Abstract
A review of the laboratory-confirmed cases of Murray Valley encephalitis (MVE) from Western Australia between 2009 and 2011 was conducted to describe the clinical, laboratory, and radiological features of the disease. The nine encephalitis patients presented with altered mental state and seizures, tremor, weakness, or paralysis. All patients developed a raised C-reactive protein, whereas most developed acute liver injury, neutrophilia, and thrombocytosis. All patients with encephalitis developed cerebral peduncle involvement on early magnetic resonance imaging (MRI). The absence of thalamic MRI hyperintensity during the acute illness, with or without leptomeningeal enhancement, predicted a better neurological outcome, whereas those patients with widespread abnormalities involving the thalamus, midbrain, and cerebral cortex or the cerebellum had devastating neurological outcomes. MRI scans repeated months after acute illness showed destruction of the thalamus and basal ganglia, cortex, or cerebellum. These findings may help clinicians predict the neurological outcome when evaluating patients with MVE.
Introduction
Of the arboviruses endemic to Australia, the neurotropic flavivirus Murray Valley encephalitis virus (MVEV) causes the most serious, sometimes fatal, illness. It belongs to the Japanese encephalitis virus (JEV) antigenic complex, which includes West Nile virus (WNV) and Kunjin virus (KUNV), a subtype of WNV found in Oceania. MVEV was first recognized to cause outbreaks of encephalitis along the east coast of Australia early in the 20th century and subsequently, caused outbreaks in the Murray-Darling River basin in 1951 and 1974. Since that time, MVEV has been maintained in enzootic waterbird–mosquito cycles in the tropical areas of northern Western Australia (WA) and the Northern Territory (NT). Human cases are reported from these areas in most years but usually in low numbers.1,2 Despite the severity of disease and the lack of an effective vaccine or treatment,3 there is relatively little documented data on the clinical, laboratory, and radiological features of MVEV disease. Moreover, it is uncertain how these features predict outcome. This uncertainty is because of the small number of cases spread over long periods of time; also, many of the earlier cases had limited investigations and little detailed clinical information.4–6
In 2011, there was widespread MVEV activity in Australia with 16 encephalitis cases, including 9 cases acquired in WA. To better define the clinical, laboratory, and radiological features predictive of neurological outcome in MVEV infection, we conducted a review of all laboratory-confirmed MVEV cases hospitalized in WA in the 5 years from 2007 to 2011. This series is the first series of hospitalized MVEV encephalitis cases to include detailed laboratory and contemporary magnetic resonance imaging (MRI).
Methods
Laboratory-confirmed cases of MVEV infection were identified at PathWest Laboratory Medicine WA, which performs all MVEV diagnostic testing for WA. A retrospective review of the medical chart, radiology, and laboratory information system was conducted for these cases. Travel history was obtained for the 4 weeks before symptom onset to encompass the incubation period. Details of clinical features on presentation, inpatient investigations, and neurological status on discharge were obtained from the medical record and the hospital laboratory information system. Radiological evaluation was performed using both a 64-slice computerized tomography (CT) scanner and a 3-Tesla MRI scanner. Pre- and post-contrast images in the axial, coronal, and sagittal planes were obtained during CT assessment. Axial T2, axial T1, axial proton density, axial gradient echo, axial diffusion weighted and apparent diffusion coefficient, sagittal and coronal fluid-attenuated inversion recovery (FLAIR), and post-gadolinium contrast-administered axial, coronal, and sagittal T1 MRI sequences were obtained.
Cases were categorized as MVEV encephalitis or non-encephalitic MVEV infection according to the Australian national notifiable diseases case definitions,7 which require the exclusion of other flaviviridae.
Serological diagnosis.
Antibody in serum was measured by a standard hemagglutination inhibition (HI) assay8 using goose red blood cells and expressed as a titer. Cerebrospinal fluid (CSF) and serum immunoglobulin M (IgM) testing for MVEV, WNV/KUNV, JEV, and dengue virus was performed using an indirect immunofluorescence immunoassay4 and reported as positive, low positive, or negative. Testing for antibody specific to MVEV and/or WNV/KUNV was performed at the Arbovirus Research and Surveillance Laboratory, Department of Microbiology, University of Western Australia using a monoclonal antibody epitope-blocking enzyme immunoassay (EIA).8 Results were expressed as percentage inhibition of binding of a monoclonal antibody (mAb) specific for flavivirus group (envelope protein) and MVEV- or WNV/KUNV-specific epitopes (non-structural protein NS1) to virus antigen after pre-incubation with patient serum. The higher the percentage blocking of the MVEV- or WNV/KUNV-specific mAb, the more type-specific antibody present in the patient serum. There was insufficient serum from case 2 available to perform monoclonal blocking EIA.
Molecular diagnosis.
RNA was extracted from CSF and used as template for a nested reverse-transcribed polymerase chain reaction (RT-PCR) using primers spanning a 273-bp region in the envelope gene of MVEV.9 The subsequent MVEV-specific DNA was detected using agarose gel electrophoresis and visualized using ethidium bromide and ultraviolet (UV) light.
This study was approved by the Human Research and Ethics Committee, Sir Charles Gairdner Hospital.
Results
There were 12 patients hospitalized in WA with laboratory-confirmed MVEV infection between 2007 and 2011: 1 patient in 2008, 3 patients in 2009, and 8 patients in 2011. A 39-year-old man hospitalized in WA with clinically diagnosed MVEV encephalitis based on a consistent exposure history, clinical picture, and MRI was excluded because of negative MVEV serology and PCR tests. The patient had received the immunosuppressive mAb rituximab, which may have prevented antibody formation as has been reported with WNV encephalitis.10 Of 12 patients with laboratory-confirmed MVEV infection, 2 patients from northern WA, a 49-year-old man diagnosed in April of 2008 and a 2-year-old girl diagnosed in March of 2009, were transferred to a hospital outside WA and were not included in the analysis.
