Abstract
Temperature is one of the most important environmental factors affecting biological processes of mosquitoes, including their interactions with viruses. In these studies, we show independent effects of rearing temperature on the immature aquatic stages and holding temperature on the adult terrestrial stage in terms of alterations in adult survival and progression of dengue-1 virus infection in the Asian tiger mosquito Aedes (Stegomyia) albopictus. Our studies show that adult survival was determined by adult-holding temperature, regardless of rearing conditions of the immature stages. In contrast, spread of virus throughout the body of the mosquito, a pre-requisite for transmission, was reduced when the immature stages were reared in cool conditions. These results show that immature-rearing temperature selectively modified mosquito traits that influence competency for viruses, and they further our understanding of the nature of temperature effects on interactions between mosquitoes and virus pathogens and risk of disease transmission.
Introduction
Biological invasions pose a major threat to ecological communities. Improving our understanding about underlying processes affecting populations of introduced species and changes to communities after introduction will assist in minimizing negative effects on native species. Invasive vector species such as mosquitoes not only have the potential to disrupt communities but also represent an epidemiological concern. Human-aided shipments of used tires have facilitated the spread of Asian tiger mosquito Aedes (Stegomyia) albopictus, an invasive species capable of transmitting numerous arthropod-borne (arbo) viruses.1 Originally found in Southeast Asia, the geographic range of Ae. albopictus has greatly expanded. In recent years, the public health concern of Ae. albopictus has become a reality as witnessed by its incrimination as the vector responsible for outbreaks of dengue in Hawaii and chikungunya virus in India, La Reunion, Italy, and South Asian countries.2–6 Ae. albopictus is considered a maintenance vector of dengue viruses, is occasionally involved in epidemics in Asia, and found to be naturally infected with dengue virus in the Americas.7,8 However, the role of Ae. albopictus in dengue virus transmission is likely to be less than the role of the primary mosquito vector Ae. (Stegomyia) aegypti.9 In North America, Ae. albopictus has been found to be infected with West Nile virus, LaCrosse virus, and Eastern equine encephalitis virus in nature.10–12 However, this species does not seem to be the predominant vector of these arboviruses in North America.
Expanded range of Ae. albopictus directly increases risk for vector-borne diseases (e.g., dengue and chikungunya viruses) but also, may indirectly alter risk of disease transmission as mediated through interactions with other mosquito species (e.g., competitive displacement of other vector species; Yellow fever mosquito Ae. aegypti).13–17 Temperature likely plays an important role in the distribution pattern of Ae. albopictus, which is by similar Northern isotherm limits of distribution in its native range in Asia and invaded range in North America.18
Temperature is regarded as one of the most important abiotic environmental factors affecting biological processes of mosquitoes, including interactions with arboviruses. Seasonal and geographic differences in temperature and anticipated climate change undoubtedly influence mosquito population dynamics, individuals' traits related to vector biology (lifespan and vector competence for arboviruses), and disease transmission patterns. Increases in adult-holding temperatures have usually been associated with enhanced vector competence.19–27 However, some studies have identified reduced vector competence and activity in nature associated with increases in temperature (western equine encephalitis virus [WEEV] and St. Louis encephalitis virus [SLEV]).28–31 Additionally, cool rearing temperature of the immature stages may be associated with reduced virus infection and dissemination in the adult stage.32,33 It has long been recognized that increases in temperature reduce the extrinsic incubation period (the time from initial acquisition of pathogens until transmission is possible).19,20 However, environmental temperature may also influence the expression of modulation, limiting virus replication and dissemination.28 Along the same lines, increases in temperature reduce the adult lifespan of mosquitoes and may impinge transmission. Temperature effects may drastically alter risk of disease transmission, especially under conditions where the extrinsic incubation period approaches the lifespan of the mosquito. For dengue viruses, several studies have identified the role of temperature in infection and transmission by Ae. albopictus and Ae. aegypti mosquitoes and its role on the incidence of dengue in nature.34–37 Despite the long history of relating temperature to vector biology, relatively few studies have evaluated the net effect of temperature on multiple traits (adult lifespan and vector competences for viruses) as it relates to risk of disease transmission.
Ae. albopictus as well as other holometabolous insect vectors of pathogens occupy entirely different environmental niches during the immature and adult stages. Because the immature stages of container mosquitoes are confined to aquatic habitats, the microclimate experienced by the immature stages of Ae. albopictus is largely determined by the placement of containers in nature and whether containers are exposed to direct sunlight for part of the day or mostly shade. In contrast, the adult stage is mobile, and diel temperature regimen is associated with mosquito activity, including movement between habitats.38–41 Thus, shifts in environmental temperature between the immature and adult stages may be common in nature. Environmental temperature acting on the immature stages shapes the adult phenotype (e.g., nutritional reserves)42–44 and therefore, may also modify traits of adults associated with ability to transmit arboviruses (e.g., lifespan and susceptibility to virus infection). Temperature-associated changes in morphology and physiology may translate to altered permissibility of midgut and salivary gland barriers that must be overcome for virus transmission to subsequent hosts.
Here, we addressed the independent effects of temperature on immature and adult stages in relation to life history traits and interactions with dengue-1 virus. Specifically, we hypothesize that cool rearing temperature of immature stages (1) buffers against life-shortening effects of warm holding conditions of adults and (2) reduces rates of dengue-1 virus infection and dissemination of adults.
