Abstract
We measured prevalence of Schistosoma haematobium, Wuchereria bancrofti, Plasmodium falciparum, hookworm, and other geohelminths among school-aged children in four endemic villages in Kwale County, Kenya and explored the relationship between multiparasite burden, undernutrition, and anemia. In 2009–2010 surveys, cross-sectional data were obtained for 2,030 children 5–18 years old. Infections were most prevalent for S. haematobium (25–62%), hookworm (11–28%), and falciparum malaria (8–24%). Over one-half of children were anemic, with high rates of acute and chronic malnutrition. Associations with infection status showed significant age and sex differences. For boys, young age, low socioeconomic standing (SES), S. haematobium, and/or malaria infections were associated with greater odds of anemia, wasting, and/or stunting; for girls, heavy S. haematobium infection and age were the significant cofactors for anemia, whereas low SES and older age were linked to stunting. The broad overlap of infection-related causes for anemia and malnutrition and the high frequency of polyparasitic infections suggest that there will be significant advantages to integrated parasite control in this area.
Introduction
Coinfection with two or more parasitic infections is very common in resource-limited areas such as rural Kenya.1 However, it was not until recently that the combined detrimental effects of polyparasitism on childhood growth and development have emerged as a research focus.2–6 The relationship between parasitic helminths and the subtle morbidities of undernutrition and anemia has been increasingly recognized in the past 20 years.4,6 Previous studies have examined the overlapping effects of infection by soil-transmitted helminths (STHs), including hookworm, Ascaris lumbricoides, and Trichuris trichiura, on these outcomes, and more recently, studies have examined the combined effects of STH with schistosomiasis.3,7,8 The public health importance of chronic malnutrition and anemia is in their intrinsically disabling effects. Related manifestations can often include reduced global functioning,9 decreased physical performance,10,11 and impaired cognition,2,12,13 resulting in decreased human capital among adults in affected populations14,15 with a related loss in years of healthy life.9 Past studies showing improvements in nutritional status and cognition after adequate antiparasitic treatment highlight the importance of effective control to prevent cognitive and growth impairment before they become irreversible.5,7,16 Catch-up growth (or increased linear growth velocity after growth insult resolves)17 can happen if inflammation is alleviated and chronic diseases are controlled before children mature. Likewise, anemia of inflammation, the leading cause of the anemia associated with schistosomiasis,18 can be significantly improved by curative therapy.19 The ensuing question is to define which parasites (or combination of parasites) are most clearly associated with growth impairment and anemia in areas with polyparasitism. In the present study, we present our findings from four villages in coastal Kenya known to be coendemic for Schistosoma haematobium, STH, Plasmodium falciparum, and Wuchereria bancrofti.1,20–24
Materials and Methods
Study area and population.
Cross-sectional data were collected from four villages, Nganja, Milalani, Vuga, and Jego, in Coast Province, Kenya. All were known to be endemic for S. haematobium and other parasites (i.e., P. falciparum malaria, W. bancrofti lymphatic filariasis [LF], and STHs, including hookworms). This study, targeting children, was part of a larger community-based project studying the ecology of vector-borne and soil-transmitted parasitic infections. All children ages 5–18 years and resident of the area for more than 2 years were eligible to participate.
Subjects were enrolled at the time of the village demography survey in February, August, and November of 2009 for Nganja, Milalani, and Vuga, respectively, and in March of 2010 for Jego. After an initial interview with the head of each household, in which general information about family structure and household living conditions were obtained, children were screened for the presence of endemic parasites, and their nutritional and fitness levels were assessed.
Ethics statement.
Before study participation, written informed consent was obtained from each subject's parent or legal guardian, and individual verbal assent was obtained from participating children who were above the age of 7 years. Ethical clearance and oversight for this study were provided by the Institutional Review Board at the University Hospitals of Cleveland, Case Medical Center, and the Ethical Review Committee at the Kenya Medical Research Institute (KEMRI). Parasitic infections detected during the course of this survey were treated with antimalarials Artemisin Combination Therapy (ACT), Diethylcarbamzine(DEC)/albendazole, albendazole, or praziquantel at age-appropriate doses, as indicated for each individual's testing outcomes.
Urine examination.
Egg burden for S. haematobium was assessed by Nuclepore urine filtration.25 The presence of gross hematuria was also recorded. The subjects provided a single mid-morning urine specimen that was processed the same day. Three intensity categories were assigned as follows: negative for detectable eggs; light for 1–50 eggs/10 mL urine; or heavy for > 50 eggs/10 mL urine.
Stool examination.
Eligible subjects were given a stool container by local community health workers the night before the parasitology survey. The following morning, stool samples were taken to the central facility and examined in duplicate by the quantitative Kato–Katz method for microscopic detection of eggs.26 For each stool specimen, eggs per 1 g feces (epg) were determined to quantify intensity of hookworm infection. The stool samples was processed and scored within 10–20 minutes to provide optimal detection of hookworm ova. Other STH eggs, such as for A. lumbricoides or T. trichiura, were scored as present or absent.
Blood collection and processing.
Finger prick blood collection was performed in all eligible children. None refused blood draw. The blood was used to measure hemoglobin (Hemocue, Ångelholm, Sweden) and perform rapid antigen testing for Pf malaria (ICT Diagnostics, Australia) and LF (Binax, Portland, ME). After hemoglobin determination, anemia and severe anemia were categorized according to World Health Organization (WHO) criteria for age and sex and scored as present or absent for each child.27
Standardized anthropometric testing.
Because growth is considered the best indicator of nutritional status in children, calibrated measurements of height and weight were used as study outcomes to assess developmental morbidity among study participants. Before the surveys, all technical staff performing anthropometric measurements received standardization training followed by independent reliability assessment. Supervised by a trained anthropometrist, trainees performed replicate measurements of height (until agreement within 0.5 cm) and weight (agreement within 1.0 kg) on 10 healthy volunteer children on the same day. The results were then compared for inter- and intraobserver reliability. The trainees' intra- and interexaminer technical errors of measurement fell within reference values28–30 and were, therefore, considered sufficiently accurate for individual morbidity assessment.