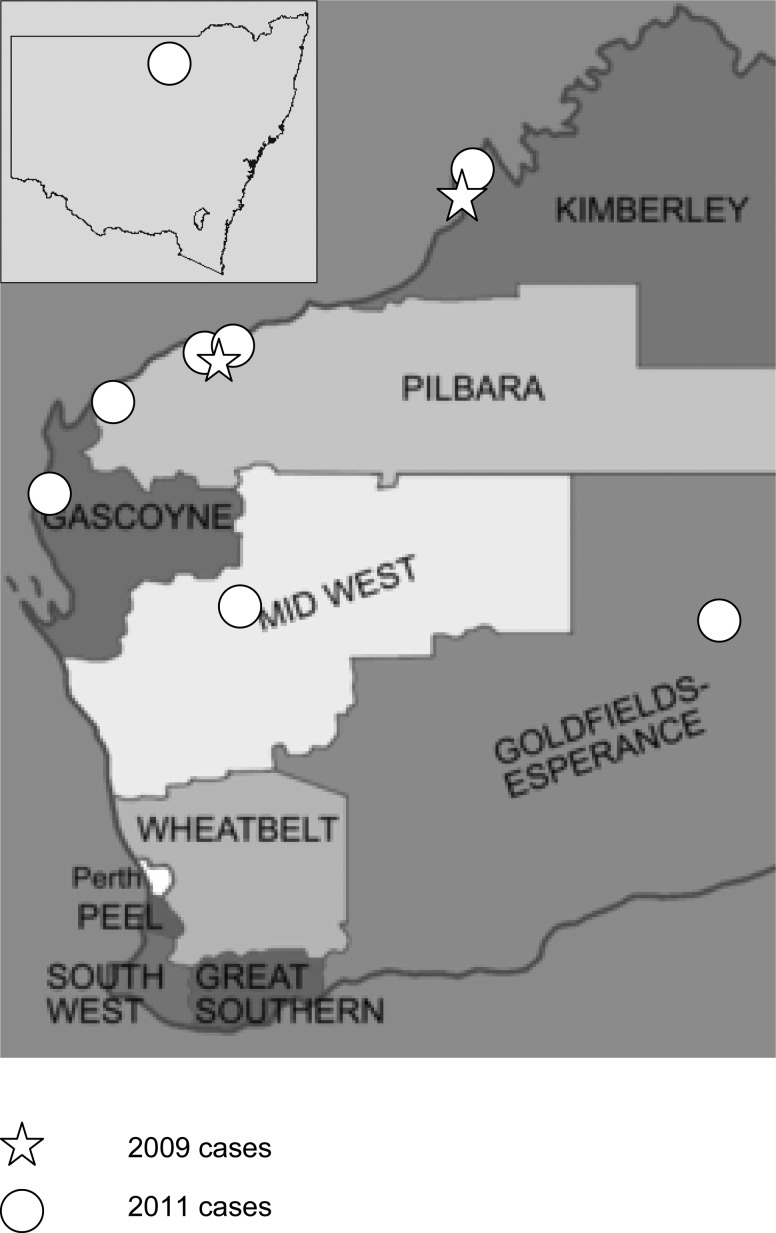
Therefore, 10 patients were included in the study (Table 1), and 9 of 10 patients were infected in WA, whereas the remaining 1 patient (case 3) was infected in New South Wales (NSW) but fell ill after traveling to WA (Figure 1). All patients acquired infection between March and May, the age range was 2–68 years, and there were two children and six males.
Table 1.
Demographics, length of stay, and investigations for hospitalized MVEV patients
| Feature | Case | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3* | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Demographics | ||||||||||
| Age (years) | 5 | 49 | 63 | 42 | 62 | 29 | 25 | 26 | 68 | 2 |
| Sex | M | M | F | F | M | M | M | M | F | F |
| Onset | March 2009 | May 2009 | March 2011 | March 2011 | April 2011 | April 2011 | April 2011 | April 2011 | May 2011 | May 2011 |
| Neurological presentation | ||||||||||
| Headache | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No |
| Confusion/disorientation | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Photophobia | Yes | No | No | No | No | Yes | No | Yes | Yes | No |
| Decreased conscious level | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Seizures | No | Yes | No | No | Yes | No | No | No | Yes | Yes |
| Meningism | No | Yes | No | No | No | No | No | No | Yes | Yes |
| Myoclonus/tremor | No | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No |
| Hyperreflexia | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Ataxia | No | Yes | No | Yes | No | N/A† | Yes | Yes | No | Yes |
| Paresis | – | Yes | No | Yes | No | – | Yes | No | No | Yes |
| Paralysis | Yes | No | No | No | No | Yes | No | No | No | No |
| Hospitalization (days) | ||||||||||
| Acute care | 102 | 85 | 9 | 30 | 18 (died) | 87 | 28 | 9 | 20 | 23 |
| Ventilation | Nil | 4 | Nil | Nil | 18 | 70 | 12 | Nil | 8 | 3 |
| Rehabilitation | 90 | 90 | Nil | 7 | 150 | 1 | 21 | 60 | Nil | |
| Investigations (RR) | ||||||||||
| Initial CSF parameters | ||||||||||
| WCC (< 5/cmm) | 47 | 98 | ND | 340 | 35 | 2 | 4,160 | 565 | 204 | 880 |
| MN/NT (%) | 50/50 | 95/5 | ND | 100/0 | 89/11 | 30/70 | 90/10 | 24/74 | 77/23 | 26/74 |
| RCC (/cmm) | 1 | 5 | ND | 540 | 3640 | 8 | 12,800 | 115 | < 1 | 16 |
| Protein (0.15–0.50 g/L) | 0.28 | 1.02 | ND | 0.64 | ND | 0.97 | 0.49 | 1 | 1.35 | 0.4 |
| Glucose (2.7–4.4 mmol/L) | 4 | 3.8 | ND | 2.6 | ND | 4.2 | 3 | 3 | 2.4 | 3.5 |
| Blood parameter peaks | ||||||||||
| Neutrophil (2.0–7.5 × 109/L) | 29.4 | 14.5 | 4.1 | 16.6 | 16.9 | 14.3 | 6.6 | 10.9 | 12.1 | 12.8 |
| Platelet (150–400 × 109/L) | 610 | 418 | 267 | 592 | 814 | 450 | 732 | 536 | 574 | 447 |
| ALT (< 40 U/L) | 23 | 175 | 89 | 120 | 709 | 212 | 560 | 102 | 64 | 58 |
| C-reactive protein (< 5 mg/L) | 52 | 310 | 19 | 71 | 91 | 200 | 430 | 30 | 89 | 168 |
| Creatine kinase (30–190 U/L) | ND | ND | ND | ND | 1,300 | 311 | 1,000 | ND | 79 | ND |
| MRI | ||||||||||
| Brain region involvement (days from onset) | Day 6: bilateral cerebral peduncle, basal ganglia and thalamus. Day 28: generalized parenchymal volume loss. Day 44: global parenchymal volume loss. | Day 9: thalamus, cerebral peduncle. Day 37: thalamus, globus pallidus. Day 630: cerebellar parenchymal volume loss. | Day 8: NAD. Day 10: NAD. Day 28: cerebral peduncle anterior, mesial temporal lobe. | Day 8: cerebral peduncle, thalamus, midbrain, mesial temporal lobe. | Day 4: thalamus, subthalamus, midbrain, corpus, callosum, middle cerebral lobe leptomeninges. Day 153: thalamus, subthalamus, cerebral peduncle, cerebral hemisphere, cerebellar hemisphere. | Day 5: leptomeninges, cerebral peduncles. | Day 21: cerebral peduncle. | Day 8: cerebral peduncle. | Day 5: leptomeninges, cerebral peduncle. | |