Materials and Methods
Field-collected larvae from discarded tires were reared to adulthood in enamel pans with ∼1.0 L tap water and 0.2 g equal mixture of brewers yeast and lactalbumin as food resources. Food resources were renewed on a weekly basis. Adults were maintained in 0.32-m3 cages with access to a 20% sucrose solution and weekly bloodmeals from guinea pigs and chickens. Eggs were collected on paper towels that lined cups with water placed inside the cages. Mosquitoes were kept in climate-controlled rooms at ∼24°C and a 14:10-hour light:dark cycle. Subsequent generations of mosquitoes were maintained using the same methodologies.
Experiment 1: Life history traits.
Ae. albopictus used were progeny of mosquitoes collected in Florida (F6–F7). Experimental units consisted of 400 mL Tri-Pour polypropylene beakers with 1.0 g Quercus virginiana live oak leaves (dried for at least 24 hours at 60°C), 0.25 g Setaria faberi giant foxtail grass, 350 mL deionized water, and 10 mL live oak leaf infusion water45; 60 newly hatched larvae less than 24 hours old were added to experimental units 5 days after setup. We manipulated temperature treatments during the immature and adult stages as 20–20°C, 20–25°C, 20–30°C, 25–25°C, 30–20°C, 30–25°C, and 30–30°C using environmental chambers where the first number refers to rearing temperature of immature stages and the second number refers to holding temperature of adults. Supplemental food resources (1.0 g oak leaves + 0.25 g foxtail grass) were added on days 7 and 14 after addition of larvae. The original water volume was maintained throughout the experiment by weekly additions of deionized water to experimental units. Each treatment was replicated eight times for a total of 56 experimental units. Pupae were transferred from experimental units to plastic vials with a cotton seal to capture adults.
On emergence, adults were recorded as male or female, and date of development to adulthood was recorded. Adult females were transferred to 16-oz cylindrical paperboard cages with screen tops and given cotton soaked in water. Mosquitoes were held in groups according to the treatment experimental units. Adult females were monitored for survival at 12-hour intervals. Dead adults were recorded and stored at −20°C. Dead females were dried at 60°C for at least 24 hours, and their dry weights were determined to the nearest milligram. For each experimental unit, we measured response variables survivorship to adulthood, development time (male and female), female weight, and female lifespan.
Experiment 2: Vector competence for dengue-1 virus.
Experimental setup and bloodfeeding adult females.
Ae. albopictus used were progeny of mosquitoes collected in Florida (F3). Experimental units consisted of plastic containers with lids, 2.0 L water, and 0.1 g equal mixture of brewers yeast and lactalbumin as food resources; 200 newly hatched larvae less than 24 hours old were added to experimental units on the same day of the experimental setup. Additions of 0.1 g supplemental food resources were added on days 2, 7, and 12 after addition of larvae. To achieve sufficient sample sizes to estimate infection and dissemination rates, we needed to increase the scale of environmental conditions (larval resources and initial number of mosquitoes) from experiment 1. We manipulated temperature treatments during the immature and adult stages as 20–20°C, 20–30°C, 25–25°C, 30–20°C, and 30–30°C using environmental chambers. Treatment temperatures were chosen based on the range of environmental temperature in tropical and subtropical dengue-endemic regions where Ae. albopictus occurs.46 Each treatment was replicated seven times for a total of 35 experimental units. Pupae were transferred from experimental units to plastic vials with a cotton seal to capture adults.
On emergence, adults were recorded as male or female; date of development to adulthood was recorded, and they were placed in cages together to facilitate mating. Mosquitoes were held together according to the treatment experimental units. Females were deprived of sucrose but not water 48 hours before bloodfeeding trials. Ages of adult females were between 7 and 10 days at the time of feeding. Ae. albopictus females were provided with blood infected with dengue-1 virus (strain BOL-KW010) isolated from a human infected in Key West, Florida in 2010 (Florida Department of Health). The virus was passaged three times in African green monkey kidney (Vero) cells. Mosquitoes were allowed to imbibe blood for 60 minutes from an artificial membrane feeding system (Hemotek, Discovery Workshops, Accrington, UK). During feeding trials, all females were kept at 30°C to maximize feeding rates. Immediately after the feeding trials, females were returned to their respective holding temperature treatments.
After bloodfeeding trials, Ae. albopictus were cold-anesthetized, and fully engorged females were transferred to 0.5-L cardboard cages with mesh screening along with a 30-mL plastic cup attached to the bottom of the cage for an oviposition site and a cotton ball soaked in 20% sucrose solution. Oviposition cups were kept moist during the time that mosquitoes underwent oviposition. Mosquitoes were then placed at the appropriate holding temperature treatment along with ∼70–80% humidity and a 14:10-hour light:dark cycle. Incubator temperatures (mean ± SD) were monitored (HOBO data logger; Onset, Bourne, MA) throughout the experiment. After 14 days of incubation, mosquitoes were killed and stored at −80°C; they were later tested for presence of dengue-1 virus RNA.
Preparation of infectious bloodmeals.
Propagation of dengue virus (DENV) for bloodmeals was accomplished by inoculating tissue culture flasks (175 cm2) with confluent monolayers of Vero cells with 250 μL virus stock (multiplicity of infection approximated at 0.0004 plaque-forming units [pfu] per cell). Dengue virus inoculum was allowed to incubate for 1 hour at 35°C with a 5% carbon dioxide atmosphere. After incubation, 25 mL media (199 media, 10% fetal bovine serum, 0.2% antimycotic, and 2% penicillin-streptomycin) were added to each flask. Infectious bloodmeals were prepared using freshly harvested media and virus from tissue culture flasks inoculated 7 days previously and combined with defibrinated bovine blood (Hemostat, Dixon, CA) in a 1:1 ratio of media/virus:blood. A total of 12 bloodmeals were provided to cohorts of mosquitoes. Aliquots of infected bloodmeals were stored at −80°C for later determination of virus titer.