Eligible study children were measured according to procedures described by Jelliffe31 while wearing a kanga—a traditional light cloth—wrapped around their bodies. Weight was obtained by digital weight scale (model 803; SECA, Hanover, MD) and rounded to the nearest 0.1 kg. Height was measured with the use of a stadiometer (model 214; SECA, Hanover, MD), and measurements were read to the nearest 1.0 cm. Instruments were calibrated daily before use. Every measurement was performed two times, and the mean values were used for analysis. Reference population Z scores were calculated for each subject's height for age (HAZ) and body mass index for age (BAZ) using international reference standards for comparison taken from the WHO's Anthro-Plus program for ages 5–19 years (WHO, Geneva, Switzerland) with reference growth standards from the year 2006.32,33
HAZ is considered an indicator of long-term linear growth, whereas BAZ variations better reflect acute changes in nutritional status. According to WHO standards,32 stunting was categorized as an observed HAZ that was 2 or more SDs below average (HAZ score ≤ −2). Children were categorized as clinically wasted if their BAZ was more than 2 SDs below average for their age (BAZ score ≤ −2). Children were further identified as severely wasted if their BAZ was ≤ −3.
Data management and statistical analysis.
Demographic data collected in the field were entered in handheld devices (Dell Axim, Round Rock, TX) using Visual CE 10 (Cambridge, MA) backed up on paper forms. Both sets of duplicate data were then transferred into ACCESS 2007 (Microsoft, Seattle, WA), and the databases were compared for errors. Parasitology and anthropometric data were similarly entered to complete the database. Exploratory analysis started with univariate distributions followed by bivariate analyses to explore the pair-wise relationships of individual outcomes. Hookworm egg counts were log-converted to adjust for their skewed distribution. Analysis of variance (ANOVA) or χ2 testing was performed to assess the significance of differences detected among the study villages. Later, multivariable analysis was used to assess the significance of associations controlling for age, sex, infection, coinfection status, and a scale of household socioeconomic standing (SES) derived using principle component analysis (PCA) of combined asset scores.10,34 As dependent variables, the presence or absence of morbidity outcomes anemia, wasting, and stunting were modeled by applying binary logistic regression with generalized estimating equation (GEE) modeling to account for household-level clustering effects. A final model for each logistic regression was chosen after all variables in the model were either statistically significant or biologically plausible and marginally significant. This latter approach was based on previous published research on the relationship between different STH, schistosomiasis, and anemia35 and the synergistic effects of malaria and schistosomiasis coinfection.36 Two analytic approaches were taken: the first approach included village as a covariate in the model to account for climatic variations, whereas the second approach clustered villages by schistosomiasis risk. In this paper, only the latter is presented in detail, because of the two approaches, it provided the best-fit parsimonious models based on information criteria.
Results
Population characteristics.
The demographic characteristics and parasitological findings for study participants are summarized in Table 1, with their hematologic and anthropometric outcomes summarized in Table 2. In all, 2,030 children, ages 5–18 years, were surveyed. Of these children, 2,013 had full parasitological and anthropometric data and were included in the final multivariable analysis; 76% of all eligible children in Nganja (235/309), 51% of all eligible children in Milalani (416/822), 74% of all eligible children in Vuga (726/983), and 74% of all eligible children in Jego (653/890) participated in the surveys.
Table 1.
Demography and distribution of parasite infection among study children in four Kwale County villages, Kenya
| Total (N = 2,030) | Nganja (N = 235) | Milalani (N = 416) | Vuga (N = 726) | Jego (N = 653) | P value* | |
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age mean in years (range) | 11.0 (5–19) | 11.2 (5–19.5) | 11.1 (5–19) | 11.6 (5–19) | 10.4 (5–18) | 0.0715 |
| Female | 48% | 45% | 51% | 51% | 46% | 0.0852 |
| Parasitology prevalence | ||||||
| S. haematobium | 37% | 62% | 62% | 25% | 25% | < 0.0001 |
| Heavy intensity (> 50 eggs/10 mL urine) | 20% | 40% | 32% | 12% | 14% | 0.0095 |
| Light intensity (1–50 eggs/10 mL urine) | 17% | 21% | 30% | 13% | 11% | < 0.0001 |
| Hookworm | 20% | 23% | 28% | 11% | 24% | < 0.0001 |
| P. falciparum (ICT card positivity) | 16.4% | 8.5% | 18% | 11% | 24% | < 0.0001 |
| W. bancrofti | 9.8% | 6.4% | 9% | 16% | 4.3% | < 0.0001 |
| A. lumbricoides | 0.5% | 0.4% | 0.7% | 0.2% | 0.3% | 0.8006 |
| T. trichiura | 18.1% | 37% | 37% | 8.9% | 10% | < 0.0001 |
| S. haematobium intensity mean eggs/10 mL | 109.4 | 195.6 | 138.3 | 52.3 | 51.4 | < 0.0001 |
| Hookworm intensity mean epg | 6.1 | 7.7 | 10.9 | 1.1 | 4.8 | < 0.0001 |
| Coinfection | ||||||
| S. haematobium–Trichuris | 9.6% | 25% | 23.5% | 2.6% | 3.2% | < 0.0001 |
| S. haematobium–hookworm | 9.3% | 15.3% | 17.5% | 4.2% | 7.6% | < 0.0001 |
| S. haematobium–Pf malaria | 6.9% | 6.4% | 14.9% | 2.9% | 6.7% | < 0.0001 |
| S. haematobium–filaria | 4.2% | 5.1% | 6.7% | 5.2% | 1.2% | < 0.0001 |
| Hookworm–Trichuris | 6% | 10.2% | 14.2% | 1.5% | 4.3% | < 0.0001 |
| Hookworm–Pf malaria | 4.7% | 3.4% | 6.9% | 1.2% | 7.6% | < 0.0001 |
| Hookworm–filaria | 1.3% | 1.3% | 2.1% | 1.2% | 1.1% | 0.4818 |
P value refers to significance of differences among the villages by ANOVA or χ2 testing.
