Abstract
In 2009, an increased proportion of suspected dengue cases reported to the surveillance system in Puerto Rico were laboratory negative. As a result, enhanced acute febrile illness (AFI) surveillance was initiated in a tertiary care hospital. Patients with fever of unknown origin for 2–7 days duration were tested for Leptospira, enteroviruses, influenza, and dengue virus. Among the 284 enrolled patients, 31 dengue, 136 influenza, and 3 enterovirus cases were confirmed. Nearly half (48%) of the confirmed dengue cases met clinical criteria for influenza. Dengue patients were more likely than influenza patients to have hemorrhage (81% versus 26%), rash (39% versus 9%), and a positive tourniquet test (52% versus 18%). Mean platelet and white blood cell count were lower among dengue patients. Clinical diagnosis can be particularly difficult when outbreaks of other AFI occur during dengue season. A complete blood count and tourniquet test may be useful to differentiate dengue from other AFIs.
Background
The infectious causes and epidemiology of acute febrile illness (AFI), defined as illness of < 1 week duration with no identified source, remain poorly characterized in many parts of the world.1,2 Previous studies performed in Egypt showed that infections, such as salmonellosis (5%), typhoid fever (18%), and brucellosis (11%), were common causes of AFI.3 In South America, infections with Leptospira, malaria, Rickettsia, dengue virus, and Venezuelan equine encephalitis virus were identified as major AFI causes.4 In some regions, such as sub-Saharan Africa and Southeast Asia, sentinel hospital-based studies have been established to obtain clinical and public health data about the causes of AFI throughout the year and to identify susceptibility patterns and clinical predictors.2,5–9 The burden of dengue is uncertain, although believed to be substantial throughout the tropics,10 and the importance of other infectious diseases such as leptospirosis is undefined.2 Lack of information about the specific etiologies that make up the differential diagnosis of dengue slows our ability to make accurate diagnoses, provide effective treatment, and effectively target public health measures.4
In Puerto Rico, dengue is endemic throughout the year; however, dengue virus (DENV) transmission increases during the period of increased ambient temperatures and rainfall, which begins in June and extends until November. The Centers for Disease Control and Prevention (CDC), in collaboration with the Puerto Rico Department of Health (PRDH), has been conducting passive dengue surveillance (PDSS) for more than 3 decades. This system records ∼3,000–5,000 suspect cases in non-epidemic years and up to 25,000 in recent epidemics. However, what is not known is the degree to which dengue is under-identified or reported among cases of AFI, and the incidence of confounding cases such as leptospirosis or influenza. Fortunately, malaria is no longer endemic in Puerto Rico as it is in neighboring islands,11 and diseases associated with poor sanitation, such as typhoid fever, are uncommon.
In April 2009, novel influenza A (H1N1) was detected in the United States12 and rapidly spread worldwide, including to Puerto Rico. The first confirmed case was detected in the southern part of Puerto Rico in June of 2009, and by early 2010, the island was one of the most heavily affected areas in the United States, with 59 influenza-related deaths among its 3.8 million inhabitants.13 The PDSS was able to detect that an outbreak of AFI was occurring in Puerto Rico.14 In May of 2009, an increase in suspected dengue cases with negative dengue diagnostic test results was detected by PDSS and these cases had an increased frequency of respiratory symptoms.
The introduction of influenza A (H1N1) 2009 virus corresponded with the onset of dengue season, which made the clinical diagnosis and differentiation between dengue fever (DF) and mild cases of influenza difficult. As a result, enhanced surveillance for AFIs was initiated at the emergency department (ED) of a large, tertiary care hospital in southern Puerto Rico. Enhanced surveillance was conducted to assess the etiology of AFI and to identify demographic and clinical features that might assist clinicians to distinguish between these different etiologies.
Patients, Materials, and Methods
Study site.
An enhanced surveillance system for AFI was established at the Saint Luke's Episcopal Hospital, in Ponce, Puerto Rico. Located ∼45 miles southwest of San Juan, Ponce is one of the 78 administrative municipalities of Puerto Rico and the second largest city (Figure 1). This hospital is a tertiary, acute-care facility that provides healthcare to patients from over 20 municipalities, has more than 54,000 patient visits to its ED annually,15 and is the main teaching affiliate of the Ponce School of Medicine.
Figure 1.
Map of Puerto Rico showing 78 municipalities.
Study population.
Patients of all ages were eligible to be enrolled if they met the following case definition of AFI: documented fever of 38.0°C or higher at presentation to the ED or history of fever that had persisted for 2–7 days without an identified source. Patients were excluded if after the initial physical examination by hospital physicians they had an identifiable source of fever including, but not limited to diagnoses of otitis media, sinusitis, bronchitis, pneumonia, cellulitis, impetigo, wound infection, urinary tract infection, osteomyelitis, or varicella.
Study protocol.
From September 29, 2009 through December 18, 2009, patients seeking medical care in the ED of St Luke's Episcopal Hospital who met the case definition for AFI were enrolled in the enhanced surveillance system (Figure 2). This surveillance included all elements of the PDSS established for decades and the addition of on-site CDC staff to help identify and collect data from AFI patients, including completion of the dengue case investigation form (DCIF), which accompanied specimens submitted to CDC's Dengue Branch for diagnostic testing. After physical examination, study personnel explained the purpose of the surveillance project and obtained verbal consent for participation. Participants were interviewed to collect DCIF data that included demographic, medical and clinical information, signs and symptoms at onset, and specimen collection dates. A tourniquet test was performed and results recorded by trained study personnel using standardized techniques as previously described.16 Two nasopharyngeal samples were collected to perform rapid antigen and reverse transcriptase-polymerase chain reaction (RT-PCR) testing for influenza. Attending physicians and patients were informed immediately of the results of the rapid influenza test that was performed on-site. One blood sample was collected for dengue diagnostic testing. Additional laboratory tests such as a complete blood count and urinalysis were performed at the discretion of the attending physician in the course of routine patient care but were not part of the study. If available, laboratory results, including white blood cell (WBC) count, platelet count, hematocrit, and albumin, were recorded on the DCIF. This protocol was reviewed and approved by the Human Subjects Institutional Review Board of the CDC and the Institutional Review Board of Ponce School of Medicine.
Figure 2.
Enrollment procedure for the Enhanced Acute Febrile Illness Surveillance Project, Ponce, Puerto Rico, 2009.
Laboratory testing.