Mosquito processing.
Mosquitoes stored at −80°C were dissected to remove their legs and one wing from the remainder of the body. Separate assays of body and legs were used to determine infection and dissemination rates of DENV as well as viral titer. Wing length measurements were used as an indicator of mosquito size. Samples were triturated separately in microcentrifuge tubes with two copper clad beads and 0.9 mL BA-1 at 25 Hz for 3 minutes (TissueLyser; Qiagen, Inc., Valencia, CA) and subsequently, were subject to centrifugation at 4°C. Nucleic acid was extracted from a 250-μL sample and eluted in 50 μL buffer using the MagNA Pure LC Total Nucleic Acid Isolation Kit (Roche Diagnostics, Indianapolis, IN). The amount of viral RNA present in samples was determined using the Superscript III One-Step Quantitative RT-PCR System (qRT-PCR; Invitrogen, Carlsbad, CA) with a Light Cycler 480 system (Roche, Mannheim, Germany) using methods described elsewhere.47 A standard curve method was used to relate the amount of DENV RNA present in samples to 10-fold serial dilutions of virus stock with known concentrations expressed in pfu per milliliter.48,49 Mosquitoes were categorized based on status of infection: disseminated infections, positively infected bodies and legs; non-disseminated infections, infected bodies but the absence of virus in legs; and uninfected, absence of virus in the body. The infection rate was the percentage of all mosquitoes tested having infected bodies. The dissemination rate was the percentage of mosquitoes with infected bodies that also had infected legs. A total of 633 individuals was assayed for dengue virus infection and dissemination. Viral titers were obtained for the same individual mosquitoes.
Statistical analyses.
Treatment effects on life history traits were analyzed using analysis of variance (ANOVA) using container microcosm as the experimental unit. We lacked the facilities to independently replicate temperature independently for each container and cage. Rather, temperature treatment was manipulated for the entire environmental chamber, and therefore, we assume that between-environmental chamber variation (other than temperature) is negligible. When significant effects were detected, we used pair-wise contrasts of means adjusted for an experiment-wise α of 0.05 (Tukey–Kramer adjustment for multiple comparisons; PROC GLM; SAS 9.22). Treatment effects on age-specific survival of adults using lifespan of adult females were compared using non-parametric survival analysis (PROC LIFETEST; SAS 9.22). When significant effects were detected, we used log-rank test statistics to compare pair-wise estimates of survival adjusting for multiple comparisons using the Sidak method. Correlation analyses were used to relate relationships between adult female lifespan and dry weight measured after death for temperature treatments. Treatment effects on vector competence (virus infection, dissemination, and titer) were analyzed similarly using ANOVA and pair-wise contrasts of means adjusting level of significance for multiple comparisons.
Results
Experiment 1: Life history traits.
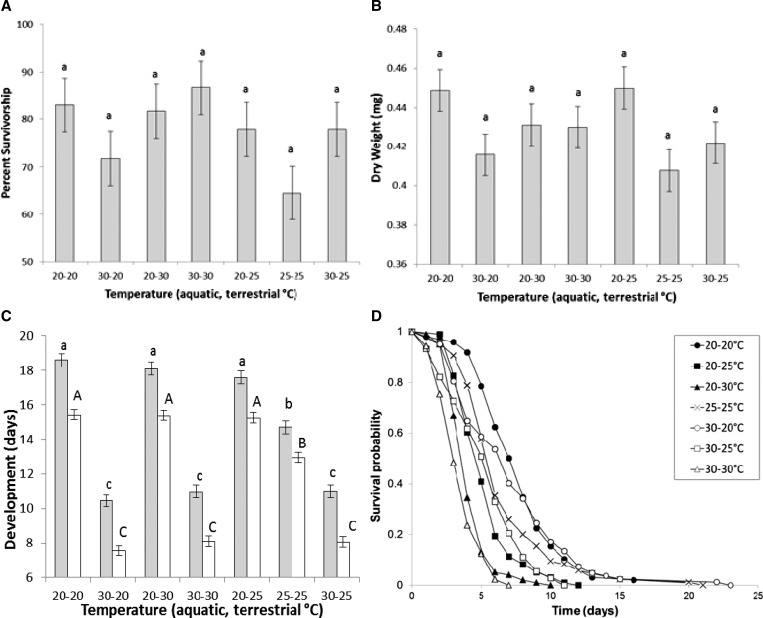
ANOVA showed no temperature treatment effects on survivorship to adulthood or dry weight of adult females (Figure 1A and B and Table 1). There was a significant effect of treatment on development time to adulthood (Table 1). For both females and males, significantly shorter development times were observed at warmer larval-rearing conditions relative to cooler conditions for all three larval-rearing temperature treatments (Figure 1C). There was a significant effect of treatment on female survival of adults (Table 1). Mosquitoes held at 20°C during the adult stage had significantly greater survival than other temperatures, regardless of rearing temperature of immature stages (Figure 1D). Mosquitoes held at 25°C during the adult stage had significantly greater survival, regardless of rearing temperature of immature stages, than individuals held at 30°C during the adult stages (Figure 1D). Adult female survival was not modified by immature-rearing temperature treatments (Figure 1D).
