areas of low prevalence (which have mostly low intensity infections), use of a less sensitive screening test can present a major barrier to efficient implementation of parasite control; the cost to obtain and process multiple daily stool specimens for testing and the need for painstaking microscopy can limit the scope and potential effectiveness of mass treatment programs.7,8 Furthermore, the logistical limitations of the test can restrict the ability of schistosomiasis mass drug administration (MDA) programs to be integrated with control and elimination programs for other neglected tropical diseases (NTDs).9,10 As a possible alternative, a point-of-care (POC) lateral flow cassette assay that diagnoses S. mansoni infection by detection of parasite circulating cathodic antigen (CCA)11–14 in patient urine is now commercially available.15–17 It has been evaluated for diagnostic accuracy in a number of single site studies performed in endemic locations, primarily in areas of high parasite prevalence and infection intensity.15–17 If shown to be generally reliable and effective, this assay could potentially replace traditional Kato-Katz stool examination for the mapping of areas at risk for intestinal schistosomiasis. Its potential benefits could include a substantial savings in time for specimen collection and specimen processing, with the results available within 20 minutes, and possibly direct savings in terms of the costs of testing and treatment delivery.7,9 An additional advantage is that urine specimens are less invasive to collect than feces and are already collected for detection of hematuria or Schistosoma haematobium eggs in areas of co-endemicity.
In 2010 the Schistosomiasis Consortium for Operational Research and Evaluation (SCORE; http://score.uga.edu/) funded investigators in five African countries (Cameroon, Côte d'Ivoire, Ethiopia, Kenya, Uganda) for a harmonized multi-center evaluation of this POC-CCA assay's performance among school-age children in areas anticipated to have high, medium, or low prevalence and intensity of S. mansoni infection. Targeted study areas also included some areas with mixed S. mansoni and S. haematobium infection. For further comparison, school-age children were also tested from an area in Ethiopia that was endemic for soil-transmitted helminths (STHs), but believed to be non-endemic for S. mansoni. Thus far, three reports have been published on SCORE-supported results for individual country locations15–17; the current work summarizes the overall results of the multi-center POC-CCA diagnostic assay testing trial.
Populations, Materials, and Methods
Ethics statement on subject recruitment.
Ethical clearance for this study was obtained from the Human Subject's Office of the University of Georgia, the National Ethics Committee of Cameroon (no. 084/CNE/DNM/09), the institutional research commission of the Swiss Tropical and Public Health Institute (Basel, Switzerland), the ethics committees of Basel (EKBB; reference no. 377/09), and of Côte d'Ivoire (reference no. 1993 MSHP/CNER), the National Council for Science and Technology of Uganda, the Institutional Review Board of Aklilu Lemma Institute of Pathobiology, Addis Ababa University of Ethiopia, and the National Ethics Review Committee of the Kenya Medical Research Institute (KEMRI-SSC no. 1768).
The objectives of the studies were explained to participating children and to their parents or guardians from whom written informed consent was obtained. Children willing to participate were then registered and tested. Each child was assigned an identification number and results were entered into a confidential database. All children who participated in the study were treated with praziquantel (40 mg/kg) free of charge.
Study design.
Each country survey was targeted to communities thought to have primarily low to moderate prevalence of S. mansoni infection prevalence based on past surveys. In the selected villages, investigators tested as many 9- to 12-year-old children as possible in local school classes. During the course of repeated daily visits, investigators collected one to three separate urine and stool specimens from each child on three consecutive days. The exception was in Uganda, where only one urine specimen was collected per child.
Stool and urine testing.
All stools were tested using a standard quantitative Kato-Katz microscopic technique1; each stool specimen was examined in at least duplicate by preparation of two individual Kato-Katz slides per stool (standard 41.7 mg/slide). In Côte d'Ivoire and Cameroon triplicate Kato-Katz thick smears per stool sample were assayed.
A subset of stool samples from Kenya was selected for multiplex real-time polymerase chain reaction (PCR) testing for detection of Schistosoma DNA.18 For POC-CCA urine assays (Rapid Medical Diagnostics; Pretoria, South Africa), the manufacturer's instructions were followed, including defining “trace” POC-CCA readings as positive results.
Statistical analysis.
For evaluation of test performance, given the imperfect sensitivity of Kato-Katz stool microscopy,4,19 latent class analysis (LCA)20–23 was used to estimate the relative sensitivities and specificities of Kato-Katz, POC-CCA, and Schistosoma-specific PCR. In LCA, the “true” disease status of each subject is treated as an unobserved binary latent variable, taking the value one if the subject is indeed infected with S. mansoni and zero if otherwise. In our adjusted estimates, the likelihood of S. mansoni infection was hypothesized to be a function of age, gender, and community to which each subject belonged. Maximum likelihood estimates of model parameters were obtained using the EM algorithm.23 (Note: The data from the area in Ethiopia thought to be non-endemic for S. mansoni were analyzed separately and were not included in any models and are not reported in the tables summarizing the five-country study.)
Logistic regression was used to assess the sensitivity of POC-CCA as a function of mean infection intensity. Standard linear regression was used to compare the prevalence estimated using a single POC-CCA urine test with that based on Kato-Katz testing of a single stool.
Results
A total of 4,305 children from 63 schools in areas endemic for schistosomiasis in the five countries were tested (Table 1). The greatest proportion of participants (43%) was from Kenya. Cameroon contributed 17%, Côte d'Ivoire 14%, Ethiopia 14%, and Uganda 12% of the test subjects included in the final analysis of the study. As expected, except in Uganda, estimated S. mansoni infection prevalence based on positive egg detection in any of three daily Kato-Katz examinations was higher than that based on a Kato-Katz examination of only the first day's stool (Table 1). In similar fashion, POC-CCA assays repeated on three consecutive daily urine specimens resulted in higher prevalence estimates of S. mansoni infection.