Two nasopharyngeal samples were obtained at the same time. The first was tested on-site with the QuickVue Influenza A + B rapid influenza test (Quidel Corporation, San Diego, CA), and the second sample was placed in viral transport medium and refrigerated until transported to the CDC's Dengue Branch, San Juan, PR, for influenza testing by the CDC RT-PCR assay.17 As per PDSS protocol, 5 to 10 mL of venous blood was collected, immediately refrigerated at 4°C, centrifuged on-site to separate serum, and transported on ice within 3 days to the CDC's Dengue Branch for further testing. Serum samples were initially tested for DENV by serotype-specific RT-PCR,18 dengue-specific non-structural protein-1 (NS1), and by DENV-specific immunoglobulin M (IgM) antibody-capture enzyme-linked immunosorbent assay (anti-DENV MAC-ELISA).19
Serum samples from patients with severe disease (i.e., hospital admission or clinical signs including plasma leakage, fluid accumulation, respiratory distress, bleeding, and organ impairment) and from those who were influenza PCR-negative were subsequently transported on ice to CDC's Bacterial Zoonosis Branch in Atlanta for Leptospira testing. Specimens were screened for IgM antibodies to Leptospira by using the rapid dipstick ELISA ImmunoDOT kit (GenBio, Inc., San Diego, CA). Specimens with positive or borderline results with the ImmunoDOT kit were further tested by using the microscopic agglutination test (MAT).20 Patient sera were serially diluted in the MAT and mixed with a panel of 20 Leptospira reference antigens representing 17 serogroups. Resulting agglutination titers were read by using dark field microscopy, and the final titer was expressed as the reciprocal of the last well that agglutinated 50% of the antigen. Samples drawn within the first 3 days from symptom onset and with sufficient serum volume were shipped frozen to the Picornavirus Laboratory at CDC, Atlanta, and tested for enteroviruses by a pan-enterovirus (EV) RT-PCR that targets the conserved 5′ non-translated region of the genome.21
Definitions:
Acute febrile illness was defined as a patient with fever of 38°C or higher at presentation to ED or history of fever that persisted for 2–7 days with no localizing source.
Laboratory-positive dengue case: a patient with one or more of the following: 1) DENV RNA detected in serum by RT-PCR, 2) negative to positive anti-DENV IgM seroconversion in paired serum specimens, 3) a single positive anti-DENV IgM result in an acute-phase or convalescent-phase specimen (positive/negative antibody ratio ≥ 2.0),22 or 4) a positive DENV antigen detection by NS1 rapid test.
Laboratory-negative dengue case: a patient who does not meet criteria for a laboratory-positive dengue case and has no anti-DENV IgM detected in a serum specimen collected 6 or more days after onset of fever.
Laboratory-indeterminate dengue case: a patient with no DENV RNA, NS1 antigen or dengue virus or anti-dengue IgM antibodies detected in the acute sample submitted for diagnostic testing and no convalescent sample submitted for diagnostic testing.
Dengue fever, dengue hemorrhagic fever (DHF), and dengue shock syndrome (DSS) were defined by using the World Health Organization (WHO) criteria.23–27
Warning signs for severe dengue were defined as those that occurred within 48 hours of defervescence (about 3 to 7 days after symptoms onset) and included: severe abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, hemorrhagic manifestation, lethargy or restlessness, and liver enlargement.28
Laboratory-positive influenza case was defined as a patient with laboratory-confirmed infection detected by the CDC RT-PCR assay.17
Laboratory-positive leptospirosis case was defined by the following: 1) having positive IgM antibodies to Leptospira with the rapid dipstick ELISA ImmunoDOT kit and 2) a positive MAT for a single titer of > 1:400 or a fourfold rise in titer between acute and convalescent phase samples.20
Laboratory-positive EV case was defined as a patient with positive EV real-time RT-PCR assay and genotype identification by EV VP1 semi-nested RT-PCR and amplicon sequencing.21,29,30
Anemia was defined as a deficiency in the total number of erythrocytes in blood. A patient was considered to be anemic if its hemoglobin was less than the 2.5 percentile for age and sex.23,24
The WBC counts were classified as leucopenia (total WBC < 5,000/mm3) or leukocytosis (WBC > 11,000/mm3).31
Hemoconcentration was defined as an increase in hematocrit ≥ 20% above average for age or a decrease in hematocrit ≥ 20% of baseline following fluid replacement therapy.22
Analyses.
Data were entered into a Microsoft Access database (Microsoft Access 2007; Microsoft, Redmond, WA) and exported to SAS version 9.2 (SAS Institute, Cary, NC) for analysis. Outcome groups were identified as having confirmed dengue, confirmed influenza, confirmed leptospirosis, confirmed EV infection, or other AFI. Categorical variables were compared by using the χ2 test or Fisher's exact test as appropriate and continuous variables were compared by using the Student's t test and the Mann-Whitney U test (Wilcoxon rank-sum test) where applicable. Results with values P ≤ 0.05 were considered statistically significant.
Results
A total of 284 patients were enrolled (Table 1), and 172 (61%) met the case definition for influenza, dengue, leptospirosis, or enteroviral disease. Influenza A was identified in 138 (49%) patients, and all except two had a positive RT-PCR for pandemic 2009 influenza A H1N1 virus. No influenza B was identified. Dengue virus was identified in 32 (11%) patients; one of these patients had a dual infection with DENV-2 and influenza A H1N1 virus. Among patients laboratory positive for dengue, 20 were DENV RNA positive; 18 had DENV-4, 1 had DENV-1, and 1 had DENV-2. DENV-3 was not found. The remaining dengue patients were NS1 antigen positive (7 [23%]) or had a single positive anti-DENV IgM positive (4, 13%). Leptospira was detected in one patient; however, this patient also had influenza A H1N1 detected by RT-PCR. Enterovirus was detected in three patients and the genotype was determined in 2 patients (coxsackievirus A2 and coxsackievirus B4). Blood cultures, urine cultures, or both were performed in only 10 of all enrolled patients. Two patients had a positive urine culture; one positive for Escherichia coli and one for Staphylococcus saprophyticus. The two patients with dual diagnoses were excluded from the rest of the analysis.
Table 1.