Figure 1.
Adult lifespan and life history traits (experiment 1). Mean (± standard error) of (A) survivorship, (B) dry weight, (C) development (females and males shown by grey and white bars, respectively), and (D) lifespan of Ae. albopictus from temperature treatments. Means followed by different letters are significantly different from one another.
Table 1.
Life history traits (experiment 1)
| Life history trait | d.f. | F | χ2 | P |
|---|---|---|---|---|
| Percent survivorship to adulthood | 6, 49 | 1.71 | – | 0.137 |
| Female dry weight | 6, 49 | 2.20 | – | 0.059 |
| Female development | 6, 49 | 95.62 | – | < 0.0001 |
| Male development | 6, 49 | 149.27 | – | < 0.0001 |
| Female adult survival | 6 | 17.36 | 149.26 | < 0.0001 |
ANOVA for temperature effects on life history traits of Ae. albopictus. d.f. = degrees of freedom.
Correlation analyses showed significant positive relationships between adult female lifespan and dry weight measured after death for temperature treatments 20–20°C (r = 0.33, N = 98, P = 0.0004), 30–20°C (r = 0.37, N = 82, P = 0.0007), and 20–30°C (r = 0.43, N = 100, P < 0.0001). All remaining comparisons were not significant after correcting P values for multiple comparisons.
Experiment 2: Vector competence for dengue-1 virus.
Life history traits.
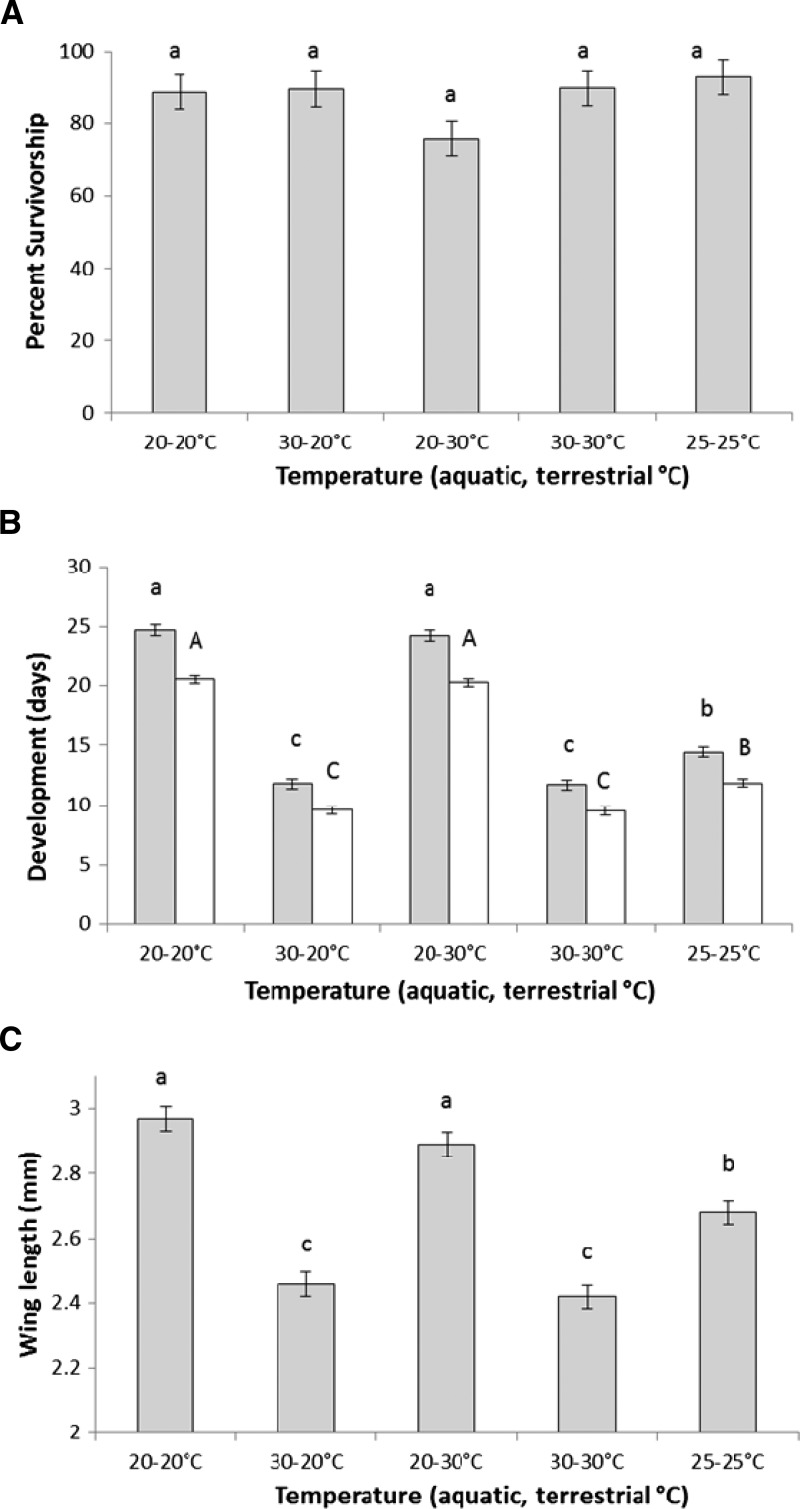
ANOVA showed no temperature treatment effects on survivorship to adulthood (Figure 2A and Table 2). There was a significant effect of treatment on female wing length and development time to adulthood (Table 2). Significantly shorter development times and wing lengths were observed at warmer larval-rearing conditions relative to cooler conditions for all three larval-rearing temperature treatments (Figure 2B and C). These treatment effects on development time were similar for both females and males.