Table 1.
School numbers and participant children surveyed in each study country*
| Country | No. schools tested | No. children tested | Overall prevalence by 1 Kato-Katz | Overall prevalence by 1 POC-CCA | Overall prevalence by 3 Kato-Katz | Overall prevalence by 3 POC-CCA | Median intensity of infections detected (EPG) [range] |
|---|---|---|---|---|---|---|---|
| Cameroon | 3 | 733 | 0.384 | 0.622 | 0.549 | 0.756 | 37 [46–590] |
| Côte d'Ivoire | 4 | 607 | 0.479 | 0.455 | 0.577 | 0.565 | 103 [161–235] |
| Ethiopia | 2 | 620 | 0.430 | 0.660 | 0.526 | 0.708 | 43 [69–153] |
| Kenya | 49 | 1845 | 0.151 | 0.499 | 0.221 | 0.657 | 14 [2–366] |
| Uganda | 5 | 500 | 0.250 | 0.626 | 0.250 | – | 32 [37–247] |
| Total or Overall | 63 | 4305 | 0.289 | 0.552 | 0.388 | 0.664 | 35 [2–590] |
Children surveyed in each study country (left columns). Center columns show observed prevalence of Schistosoma mansoni infection, as detected either in 1 or 3 daily stool specimens using standard Kato-Katz microscopy, or in 1 or 3 daily urine specimens using point-of-care assays for parasite circulating cathodic antigen (POC-CCA). The country-level median intensity of Kato-Katz-detected infections (in eggs per gram of feces, EPG) is indicated in the right-most column.
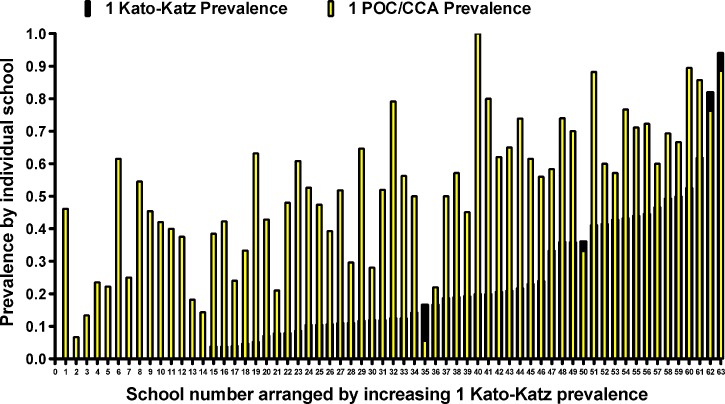
To show the range of infection prevalence measured in participating schools, Figure 1 presents the standard prevalence for each school tested, as determined by either a single Kato-Katz or a single POC-CCA test, in rank order of the 1-day Kato-Katz results. Participating study schools had a broad range of both prevalence and intensity of infection (Figure 1; Table 1). Most of the data for very low prevalence (< 10%) schools came from Kenya, but schools from other countries were also represented across a range of prevalence levels. In all but four of the 63 schools, prevalence based on a single POC-CCA was higher than that found by a single Kato-Katz examination, often considerably higher. The difference between estimates by POC-CCA and Kato-Katz was not consistent across all schools (Figure 1).
Figure 1.
Range and variation of Schistosoma mansoni prevalence of infection in 63 participant schools. Dark bars indicate prevalence as determined by standard Kato-Katz microscopy of the first daily stool specimen; superimposed light bars indicate prevalence measured using concurrent point-of-care circulating cathodic antigen (POC-CCA) detection in the first urine specimen. Bars are arranged in order of ascending prevalence based on the Kato-Katz results.
A comparison of the results of the first day's POC-CCA with the first day's Kato-Katz test result for each child, organized by country, indicates that there were differences in test performance among countries (Table 2). For example, in Uganda, 53% of POC-CCA-positive individuals were Kato-Katz negative, whereas this discordant outcome was seen in only 14% of children in Côte d'Ivoire. In an analysis combining all the results, 10.6% of children whose first stool was positive using Kato-Katz had their first POC-CCA test reported as negative, and 43% of children whose first stool was negative had a positive POC-CCA result on their first urine.
Table 2.
Results of concurrent POC-CCA and Kato-Katz testing on the first daily specimens (urine and stool, respectively) obtained from enrolled 9- to 12-year-old study subjects living in Schistosoma mansoni-endemic areas of five African countries*
| Kato-Katz negative | Kato-Katz positive | ||
|---|---|---|---|
| Cameroon | POC-CCA− | 231 (52.6%) | 27 (9.8%) |
| POC-CCA+ | 208 (47.4%) | 247 (90.2%) | |
| Côte d'Ivoire | POC-CCA− | 249 (85.6%) | 38 (14.2%) |
| POC-CCA+ | 42 (14.4%) | 230 (85.8%) | |
| Ethiopia | POC-CCA− | 195 (55.2%) | 16 (6.0%) |
| POC-CAA+ | 158 (44.8%) | 251 (94.0%) | |
| Kenya | POC-CCA− | 833 (55.6%) | 35 (13.2%) |
| POC-CCA+ | 664 (44.4%) | 231 (86.8%) | |
| Uganda | POC-CCA− | 176 (46.9%) | 11 (8.8%) |
| POC-CCA+ | 199 (53.1%) | 114 (91.2%) | |
| All 5 Countries | POC-CCA− | 1684 (57.0%) | 127 (10.6%) |
| POC-CCA+ | 1271 (43.0%) | 1073 (89.4%) | |
Numbers in parentheses indicate column percentages for each 2 × 2 sub-table.