ICT = rapid immuochromatography test for malaria. High- and low-risk villages refer to high and low S. haematobium prevalence, respectively.
Table 2.
Hematologic and anthropometric characteristics of children surveyed in Kwale County, Kenya
| Total (N = 2,030) | Nganja (N = 235) | Milalani (N = 416) | Vuga (N = 726) | Jego (N = 653) | P value* | |
|---|---|---|---|---|---|---|
| Hematology | ||||||
| Percent anemic† | 50.8% | 47.5% | 50.7% | 45.2% | 58.4% | 0.0567 |
| Severely anemic‡ | 1.1% | 2.1% | 1.9% | 0.8% | 0.6% | 0.0961 |
| Mean hemoglobin (range) | 11.7 (3.4, 15.8) | 11.9 (4.8, 17) | 11.8 (6.3, 15.7) | 11.9 (5.2, 15.9) | 11.9 (3.4, 15.8) | 0.0217 |
| Anthropometrics | ||||||
| Mean HAZ (range) | −1.4 (−6.6, 7) | −1.9 (−5.7, 1.9) | −0.98 (−3, 1.9) | −1.37 (−6, 7) | −1.4 (−6.6, 2.6) | 0.0008 |
| Mean BAZ (range) | 0.99 (−6, 4.1) | −1.2 (−3.7, 0.9) | −0.83 (−4.2, 2.8) | −1.15 (−4.8, 4.1) | −0.8 (−6, 4.6) | 0.0019 |
| Wasting§ (%) | ||||||
| WHO reference standards∥ | 19.2% | 18.7% | 12.9% | 30.8% | 10.4% | < 0.0001 |
| Stunting¶ (%) | ||||||
| WHO reference standards∥ | 36% | 45.4% | 35.3% | 43.0% | 25.5% | < 0.0001 |
| Severely wasted** (%) | ||||||
| WHO reference standards∥ | 6.4% | 3.3% | 2.8% | 13.6% | 2.0% | < 0.0001 |
P value refers to significance of differences in prevalence among the villages by ANOVA or χ2 testing.
Anemia: hemoglobin (Hb): for age < 12 years, Hb < 11.5 g/dL; for age > 12 years, Hb < 12 g/dL, except for males > 15 years, Hb < 13 g/dL.
Severely anemic: Hb < 8 g/dL.
Wasting: BAZ ≤ −2.
WHO 2007 growth reference charts.
Stunting: HAZ ≤ −2.
Severely wasted: BAZ ≤ −3.
Parasite burden and polyparasitism.
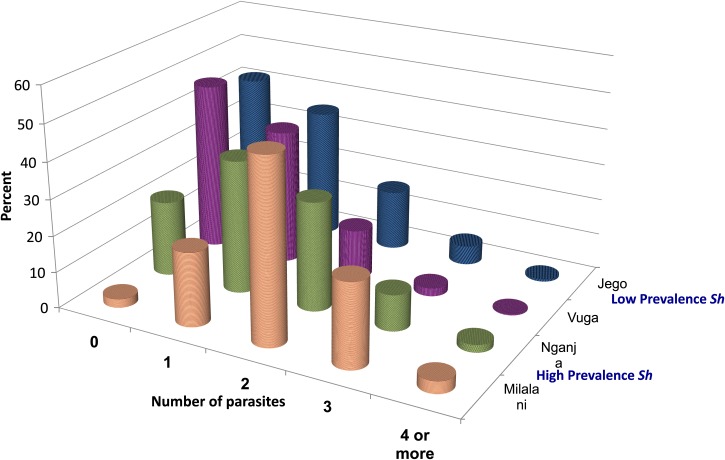
There were significant differences among villages in terms of S. haematobium prevalence. Two of them (Nganja and Milalani) had significantly higher prevalence of active Schistosoma infection, with over 60% of school-aged children positive on urine filtration testing, whereas the other two villages (Vuga and Jego) had lower prevalence (25%) (Table 1). The high-prevalence villages also had the greatest prevalence of polyparasitism, with over 30% of children coinfected with S. haematobium and one or more STH (Figure 1 and Table 1). Figure 1 shows the proportion of children infected with one, two, three, or four or more parasites in the different study villages.
Figure 1.
Percentage of children per village coinfected with different parasites.
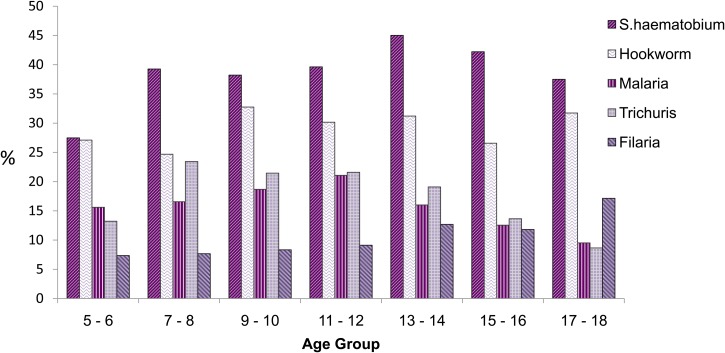
The age distribution of the different parasite infections is shown in Figure 2. S. haematobium was the most common infection in all age groups followed by hookworm and Trichuris. P. falciparum malaria was common in all ages, but most prevalent among 11- to 12-year-old children. LF was most common among older children, particularly 17- to 18-year-old children, perhaps reflecting the impact of LF elimination campaigns that have been active in the area since 2003.