Pathogens identified among enrolled patients, Enhanced Acute Febrile Illness Surveillance Project, Ponce Puerto Rico, 2009*
| Infectious agent | Number of positive cases | Number of negative cases | Number of not tested, indeterminate, or invalid* | Percent positive of tested | Percent positive of all samples | Total tested |
|---|---|---|---|---|---|---|
| Influenza virus only | 136 | 144 | 2 | 48.2 | 47.8 | 283† |
| Dengue virus only | 31 | 249 | 3 | 10.9 | 10.9 | 283† |
| Leptospirosis only | 0 | 133 | 150 | 0.7 | 0.3 | 283† |
| Enterovirus only | 3 | 73 | 208 | 4.1 | 1.4 | |
| Dual infection | 2† | – | – | – | 0.7 | |
| None‡ | 112 | – | – | – | – | |
| Total | 284 |
A total of 282 were considered for further analysis. Two dual infections were excluded; one with influenza A H1 N1 and dengue virus and the other with influenza A H1 N1 and Leptospira. All initial participants could have been tested for more than one test.
Not tested: no sample volume available for testing; indeterminate: negative results in the acute sample but no convalescent sample were submitted for diagnostic testing; invalid: insufficient sample volume for testing.
None, no etiological agent was identified after testing.
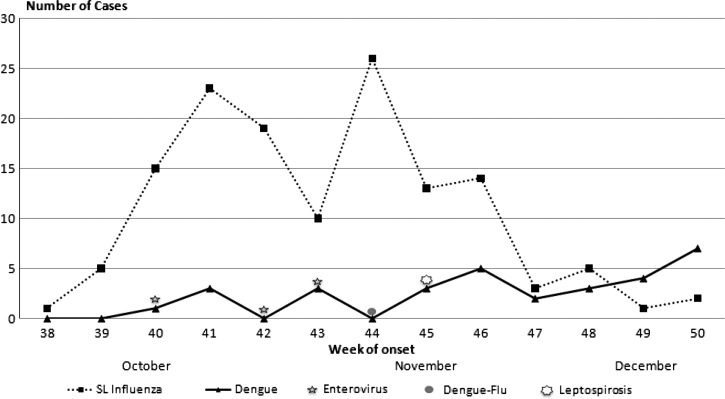
Influenza cases were more likely to be detected earlier in the study period than laboratory-positive dengue cases; most influenza cases were detected from October 1 through November 18, 2009, versus November 5 through December 16, 2009, for dengue (Figure 3). The three EV cases occurred during October. Overall, the patients ranged in age from 6 months to 82 years with a median age of 17.9 years (Table 2). Median age for patients with other AFI was 15.4 years compared with 20.3 years for dengue patients and 18.7 years for influenza patients. Overall, 55% of enrolled patients were female; however, more than half (55%) of those with dengue were male. Most enrolled patients lived in the neighboring municipalities of Ponce (128 [45%]), Villalba (40 [14%]), and Juana Diaz (38 [13%],). However, dengue cases were predominately residents of Villalba (18 [58%]), whereas most influenza cases were from Ponce (74 [54%]).
Figure 3.
Number of laboratory-positive cases of influenza A, dengue, enterovirus, and leptospirosis by week of symptom onset; Enhanced Acute Febrile Illness Surveillance Project, Ponce Puerto Rico, 2009.
Table 2.
Patient demographic characteristics by pathogen identified at initial presentation, Enhanced Acute Febrile Illness (AFI) Surveillance Project, Ponce Puerto Rico, 2009
| Characteristic | All patients | Laboratory-positive Dengue | Laboratory-positive Influenza | Laboratory-positive Enterovirus | Other AFI | Significance | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (N = 282)* | (N = 31) | (N = 136) | (N = 3) | (N = 112)† | P value | ||||||
| % Female | 54.9 | 45.2 | 55.9 | 33.0 | 57.1 | 0.08 | |||||
| Age, years | |||||||||||
| Median (range) | 17.9 (0.5–82.4) | 20.3 (3.3–64.4) | 18.7 (0.8–61.5) | 5 (0.64–7.6) | 15.4 (0.5–82.4) | 0.81 | |||||
| n | % | n | % | n | % | n | % | n | % | ||
| 0–4 | 41 | 14.5 | 1 | 3.2 | 9 | 6.6 | 1 | 33.3 | 30 | 26.8 | 0.47 |
| 5–9 | 40 | 14.2 | 3 | 9.7 | 21 | 15.4 | 2 | 66.7 | 14 | 12.5 | 0.41 |
| 10–14 | 41 | 14.5 | 5 | 16.1 | 25 | 18.4 | – | 11 | 9.8 | 0.76 | |
| 15–19 | 27 | 9.6 | 6 | 19.4 | 18 | 13.2 | – | 3 | 2.7 | 0.37 | |
| 20–24 | 16 | 5.7 | 5 | 16.1 | 8 | 5.9 | – | 3 | 2.7 | 0.05 | |
| 25–29 | 23 | 8.2 | 2 | 2.5 | 15 | 11.0 | – | 6 | 5.4 | 0.14 | |
| 30–34 | 24 | 8.5 | 2 | 6.5 | 8 | 5.9 | – | 14 | 12.5 | 0.90 | |
| 35–39 | 23 | 8.2 | 4 | 12.9 | 10 | 7.4 | – | 9 | 8.0 | 0.32 | |
| 40–44 | 13 | 4.6 | – | 9 | 6.6 | – | 4 | 3.6 | |||
| 45–49 | 11 | 3.9 | 2 | 6.5 | 3 | 2.2 | – | 6 | 5.4 | 0.21 | |
| 50–54 | 7 | 2.5 | – | 5 | 3.7 | – | 2 | 1.8 | |||
| 55–59 | 8 | 2.8 | – | 4 | 2.9 | – | 4 | 3.6 | 0.34 | ||
| 60–64 | 4 | 1.4 | 1 | 3.2 | 1 | 0.7 | – | 2 | 1.8 | 0.24 | |
| 65–69 | 2 | 0.71 | – | – | – | 2 | 1.8 | – | |||
| 70+ | 2 | 0.71 | – | – | 2 | 1.8 | – | ||||
| Residence, municipality | |||||||||||
| Ponce | 128 | 45.1 | 1 | 3.2 | 74 | 54.4 | 0 | 53 | 47.8 | < 0.0001 | |
| Villalba | 40 | 14.1 | 18 | 58.1 | 9 | 6.6 | 1 | 33.3 | 12 | 10.7 | < 0.0001 |
| Juana Diaz | 38 | 13.4 | 2 | 6.5 | 18 | 13.6 | 1 | 33.3 | 17 | 15.2 | 0.28 |
| Coamo | 21 | 7.4 | 5 | 16.1 | 11 | 8.1 | 0 | 5 | 4.5 | 0.17 | |
| Peñuelas | 16 | 5.7 | 0 | 0.0 | 7 | 5.2 | 0 | 9 | 8.0 | 0.19 | |
| Santa Isabel | 10 | 3.5 | 1 | 3.2 | 5 | 3.7 | 1 | 33.3 | 3 | 2.7 | 0.89 |
P value: compares laboratory positive dengue with laboratory positive influenza patients.