| Neurological deficits at discharge | Spastic quadriplegia, aphasia, dystonia | Ataxia, dysarthria, expressive dysphasia, impulsiveness | Nil | Cognitive impairment, sixth and seventh cranial nerve palsy, hypertonia | Died | Right upper limb flaccid, left upper limb and lower limb extensor posturing and hypertonicity, wheelchair-bound | Slowed cognition, poor gait, dysphagia, severe memory impairment | Poor memory, impaired mobility | Impaired cognition, mild dysphagia | Nil |
ALT = alanine aminotransferase; F = female; M = male; MN/NT = mononuclear/neutrophil ratio; NAD = no abnormality detected; RCC = red cell count; RR = reference range; WCC = white cell count.
Non-encephalitic case.
Paralysis too severe to assess.
Figure 1.
Location of MVEV acquisition.
Clinical findings.
All but one patient presented to the hospital within 5 days of illness onset, and the remaining adult suffered a prolonged headache for 2 weeks before presentation. The patient without encephalitis from NSW suffered prolonged fever but made a full recovery with no neurological sequelae after 9 days of acute care. Among the patients with encephalitis, altered mental state was universal, and the majority was febrile with shivers, headache, fatigue, and anorexia. Meningism was uncommon, but eight patients developed decreased conscious state.
Neither child developed tremor. One child (case 10) made a full recovery after intubation for 3 days during 23 days of acute care for intractable seizures. The second child (case 1) did not have seizures or require intubation but developed significant spastic quadriplegia necessitating 102 days of acute care and 90 days of inpatient rehabilitation.
Of seven adults with encephalitis, six developed tremor, six developed limb paresis or paralysis, three developed seizures, and two developed cranial nerve palsies. The period of acute care was variable (9–87 days), but six survivors required inpatient rehabilitation from 1 to 12 weeks. There was one fatality, with brain death declared after 18 days of illness. The surviving adults all suffered neurological deficits of varying severity.
Electroencephalographic findings.
Six adults and one child underwent electroencephalography (EEG) 6–18 days into illness, showing diffuse slowing without focal epileptiform features consistent with a generalized encephalopathy.
Laboratory findings.
Two children and two adults presented with a mild neutropenia without thrombocytopenia. Most cases progressed to neutrophilia and thrombocytosis with mild hyponatreamia but without significant renal impairment (Table 1). All cases except one child presented with or developed an elevated alanine aminotransferase (ALT) within days of admission associated with raised γ-glutamyl transferase. In contrast, the alkaline phosphatase was raised in only the fatal case with severe liver function derangement. Several patients developed significantly raised creatinine kinase levels to 1,000 U/L. The C-reactive protein was raised in all patients, often at presentation, peaking several days into hospital admission.
All but one patient with encephalitis had a CSF pleocytosis at the time of first lumbar puncture, but only six of nine patients showed a mononuclear predominance (Table 1). Protein levels in the CSF were mildly raised, and the average CSF glucose level was 2.9 mmol/L (41% of the corresponding blood level).
Anti-MVEV IgM was detected in the CSF from four patients but from the serum of all patients, and it was present for over 1 month in the adult and child with available convalescent blood (Table 2). MVEV-IgM but not WNV/KUNV-IgM was detected in the blood of one patient (case 4) but not in the CSF. Both MVEV and WNV/KUNV HI antibody and MVEV and WNV/KUNV-specific antibody using the monoclonal blocking EIA was present in the serum. This patient's serology suggested past WNV/KUNV and recent MVEV infection. All patients developed detectable HI antibody after 3–10 days, except for the fatality. Nested MVEV RT-PCR was attempted from this patient and three others with available CSF, but all were negative.