POC-CCA = point-of-care assays for parasite circulating cathodic antigen.
Seven villages in Cameroon and Côte d'Ivoire included children who had mixed infection with both S. mansoni and S. haematobium. The rate of positivity of POC-CCA was 86.5% among the 525 children who had S. mansoni only (by Kato-Katz), compared with 90.3% among the 248 children who had mixed infections (as determined by Kato-Katz positivity plus urine filtration positivity24 or hematuria detected by reagent strip testing [i.e., Hemastix]). Thus, mixed infection with both S. mansoni and S. haematobium did not alter the ability to detect S. mansoni infection using the POC-CCA assay. Furthermore, in a control village in Ethiopia endemic for STHs but predicted and subsequently shown to be non-endemic for schistosomiasis, among 100 children tested, prevalence of at least one STH (by Kato-Katz stool examination) was 50%, whereas the prevalence of S. mansoni by Kato-Katz was 0%, and by POC-CCA 1%, with only a single positive POC-CCA reading, which was recorded as “trace.” The children in this study were born and brought up in that area and had never traveled to areas endemic for schistosomiasis.
Specimens concordant or discordant for first day Kato-Katz and the first day POC-CCA outcomes were tested by PCR of the first day stool to assist with interpretation of discordant results. These results also contributed to estimates derived from LCA models. Of the 905 specimens tested by PCR, 18% were positive by both Kato-Katz and POC-CCA, 38% were negative by both methods, 2% were Kato-Katz positive but POC-CCA negative, and 42% were Kato-Katz negative but POC-CCA positive. Summarizing the detailed results shown in Table 3, 89% of the 176 stools positive for S. mansoni eggs by Kato-Katz were also positive for Schistosoma DNA by PCR. However, only 47% of the 539 children with positive POC-CCA urine assays were seen as stool-positive by PCR. By contrast, 86% of the 366 who were POC-CCA-negative were also negative by PCR (Table 3).
Table 3.
Results of PCR testing for Schistosoma DNA in stool specimens performed on a subset of 905 Kenyan subjects*
| Test results group | Total | PCR positive | PCR negative |
|---|---|---|---|
| Kato-Katz and POC/CCA both positive | 158 | 146 (92%) | 12 (8%) |
| Kato-Katz and POC/CCA both negative | 348 | 42 (12%) | 306 (88%) |
| Kato-Katz positive, POC/CCA negative | 18 | 11 (61%) | 7 (39%) |
| Kato-Katz negative, POC/CCA positive | 381 | 105 (28%) | 276 (72%) |
| Total | 905 | 318 (33%) | 633 (67%) |
All data are from the first day stool and urine specimens. Columns show positive and negative polymerase chain reaction (PCR) results for four subgroups defined by the subject's Kato-Katz and point-of-care assays for parasite circulating cathodic antigen (POC/CCA) test results. Numbers in parentheses indicate row percentages.
Assuming 100% specificity for a Kato-Katz test (i.e., any S. mansoni egg visualization represents an infection), our LCA using data from all available POC-CCA, Kato-Katz and PCR first day assays (except those from the non-endemic area of Ethiopia) indicated that a single POC-CCA test was more sensitive for diagnosis of S. mansoni infection than a single stool tested by Kato-Katz examination—86% versus 62%, respectively (Table 4). The overall specificity of the POC-CCA test, as estimated by LCA, was 72%, compared with the assumed specificity of 100% for the Kato-Katz. There was, however, considerable variation among countries in the LCA-estimated sensitivity of the Kato-Katz and in the specificity and sensitivity of the POC-CCA, with Côte d'Ivoire experiencing the lowest POC-CCA test sensitivity (78%) with high specificity (92%), and Uganda having the lowest specificity for the POC-CCA (56%). Ethiopia had the highest estimated test specificity (92%) and sensitivity (94%) for POC-CCA. By LCA, the estimated sensitivity and specificity for the PCR stool assays were 69% and 91%, respectively. Slight differences in the model outcomes for Kenya are seen if the LCA model is run with the specificities of both the Kato-Katz and the PCR set at 100%. In this case the Kenyan Kato-Katz sensitivity decreases from 44% to 38%, whereas the POC-CCA sensitivity decreases from 86% to 82% and the POC-CCA specificity increases from 70% to 72%. The sensitivity of the PCR remains the same at 69% under these conditions.
Table 4.
As estimated by latent class analysis of all available subject data, test sensitivity, and specificity of Kato-Katz microscopy of a single stool, POC-CCA testing of a single urine, and of PCR testing of a single stool in the Schistosoma mansoni-endemic areas included in this study*
| Assay | Criterion | Countries | |||||
|---|---|---|---|---|---|---|---|
| Cameroon | Ethiopia | Côte d'Ivoire | Kenya | Uganda | Overall | ||
| Kato-Katz | Sensitivity | 64% | 59% | 77% | 44% | 61% | 62% |
| POC-CCA | Sensitivity | 87% | 92% | 78% | 86% | 89% | 86% |
| Specificity | 74% | 94% | 92% | 70% | 56% | 72% | |
| PCR | Sensitivity | 69% | |||||
| Specificity | 91% | ||||||
Results are listed for each participating study country and for all countries combined.
POC-CCA = point-of-care assays for parasite circulating cathodic antigen; PCR = polymerase chain reaction.