Figure 2.
Adult life history traits for mosquitoes exposed to dengue-1 virus (experiment 2). Mean (± standard error) of (A) survivorship, (B) development (females and males shown by grey and white bars, respectively), and (C) wing length of Ae. albopictus from temperature treatments. Means followed by different letters are significantly different from one another.
Table 2.
Vector competence for dengue-1 virus (experiment 2)
| Response variable | d.f. | F | P |
|---|---|---|---|
| Life history trait | |||
| Survivorship | 4, 30 | 1.97 | 0.123 |
| Female wing length | 4, 30 | 44.47 | < 0.0001 |
| Female development | 4, 30 | 237.94 | < 0.0001 |
| Male development | 4, 30 | 266.51 | < 0.0001 |
| Vector competence | |||
| Virus infection | 4, 30 | 1.29 | 0.2975 |
| Virus dissemination | 4, 30 | 72.89 | < 0.0001 |
| Body titer (non-disseminated infection) | 4, 24 | 6.77 | 0.0008 |
| Body titer (disseminated infection) | 4, 24 | 7.15 | 0.0006 |
| Leg titer (disseminated infection) | 4, 24 | 6.45 | 0.0011 |
ANOVA for temperature effects on life history traits and vector competence of Ae. albopictus for dengue-1 virus. d.f. = degrees of freedom.
Susceptibility to dengue virus infection and dissemination.
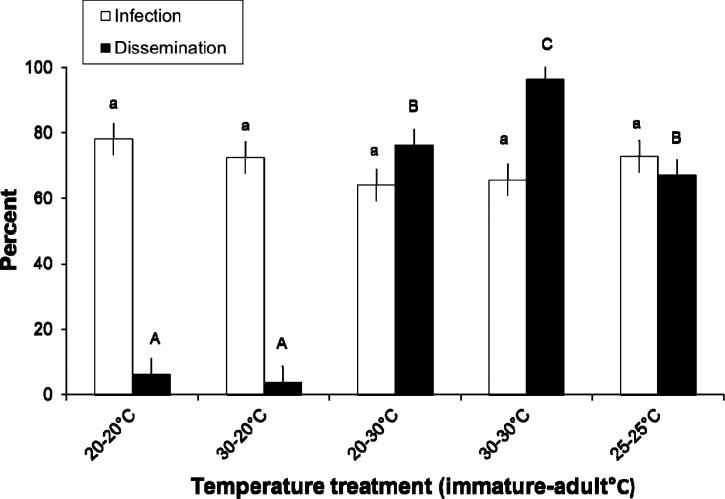
Bloodmeal titers estimated from an aliquot of the suspension were 7.09 ± 0.14 log10 pfu DENV/ml (mean ± SD). ANOVA showed no significant temperature treatment effect on susceptibility to dengue virus infection 14 days after imbibing dengue-infected blood (Table 2). There was a significant effect of treatment on virus dissemination 14 days after imbibing dengue-infected blood (Table 2). Mosquitoes held at 20°C during the adult stage had significantly lower dissemination rates than all other temperature treatments, regardless of rearing temperature of immature stages (Figure 3). Mosquitoes held at 25°C during the adult stage had significantly lower dissemination rates than individuals maintained at 30–30°C but not 20–30°C treatments (Figure 3). Individuals maintained at the 20–30°C treatment had significantly lower disseminations than individuals maintained at 30–30°C (Figure 3).
Figure 3.
Vector competence for mosquitoes exposed to dengue-1 virus-infected blood (experiment 2). Mean (± standard error) susceptibility to dengue virus infection and dissemination of Ae. albopictus from temperature treatments. The numbers of mosquitoes assayed to determine infection and dissemination rates were 141 (20–20°C), 144 (30–20°C), 85 (20–30°C), 125 (30–30°C), and 138 (25–25°C). Means followed by different letters are significantly different from one another.
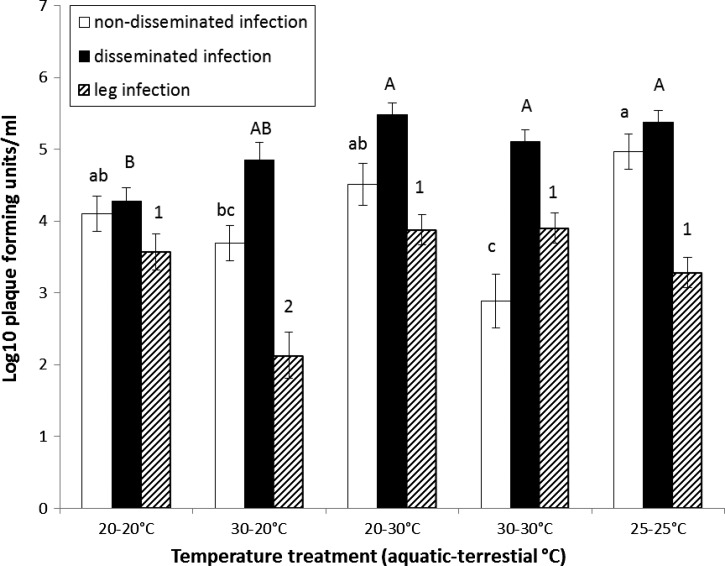
ANOVA on body titer of mosquitoes with non-disseminated infections showed that individuals in the 25–25°C treatment had significantly higher or similar body titers than individuals in the 20–20°C, 30–20°C, and 20–30°C treatments 14 days after imbibing dengue-infected blood (Figure 4 and Table 2). The body titers of mosquitoes from the 30–30°C treatments were significantly lower or similar than all other treatments. For mosquitoes with disseminated infections, ANOVA showed that body titers in the 20–30°C, 30–30°C, and 25–25°C treatments were significantly higher or equal to all other treatments (Figure 4 and Table 2). The lowest body titers were observed in 20–20°C and 30–20°C treatments, which were similar to each other (Figure 4). Leg titers were similar for all treatment groups except for the 30–20°C treatment, which was significantly lower than all other treatments (Figure 4 and Table 2).