Figure 2.
Parasite prevalence by age groups.
Morbidity outcomes: anemia, stunting, and wasting.
Table 2 summarizes the hematologic and anthropometric findings of the children surveyed. Over one-half (50.8%) of the children studied were anemic, and 1.1% were severely anemic. Hemoglobin levels increased with age but varied inversely with intensity of S. haematobium infection. Overall, the nutritional status of the children was poor, with a high prevalence of both acute and chronic undernutrition reflected as wasting (BAZ ≤ −2) and stunting (HAZ ≤ −2), respectively. The prevalence of wasting and anemia was significantly higher among boys than girls in all villages (see below). In multivariable analysis, the significant interaction of sex with other covariates of our morbidity outcomes led us to stratify all subsequent analysis by sex.
Anemia.
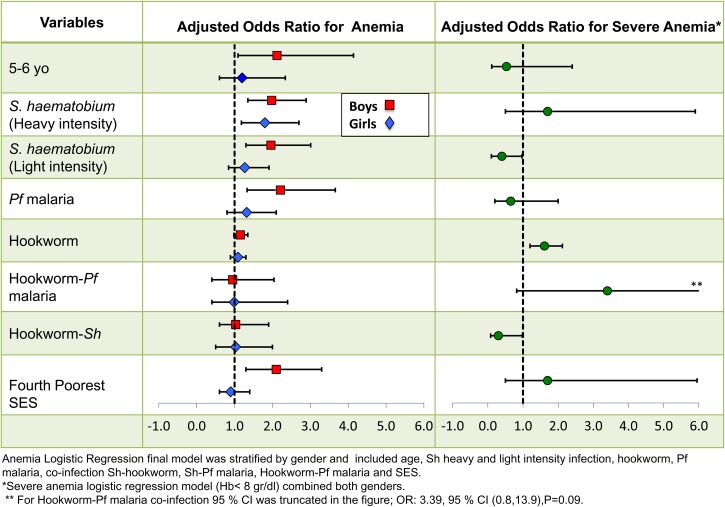
For both boys and girls, significant bivariate associations were found between anemia and age, single infections (Pf malaria, S. haematobium, and hookworm), and polyparasitic infections (S. haematobium–hookworm, S. haematobium–Pf malaria, and hookworm–Pf malaria). Results are summarized in Figure 4 and Supplemental Table 1. Multivariable logistic regression modeling, accounting for household clustering, indicated that younger boys (5–6 years) were significantly more anemic than the reference group of 17- to 18-year-old children (P = 0.026). In addition, both heavy- and light-intensity S. haematobium infection and Pf malaria were independently associated with anemia among boys. With respect to household resources, boys belonging to the fourth poorest stratum of households were also significantly more anemic. Although hookworm infection and combined infections with S. haematobium–hookworm, S. haematobium–Pf malaria, and hookworm–Pf malaria were significantly associated with anemia among boys in the unadjusted analysis, this significance was not retained after multiple adjustment in the multivariable model.
Figure 4.
Adjusted ORs for anemia and severe anemia.
Among girls, odds of anemia were significantly greater in older girls (17–18 years) compared with 7- to 8-year-old children. Like among boys, heavy-intensity Schistosoma infection was independently associated with girls' anemia. Different from what was found among boys, for girls, light S. haematobium infection was not a significant covariate for anemia. In bivariate analysis, coinfection with S. haematobium–hookworm or S. haematobium–Pf malaria was linked to anemia in girls, but this relationship was no longer significant when adjusted for other covariates.
Severe anemia.
Bivariate analysis indicated significant associations between S. haematobium infection (either heavy or light intensity) and severe anemia. A significant link was seen for hookworm infection as well. Among boys, a strong interaction was seen between hookworm and Pf malaria as predictors of severe anemia (odds ratio [OR] = 5.7, 95% confidence interval [CI] = 1.6, 20.1, P = 0.0007) that was not found among girls. Being resident of a high-risk village was a significant predictor of severe anemia among girls (OR = 3.7, 95% CI = 1.4, 9.6, P < 0.0001). However, after adjusting for other single and multiple infections, these associations were no longer significant. Figure 4 summarizes the findings.
Acute malnutrition (wasting).
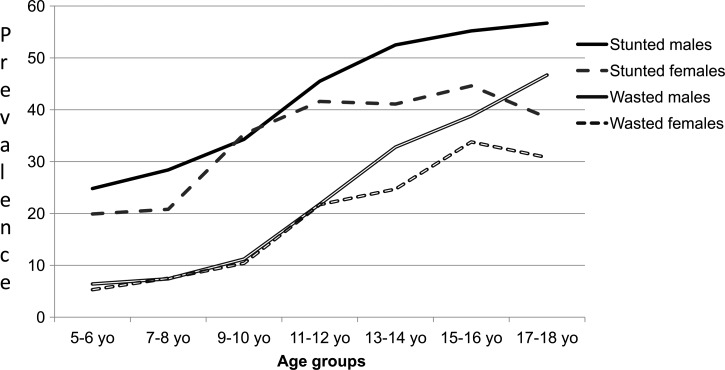
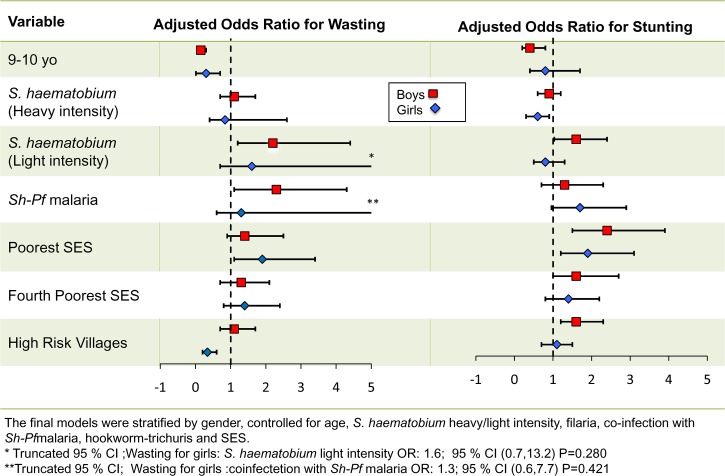
In exploratory bivariate analysis, both boys and girls with S. haematobium infection, Pf malaria, filaria, or Trichuris (data not shown) and boys infected with Pf malaria and coinfected with S. haematobium–filaria were likely to be wasted. Overall, there were increased levels of malnutrition in older children, which was more evident in boys than girls (Figure 3 ). As shown in Figure 5 and Supplemental Table 2, on multivariable adjustment and stratification by sex, only boys had greater odds of wasting in the presence of light-intensity Schistosoma infection. An independent additive effect was significant when boys were coinfected with S. haematobium and Pf malaria (P = 0.015). Older age (> 10 years) was a significant correlate of wasting in both boys and girls. Girls who were (1) older than 10 years, (2) residents of a low-risk village, or (3) or residents of the poorest stratum of households were significantly more likely to be wasted.