Table 2.
Flavivirus serology results for hospitalized MVEV
| Days from illness onset | CSF | Serum | Epitope-blocking EIA* (percent inhibition) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MVEV IgM | WNV/KUNV IgM | MVEV IgM | MVEV HI | WNV/KUNV IgM | WNV/KUNV HI | FLAVIVIRUS | MVEV | WNV/KUNV | |
| 1 | |||||||||
| 7 | POS | 1:80 | NEG | 1:40 | |||||
| 16 | POS | NEG | POS | 1:80 | NEG | 1:40 | |||
| 22 | POS | 1:640 | NEG | 1:160 | 38 | 41 | NEG | ||
| 38 | POS | 1:320 | NEG | 1:160 | |||||
| 2 | |||||||||
| 3 | NEG | NEG | POS | 1:80 | NEG | 1:80 | |||
| 9 | NEG | NEG | POS | 1:40 | NEG | 1:80 | |||
| 14 | POS | 1:80 | NEG | 1:80 | |||||
| 3 | |||||||||
| 8 | POS | 1:2,560 | NEG | ||||||
| 13 | POS | 1:2,560 | NEG | 1:20 | |||||
| 19 | 1:1,280 | 1:20 | 46 | 23 | NEG | ||||
| 4 | |||||||||
| 4 | LOW POS | 1:320 | NEG | 1:640 | |||||
| 5 | NEG | NEG | |||||||
| 12 | LOW POS | 1:640 | NEG | 1:20,480 | 94 | 72 | 81 | ||
| 5 | |||||||||
| 6 | LOW POS | < 1:10 | NEG | < 1:10 | |||||
| 10 | POS | 1:10 | NEG | 1:20 | |||||
| 13 | POS | NEG | < 1:10 | < 1:10 | 30 | 58 | 24 | ||
| 6 | |||||||||
| 2 | NEG | NEG | |||||||
| 3 | LOW POS | < 1:10 | NEG | < 1:10 | |||||
| 4 | POS | < 1:10 | NEG | < 1:10 | |||||
| 10 | POS | 1:20 | POS | 1:20 | |||||
| 18 | 1:80 | 1:80 | 48 | 71 | 25 | ||||
| 7 | |||||||||
| 4 | NEG | NEG | |||||||
| 6 | POS | 1:10 | NEG | 1:20 | |||||
| 10 | POS | 1:80 | NEG | 1:160 | 50 | 90 | 5 | ||
| 8 | |||||||||
| 12 | POS | NEG | POS | 1:40 | NEG | 1:10 | |||
| 16 | POS | 1:320 | 1:40 | ||||||
| 37 | POS | 1:160 | NEG | 1:40 | 55 | 74 | NEG | ||
| 9 | |||||||||
| 3 | NEG | NEG | |||||||
| 7 | POS | 1:320 | NEG | 1:320 | 73 | 4 | NEG | ||
| 10 | |||||||||
| 1 | POS | < 1:10 | NEG | < 1:10 | |||||
| 5 | POS | NEG | |||||||
| 8 | POS | 1:20 | NEG | 1:10 | 50 | 73 | NEG | ||
| 17 | 1:320 | 1:80 | |||||||
LOW POS = low positive; NEG = negative; POS = positive.
Insufficient serum for case 2.
Radiological findings and neurological outcomes.
None of the seven patients who underwent CT scanning in the first week of illness had focal abnormalities, but one child's CT (case 10) showed leptomeningeal enhancement. In contrast, abnormal MRI signal characteristics affecting the cerebral peduncles bilaterally were shown in all encephalitic patients. In two adults (cases 8 and 9), this abnormality was the only MRI abnormality. Both patients experienced mild neurological deficits, which included cognitive/memory impairment, dysphagia, and gait abnormalities.
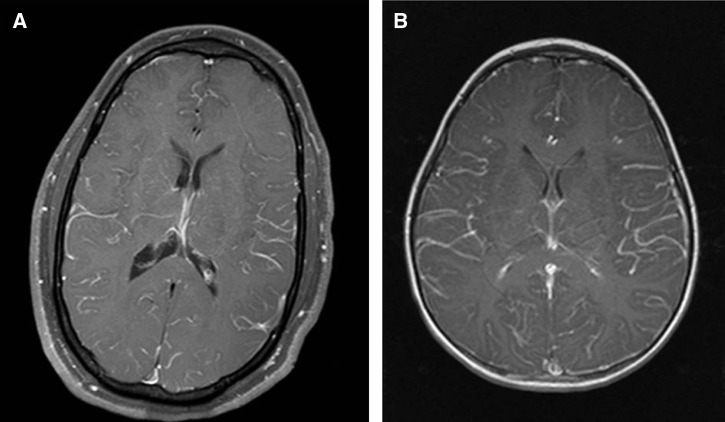
Diffuse MRI leptomeningeal enhancement (Figure 2) was shown in an adult and child. The child (case 10) had seizures with secondary Todd's paresis but no neurological deficits at discharge, whereas the adult (case 7) experienced slowed cognition, poor gait, memory impairment, and dysphagia. Neither of these patients required inpatient rehabilitation after acute care.
Figure 2.
Leptomeningeal involvement in MVEV. (A) Case 7 T1 post-contrast MRI and (B) case 10 T1 post-contrast MRI 5 days after illness onset showing generalized leptomeningeal enhancement.