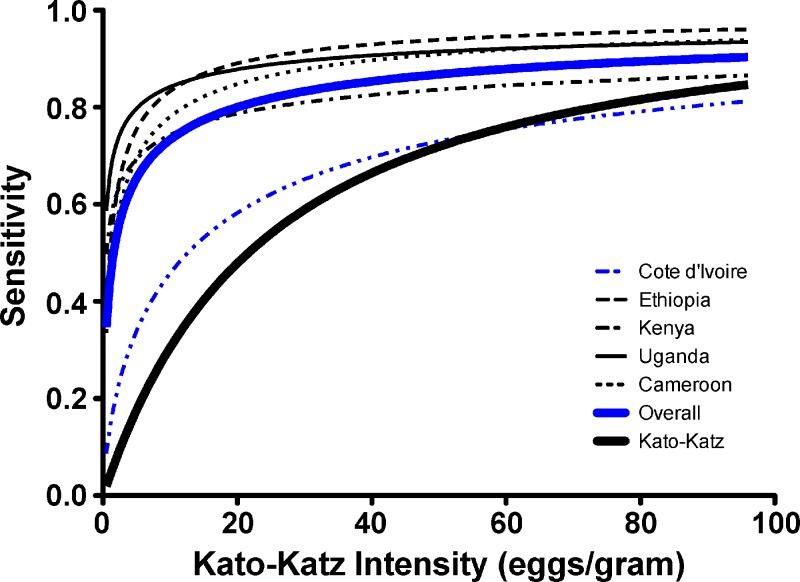
Figure 2 presents the results of our location-adjusted logistic modeling estimates of the sensitivity of a single POC-CCA or a single Kato-Katz, as a function of the intensity of S. mansoni infection, for each of the five country partners in the study and for all countries combined. The results imply that a Kato-Katz test of a single stool is consistently less sensitive for S. mansoni diagnosis at lower intensities of infection, whereas its sensitivity is 80% or greater at infection intensities above 100 eggs per gram of feces (EPG). When data from all countries were combined, the estimated POC-CCA sensitivity approached 80% as the infection intensity rose above 20 EPG. When analyzed individually, three of the five country test groups approached or exceeded 80% sensitivity at intensities of 10 EPG.
Figure 2.
Modeled estimation of the sensitivity of a single point-of-care circulating cathodic antigen (POC-CCA) in the five study countries (as shown in the variously thin or dotted lines as indicated in the Figure) or the five taken together (thick gray line), according to the “true” local Schistosoma mansoni prevalence of infection among 9- to 12-year-old children. Comparison is shown to results for a single Kato-Katz stool examination (thick black line). The model is based on logistic regression in which “true” disease status (yes or no) of each subject is treated as a binary latent variable, and the likelihood of schistosomiasis was hypothesized to be a function of age, gender, and community to which each subject belonged.
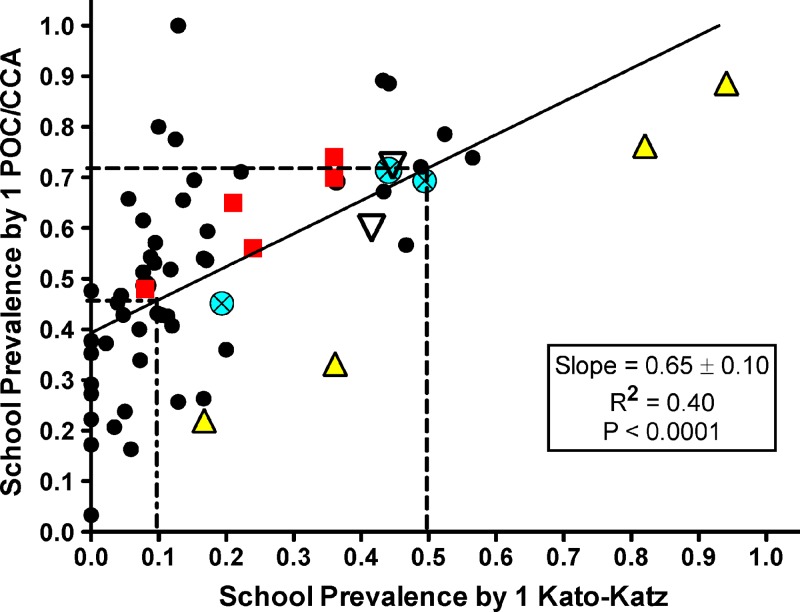
Figure 3 presents a comparison of individual school prevalence levels measured by single specimen Kato-Katz testing versus single-specimen POC-CCA testing. Based on a linear regression model, prevalence of 46% using POC-CCA corresponded to a prevalence of 10% using Kato-Katz, and a prevalence of 72% by POC-CCA corresponded to a prevalence of 50% by Kato-Katz testing.
Figure 3.
Correlation of prevalence in individual schools (based on a single Kato-Katz examination) with that from a single point-of-care circulating cathodic antigen (POC-CCA) urine testing. Linear regression parameters are shown in the legend. Small black circles, Kenya; large triangles, Côte d'Ivoire; squares, Uganda; large circles with an X, Cameroon; large inverted triangles, Ethiopia. Vertical dotted lines represent school-age prevalence Kato-Katz thresholds used in the World Health Organization (WHO) guidelines for timing and coverage of mass drug administration (MDA)-based schistosomiasis control programs and horizontal dotted lines show the corresponding cutoffs for prevalence determined by the POC-CCA assay.