Figure 4.
Viral titers in mosquitoes with non-disseminated and disseminated dengue-1 virus infection (experiment 2). Mean (± standard error) viral titers of individual adult female Ae. albopictus from temperature treatments. Means followed by different lower and upper case letters denote significant differences of body titer for non-disseminated and disseminated infections, respectively. Means followed by different numbers show significant differences of leg titers. No comparisons are made between body titers of individuals with non-disseminated and disseminated infections or leg titers.
Discussion
Survivorship to adulthood was similar between the larval-rearing temperature range of 20°C and 30°C, suggesting that these temperatures did not impose substantial stress to induce mortality in Ae. albopictus. Similar results have been observed for temperatures in this range for Ae. albopictus,32,33,50 although wider ranges of temperature have been shown to influence survivorship to adulthood.51,52 Development time increased with decreasing temperatures for both males and females, with temperature-specific rates being similar for both experiments, despite several differences in experimental setup between the experiments (water volume, nutrients, and larval density). Development time to adulthood was shorter for males than females, likely attributable to lower nutritional thresholds for males of most mosquito species. Because development times were determined on emergence to adulthood, only environmental temperature of immature stages contributed to observed temperature effects on development.
Temperature-dependent differences were observed in wing lengths but not dry weight of adult females from the two experiments. The lack of an effect of temperature on dry weight is surprising given that other studies have identified differences in masses of adult Ae. albopictus reared within this range of environmental temperatures.33 In experiment 1, mosquitoes were deprived access to adult nutrition and therefore, forced to rely on nutrition acquired in the immature environment. Depletion of nutritional reserves and perhaps, associated biomass may obscure differences in dry weight between temperature treatments. In addition, recent studies have shown differences in temperature-dependent allometric relationship between body mass and wing length in Ae. albopictus,53 accounting, in part, for discrepancies in temperature effects on size reported here.
Cooler holding temperatures of adult females were associated with greater survival relative to warmer temperatures, regardless of rearing temperature of the immature stages (20°C > 25°C > 30°C). Temperature during the immature stages did not impinge on adult survival. This result was somewhat surprising given that immature-rearing temperature determines the phenotypic traits of adults (e.g., nutritional reserves42–44). These results suggest that cool larval-rearing temperature does not buffer against life-shortening effects of warm holding conditions of the adults. Rather, female adult survival is robust to temperature experienced during the immature stages. It is plausible that longer development times associated with cool immature-rearing conditions facilitate the production of large-sized adults because of greater nutrient uptake and energy reserves at emergence to adulthood,42–44 perhaps extending survival of adults. However, we can exclude this explanation for the current study, because adult female size (dry weight) was similar across temperature treatments. Despite the lack of treatment effects on female weight, heavier adult female mosquitoes did experience greater survival in some treatments where either immature-rearing or adult-holding conditions included 20°C, suggesting that increased size has life-lengthening effects in some instances.54–56
We observed temperature treatment effects on Ae. albopictus vector competence for dengue-1 virus. Regardless of immature-rearing or adult-holding temperature, susceptibility to viral infection was similar for all temperature treatments. These results suggest that barriers to midgut infection were robust to a range of temperatures. We are unable to rule out the possibility that a common environmental temperature of 30°C during bloodfeeding trials may have contributed, in part, to similar infection rates. However, mosquitoes were only exposed to a common temperature of 30°C for a short period of time during bloodfeeding trials (60 minutes), after which time they were immediately returned to their respective temperature treatments.
In contrast to infection rates, midgut escape barriers preventing dissemination were strongly influences by both rearing temperature of immature stages and holding temperature of the adult stage. A holding temperature of 20°C during the adult stage resulted in the lowest rates of viral dissemination. Rates of dissemination were higher at 25°C and still higher at 30°C relative to cooler holding temperatures of adults. These results corroborate observations found for laboratory studies examining susceptibility to dengue virus infection and length of the extrinsic incubation period in Ae. albopictus and Ae. aegypti37,57–59 as well as the relationship between temperature and the occurrence of dengue in nature.34–37 The current experiment did not test mosquitoes at multiple time points post-exposure to dengue virus to identify the extrinsic incubation period (the time between initial acquisition of the pathogen and when transmission is possible). The extrinsic incubation period of dengue virus depends on temperature, and therefore, it is unclear whether sampling at later than 14 days post-exposure to dengue virus may have resulted in higher rates of dissemination, especially at cooler temperatures known to slow the progression of virus infection in the mosquito.37 We also identified that the temperature during the immature stages influences rates of dengue-1 virus dissemination independent of the adult-holding temperature. Specifically, mosquitoes reared at a cool temperature during the immature stage but held at high temperature at the adult stage (20–30°C) had approximately 21% reduction in rates of viral dissemination relative to females maintained at warm temperatures during both the immature and adult stages (30–30°C). These results suggest long-lasting effects of the immature stage environment on phenotypes of adults related to progression of viral infection. In particular, the efficacy of the midgut escape barrier to dengue virus is substantially improved by cooler rearing conditions of the immature stages. Although the mechanism responsible for alterations in the midgut escape barrier is unclear, these results provide a direct association between immature-stage rearing temperature and progression of viral infection in adults. Previous studies have suggested that differences in the thickness of the basal lamina in response to larval conditions may influence the efficiency of arbovirus dissemination.60 However, there are numerous other aspects of the mosquito biology beyond morphology that may affect the efficacy of the midgut escape barrier. The current experiment used nutrient-rich resources (yeast and lactalbumin) that do not mimic natural basal resources in container habitats (plant and invertebrate detritus). Future experiments will need to assess whether the observed temperature effects in this study translate to resources conditions found in nature.