Figure 3.
Malnutrition distribution by age and sex.
Figure 5.
Adjusted ORs for acute (wasting) and chronic malnutrition (stunting).
Chronic malnutrition (stunting).
In bivariate analysis, older age and S. haematobium infection were found to be associated with chronic undernutrition. Low SES and village were predictors of stunting in both boys and girls in unadjusted analysis. After sex-stratified multivariable analysis (Figure 5 and Supplemental Table 3), age over 8 years and belonging to a poor (lowest SES) household were significant predictors of stunting for both boys and girls. Only girls (but not boys) coinfected with S. haematobium–Pf malaria were more likely to be stunted. However, this effect was marginally significant in multivariable analysis (P = 0.06). In assessing the impact of individual infections, analysis of the effects of infection by S. haematobium yielded opposite results for boys and girls: among boys, light-intensity Schistosoma infection was associated with stunting, whereas uninfected girls were more likely to be stunted. We also found that boys who resided in high-risk villages were more likely to be chronically undernourished.
Discussion
Among children, anemia and undernutrition can occur through many possible pathways. Although much of impaired early childhood development in sub-Saharan Africa has been ascribed to protein, calorie, and micronutrient deficiencies, it is becoming increasingly apparent that the process of chronic parasitic infection is associated with continuing inflammation that can limit childhood growth6,7,37 and cause persistent or recurrent anemia of chronic inflammation.18 Despite the difficulties in isolating individual causes of undernutrition and anemia in developing areas, we were able to show a clear association between infection and both anemia and undernutrition after adjusting for sex-specific effects and possible SES and environmental confounders.
Results of our surveys show an alarmingly high prevalence of anemia in an area where malaria, a major contributor to childhood anemia, is now decreasing in frequency.38 Anemia has been acknowledged as a major public health threat,27 with multiple measurable downstream effects on physical and cognitive function in children as well as risk for low-birth weight pregnancies and increased prematurity. Iron deficiency is typically considered the most common etiology of acquired anemia. However, in rural areas such as Kwale County, other mechanisms must be considered, particularly those mechanisms related to inflammation caused by endemic parasite infections.18 Boys surveyed in our study showed a consistent association between light-intensity Schistosoma infection, anemia, and both acute and chronic undernutrition. These findings suggest that a proinflammatory state, occurring even with low-level parasite burden, can lead to chronic morbidity.19 This process could undoubtedly lead to development of irreversible pathology beginning in early life. The underlying mechanisms for this process are not fully explored.39 Parasite migration, persistence in the circulation, egg retention, and consequent inflammatory response by the host is a plausible mechanistic pathway; however, more research is needed in this area.
We found important age and sex differences in the association between infection, environmental effects, and anemia. Younger boys (5–6 years) and older girls (17–18 years) were more likely to be anemic. Post-menarchal girls presumably have a higher blood loss during their monthly cycle, which could exacerbate the mixed anemia caused by Schistosoma infection.40 If anemia is present in young school-aged boys (5–6 years), it is more than likely caused by events occurring at a younger age. The reality of early childhood helminth infection is gaining better recognition, because recent surveys in Uganda, Kenya, Niger, and Ghana show a Schistosoma egg shedding prevalence of over 50% in pre-school–aged children in high-risk areas.41–44 Of note, this population has not been reliably included in schistosomiasis control programs.45 It is also important to note that adolescents require attention in this respect as well,46 which is highlighted in our results for older girls. In our study, we noted that severely anemic boys were more likely to be coinfected with hookworm and Pf malaria, indicating the need for comprehensive intervention to prevent severe pathologic outcomes.
Polyparasitic interactions have been previously shown to negatively affect the growth of children,47 although the relationship with chronic growth failure (stunting) has not been as clear.47,48 This gap in the association with chronic parasitic infection is probably caused by the prolonged lag (time elapsed) between initial infection and its effect on linear growth and the detection of growth failure (HAZ < −2), which takes months to years to become manifest.37 Causality is, therefore, difficult to establish because of a multiplicity of potential confounders, particularly those confounders associated with poor diet, poverty, and low SES.49 In our study, unmeasured variation in cofactors among the different villages and lower participation in Milalani could have added undetected bias. Seasonal variation in malaria transmission risk could have affected the observed outcomes. We had previously found no predictable seasonal pattern in 2009–2010 malaria transmission based on monthly or quarterly rainfall.38 Other location-specific climatic variations are expected to be reflected embedded in the village-level factors included in the analysis.