Excludes two patients with dual infections: dengue/influenza and influenza/leptospirosis.
Includes two positive urine cultures.
In addition to fever, most enrolled patients reported headache (81%), cough (79%), body pain (78%), nasal congestion (66%), eye pain (61%), arthralgia (59%), and sore throat (57%) (Table 3). The most common symptoms among influenza patients were cough (92%), headache (85%), nasal congestion (83%), and body pain (82%). Cough and sore throat were more common among influenza patients than among patients with other etiologies (P ≤ 0.0004). Although most (120 [88%]) influenza patients met the CDC criteria for influenza-like illness (i.e., fever with cough or sore throat), so did half (15 [48.4%]) of the dengue cases and most (93 [83%]) of patients with unidentified febrile illness. In addition, most (107 [79%]) influenza patients met WHO criteria for DF; 15 (11%) presented with clinical bleeding, and 21 (18%) had a positive tourniquet test.
Table 3.
Signs and symptoms at initial presentation, Enhanced Acute Febrile Illness (AFI) Surveillance Project, Ponce, Puerto Rico, 2009
| Characteristic | All patients | Laboratory-positive Dengue | Laboratory-positive Influenza | Laboratory-positive Enterovirus | Other AFI | Significance P value* | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (N = 282)† | (N = 31) | (N = 136) | (N = 3) | (N = 112) | |||||||
| n | % | n | % | n | % | n | % | n | % | ||
| Signs and Symptoms | |||||||||||
| Fever | 248 | 87.9 | 30 | 96.8 | 120 | 88.2 | 3 | 100 | 95 | 84.8 | 0.15 |
| Rash | 41 | 14.7 | 12 | 38.7 | 12 | 8.8 | 1 | 33.3 | 16 | 14.7 | < 0.0001 |
| Headache | 229 | 81.2 | 29 | 93.6 | 115 | 84.6 | 3 | 100 | 82 | 73.2 | 0.19 |
| Eye pain | 170 | 60.5 | 21 | 67.7 | 89 | 65.4 | 0 | 60 | 54.1 | 0.81 | |
| Body pain | 221 | 78.4 | 26 | 83.9 | 112 | 82.4 | 1 | 33.3 | 82 | 73.2 | 0.84 |
| Arthalgia | 167 | 59.2 | 23 | 74.2 | 85 | 62.5 | 0 | 59 | 52.7 | 0.22 | |
| Cough | 224 | 79.4 | 11 | 35.5 | 125 | 91.9 | 1 | 33.3 | 87 | 77.7 | < 0.0001 |
| Nasal congestion | 187 | 66.3 | 7 | 22.6 | 112 | 82.5 | 1 | 33.3 | 67 | 59.8 | < 0.0001 |
| Sore throat | 161 | 57.1 | 8 | 25.8 | 83 | 61 | 1 | 33.3 | 69 | 61.6 | 0.0004 |
| Hemorrhagic manifestations | |||||||||||
| Positive tourniquet test (TT) | 54 | 21.9 | 16 | 51.6 | 21 | 17.8 | 0 | 17 | 17.7 | 0.0001 | |
| Hemorrhage with TT | 96 | 34.0 | 25 | 80.6 | 36 | 26.5 | 2 | 66.7 | 33 | 29.5 | < 0.0001 |
| Hemorrhage without TT | 42 | 14.9 | 9 | 29.0 | 15 | 11.0 | 2 | 66.7 | 16 | 14.3 | < 0.0001 |
| Warning signs for severe disease | |||||||||||
| Persistent vomiting | 35 | 12.6 | 5 | 16.1 | 15 | 11.2 | 0 | 15 | 13.6 | 0.45 | |
| Abdominal pain | 95 | 33.7 | 18 | 58.1 | 38 | 27.9 | 0 | 39 | 34.8 | 0.003 | |
| Liver enlargement | 2 | 0.7 | 1 | 3.2 | 1 | 0.8 | 0 | 0 | 0.28 | ||
P value: compares laboratory-positive dengue with laboratory-positive influenza patients.
Excludes two patients with dual infections: dengue/influenza and influenza/leptospirosis.
Dengue patients most commonly had headache (29 [94%]); body pain (26 [84%]); a positive tourniquet test or other hemorrhagic manifestations (25 [81%]); arthralgia (23 [74%]); and eye pain (21 [68%]) (Table 3). Rash was less common than other mentioned signs and symptoms, however more likely to be present among dengue patients than among patients with influenza (39% versus 9% P ≤ 0.0001) or with other AFI (39% versus 15% P ≤ 0.003). Dengue patients were more likely than influenza patients to have a positive tourniquet test (52% versus 18%, P ≤ 0.0001) or other hemorrhagic manifestations (29% versus 11%, P ≤ 0.0001). Other hemorrhagic manifestations included petechiae (48% versus 7%, P ≤ 0.0001), purpura (13% versus 2%), epistaxis (6% versus 2%), bleeding gums (19% versus 3%, P ≤ 0.003), blood in stool (3% versus 2%), hematemesis (6% versus 2%), menorrhagia (10% versus 0%), and hematuria (23% versus 4%). All but one dengue patient (30 [97%]) met WHO criteria for DF; 18 (58%) patients met criteria for DF with at least one warning sign for severe dengue, and 4 (13%) met criteria for DHF or DSS. Dengue patients were also more likely than influenza patients to report warning signs for severe dengue such as persistent vomiting (16% versus 11%), abdominal pain (58% versus 28%, P ≤ 0.003), and liver enlargement (3% versus 0.8%).