Discussion
Our analysis of a coordinated set of diagnostic test comparisons carried out in endemic settings in five African countries indicated that for diagnosis of S. mansoni on an individual and community basis, the POC-CCA urine test appears to be a more sensitive test than the Kato-Katz stool examination technique. The results for the combined country-level data are consistent with those reported earlier for POC-CCA testing in several individual study locations across different S. mansoni endemicity levels, some of which had mixed infections with S. haematobium.15–17 Our results therefore corroborate with previous diagnostic studies using the now discontinued CCA dipstick test12,14,16 and address some of the issues by Stothard25 on how rapid diagnostic tests could improve control of schistosomiasis. The increased diagnostic sensitivity of the POC-CCA seen in our study was evident across a wide range of community prevalence levels (1–60% S. mansoni infection prevalence as determined by a single stool Kato-Katz screening). The increased sensitivity was particularly notable among schools having between 10% and 25% prevalence (Figure 3), where more of the infections were of light intensity. Testing one urine specimen by POC-CCA yielded a higher prevalence estimate than testing one stool by Kato-Katz in nearly all schools. Three of four schools in which Kato-Katz prevalence was higher than POC-CCA prevalence had very high S. mansoni prevalence of infection and high intensity of infection (arithmetic mean fecal egg counts: 116, 460, and 590 EPG), where sensitivity performance of Kato-Katz testing would be expected to be much better.26
In general, the stool PCR testing results were in better agreement with Kato-Katz results than with the POC-CCA results. This may not be surprising, as Kato-Katz and PCR are likely to be similarly limited by the small amounts of stool actually tested, with the resulting potential for sampling errors. It is not yet clear how to interpret discordant results between POC-CCA and Kato-Katz. The majority of discordant results were POC-CCA positive and Kato-Katz negative, in large measure likely reflecting the incomplete sensitivity of the Kato-Katz technique. However, false positive POC-CCA results could occur because of urinary tract inflammation (hematuria or pyuria) or as a result of mislabeling of specimens or unobserved “specimen sharing” among study subjects, who were not observed during sample collection. In the absence of a diagnostic “gold” standard for S. mansoni, LCA modeling provides a sound statistical estimate of the performance characteristics of the diagnostic tests included in this study. The LCA suggests that the POC-CCA test can provide false positive results. However, the finding of only 1% POC-CCA positivity in an Ethiopian area non-endemic for S. mansoni, and prior evaluations of Kato-Katz performance characteristics,4,5,27–30 suggest that the POC-CCA is likely to be more specific than estimated by the LCA model. Assuming no reader error or quality control problems, the finding of several Kato-Katz positive/POC-CCA negative results indicates that POC-CCA testing can also yield false negative results. As several studies have found the POC-CCA to be erratic in detection of S. haematobium infections,12,31 we sought to determine if mixed infections of S. haematobium and S. mansoni in some way led to erratic detection of S. mansoni infections. Based on results from seven schools where children had mixed infections, the ability of the POC-CCA assay to detect S. mansoni in mixed infections was unimpeded.
In general, based on LCA modeling of the combined five country data, the POC-CCA test appeared to be more sensitive and less specific than Kato-Katz. Using a single urine specimen, POC-CCA specificity in the various countries ranged from 56% to 94%, with an estimated overall specificity of 72%. Although the Kato-Katz assay was considered in this study to be 100% specific, that could be an overestimate, if, for example, laboratory errors occur caused by contamination with residual eggs or reader/data entry errors. Such “small” errors could have disproportionately affected the specificity estimates (for all tests) as estimated by the LCA. SCORE is supporting other work to develop more sensitive and specific laboratory-based diagnostics, which will allow a more refined estimated of the diagnostic performance characteristics of the POC-CCA detection approach.
Our regression analysis suggests that separate estimates of school prevalence by Kato-Katz and by POC-CCA are significantly correlated (r = 0.63, P < 0.0001). This suggests that measured POC-CCA prevalence could be correlated with the prevalence cutoffs established by WHO for treatment allocation according to high, medium, or low prevalence in individual communities2; however, we note that the test–test regression line does not pass through the origin. This is a combined result of the lower sensitivity of the Kato-Katz and the limited number of communities having “truly” minimal prevalence in our study. If our studies included a higher proportion of schools similar to the STH-endemic/S. mansoni-negative area we studied in Ethiopia, the regression intercept would have been lower and the curve less linear.
Test performance varied across the five countries. Some of this may be related to different average infection intensities in the different countries, however there may also be other sources of variation, such as the working threshold for calling a cassette reading as trace positive. Other research has shown an association between intensity of an individual's S. mansoni infection (as expressed in EPG) and intensity of his or her CCA reaction in screening tests.16 Differences in intensity readings could be rectified by training or by changes in the cassette format, perhaps by use of a CCA standard or addition of a standardized “trace positive” comparison line.
It is important to emphasize that our test evaluation is based only on 63 schools in five countries where the surveys were conducted. Additional data from additional locations having very low prevalence and from more control areas not endemic for schistosomiasis would be helpful to further determine the usefulness of the POC-CCA in areas where the prevalence is expected to be from 1% to 15%, and the intensities of infection < 20 EPG. However, our data indicate that the POC-CCA could be a useful tool in medium-to-high prevalence areas that are presently working to control morbidity from S. mansoni infection, the first step of which is to do prevalence mapping to determine where and to what degree PCT should be implemented.