Despite the above limitations, our results clearly show (1) a marked sex difference, with boys being more undernourished than girls, which is in agreement with other studies,8 and (2) that boys and girls with polyparasitic infections (Pf malaria–S. haematobium) had higher odds of being wasted and stunted, respectively. The impact of SES in all of our study's morbidity outcomes points to a social stratification in an already impoverished area. These effects were better seen in acute undernutrition rather than stunting, likely because of the shorter time from infection to disease for the wasting outcome.
One limitation of our surveys is the lack of data regarding enteric infections. The relationship between diarrheal diseases and stunting is well-known50,51 and could potentially have served as additional causes of stunting in our study communities. Also, single urine and stool sampling is likely to miss a variable proportion of each helminth infection, leading to some misclassification bias. Causality cannot be established with cross-sectional data, which was presented in our study. However, there is a strong suggestion that parasitic infections in childhood have a consistent association with growth and hemoglobin status in the school-aged child. Our previous report has detailed the impact of anemia and stunting on the physical fitness of Kwale County children.10 We believe that these effects can be reversed if adequate treatment is in place, particularly if it is associated with decreased risk for reinfection,37 because children have a limited window for growth rebound (catch-up growth) before reaching puberty that would allow them to achieve normal or nearly normal adult physical growth parameters if they remain free from infection.17,37,52
The high prevalence of anemia and malnutrition in our villages and the associations found between single or multiparasite infections and childhood morbidity suggest a serious need for integrated parasite control efforts. The reversibility of the morbidity outcomes presented here will only be determined with adequate antiparasitic treatment delivered through committed, integrated control programs that are, to date, still absent from this area.
Supplementary Material
ACKNOWLEDGMENTS
The authors want to specially acknowledge our energetic field workers, Joyce Bongo, Phyllis Mutemi, and Nancy Halloway, for their dedication and meticulous anthropometric measurements and exercise test recording. We also thank the many Division of Vector Borne Neglected Diseases (DVBND) laboratory technicians who provided parasitology results. We warmly thank the many children of Nganja, Milalani, Vuga, and Jego who willingly participated in the study.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Financial support: This work was supported by National Institutes of Health Research Grant R01TW008067 funded by the Ecology of Infectious Diseases Program of the Fogarty International Center. Funding support was also provided through a National Institutes of Health T32 Ruth L. Kirschstein National Service Research Award Training Grant (to A.L.B.).
Authors' addresses: Amaya L. Bustinduy, CTID Building, Liverpool School of Tropical Medicine, Liverpool, United Kingdom, E-mail: Amaya.Bustinduy@liverpool.ac.uk. Isabel M. Parraga, Department of Nutrition, Case Western Reserve University, Cleveland, OH, E-mail: imp@case.edu. Charles L. Thomas, Center for Health Care Research and Policy, MetroHealth Medical Center, Cleveland, OH, E-mail: clt6@cwru.edu. Peter L. Mungai and Charles H. King, Center for Global Health and Diseases, Case Western Reserve University, Cleveland, OH, E-mails: plmungai@yahoo.com and chk@cwru.edu. Francis Mutuku and Uriel Kitron, Department of Environmental Studies, Emory University, Atlanta, GA, E-mails: fmutuku73@gmail.com and ukitron@emory.edu. Eric M. Muchiri, Division of Vector Borne Neglected and Tropical Diseases, Ministry of Public Health and Sanitation, Nairobi, Kenya, E-mail: ericmmuchiri@gmail.com.
References
- 1.Ashford RW, Craig PS, Oppenheimer SJ. Polyparasitism on the Kenya coast. 1. Prevalence, and association between parasitic infections. Ann Trop Med Parasitol. 1992;86:671–679. doi: 10.1080/00034983.1992.11812724. [DOI] [PubMed] [Google Scholar]
- 2.Ezeamama AE, Friedman JF, Acosta LP, Bellinger DC, Langdon GC, Manalo DL, Olveda RM, Kurtis JD, McGarvey ST. Helminth infection and cognitive impairment among Filipino children. Am J Trop Med Hyg. 2005;72:540–548. [PMC free article] [PubMed] [Google Scholar]
- 3.Ezeamama AE, McGarvey ST, Acosta LP, Zierler S, Manalo DL, Wu HW, Kurtis JD, Mor V, Olveda RM, Friedman JF. The synergistic effect of concomitant schistosomiasis, hookworm, and Trichuris infections on children's anemia burden. PLoS Negl Trop Dis. 2008;2:e245. doi: 10.1371/journal.pntd.0000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephenson LS, Latham MC, Adams EJ, Kinoti SN, Pertet A. Physical fitness, growth and appetite of Kenyan school boys with hookworm, Trichuris trichiura and Ascaris lumbricoides infections are improved four months after a single dose of albendazole. J Nutr. 1993;123:1036–1046. doi: 10.1093/jn/123.6.1036. [DOI] [PubMed] [Google Scholar]
- 5.Stephenson LS, Latham MC, Kurz KM, Kinoti SN, Oduori ML, Crompton DW. Relationships of Schistosoma hematobium, hookworm and malarial infections and metrifonate treatment to hemoglobin level in Kenyan school children. Am J Trop Med Hyg. 1985;34:519–528. doi: 10.4269/ajtmh.1985.34.519. [DOI] [PubMed] [Google Scholar]