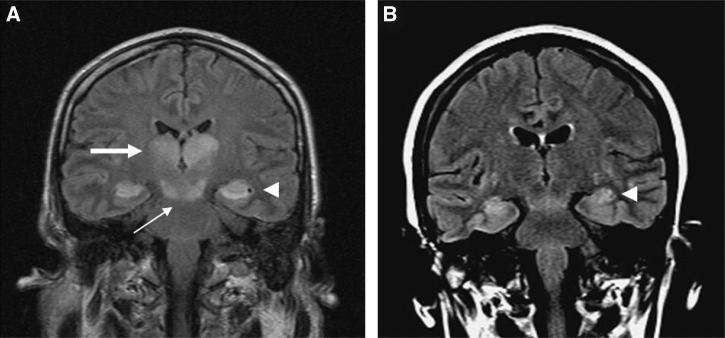
One adult (case 4) had an unremarkable MRI on days 8 and 10, but a repeat MRI at 28 days showed signal changes within the cerebral peduncles, the anterior temporal lobes, and right mesial temporal lobe (Figure 3) with non-specific subcortical white matter signal. This patient developed a sixth and seventh cranial nerve palsy acutely and had residual cognitive dysfunction and hypertonia.
Figure 3.
MVEV of the temporal lobes. (A) Case 5 FLAIR MRI 8 days after illness onset showing bithalamic (large arrow), midbrain (small arrow), and mesial temporal lobe (arrowhead) involvement. (B) Case 4 FLAIR MRI 28 days after illness onset showing anterior temporal lobe involvement (arrowhead).
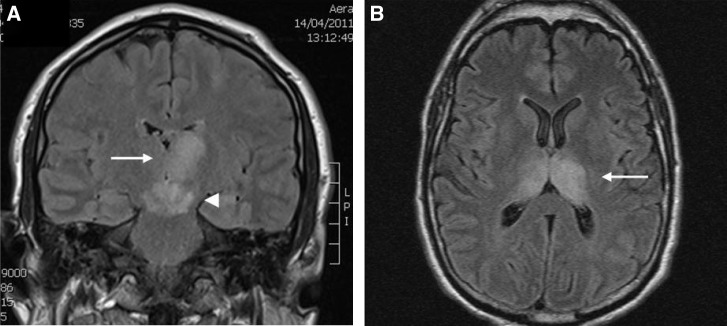
More severe neurological outcomes were associated with unilateral or bilateral thalamic T2 signal hyperintensity 4–9 days after illness onset (Figure 4) in conjunction with basal ganglia and cerebellar (case 2) or cortical (cases 1, 5, and 6) abnormalities. In one patient (case 2), globus pallidum involvement was detected 28 days after the initial MRI showed thalamic and cerebral peduncle abnormalities. This patient required prolonged acute care and inpatient rehabilitation for ataxia, dysarthria, expressive dysphasia, and impulsiveness. MRI 21 months later showed cerebellar parenchymal volume loss.
Figure 4.
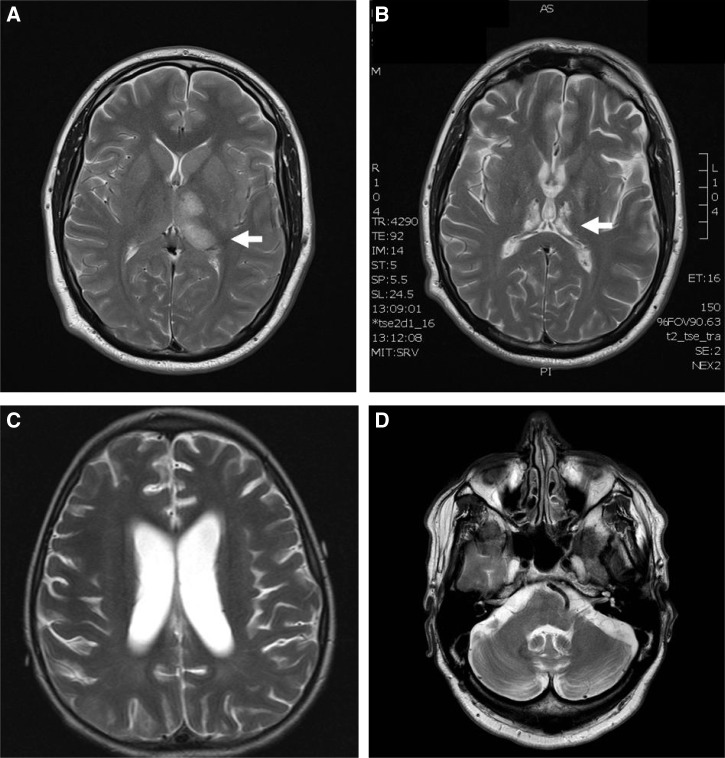
MRI images from severe MVEV. (A) Case 6 FLAIR MRI 4 days and (B) case 2 FLAIR MRI 37 days after illness onset showing high signal in the thalami (arrows), subthalamus, red nuclei, and substantia nigra (arrowhead).
Patients with widespread brainstem and cortical MRI abnormalities had devastating neurological outcomes. The child with bilateral thalamic and cerebral peduncle involvement on MRI 6 days after illness onset (case 1) progressed to global parenchymal volume loss of the cortex, white matter, and deep grey matter 5 weeks later. He required prolonged acute care and inpatient rehabilitation for spastic quadriplegia, aphasia, and dystonia. The adult (case 5) with extensive bilateral thalamic signal abnormality extending into the midbrain and the mesial aspect of temporal lobes bilaterally (Figure 3) died 18 days after illness onset. The second adult (case 6) showed T2 hyperintensity involving the left thalamus, the subthalamic nuclei, the red nuclei, and the substantia nigra within the midbrain bilaterally with additional foci within the splenium of the corpus callosum and the cortex of the left middle frontal gyrus. An MRI study performed 5 months later showed bithalamic and subthalamic nuclei destruction in conjunction with moderate cerebral and cerebellar parenchymal volume loss (Figure 5). This patient remained wheelchair-bound with unilateral upper limb flaccid paralysis and lower limb hypertonic paresis 8 months after illness onset.