The use of the POC-CCA test for PCT decision-making will require the addition of POC-CCA-specific criteria to the WHO guidelines, which currently are based on cutoffs taken from Kato-Katz-based screening values (or urine filtration or school-based questionnaires for S. haematobium).32 These WHO guidelines for MDA have set thresholds of < 10% (low prevalence), 10–50% (moderate prevalence), and > 50% (high prevalence) by single-stool Kato-Katz screening in 9- to 12-year-old children. Based on our findings, 10% prevalence by Kato-Katz would be equivalent to 46% prevalence by POC-CCA, whereas 50% prevalence by Kato-Katz would be equivalent to 72% by POC-CCA. In our study communities, based on current Kato-Katz-based WHO cutoff values, there are 26 schools categorized as low-prevalence, 33 as moderate, and 4 as high. Using our derived POC-CCA cutoffs based on Figure 3, the numbers of schools in the low, moderate, and high categories would be 25, 28, and 10, respectively. Thus, with Kato-Katz screening, prevalence in four schools would indicate the need for community-wide PCT, whereas with POC-CCA, 10 villages would require community-wide treatment. Although the increased number of communities requiring PCT based on POC-CCA mapping would increase the cost to a national program, over time, policy decision-making based on more accurate diagnosis should lead to more effective schistosomiasis control.
Several issues affect the potential for widespread adoption of POC-CCA testing for mapping of S. mansoni burden. Among the most important is cost. The POC-CCA cassettes used in these studies cost ∼US$1.75 per cassette. Based on published33 and unpublished cost data from implementation of recent Schistosomiasis Control Initiative (SCI) and SCORE control programs, the running costs of doing Kato-Katz assays in screening surveys are now similar to this cost of buying and performing POC-CCA (Worrell, personal communication; Kabatereine, personal communication). In considering the use of POC-CCA for mapping S. mansoni in integrated NTD programs, the use of urine instead of stool for parasite mapping eliminates the ability to simultaneously test for the STHs. This needs to be considered, however may not be an actual impediment to use of the POC-CCA because STH mapping is often done on younger children, and PCT for the STHs is often done without prior mapping. Nevertheless, all programmatic impacts, such as the ease of performing the assays by teachers or village volunteers and transport costs, should be assessed before any recommendation for widespread adoption of POC-CCA.
An important next step will be to collect additional data on POC-CCA performance at other sites and compare the data from our sites with those from other regions. This should allow greater refinement of the cutoff values for decision-making related to PCT allocation. Efforts should also be made to improve POC-CCA read-outs and establish better consistency in performance among countries. Remaining questions include whether the intensity/density of a positive line on the cassette can be used as a correlate of S. mansoni infection intensity, whether the test can determine if a person has been cured after treatment, and whether the test works equally well for preschool-age children (Coulibaly, personal communication).16
Based on the merged data from this five-country study, SCORE has recommended to WHO/NTD that the commercially produced, urine-based POC-CCA test for S. mansoni is sufficiently sensitive and specific that it can be used as a mapping tool for determining prevalence among school-age children and deciding PCT strategies. On 25 May 2012, World Health Assembly 65 endorsed a resolution that morbidity control of schistosomiasis should be continued and enhanced, and in addition, that elimination—that is, the interruption of transmission34—should be pursued wherever possible. This is a major leap forward in the thinking in regard to public health as related to schistosomiasis. The data in this study would indicate that the POC-CCA assay will not be sufficiently specific and sensitive at very low levels of egg output to support the “end-game” of elimination, but that it can contribute greatly to the identification of endemic locations, thereby providing the disease mapping needed to properly plan strategies for gaining and sustaining control, with the ultimate objective of moving toward total elimination.
ACKNOWLEDGMENTS
We acknowledge all the laboratory and field staff in each of the collaborating countries, as well as their colleagues who formed the SCORE POC-CCA teams and the valuable cooperation of the district education and health bureaus, school directors and teachers, parents/guardians and participating school children. In addition, Eric Brienen performed the PCR assays, Shiyo Wang and Chunla He assisted with statistical analyses, and Tammy Andros provided critical editorial and citation assistance.
Footnotes
Financial support: This study received financial support from the University of Georgia Research Foundation, Inc., which was funded by the Bill & Melinda Gates Foundation for the SCORE project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors' addresses: Daniel G. Colley, Center for Tropical and Emerging Global Diseases and the Department of Microbiology, University of Georgia, Athens, GA, E-mail: dcolley@uga.edu. Sue Binder, Sue Binder Consulting, Atlanta, GA, E-mail: scbinder@bellsouth.net. Carl Campbell, Center for Tropical and Emerging Global Diseases, University of Georgia, Athens, GA, E-mail: ccamp@uga.edu. Charles H. King, Center for Global Health and Diseases, Case Western Reserve University School of Medicine, Cleveland, OH, E-mail: chk@cwru.edu. Louis-Albert Tchuem Tchuenté, Centre for Schistosomiasis and Parasitology, Laboratory of Parasitology and Ecology, University of Yaoundé I, Yaoundé, Cameroon, E-mail: tchuemtchuente@schisto.com. Eliézer K. N'Goran, Laboratoire de Zoologie Biologie Animale, Unité de Recherche et de Formation Biosciences, Université de Cocody, Abidjan, Côte d'Ivoire, E-mail: eliezerngoran@yahoo.fr. Berhanu Erko, Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Addis Ababa, Ethiopia, E-mail: berhanue@yahoo.com. Diana M. S. Karanja, Kenya Medical Research Institute, Center for Global Health Research, Kisumu, Kenya, E-mail: dkaranja@kemricdc.org. Narcis B. Kabatereine, Schistosomiasis Control Initiative (SCI), based at Vector Control Division of Ministry of Health Uganda, Kampala, E-mail: vcdmoh@gmail.com. Lisette van Lieshout, Leiden University Medical Center, Department of Parasitology (zone P4-P), Leiden, The Netherlands, E-mail: lvanlieshout@lumc.nl. Stephen Rathbun, Department of Epidemiology and Biostatistics, University of Georgia, Athens, GA, E-mail: rathbun@uga.edu.