Observations reported here are consistent with studies investigating the effects of heat shock and elevated temperature during immature stages and susceptibility to infection and dissemination of dengue and Sindbis viruses in Ae. aegypti.32,33,61 In contrast, laboratory studies using other Alphaviruses and Bunyaviruses show enhanced viral infection or dissemination of mosquitoes reared in cool ambient temperatures (Chikungunya virus52, Rift Valley Fever virus, and Venezuelan equine encephalitis virus23) relative to warmer conditions. Similarly, enhanced seasonal activity of Alphavirus Western equine encephalitis virus, but not Flavivirus St. Louis encephalitis virus, correlates to cool ambient temperature.29 Taken together, these observations suggest that temperature effects on vector efficiency depend on the particular vector–virus system. Given these observations, it is tempting to draw the conclusions that warmer rearing conditions may enhance competence of mosquitoes for Flaviviruses but depress competence for other arboviruses (Alphaviruses and Bunyaviruses). However, caution is advised in interpreting potential fundamental differences in the influence of temperature on mosquito–virus interactions because of inconsistencies in observed temperature effects (e.g., warm larval-rearing conditions increased susceptibility to infection and dissemination for Alphavirus Sindbis33,62; cool larval-rearing conditions enhanced horizontal and vertical transmission of St. Louis encephalitis and Murray Valley encephalitis Flaviviruses22,31). The constant temperatures used here do not capture the daily temperature regimen in nature known to influence dengue virus63 and malaria transmission,64,65 an important consideration for predictive models and control efforts. Our experiment was designed as a general test of separating temperature effects acting on the immature and adult stages. An intriguing possibility for future work is an investigation to determine whether the drastic temperature shifts (up to 10°C) between the immature and adult stages have similar consequences on longevity and susceptibility to dengue virus infection in nature, where diel temperature is variable. Regardless, the current studies results may serve as a starting point in improving predictive models assessing the risk of dengue virus transmission and control efforts by incorporating stage dependency in the manner by which temperature influences transmission.63,66,67
For mosquitoes with disseminated infections, moderate to high holding temperatures as adults (25°C and 30°C) resulted in the highest viral titers, regardless of immature-rearing temperature. Mosquitoes held at low temperature as adults (20°C) had the lowest viral titers. These observed results are consistent with higher titers of dengue-2 virus in Ae. aegypti maintained at high temperatures37 and the anticipated direct relationship between viral titer and duration of the extrinsic incubation period.37,68 However, in the current study, this latter effect was modified by the immature temperature environment. Specifically, higher immature temperatures (30°C) resulted in viral titers similar to viral titers observed in mosquitoes held at 30°C as adults, despite being maintained at low adult-holding temperature (20°C). Thus, it seems that warm conditions experienced during the immature and adult stages may enhance viral replication in mosquitoes with disseminated infections as indicated by higher titers in these individuals. However, these effects do not translate to all mosquito tissues given that the opposite result was observed in the legs of individuals with disseminated infections for mosquitoes from the 30–20°C treatment. The explanation for these observed effects in body versus leg titers of mosquitoes with disseminated infections is not entirely clear, but it does suggest complex effects of temperature on virus replication and by extension, mosquito immune function69 contingent on both immature- and adult-rearing environment. High viral loads and rates of dissemination associated with warm conditions may favor vertical transmission, allow dengue virus to persist during interepidemic periods,70 and contribute to the epidemiology of dengue. In Thailand, where all four serotypes of dengue virus co-occur, a field study showed direct trends in increases in vertical transmission of dengue virus and temperature preceding increases of incidence of human infections.71 However, in the current study, for mosquitoes with non-disseminated infections, individuals reared at 20°C and 25°C during their immature stages tended to have the highest body titers, whereas mosquitoes from elevated temperatures during the immature stages tended to have the lowest body titers. These results suggest complex relationships between temperature-dependent viral titers, which depend on progression of infection beyond the midgut of mosquitoes (non-disseminated versus disseminated infections).
The current study underscores the importance of the environmental temperature experienced by immature stages in shaping adult phenotypes. Environmental temperature of immature stages selectively modified traits of adult mosquitoes related to virus transmission, furthering our understanding of the nature of temperature effects on interactions between mosquitoes and virus pathogens and risk of disease transmission. We show that rearing temperature of immature stages affects adult mosquito interactions with dengue virus, most likely attributable to alterations in viral replication and efficacy of the midgut escape barrier.