Overall, patients sought medical care early after symptom onset (mean = 3.3; SD = 2.7 days), which was especially true for influenza patients who sought care a mean of 2.8 days after symptom onset. Nearly one in five (19%) enrolled patients were admitted to the hospital, including 22 (71%) of the dengue patients. Dengue patients were more likely than influenza patients to present later and to be admitted to the hospital. The median length of hospital stay was 4 days (range: 2–77 days). There was no statistically significant difference in length of hospital stay between patients with dengue and with those with influenza. Ten percent of dengue patients were admitted to an intensive care unit, which was a higher rate than for all admitted patients (3.2%; P ≤ 0.008).
Upon initial presentation, the median platelet count of participants was 198,000 (range 10,000–449,000), and 13% had thrombocytopenia (platelet count ≤ 100,000) (Table 4). Patients with dengue had a lower median platelet count of 52,000 (range 10,000–227,000), whereas influenza patients had a median platelet count of 189,000 (range 39,000–369,000) (P ≤ 0.0001). Most (22 [71%]) dengue patients were thrombocytopenic and nearly all (28 [90%)] of them had platelet counts ≤ 150,000 compared with 8 (6%) and 28 (21%) of influenza patients, respectively (P ≤ 0.0001). Overall, the median WBC count of enrolled patients was 6.2×103/mm3 (range 0.9–27.2×103/mm3). Dengue patients had significantly lower median white cell counts when compared with influenza patients (3.1×103/mm3 versus 5.6×103/mm3, P ≤ 0.0001). A higher proportion of dengue patients than influenza patients were leucopenic (87.1% versus 44.1%, P ≤ 0.0001), and none of the dengue patients had elevated WBC counts, whereas 5% of influenza patients and 21% of other patients had elevated white cell counts. Overall, the initial median hematocrit value for enrolled patients was 39.0 (range: 12.5–56.4); dengue patients had higher median hematocrit values (43.2, range: 26.2–56.4) than influenza patients (39.3, range: 12.5–50.6) (P ≤ 0.010). However, only 3% of dengue patients and < 1% of influenza were hemoconcentrated. Hyponatremia (Na+ concentration < 135 mEq/L) was detected in nearly 40% of dengue patients at initial presentation, a proportion significantly higher (P ≤ 0.0001) in comparison with patients with influenza. Finally, dengue patients had higher median alanine aminotransferase levels (119 versus 56 U/L, P ≤ 0.01) and asparate aminotransferase (181 versus 40 U/L, P ≤ 0.001) levels than patients with influenza.
Table 4.
Laboratory findings at initial presentation, Enhanced Acute Febrile Illness (AFI) Surveillance Project, Ponce Puerto Rico, 2009.
| Characteristic | All patients | Laboratory-positive Dengue | Laboratory-positive Influenza | Laboratory-positive Enterovirus | Other AFI | Significance P value* | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (N = 282)* | (N = 31) | (N = 136) | (N = 3) | (N = 112) | |||||||
| Platelet count per mm3 | |||||||||||
| Mean (SD) | 198,281 (84,000) | 74,484 (58,000) | 189,639 (57,400) | 290,000 (74,100) | 241,118 (80,600) | 0.0001 | |||||
| Median (range) | 198,000 (10–449 × 103) | 52,000 (10–227 × 103) | 189,000 (39–369 × 103) | 256,000 (239–375 × 103) | 240,500 (43–449 × 103) | 0.0001 | |||||
| < 100,000 (no. per %) | 35 | 12.4 | 22 | 71.0 | 8 | 5.9 | 0 | – | 5 | 4.6 | 0.0001 |
| < 150,000 (no. per %) | 71 | 25.2 | 28 | 90.3 | 28 | 21.1 | 0 | – | 15 | 13.6 | 0.0001 |
| White cell count per mm3 | |||||||||||
| Mean (±2 SD) | 6.28 (3.8) | 3.3 (1.5) | 5.8 (2.8) | 8 (3.6) | 8.9 (4.1) | 0.0001 | |||||
| Median (range) | 6.2 (0.9–27.2) | 3.1 (1.9–6.7) | 5.6 (0.9–19.9) | 5.9 (5.9–12.2) | 8.4 (1.7–27.2) | 0.0001 | |||||
| < 5000 (no. per %) | 102 | 36 | 27 | 87.1 | 60 | 44.1 | 0 | 13 | 11.8 | 0.0001 | |
| > 11000 (no. per %) | 32 | 11 | 0 | 7 | 5.2 | 2 | 50 | 24 | 21.4 | 0.0001 | |
| Hematocrit | |||||||||||
| Median (min-max) | 39 (12.5–56.4) | 43.2 (26.2–56.4) | 39.3 (12.5–50.6) | 37.6 (34.3–43.9) | 37.5 (28.8–48.2) | 0.01 | |||||
| Hemoconcentrated (no. per %) | 2 | 0.7 | 1 | 3.2 | 1 | 0.7 | 0 | 0 | 0 | 0 | – |
| Chemistry (no. per %) | |||||||||||
| Hyponatremia (< 135) | 18 | 14.9 | 10 | 38.5 | 3 | 6 | 0 | 0 | 5 | 11.1 | 0.004 |
| Hyperkalemia (> 5) | 13 | 10.8 | 2 | 7.7 | 5 | 10.2 | 0 | 0 | 6 | 13.3 | 0.72 |
P value: compares laboratory-positive dengue with laboratory-positive influenza patients.