References
- 1.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 2.WHO . Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual For Health Professionals and Programme Managers. Geneva: World Health Organization; 2006. [Google Scholar]
- 3.WHO . First WHO Report on Neglected Tropical Diseases 2010: Working to Overcome the Global Impact of Neglected Tropical Diseases. Geneva: World Health Organization; 2010. [Google Scholar]
- 4.de Vlas SJ, Gryseels B. Underestimation of Schistosoma mansoni prevalences. Parasitol Today. 1992;8:274–277. doi: 10.1016/0169-4758(92)90144-q. [DOI] [PubMed] [Google Scholar]
- 5.Utzinger J, Booth M, N'Goran EK, Muller I, Tanner M, Lengeler C. Relative contribution of day-to-day and intra-specimen variation in fecal egg counts of Schistosoma mansoni before and after treatment with praziquantel. Parasitology. 2001;122:537–544. doi: 10.1017/s0031182001007752. [DOI] [PubMed] [Google Scholar]
- 6.Deelder AM, van Dam GJ, van Lieshout L. Response to: accuracy of circulating cathodic antigen tests for rapid mapping of Schistosoma mansoni and S. haematobium infections in southern Sudan by RA Ashton et al. (2011) Trop Med Int Health 16, pp. 1099–1103. Trop Med Int Health. 2012;17:402–403. doi: 10.1111/j.1365-3156.2011.02930.x. [DOI] [PubMed] [Google Scholar]
- 7.Brooker S, Kabatereine NB, Myatt M, Russell Stothard J, Fenwick A. Rapid assessment of Schistosoma mansoni: the validity, applicability and cost-effectiveness of the Lot Quality Assurance Sampling method in Uganda. Trop Med Int Health. 2005;10:647–658. doi: 10.1111/j.1365-3156.2005.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergquist R, Johansen MV, Utzinger J. Diagnostic dilemmas in helminthology: what tools to use and when? Trends Parasitol. 2009;25:151–156. doi: 10.1016/j.pt.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Richards FO, Jr, Eigege A, Miri ES, Jinadu MY, Hopkins DR. Integration of mass drug administration programmes in Nigeria: the challenge of schistosomiasis. Bull World Health Organ. 2006;84:673–676. doi: 10.2471/blt.06.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon AW, Engels D, Bailey RL, Blake IM, Brooker S, Chen J-X, Chen J-H, Churcher TS, Drakeley CJ, Edwards T, Fenwick A, French M, Gabrielli AF, Grassly NC, Harding-Esch EM, Holland MJ, Koukounari A, Lammie PJ, Leslie J, Mabey DC, Rhajaoui M, Secor WE, Stothard JR, Wei H, Zhou XN, Peeling RW. A diagnostics platform for the integrated mapping, monitoring, and surveillance of neglected tropical diseases: rationale and target product profiles. PLoS Negl Trop Dis. 2012;6:e1746. doi: 10.1371/journal.pntd.0001746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Lieshout L, Polderman AM, Deelder AM. Immunodiagnosis of schistosomiasis by determination of the circulating antigens CAA and CCA, in particular in individuals with recent or light infections. Acta Trop. 2000;77:69–80. doi: 10.1016/s0001-706x(00)00115-7. [DOI] [PubMed] [Google Scholar]
- 12.Stothard JR, Kabatereine NB, Tukahebwa EM, Kazibwe F, Rollinson D, Mathieson W, Webster JP, Fenwick A. Use of circulating cathodic antigen (CCA) dipsticks for detection of intestinal and urinary schistosomiasis. Acta Trop. 2006;97:219–228. doi: 10.1016/j.actatropica.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 13.van Dam GJ, Wichers JH, Ferreira TM, Ghati D, van Amerongen A, Deelder AM. Diagnosis of schistosomiasis by reagent strip test for detection of circulating cathodic antigen. J Clin Microbiol. 2004;42:5458–5461. doi: 10.1128/JCM.42.12.5458-5461.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Legesse M, Erko B. Field-based evaluation of a reagent strip test for diagnosis of Schistosoma mansoni by detecting circulating cathodic antigen in urine before and after chemotherapy. Trans R Soc Trop Med Hyg. 2007;101:668–673. doi: 10.1016/j.trstmh.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Coulibaly JT, Knopp S, N'Guessan NA, Silué KD, Fürst T, Lohourignon LK, Brou JK, N'Gbesso YK, Vounatsou P, N'Goran EK, Utzinger J. Accuracy of urine circulating cathodic antigen (CCA) test for Schistosoma mansoni diagnosis in different settings of Côte d'Ivoire. PLoS Negl Trop Dis. 2011;5:e1384. doi: 10.1371/journal.pntd.0001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shane HL, Verani JR, Abudho B, Montgomery SP, Blackstock AJ, Mwinzi PN, Butler SE, Karanja DM, Secor WE. Evaluation of urine CCA assays for detection of Schistosoma mansoni infection in western Kenya. PLoS Negl Trop Dis. 2011;5:e591. doi: 10.1371/journal.pntd.0000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tchuem Tchuenté L-A, Kuete Fouodo CJ, Kamwa Ngassam RI, Sumo L, Dongmo Noumedem C, Kenfack CM, Gipwe NF, Nana ED, Stothard JR, Rollinson D. Evaluation of circulating cathodic antigen (CCA) urine-tests for diagnosis of Schistosoma mansoni infection in Cameroon. PLoS Negl Trop Dis. 2012;6:e1758. doi: 10.1371/journal.pntd.0001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obeng BB, Aryeetey YA, de Dood CJ, Amoah AS, Larbi IA, Deelder AM, Yazdanbakhsh M, Hartgers FC, Boakye DA, Verweij JJ, van Dam GJ, van Lieshout L. Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana. Ann Trop Med Parasitol. 2008;102:625–633. doi: 10.1179/136485908X337490. [DOI] [PubMed] [Google Scholar]