Figure 5.
Longer-term changes in MVEV. (A) Case 6 T2 MRI 4 days and (B) 5 months after illness onset showing progression from bithalamic inflammation to parenchymal volume loss (arrows). (C) Case 1 T2 MRI 44 days after illness onset showing widespread grey and white matter cerebral parenchymal volume loss. (D) Case 2 T2 MRI 21 months after illness onset showing cerebellar parenchymal volume loss.
Discussion
MVEV is enzootic in the sparsely populated northern WA and NT regions of Australia, where significant water bodies are present and the ambient temperature is sufficient for the mosquito breeding cycle and virus replication.6,11 In contrast to WNV and JEV, the number of symptomatic MVEV infections after seasonal heavy rainfall from January to May12 each year is small.
Heavy rainfall in traditionally arid regions can extend the risk area of MVEV to more southern reaches of Australia, which happened in 20006,13 and again, in 2011, when 16 confirmed MVEV cases occurred across Australia.3 This series is the first series detailing laboratory investigations and MRI in hospitalized MVEV and complements the individual cases published to date.14–18
The clinical prodrome, neurological findings, EEG, CSF, and serological parameters were similar to previous MVEV case series,4–6,13,19 illustrating how MVEV encephalitis can present with or without either a CSF neutrophilic or lymphocytic pleocytosis. In the 54 MVEV cases reported since 1978, seizures have been prominent in children but reported in only two adults.4,5 Three of our adults with encephalitis developed seizures, suggesting that this presentation may be more common in adults than originally thought.
We found that anti-MVEV IgM was more prevalent in serum than CSF and appeared before HI antibody, confirming that serum anti-MVEV IgM is the most sensitive antibody test early in MVEV disease.5 One patient showed the serological profile of recent MVEV and previous WNV/KUNV infection confirmed by monoclonal-blocking EIA. Nested MVEV RT-PCR was attempted from CSF from four patients with sufficient CSF available, but none were positive. This difficulty in detecting MVEV RNA from CSF has been found by others,14,20,21 suggesting low virus concentrations during acute encephalitis.
In the two previous MVEV cases documenting biochemical and hematological parameters, peripheral leukocytosis developed in both,14,15 but liver function and C-reactive protein were reported as normal. In contrast, we found peripheral neutrophilia to be a common feature, often occurring with thrombocytosis, along with raised creatine kinase levels, including in those patients without seizures. All of our patients developed raised C-reactive protein, and in all but one child, acute liver injury manifested by raised ALT and γ-glutamyl transferase. Interestingly, the alkaline phosphatase was raised only in the fatal case, where significant liver function derangement occurred before death. These abnormalities have not been previously described in MVEV infection. Acute liver injury, raised creatine kinase, and peripheral leukocytosis are common features of WNV encephalitis,22 and raised ALT levels have been reported in JEV infection.23
Cranial CT scans are often normal at the time of presentation in flavivirus encephalitis,5,14,15,17,18,24 but they may show reduced attenuation in the thalami16,17 or temporal lobes15 or show diffuse cerebral edema.25 Only one of our patients had an abnormal CT scan early in the illness, confirming that this modality lacks the sensitivity to detect the early changes of MVEV encephalitis.
Five recent MVEV cases15–18,25 had variable acute MRI changes of thalamic, basal ganglia, brainstem, subcortical structures, and temporal lobe focal T2 hyperintensity. All of our encephalitic patients had bilateral cerebral peduncle MRI involvement and when found in isolation or with leptomeningeal enhancement, correlated with mild to moderate neurological deficits. Ali and others,26 in their series of adult WNV encephalitis, found that leptomeningeal enhancement correlated with more neurological deficits, including memory impairment. This finding was true for our adult patient but not the child, suggesting that this finding may be an age-related phenomenon. One of our patients had temporal lobe but not thalamic involvement, resulting in cognitive difficulties. This presentation, more characteristic of herpes simplex encephalitis, may occur in MVEV15 and JEV27 infection and is less likely to result in motor disturbances.
Unilateral or bilateral thalamic involvement on T2 and FLAIR MRI is a characteristic feature of flavivirus encephalitis23,26–28 and correlates with MVEV encephalitis autopsy findings of focal thalamic destruction.29 Almost one-half of our encephalitis patients showed unilateral or bilateral thalamic high T2 signal intensity with variable extension to the globus pallidi, red nuclei, and substantia nigra within the midbrain, the corpus callosum, and the temporal or frontal cortex. The extent of involvement beyond the thalami correlated with the severity of neurological compromise, but thalamic involvement indicated at least moderate neurological deficits.
Widespread early MRI abnormalities of the thalami, brainstem, and cortical and subcortical structures indicated a devastating neurological outcome with either spastic quadriparesis or brain death. Flaccid paralysis in an adult18 and spastic quadriparesis in a child20 have been found in MVEV infection and are known to occur in 5–15% of neuroinvasive WNV infection.30 Early brainstem-increased T2 signal followed by global parenchymal cortical, white matter, and deep grey matter volume loss after 5 weeks likely represent the MRI evolution of MVEV neurological disease. The one fatality among our patients is consistent with the reported 20% MVEV encephalitis mortality rate.2
Repeat MRI several weeks after initial scanning is recommended to better show the true extent of brain involvement, because this MRI showed new signal abnormalities in several of our patients. More MRI months after acute illness better represents the more permanent neurological deficits with destruction and parenchymal volume loss of the thalami and basal ganglia, cerebral cortex, or cerebellum seen in our severely affected patients.