Discussion
In this study of the etiology of AFI during a ∼3-month period in the fall of 2009, we found that most patients presenting to a tertiary hospital in southern Puerto Rico had influenza A (H1N1) or dengue. We found that in the context of these two diseases occurring at the same time, clinical diagnosis was challenging because most influenza patients met the case definition for dengue and vice versa. This was not the first time that dengue and influenza have occurred simultaneously in Puerto Rico31–33 or in other locations.34 In Puerto Rico in 1977, simultaneous outbreaks of influenza and dengue caused considerable diagnostic confusion as nearly one in four suspected dengue cases reported to PDSS were found to have influenza A/Texas/1/77, and only nasal congestion/rhinitis distinguished influenza from dengue patients.33 In addition, hemorrhagic manifestations were reported in an equal proportion (∼10%) of laboratory-confirmed and laboratory-negative dengue cases.33
One striking characteristic of our patients with influenza H1N1 was a relatively high proportion with a hemorrhagic manifestation or thrombocytopenia. In addition, the mean platelet count was considerably lower in our group of H1N1 patients in comparison with patients in previous investigations.34–37 Influenza, however, has been associated with hemorrhagic manifestations, including a positive tourniquet test.32,38–40 In 1971, during an investigation of suspected DHF among children hospitalized in Burma, influenza A was found to be the etiologic agent in 58% of the 278 cases investigated.38 Of the 139 influenza cases for which clinical features were described, most had at least one hemorrhagic manifestation including hematemesis (89%), positive tourniquet test (47%), epistaxis (22%), and melena (12%). However, the authors of that study hypothesized that their findings may have been due in part to the wide-spread use of a traditional Burmese medicine containing salicylates, as patients with Chikungunya, measles, and other AFIs also had hemorrhagic manifestations.39 In 1975, several influenza A cases with hemorrhagic manifestations were identified during a DF outbreak in Fiji.40 And more recently, a simultaneous outbreak of dengue and influenza A (H1N1) was detected in rural Thailand.34
Although there can be considerable diagnostic confusion among dengue-like AFIs, an enhanced surveillance system can be useful to test for diseases that are not routinely surveyed and obtain laboratory confirmation to differentiate between etiologies during low incidence periods in endemic areas. On the other hand, knowledge of local dengue outbreaks can assist clinicians by increasing their index of suspicion for dengue among patients presenting from the affected sites. In our case, most laboratory-confirmed dengue cases resided in Villalba, a small municipality that is physically isolated from Ponce and the surrounding municipalities by a mountain range. Interestingly, DENV-4 was the most common serotype identified in our study, whereas DENV-1 was the most common serotype detected islandwide in 2009. This was probably because before 2009, DENV-4 had not been detected in southern Puerto Rico (and specifically Villalba) for almost 10 years,33,35 and because of this, residents < 10 years of age may have been particularly susceptible. In this study, most dengue patients ranged in age from 2 to 18 years.
Early clinical manifestations of leptospirosis are non-specific and similar to other AFI including dengue. In Puerto Rico, the burden of leptospirosis is not well defined. Surveillance is passive and intermittent, case confirmation with laboratory tests is uncommon, and only a small proportion of suspected cases are thought to be reported to the Puerto Rico Department of Health.41 A study conducted in 1997 estimated an overall annual incidence of 1 case per 100,000 inhabitants, and municipalities in the Ponce area, including Orocovis, Patillas, Las Marias, and Adjuntas, were thought to have the highest incidence of leptospirosis.41 In our study, we identified one case of leptospirosis. One limitation was that samples with insufficient volume remaining after dengue diagnostic testing and those that were positive for influenza virus were not tested for Leptospira. Nevertheless, leptospirosis is thought to be an important AFI in the differential diagnosis of dengue in Puerto Rico, as unrecognized leptospirosis is often identified as the cause of laboratory-negative dengue deaths.35 Because rapid diagnostic testing is limited for Leptospira, it is important for clinicians to identify risk factors for leptospirosis, such as contact with contaminated water, which may help to distinguish this condition from dengue.
This study has a number of limitations: 1) hospital-based surveillance underestimates the disease incidence, and the true burden of disease cannot be well characterized because not all patients seek medical attention at a hospital; 2) recruitment was limited in duration of time, which could have affected the observed incidence of some illnesses whose frequency vary throughout the year; 3) leptospirosis and EV could be underdiagnosed because only a subgroup of our patients were tested for these two pathogens; 4) patients with < 48 hours of fever were excluded; therefore, patients in the early stage of the disease were not recruited, with the exception of those who subsequently returned to the ED; 5) data are representative of only one hospital that receives patients mostly from one health region and does not represent the entire Puerto Rican population; and 6) data concerning the tourniquet test and laboratory test results were missing for a small group of patients.
In Puerto Rico, dengue seasonality follows a pattern with a high transmission period that begins as early as June and lasts until the end of November however information about the seasonality of other AFI is limited. The underlying causes of AFI are likely to vary substantially throughout the year and between years and in different geographic areas. One of our next steps is to establish an enhanced surveillance system for dengue-like illness in several hospitals in Puerto Rico during a longer period of time to determine the differential diagnosis of dengue and to establish the incidence, seasonality, and burden of these diseases. This system will be used as a platform to conduct epidemiologic, clinical, and laboratory research about pathogenesis, clinical outcomes, and approaches to therapy. Although many dengue-endemic countries have malaria, measles, rubella, and typhoid on the differential diagnosis of dengue, these conditions are uncommon in Puerto Rico because of eradication, immunization, improvements in water and sanitation.11,31,32,42–45 However, there are other conditions in which information is limited, including co-infections. Recently, a study conducted in Cambodia identified unique co-infections such as dengue and Burkholderia pseudomallei and Salmonella typhi46; through an enhanced surveillance system co-infections could be identified and data could be used to better understand clinical outcomes of patients who were laboratory positive for multiple etiologies. In addition, surveillance and improved knowledge among clinicians of patients' final diagnoses can be useful in improving physicians' clinical diagnosis skills and broadening their differential diagnosis, and assisting public health authorities in prioritizing limited resources.
Footnotes
Authors' addresses: Olga D. Lorenzi, Christopher J. Gregory, Luis Manuel Santiago, Elizabeth Hunsperger, Jorge Muñoz, and Kay M. Tomashek, Dengue Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, San Juan, Puerto Rico, E-mails: oal9@cdc.gov, hgk4@cdc.gov, zwt3@cdc.gov, enh4@cdc.gov, ckq2@cdc.gov, and kct9@cdc.gov. Héctor Acosta and Carlos García-Gubern, Ponce School of Medicine, Emergency Medicine Residency Program, Puerto Rico, E-mails: hacosta73@gmail.com and cgarcia@psm.edu. Ivonne E. Galarza, Ponce School of Medicine, Pediatric Residency Program, Ponce, Puerto Rico, E-mail: ivonnegalarza@hotmail.com. Duy M. Bui, Centers for Disease Control and Prevention, Zoonotic and Select Agent Laboratory, Atlanta, GA, E-mail: dbui85@gmail.com. M. Steven Oberste, Centers for Disease Control and Prevention, Respiratory, and Enteric Viruses Branch, Atlanta, GA, E-mail: mbo2@cdc.gov. Silvia Peñaranda, Centers for Disease Control and Prevention, Polio and Picornavirus Branch, Atlanta, GA, E-mail: sxp9@cdc.gov.