- 19.de Vlas SJ, Engels D, Rabello ALT, Oostburg BFI, Van Lieshout L, Polderman AM, Van Oortmarssen GJ, Habbema JD, Gryseels B. Validation of a chart to estimate true Schistosoma mansoni prevalences from simple egg counts. Parasitology. 1997;114:113–121. doi: 10.1017/s0031182096008207. [DOI] [PubMed] [Google Scholar]
- 20.Rindskopf D, Rindskopf W. The value of latent class analysis in medical diagnosis. Stat Med. 1986;5:21–27. doi: 10.1002/sim.4780050105. [DOI] [PubMed] [Google Scholar]
- 21.Espeland MA, Handelman SL. Using latent class models to characterize and assess relative error in discrete measurements. Biometrics. 1989;45:587–599. [PubMed] [Google Scholar]
- 22.Utzinger J, Vounatsou P, N'Goran EK, Tanner M, Booth M. Reduction in the prevalence and intensity of hookworm infections after praziquantel treatment for schistosomiasis infection. Int J Parasitol. 2002;32:759–765. doi: 10.1016/s0020-7519(02)00012-7. [DOI] [PubMed] [Google Scholar]
- 23.Koukounari A, Webster JP, Donnelly CA, Bray BC, Naples J, Bosompem K, Shiff C. Sensitivities and specificities of diagnostic tests and infection prevalence of Schistosoma haematobium estimated from data on adults in villages northwest of Accra, Ghana. Am J Trop Med Hyg. 2009;80:435–441. [PMC free article] [PubMed] [Google Scholar]
- 24.Peters PA, Warren KS, Mahmoud AA. Rapid, accurate quantification of schistosome eggs via nuclepore filters. J Parasitol. 1976;62:154–155. [PubMed] [Google Scholar]
- 25.Stothard JR. Improving control of African schistosomiasis: towards effective use of rapid diagnostic tests within an appropriate disease surveillance model. Trans R Soc Trop Med Hyg. 2009;103:325–332. doi: 10.1016/j.trstmh.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Polman K, Stelma FF, Gryseels B, Van Dam GJ, Talla I, Niang M, Van Lieshout L, Deelder AM. Epidemiologic application of circulating antigen detection in a recent Schistosoma mansoni focus in northern Senegal. Am J Trop Med Hyg. 1995;53:152–157. doi: 10.4269/ajtmh.1995.53.152. [DOI] [PubMed] [Google Scholar]
- 27.Kongs A, Marks G, Verlé P, Van der Stuyft P. The unreliability of the Kato-Katz technique limits its usefulness for evaluating S. mansoni infections. Trop Med Int Health. 2001;6:163–169. doi: 10.1046/j.1365-3156.2001.00687.x. [DOI] [PubMed] [Google Scholar]
- 28.Booth M, Vounatsou P, N'Goran EK, Tanner M, Utzinger J. The influence of sampling effort and the performance of the Kato-Katz technique in diagnosing Schistosoma mansoni and hookworm co-infections in rural Côte d'Ivoire. Parasitology. 2003;127:525–531. doi: 10.1017/s0031182003004128. [DOI] [PubMed] [Google Scholar]
- 29.Berhe N, Medhin G, Erko B, Smith T, Gedamu S, Bereded D, Moore R, Habte E, Redda A, Gebre-Michael T, Gundersen SG. Variations in helminth fecal egg counts in Kato-Katz thick smears and their implications in assessing infection status with Schistosoma mansoni. Acta Trop. 2004;92:205–212. doi: 10.1016/j.actatropica.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Enk MJ, Lima AC, Drummond SC, Schall VT, Coelho PM. The effect of the number of stool samples on the observed prevalence and the infection intensity with Schistosoma mansoni among a population in an area of low transmission. Acta Trop. 2008;108:222–228. doi: 10.1016/j.actatropica.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Stothard JR, Sousa-Figueiredo JC, Standley C, Van Dam GJ, Knopp S, Utzinger J, Ameri H, Khamis AN, Khamis IS, Deelder AM, Mohammed KA, Rollinson D. An evaluation of urine-CCA strip test and fingerprick blood SEA-ELISA for detection of urinary schistosomiasis in schoolchildren in Zanzibar. Acta Trop. 2009;111:64–70. doi: 10.1016/j.actatropica.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 32.WHO . Prevention and Control of Schistosomiasis and Soil Transmitted Helminthiasis: Report of a WHO Expert Committee. Geneva: World Health Organization; 2002. WHO Technical Report Series 912. [PubMed] [Google Scholar]
- 33.Speich B, Knopp S, Mohammed KA, Khamis IS, Rinaldi L, Cringoli G, Rollinson D, Utzinger J. Comparative cost assessment of the Kato-Katz and FLOTAC techniques for soil-transmitted helminth diagnosis in epidemiological surveys. Parasites & Vectors. 2010;3:71. doi: 10.1186/1756-3305-3-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuenté L-A, Garba A, Mohammed KA, Schur N, Person B, Colley DG, Utzinger J. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2012;44:221–225. doi: 10.1016/j.actatropica.2012.04.013. [DOI] [PubMed] [Google Scholar]