In summary, there were similarities to the disease patterns and MRI seen with other neurotropic flaviviridae, such as WNV and JEV. However, we found peripheral blood neutrophilia, thrombocytosis, raised ALT, and creatine kinase to be common and raised C-reactive protein to be universal. In addition, there was universal involvement of the cerebral peduncles with thalamic focal FLAIR and T2 hyperintensity on MRI early in the disease process associated with more significant neurological compromise. More extensive MRI signal abnormalities beyond the thalami involving the basal ganglia and cortical structures predicted devastating neurological outcomes.
Long-term inpatient rehabilitation is recommended for those MVEV cases with significant neurological compromise, because it has been shown to significantly improve function after severe WNV encephalitis.31
These findings may help clinicians in terms of prognosis in acute MVEV encephalitis.
ACKNOWLEDGMENTS
The authors thank the Arbovirus Research and Surveillance Laboratory, Department of Microbiology, University of Western Australia for performance of the monoclonal epitope-blocking EIA testing and Dr. Bradley Wood for help with the MRI interpretations.
Footnotes
Authors' addresses: David J. Speers, Department of Microbiology, PathWest Laboratory Medicine WA, Nedlands, Western Australia, Australia, and School of Medicine and Pharmacology, University of Western Australia, Crawley, Western Australia, Australia, E-mail: david.speers@health.wa.gov.au. James Flexman, Department of Microbiology, PathWest Laboratory Medicine WA, Royal Perth Hospital, Perth, Western Australia, Australia, E-mail: james.flexman@health.wa.gov.au. Christopher C. Blyth, Princess Margaret Hospital for Children, Subiaco, Western Australia, Australia, and School of Paediatrics and Child Health, University of Western Australia, Crawley, Western Australia, Australia, E-mail: christopher.blyth@uwa.edu.au. Nirooshan Rooban, Sir Charles Gairdner Hospital, Nedlands, Western Australia, Australia, E-mail: nirooshan@doctors.org.uk. Edward Raby, Department of Microbiology, PathWest Laboratory Medicine WA, Fremantle Hospital, Fremantle, Western Australia, Australia, E-mail: edward.raby@health.wa.gov.au. Ganesh Ramaseshan, Fremantle Hospital, Fremantle, Western Australia, Australia, E-mail: katcheri@yahoo.com.au. Susan Benson, School of Pathology and Laboratory Medicine, University of Western Australia, Crawley, Western Australia, Australia, E-mail: susan.benson@iinet.net.au. David W. Smith, Department of Microbiology, PathWest Laboratory Medicine WA, Nedlands, Western Australia, Australia, and School of Pathology and Laboratory Medicine, University of Western Australia, Crawley, Western Australia, Australia, E-mail: david.smith@health.wa.gov.au.
References
- 1.Spencer JD, Azoulas J, Broom AK, Buick TD, Currie B, Daniels PW, Doggett SL, Hapgood GD, Jarrett PJ, Lindsay MD, Lloyd G, Mackenzie JS, Merianos A, Moran RJ, Ritchie SA, Russell RC, Smith DW, Stenhouse FO, Whelan PI. Murray Valley encephalitis virus surveillance and control initiatives in Australia. National Arbovirus Advisory Committee of the Communicable Diseases Network Australia. Commun Dis Intell. 2001;25:33–47. [PubMed] [Google Scholar]
- 2.Smith DW, Speers DJ, Mackenzie JS. The viruses of Australia and the risk to tourists. Travel Med Infect Dis. 2011;9:113–125. doi: 10.1016/j.tmaid.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Knox J, Cowan RU, Doyle JS, Ligtermoet MK, Archer JS, Burrow JNC, Tong SYC, Currie BJ, Mackenzie JS, Smith DW, Catton M, Moran RJ, Aboltins CA, Richards JS. Murray Valley encephalitis: a review of clinical features, diagnosis and treatment. Med J Aust. 2012;196:322–325. doi: 10.5694/mja11.11026. [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie JS, Smith DW, Broom AK, Bucens MR. Australian encephalitis in Western Australia, 1978–1991. Med J Aust. 1993;158:591–595. doi: 10.5694/j.1326-5377.1993.tb137623.x. [DOI] [PubMed] [Google Scholar]
- 5.Burrow JN, Whelan PI, Kilburn CJ, Fisher DA, Currie BJ, Smith DW. Austalian encephalitis in the Northern Territory: clinical and epidemiological features, 1987–1996. Aust N Z J Med. 1998;28:590–596. doi: 10.1111/j.1445-5994.1998.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 6.Cordova SP, Smith DW, Broom AK, Lindsay MD, Dowse GK, Beers MY. Murray Valley encephalitis in Western Australia in 2000, with evidence of southerly spread. Commun Dis Intell. 2000;24:368–372. [PubMed] [Google Scholar]
- 7.Communicable Diseases Network of Australia Australian National Notifiable Diseases Case Definitions. Commonwealth Department of Health and Ageing. 2012. http://www.health.gov.au/internet/main/publishing.nsf/content/cdna-casedefinitions.htm Available at. Accessed March 5, 2012.