References
- 1.Afifi S, Earhart K, Azab M, Youssef F, Sakka H, Wasfy M, Mansour H, Oun SE, Rakha M, Mahoney F. Hospital-based surveillance for acute illness in Egypt: a focus on community-acquired bloodstream infections. Am J Trop Med Hyg. 2005;73:392–399. [PubMed] [Google Scholar]
- 2.Murdoch D, Woods C, Zimmerman M, Dull P, Belbase R, Keenan A, Scott RM, Archibald LK, Reller LB. The etiology of febrile illness in adults presenting to Patan Hospital in Kathmandu, Nepal. Am J Trop Med Hyg. 2004;70:670–675. [PubMed] [Google Scholar]
- 3.Parker T, Murray C, Richards A, Samir A, Ismail T, Fadeel M, Jiang J, Wasfy MO, Pimentel G. Concurrent infections in acute febrile illness patients in Egypt. Am J Trop Med Hyg. 2007;77:390–392. [PubMed] [Google Scholar]
- 4.Manock S, Jacobsen K, Brito N, Russell K, Negrete M, Olson J, Sanchez JL, Blair PJ, Smalligan RD, Quist BK, Espín JF, Espinoza WR, MacCormick F, Fleming LC, Kochel T. Etiology of acute undifferentiated febrile illness in the Amazon Basin of Ecuador. Am J Trop Med Hyg. 2009;81:146–151. [PubMed] [Google Scholar]
- 5.Archibald LK, den Dulk MO, Pallangyo KJ, Reller LB. Fatal Mycobacterium tuberculosis bloodstream infections in febrile hospitalized adults in Dar es Salaam, Tanzania. Clin Infect Dis. 1998;26:290–296. doi: 10.1086/516297. [DOI] [PubMed] [Google Scholar]
- 6.Ssali FN, Kamya MR, Wabwire-Mangen F, Kasasa S, Joloba M, Williams D, Mugerwa RD, Ellner JJ, Johnson JL. A prospective study of community-acquired bloodstream infections among febrile adults admitted to Mulago Hospital in Kampala, Uganda. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:484–489. doi: 10.1097/00042560-199812150-00007. [DOI] [PubMed] [Google Scholar]
- 7.Archibald LK, McDonald LC, Rheanpumikankit S, Tansuphaswadikul S, Chaovanich A, Eampokalap B, Banerjee SN, Reller LB, Jarvis WR. Fever and human immunodeficiency virus infection as sentinels for emerging mycobacterial and fungal bloodstream infections in hospitalized patients 15 years old, Bangkok. J Infect Dis. 1999;180:87–92. doi: 10.1086/314836. [DOI] [PubMed] [Google Scholar]
- 8.Archibald LK, McDonald LC, Nwanyanwu O, Kazembe P, Dobbie H, Tokars J, Reller LB, Jarvis WR. A hospital-based prevalence survey of bloodstream infections in febrile patients in Malawi: implications for diagnosis and therapy. J Infect Dis. 2000;181:1414–1420. doi: 10.1086/315367. [DOI] [PubMed] [Google Scholar]
- 9.Bell M, Archibald LK, Nwanyanwu O, Dobbie H, Tokars J, Kazembe PN, Reller LB, Jarvis WR. Seasonal variation in the etiology of bloodstream infections in a febrile inpatient population in a developing country. Int J Infect Dis. 2001;5:63–69. doi: 10.1016/s1201-9712(01)90027-x. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization Impact of Dengue, 2010. 2010. http://www.who.int/csr/disease/dengue/impact/en/index/html Global Alert and Response (GAR) Available at. Accessed June 2, 2010.
- 11.Franco RM, Casta Vélez A. Eradication of malaria in Puerto Rico [in Spanish] Am J Public Health. 1997;2:146–150. [PubMed] [Google Scholar]
- 12.Novel Swine-Origin Influenza A (H1N1), Virus Investigation Team Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 13.López-Rodríguez E, Tomashek K, Gregory CJ, Munoz J, Hunsperger E, Lorenzi OD, Irizarry JG, Garcia-Gubern C. Co-infection with dengue virus and pandemic (H1N1) 2009 virus. EID. 2010;16:882–883. doi: 10.3201/eid1605.091920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Passive Dengue Surveillance System Data. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 15.Caribbean Business Puerto Rico's Largest Private Hospitals. 2010. http://www.caribbeanbusinesspr.com Available at. Accessed June 1, 2010.
- 16.Kalayanarooj S, Vaughn DW, Nimmannitya S, Green S, Suntayakorn S, Kumentrasai N. Early clincal and laboratory indicators of acute dengue illness. JID. 1997;176:313–321. doi: 10.1086/514047. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization WHO Information for Laboratory Diagnosis of Pandemic (H1N1) 2009 Virus in Humans. 2009. http://www.who.int/csr/resources/publications/swineflu/WHO_Diagnostic_RecomendationsH1N1_ 20090521.pdf Revised 2009. Available at. Accessed July 31, 2010.