- 6.Stephenson LS, Latham MC, Ottesen EA. Malnutrition and parasitic helminth infections. Parasitology. 2000;121((Suppl)):S23–S38. doi: 10.1017/s0031182000006491. [DOI] [PubMed] [Google Scholar]
- 7.Coutinho HM, Acosta LP, McGarvey ST, Jarilla B, Jiz M, Pablo A, Su L, Manalo DL, Olveda RM, Kurtis JD, Friedman JF. Nutritional status improves after treatment of Schistosoma japonicum-infected children and adolescents. J Nutr. 2006;136:183–188. doi: 10.1093/jn/136.1.183. [DOI] [PubMed] [Google Scholar]
- 8.Parraga IM, Assis AM, Prado MS, Barreto ML, Reis MG, King CH, Blanton RE. Gender differences in growth of school-aged children with schistosomiasis and geohelminth infection. Am J Trop Med Hyg. 1996;55:150–156. doi: 10.4269/ajtmh.1996.55.150. [DOI] [PubMed] [Google Scholar]
- 9.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 10.Bustinduy AL, Thomas CL, Fiutem JJ, Parraga IM, Mungai PL, Muchiri EM, Mutuku F, Kitron U, King CH. Measuring fitness of Kenyan children with polyparasitic infections using the 20-meter shuttle run test as a morbidity metric. PLoS Negl Trop Dis. 2011;5:e1213. doi: 10.1371/journal.pntd.0001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yap P, Du ZW, Chen R, Zhang LP, Wu FW, Wang J, Wang XZ, Zhou H, Zhou XN, Utzinger J, Steinmann P. Soil-transmitted helminth infections and physical fitness in school-aged Bulang children in southwest China: results from a cross-sectional survey. Parasit Vectors. 2012;5:50. doi: 10.1186/1756-3305-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131:649S–666S. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- 13.Nokes C, McGarvey ST, Shiue L, Wu G, Wu H, Bundy DA, Olds GR. Evidence for an improvement in cognitive function following treatment of Schistosoma japonicum infection in Chinese primary schoolchildren. Am J Trop Med Hyg. 1999;60:556–565. doi: 10.4269/ajtmh.1999.60.556. [DOI] [PubMed] [Google Scholar]
- 14.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 2008;4:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- 16.WHO . Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Geneva: World Health Organization; 2006. [Google Scholar]
- 17.Golden MH. Is complete catch-up possible for stunted malnourished children? Eur J Clin Nutr. 1994;48((Suppl 1)):S58–S70. [PubMed] [Google Scholar]
- 18.Friedman JF, Kanzaria HK, McGarvey ST. Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends Parasitol. 2005;21:386–392. doi: 10.1016/j.pt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Leenstra T, Coutinho HM, Acosta LP, Langdon GC, Su L, Olveda RM, McGarvey ST, Kurtis JD, Friedman JF. Schistosoma japonicum reinfection after praziquantel treatment causes anemia associated with inflammation. Infect Immun. 2006;74:6398–6407. doi: 10.1128/IAI.00757-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King CH. Long-term outcomes of school-based treatment for control of urinary schistosomiasis: a review of experience in Coast Province, Kenya. Mem Inst Oswaldo Cruz. 2006;101((Suppl 1)):299–306. doi: 10.1590/s0074-02762006000900047. [DOI] [PubMed] [Google Scholar]
- 21.King CH, Muchiri E, Ouma JH, Koech D. Chemotherapy-based control of schistosomiasis haematobia. IV. Impact of repeated annual chemotherapy on prevalence and intensity of Schistosoma haematobium infection in an endemic area of Kenya. Am J Trop Med Hyg. 1991;45:498–508. doi: 10.4269/ajtmh.1991.45.498. [DOI] [PubMed] [Google Scholar]
- 22.Ouma JH, King CH, Muchiri EM, Mungai P, Koech DK, Ireri E, Magak P, Kadzo H. Late benefits 10–18 years after drug therapy for infection with Schistosoma haematobium in Kwale District, Coast Province, Kenya. Am J Trop Med Hyg. 2005;73:359–364. [PubMed] [Google Scholar]
- 23.King CH, Keating CE, Muruka JF, Ouma JH, Houser H, Siongok TK, Mahmoud AA. Urinary tract morbidity in schistosomiasis haematobia: associations with age and intensity of infection in an endemic area of Coast Province, Kenya. Am J Trop Med Hyg. 1988;39:361–368. doi: 10.4269/ajtmh.1988.39.361. [DOI] [PubMed] [Google Scholar]
- 24.Mutuku FM, King CH, Bustinduy AL, Mungai PL, Muchiri EM, Kitron U. Impact of drought on the spatial pattern of transmission of Schistosoma haematobium in coastal Kenya. Am J Trop Med Hyg. 2011;85:1065–1070. doi: 10.4269/ajtmh.2011.11-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters PAS, Kazura JW. Update on diagnostic methods for schistosomiasis. In: Mahmoud AA, editor. Balliere's Clinical Tropical Medicine and Communicable Diseases, Schistosomiasis. London, UK: Bailliere Tindall; 1987. pp. 419–433. [Google Scholar]
- 26.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 27.WHO/CDC . Assessing the Iron Status of Populations. Geneva: World Health Organization; 2007. [Google Scholar]
- 28.Frisancho A. Anthropometric Standards for the Assessment of Growth and Nutrition Status. Ann Arbor, MI: The University of Michigan Press; 1990. [Google Scholar]
- 29.Johnston FE, Hamill PV, Lemeshow S. Skinfold thickness of children 6–11 years, United States. Vital Health Stat. 1972;11(120):1–60. [PubMed] [Google Scholar]
- 30.Zerfas A. Checking Continuous Measurements: Manual for Anthropometry. Los Angeles, CA: Division of Epidemiology, School of Public Health, University of California; 1985. [Google Scholar]
- 31.Jelliffe DB. The assessment of the nutritional status of the community (with special reference to field surveys in developing regions of the world) Monogr Ser World Health Organ. 1966;53:3–271. [PubMed] [Google Scholar]