- 8.Hall RA, Broom AK, Hartnett AC, Howard MJ, Mackenzie JS. Immunodominant epitopes on the NS1 protein of MVE and KUN viruses serve as targets for a blocking ELISA to detect virus-specific antibodies in sentinel animal serum. J Virol Methods. 1995;51:201–210. doi: 10.1016/0166-0934(94)00105-p. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka M. Rapid identification of flavivirus using the polymerase chain reaction. J Virol Methods. 1993;41:311–322. doi: 10.1016/0166-0934(93)90020-r. [DOI] [PubMed] [Google Scholar]
- 10.Levi ME, Quan D, Ho JT, Kleinschmidt-DeMasters BK, Tyler KL, Grazia TJ. Impact of rituximab-associated B-cell defects on West Nile virus meningoencephalitis in solid organ transplant recipients. Clin Transplant. 2010;24:223–228. doi: 10.1111/j.1399-0012.2009.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broom AK, Lindsay MD, Harrington SA, Smith DW. Investigation of the southern limits of Murray Valley encephalitis activity in Western Australia during the 2000 wet season. Vector Borne Zoonotic Dis. 2002;2:87–95. doi: 10.1089/153036602321131887. [DOI] [PubMed] [Google Scholar]
- 12.Schuster G, Ebert EE, Stevenson MA, Corner RJ, Johansen CA. Application of satellite precipitation data to analyse and model arbovirus activity in the tropics. Int J Health Geogr. 2011;10:8. doi: 10.1186/1476-072X-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown A, Bolisetty S, Whelan P, Smith D, Wheaton G. Reappearance of human cases due to Murray Valley encephalitis virus and Kunjin virus in Central Australia after an absence of 26 years. Commun Dis Intell. 2002;26:39–44. [PubMed] [Google Scholar]
- 14.Stich A, Gunther S, Drosten C, Emmerich P, Dwyer DE, Hueston L, Hetzel W, Kirschner A, Fleischer K. Clinical and laboratory findings on the first imported case of Murray Valley encephalitis in Europe. Clin Infect Dis. 2003;37:19–21. doi: 10.1086/375068. [DOI] [PubMed] [Google Scholar]
- 15.Wong SH, Smith DW, Fallon MJ, Kermode AG. Murray Valley encephalitis mimicking herpes simplex encephalitis. J Clin Neurosci. 2005;12:822–824. doi: 10.1016/j.jocn.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Kienzle N, Boyes L. Murray Valley encephalitis: case report and review of neuroradiological features. Australas Radiol. 2003;47:61–63. doi: 10.1046/j.1440-1673.2003.01105.x. [DOI] [PubMed] [Google Scholar]
- 17.Einsiedel L, Kat E, Ravindran J, Slavotinek J, Gordon DL. MR findings in Murray Valley encephalitis. AJNR Am J Neuroradiol. 2003;24:1379–1382. [PMC free article] [PubMed] [Google Scholar]
- 18.Douglas MW, Stephens DP, Burrow JNC, Anstey NM, Talbot K, Currie BJ. Murray Valley encephalitis in an adult traveler complicated by long-term flaccid paralysis: case report and review of the literature. Trans R Soc Trop Med Hyg. 2007;101:284–288. doi: 10.1016/j.trstmh.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Mackenzie JS, Smith DW, Broom AK, Bucens MR. Australian encephalitis in Western Australia, 1978–1991. Med J Aust. 1993;158:591–595. doi: 10.5694/j.1326-5377.1993.tb137623.x. [DOI] [PubMed] [Google Scholar]
- 20.Hills S. Murray Valley encephalitis in Mt Isa, north Queensland. Commun Dis Intell. 2001;25:48. [PubMed] [Google Scholar]
- 21.Bennett NM. Murray Valley encephalitis. Commun Dis Intell. 2001;25:154–155. [PubMed] [Google Scholar]
- 22.Murray KO, Baraniuk S, Resnick M, Arafat R, Kilborn C, Shallenberger R, York TL, Martinez D, Malkoff M, Elgawley N, Mcneely W, Khuwaja SA. Clinical investigation of hospitalized human cases of West Nile virus infection in Houston, Texas, 2002–2004. Vector Borne Zoonotic Dis. 2008;8:167–174. doi: 10.1089/vbz.2007.0109. [DOI] [PubMed] [Google Scholar]
- 23.Misra UK, Kalita J, Goel D, Mathur A. Clinical, radiological and neurophysiological spectrum of JEV encephalitis and other non-specific encephalitis during post-monsoon period in India. Neurol India. 2003;51:55–59. [PubMed] [Google Scholar]
- 24.Zak IT, Altinok D, Merline JR, Chander S, Kish KK. West Nile virus infection. Am J Roentgenol. 2005;184:957–961. doi: 10.2214/ajr.184.3.01840957. [DOI] [PubMed] [Google Scholar]
- 25.McMinn P, Carman P, Smith DW. Early diagnosis of Murray Valley encephalitis by reverse transcriptase-polymerase chain reaction. Pathology. 2000;32:49–51. doi: 10.1080/003130200104583. [DOI] [PubMed] [Google Scholar]
- 26.Ali M, Safriel Y, Sohi J, Llave A, Weathers S. West Nile virus infection: MR imaging findings in the nervous system. AJNR Am J Neuroradiol. 2005;26:289–297. [PMC free article] [PubMed] [Google Scholar]
- 27.Handique SK, Das RR, Barman K, Medhi N, Saharia B, Saikia P, Ahmed SA. Temporal lobe involvement in japanese encephalitis: problems in differential diagnosis. AJNR Am J Neuroradiol. 2006;27:1027–1031. [PMC free article] [PubMed] [Google Scholar]
- 28.Petropoulou KA, Gordon SM, Prayson RA, Ruggierri PM. West Nile virus meningoencephalitis: MR imaging findings. AJNR Am J Neuroradiol. 2005;26:1986–1995. [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson EG. Murray Valley encephalitis; pathological aspects. Med J Aust. 1952;1:107–110. [PubMed] [Google Scholar]
- 30.Sejvar JJ, Haddad MB, Tierney BC. Neurologic manifestations and outcome of West Nile virus infection. JAMA. 2003;290:511–515. doi: 10.1001/jama.290.4.511. [DOI] [PubMed] [Google Scholar]
- 31.Rao N, Char D, Gnatz S. Rehabilitation outcomes of 5 patients with severe West Nile infection: a case series. Arch Phys Med Rehabil. 2005;86:449–452. doi: 10.1016/j.apmr.2004.10.019. [DOI] [PubMed] [Google Scholar]