- 18.Johnson BW, Russell BJ, Lanciotti RS. Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. J Clin Microbiol. 2005;43:4977–4983. doi: 10.1128/JCM.43.10.4977-4983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bessoff K, Phoutrides E, Delorey M, Acosta LN, Hunsperger E. Defining the utility of a commercial NS1 antigen capture kit as a dengue virus (DENV) diagnostic test. Clin Vaccine Immunol. 2010;17:949–953. doi: 10.1128/CVI.00041-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dikken H, Kmety E. Serological typing methods of leptospires. In: Bergan T, Norris JR, editors. Methods in Microbiology. London: Academic Press; (1978). pp. 259–307. [Google Scholar]
- 21.Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol. 2006;44:2698–2704. doi: 10.1128/JCM.00542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miagostovich MP, Nogueira RM, dos Santos FB, Schatzmayr HG, Araújo ES, Vorndam V. Evaluation of an IgG enzyme-linked immunosorbent assay for dengue diagnosis. J Clin Virol. 1999;14:183–189. doi: 10.1016/s1386-6532(99)00059-1. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization . Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. Second edition. Geneva: World Health Organization; 1997. [Google Scholar]
- 24.The Harriet Lane Handbook . A Manual for Pediatric House Officers. Sixteenth edition. Philadelphia, PA: Mosby, Inc; 2002. [Google Scholar]
- 25.Hollowell JG, van Assendelft OW, Gunter EW, Lewis BG, Najjar M, Pfeiffer C. Hematological and iron-related analytes: reference data for persons aged 1 year and over: United States, 1988–94. Vital Health Stat. 2005;11:1–156. [PubMed] [Google Scholar]
- 26.Tietz Textbook of Clinical Chemistry. Second edition. St. Louis, MO: W.B. Saunders; 1999. [Google Scholar]
- 27.Lockitch G, Halstead AC, Quigley G, MacCallum C. Age- and sex-specific pediatric reference intervals: study design and methods illustrated by measurement of serum proteins with the Behring LN Nephelometer. Clin Chem. 1988;34:1618–1621. [PubMed] [Google Scholar]
- 28.World Health Organization . Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: World Health Organization; 2009. [PubMed] [Google Scholar]
- 29.Oberste MS, Peñaranda S, Rogers SL, Henderson E, Nix WA. Comparative evaluation of Taqman real-time PCR and semi-nested VP1 PCR for detection of enterovirus in clinical specimens. J Clin Virol. 2010;49:73–74. doi: 10.1016/j.jcv.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Romero JR. Reverse-transcription polymerase chain reaction detection of the enterovirus. Arch Pathol Lab Med. 1999;123:1161–1169. doi: 10.5858/1999-123-1161-RTPCRD. [DOI] [PubMed] [Google Scholar]
- 31.Gregory CJ, Santiago LM, Argüello DF, Hunsperger E, Tomashek K. Clinical and laboratory features that differentiate dengue from other febrile illness in an endemic area-Puerto Rico, 2007–2008. Am J Trop Med Hyg. 2010;82:922–929. doi: 10.4269/ajtmh.2010.09-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morens DM, Rigau-Perez JG, Lopez-Correa RH, Moore CG, Ruiz-Tiben EE, Sather GE, Chiriboga J, Eliason DA, Casta-Velez A, Woodall JP. Dengue in Puerto Rico, 1977: public health response to characterize and control an epidemic of multiple serotypes. Am J Trop Med Hyg. 1986;35:197–211. doi: 10.4269/ajtmh.1986.35.197. [DOI] [PubMed] [Google Scholar]
- 33.López-Correa RH, Moore CG, Sather GE, Morens DM, Chiriboga J, Banegura F, Woodall JP. The 1977 dengue epidemic in Puerto Rico: epidemiologic and clinical observations. Pan American Health Organization Scientific Publication. 1979;375:60–67. [Google Scholar]
- 34.Silarug N, Foy HM, Kudradinon S, Rojanasuphot S, Nisalak A, Pongsuwant Y. Epidemic of fever of unknown origin in rural Thailand, cause by influenza A (H1N1) and dengue fever. Southeast Asian J Trop Med Public Health. 1990;21:61–67. [PubMed] [Google Scholar]
- 35.Tomashek K, Rivera A, Muñoz-Jordan J, Hunsperger E, Santiago L, Padro O, Garcia E, Sun W. Description of a large island-wide outbreak of dengue in Puerto Rico, 2007. Am J Trop Med Hyg. 2009;81:467–474. [PubMed] [Google Scholar]
- 36.Kumar A, Zarychanski R, Pinto R, Cook D, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, Turgeon AF, Lapinsky S, Ahern SP, Smith O, Siddiqui F, Jouvet P, Khwaja K, McIntyre L, Menon K, Hutchison J, Hornstein D, Joffe A, Lauzier F, Singh J, Karachi T, Wiebe K, Olafson K, Ramsey C, Sharma S, Dodek P, Meade M, Hall R, Fowler RA. Canadian Critical Care Trials Group H1N1 Collaborative Critically ill patients with 2009 influenza A (H1N1) infection in Canada. JAMA. 2009;302:E1–E8. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 37.Dominguez-Cherit G, Lapinski S, Macias A, Pinto R, Espinoza-Pérez L, de la Torre A, Poblano-Morales M, Baltazar-Torres JA, Bautista E, Martinez A, Martinez MA, Rivero E, Valdez R, Ruiz-Palacios G, Hernández M, Stewart TE, Fowler RA. Critically ill patients with 2009 influenza A (H1N1) in Mexico. JAMA. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 38.Thein S, Ming CK, Thaung U, Shwe TN, Heng SB, Halstead SB. Hemorrhagic manifestations of influenza A infection in children. J Trop Med Hyg. 1975;78:78–80. [PubMed] [Google Scholar]
- 39.Thaung U, Ming CK, Thein S. Dengue hemorrhagic fever in Burma. Southeast Asian J Trop Med Public Health. 1975;6:580–591. [PubMed] [Google Scholar]
- 40.Kuberski TT. Hemorrhage associated with influenza. Lancet. 1977;1:709. doi: 10.1016/s0140-6736(77)92156-0. [DOI] [PubMed] [Google Scholar]
- 41.Bruce M, Sanders EJ, Leake JAD, Zaidel O, Bragg SL, Aye T, Shutt KA, Deseda CC, Rigau-Perez JG, Tappero JW, Perkins BA, Spiegel RA, Ashford DA. Leptospirosis among patients presenting with dengue-like illness in Puerto Rico. Acta Trop. 2005;96:36–46. doi: 10.1016/j.actatropica.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Lynch MF, Blanton EM, Bulens S, Polyak C, Vojdani J, Stevenson J, Medalla F, Barzilay E, Joyce K, Barrett T, Mintz ED. Typhoid fever in the United States, 1999–2006. JAMA. 2009;302:859–865. doi: 10.1001/jama.2009.1229. [DOI] [PubMed] [Google Scholar]
- 43.Measles occurrence in the Caribbean prior to the measles elimination campaign. CAREC Surveillance Report. 1991;17:1–6. [Google Scholar]
- 44.CDC Measles – Puerto Rico 1993, and the measles elimination program. Morb Mortal Wkly Rep. 1994;43:171–173. [PubMed] [Google Scholar]
- 45.Dietz VJ, Nieburg P, Gubler DJ, Gomez I. Diagnosis of measles by clinical case definition in dengue-endemic areas: implication for measles surveillance and control. Bull World Health Organ. 1992;70:745–750. [PMC free article] [PubMed] [Google Scholar]
- 46.Kasper MR, Blair PJ, Touch S, Sokhal B, Yasuda CY, Williams M, Richards AL, Burgess TH, Wierzba TF, Putnam SD. Infectious etiologies of acute febrile illness among patients seeking health care in south-central Cambodia. Am J Trop Med Hyg. 2012;86:246–253. doi: 10.4269/ajtmh.2012.11-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]