- 32.WHO . WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Growth Velocity Based on Weight, Length and Head Circumference: Methods and Development. Geneva: World Health Organization; 2009. [Google Scholar]
- 33.de Onis M. Growth curves for school age children and adolescents. Indian Pediatr. 2009;46:463–465. [PubMed] [Google Scholar]
- 34.Gwatkin DR, Rustein S, Johnson K, Pande R, Wagstaff A. Socio-Economic Differences in Health, Nutrition, and Population in Kenya. Washington, DC: World Bank; 2000. [PubMed] [Google Scholar]
- 35.Ezeamama AE, Friedman JF, Olveda RM, Acosta LP, Kurtis JD, Mor V, McGarvey ST. Functional significance of low-intensity polyparasite helminth infections in anemia. J Infect Dis. 2005;192:2160–2170. doi: 10.1086/498219. [DOI] [PubMed] [Google Scholar]
- 36.Florey LS, King CH, Van Dyke MK, Muchiri EM, Mungai PL, Zimmerman PA, Wilson ML. Partnering parasites: evidence of synergism between heavy Schistosoma haematobium and Plasmodium species infections in Kenyan children. PLoS Negl Trop Dis. 2012;6:e1723. doi: 10.1371/journal.pntd.0001723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurarie D, Wang X, Bustinduy AL, King CH. Modeling the effect of chronic schistosomiasis on childhood development and the potential for catch-up growth with different drug treatment strategies promoted for control of endemic schistosomiasis. Am J Trop Med Hyg. 2011;84:773–781. doi: 10.4269/ajtmh.2011.10-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mutuku FM, King CH, Mungai P, Mbogo C, Mwangangi J, Muchiri EM, Walker ED, Kitron U. Impact of insecticide-treated bed nets on malaria transmission indices on the south coast of Kenya. Malar J. 2011;10:356. doi: 10.1186/1475-2875-10-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coutinho HM, Leenstra T, Acosta LP, Su L, Jarilla B, Jiz MA, Langdon GC, Olveda RM, McGarvey ST, Kurtis JD, Friedman JF. Pro-inflammatory cytokines and C-reactive protein are associated with undernutrition in the context of Schistosoma japonicum infection. Am J Trop Med Hyg. 2006;75:720–726. [PubMed] [Google Scholar]
- 40.Leenstra T, Acosta LP, Langdon GC, Manalo DL, Su L, Olveda RM, McGarvey ST, Kurtis JD, Friedman JF. Schistosomiasis japonica, anemia, and iron status in children, adolescents, and young adults in Leyte, Philippines. Am J Clin Nutr. 2006;83:371–379. doi: 10.1093/ajcn/83.2.371. [DOI] [PubMed] [Google Scholar]
- 41.Verani JR, Abudho B, Montgomery SP, Mwinzi PN, Shane HL, Butler SE, Karanja DM, Secor WE. Schistosomiasis among young children in Usoma, Kenya. Am J Trop Med Hyg. 2011;84:787–791. doi: 10.4269/ajtmh.2011.10-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Odogwu SE, Ramamurthy NK, Kabatereine NB, Kazibwe F, Tukahebwa E, Webster JP, Fenwick A, Stothard JR. Schistosoma mansoni in infants (aged < 3 years) along the Ugandan shoreline of Lake Victoria. Ann Trop Med Parasitol. 2006;100:315–326. doi: 10.1179/136485906X105552. [DOI] [PubMed] [Google Scholar]
- 43.Bosompem KM, Bentum IA, Otchere J, Anyan WK, Brown CA, Osada Y, Takeo S, Kojima S, Ohta N. Infant schistosomiasis in Ghana: a survey in an irrigation community. Trop Med Int Health. 2004;9:917–922. doi: 10.1111/j.1365-3156.2004.01282.x. [DOI] [PubMed] [Google Scholar]
- 44.Garba A, Barkire N, Djibo A, Lamine MS, Sofo B, Gouvras AN, Bosque-Oliva E, Webster JP, Stothard JR, Utzinger J, Fenwick A. Schistosomiasis in infants and preschool-aged children: infection in a single Schistosoma haematobium and a mixed S. haematobium-S. mansoni foci of Niger. Acta Trop. 2010;115:212–219. doi: 10.1016/j.actatropica.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 45.WHO . Report of a Meeting to Review the Results of Studies on the Treatment of Schistosomiasis in Preschool-Aged Children. Geneva: World Health Organization; 2011. [Google Scholar]
- 46.UNICEF . The State of the World's Children 2011. Adolescence. An Age of Opportunity. Geneva: World Health Organization; 2011. [Google Scholar]
- 47.Mupfasoni D, Karibushi B, Koukounari A, Ruberanziza E, Kaberuka T, Kramer MH, Mukabayire O, Kabera M, Nizeyimana V, Deville MA, Ruxin J, Webster JP, Fenwick A. Polyparasite helminth infections and their association to anaemia and undernutrition in northern Rwanda. PLoS Negl Trop Dis. 2009;3:e517. doi: 10.1371/journal.pntd.0000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koukounari A, Gabrielli AF, Toure S, Bosque-Oliva E, Zhang Y, Sellin B, Donnelly CA, Fenwick A, Webster JP. Schistosoma haematobium infection and morbidity before and after large-scale administration of praziquantel in Burkina Faso. J Infect Dis. 2007;196:659–669. doi: 10.1086/520515. [DOI] [PubMed] [Google Scholar]
- 49.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 50.Mondal D, Minak J, Alam M, Liu Y, Dai J, Korpe P, Liu L, Haque R, Petri WA., Jr Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis. 2012;54:185–192. doi: 10.1093/cid/cir807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guerrant DI, Moore SR, Lima AA, Patrick PD, Schorling JB, Guerrant RL. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. Am J Trop Med Hyg. 1999;61:707–713. doi: 10.4269/ajtmh.1999.61.707. [DOI] [PubMed] [Google Scholar]
- 52.Coly AN, Milet J, Diallo A, Ndiaye T, Benefice E, Simondon F, Wade S, Simondon KB. Preschool stunting, adolescent migration, catch-up growth, and adult height in young Senegalese men and women of rural origin. J Nutr. 2006;136:2412–2420. doi: 10.1093/jn/136.9.2412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.