ACKNOWLEDGMENTS
The authors thank C. C. Lord for use of environmental chambers. Dengue-1 virus (strain BOL-KW010) was kindly provided by the Florida Department of Health Bureau of Laboratories. The authors thank L. P. Lounibos and J. R. Rey for reviewing earlier versions of the manuscript.
Footnotes
Financial support: These studies were supported by Florida Department of Agricultural and Consumer Services Project No. 00090369, Illinois Waste Tire and Emergency Public Health Funds, and Dean's research grant for undergraduate students from the University of Florida—Institute of Food and Agricultural Sciences (UF-IFAS), and startup funds from UF-IFAS.
Authors' addresses: Barry W. Alto, Florida Medical Entomology Laboratory, University of Florida, Vero Beach, FL, E-mail: bwalto@ufl.edu. David Bettinardi, Department of Molecular and Cellular Biology, University of Illinois, Urbana, IL, E-mail: david.j.bettinardi@gmail.com.
References
- 1.Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007;7:76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Effler PW, Pang L, Kitsutani P, Vorndam V, Nakata M, Ayers T, Elm J, Tom T, Reiter P, Rigau-Perez JG, Hayes JM, Mills K, Napier M, Clark GG, Gubler DJ. Hawaii Dengue Outbreak Investigation Team Dengue fever, Hawaii, 2001–2002. Emerg Infect Dis. 2005;11:742–749. doi: 10.3201/eid1105.041063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arankalle VA, Shrivastava S, Cherian S, Gunjikar RS, Walimbe AM, Jadhav SM, Sudeep AB, Mishra AC. Genetic divergence of chikungunya viruses in India (1963–2006) with special reference to the 2005–2006 explosive epidemic. J Gen Virol. 2007;88:1967–1976. doi: 10.1099/vir.0.82714-0. [DOI] [PubMed] [Google Scholar]
- 4.Paquet C, Quatresovs I, Solet J-L, Sissoko D, Renault P, Pierre V, Cordel H, Lassalle C, Thiria J, Zeller H. Chikungunya outbreak in reunion: epidemiology and surveillance, 2005 to early January 2006. Euro Surveill. 2006;11:E0602023. doi: 10.2807/esw.11.05.02891-en. [DOI] [PubMed] [Google Scholar]
- 5.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, Cordioli P, Fortuna C, Boros S, Magurano F, Silvi G, Angelini P, Dottori M, Ciufolini MG, Majori GC, Cassone A. CHIKV study group Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 6.Seneviratne SL, Gurugama P, Perera J. Chikungunya viral infections: an emerging problem. J Travel Med. 2007;14:320–325. doi: 10.1111/j.1708-8305.2007.00135.x. [DOI] [PubMed] [Google Scholar]
- 7.Ibañez-Bernal S, Briseño B, Mutebi J-P, Argot E, Rodriguez G, Martinez-Camposc C, Paz R, de la Fuente-San Roman P, Tapia-Conyer R, Flisser A. First record in America of Aedes albopictus naturally infected with dengue virus during the 1995 outbreak at Reynosa, Mexico. Med Vet Entomol. 1997;11:305–309. doi: 10.1111/j.1365-2915.1997.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 8.Méndez F, Barreto M, Arias JF, Renfigo G, Muñoz J, Burbano ME, Parra B. Human and mosquito infections by dengue viruses during and after epidemics in a dengue-endemic region of Colombia. Am J Trop Med Hyg. 2006;74:678–683. [PubMed] [Google Scholar]
- 9.Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis. 2010;4:e646. doi: 10.1371/journal.pntd.0000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holick J, Kyle A, Ferraro W, Delaney RR, Iwaseczko M. Discovery of Aedes albopictus infected with West Nile virus in southeastern Pennsylvania. J Am Mosq Control Assoc. 2002;18:131. [PubMed] [Google Scholar]
- 11.Gerhardt RR, Gottfied KL, Apperson CS, Davis BS, Erwin PC, Smith AB, Panella NA, Powell EE, Nasci RS. First isolation of La Crosse virus from naturally infected Aedes albopictus. Emerg Infect Dis. 2001;7:807–811. doi: 10.3201/eid0705.017506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell CJ, Niebylski ML, Karabatsos N, Martin D, Mutebi J-P, Craig GB, Mahler MJ. Isolation of eastern equine encephalitis from Aedes albopictus in Florida. Science. 1992;257:526–527. doi: 10.1126/science.1321985. [DOI] [PubMed] [Google Scholar]
- 13.Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecol Entomol. 1996;21:117–127. [Google Scholar]
- 14.Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- 15.Braks MAH, Honório NA, Lounibos LP, Lourenco-de-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Ann Entomol Soc Am. 2004;97:130–139. [Google Scholar]
- 16.Juliano SA, Lounibos LP, O'Meara GF. A field test for competitive effects of Aedes albopictus on Aedes aegypti in south Florida: differences between sites of coexistence and exclusion? Oecologia. 2004;139:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murrell EG, Juliano SA. Detritus type alters the outcome of interspecific competition between Aedes aegypti and Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2008;45:375–383. doi: 10.1603/0022-2585(2008)45[375:dtatoo]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nawrocki SJ, Hawley WA. Estimation of the northern limits of distribution of Aedes albopictus in North America. J Am Mosq Control Assoc. 1987;3:314–317. [PubMed] [Google Scholar]
- 19.Davis NC. The effect of various temperatures in modifying the extrinsic incubation period of the yellow fever virus in Aedes aegypti. Am J Hyg. 1932;16:163–176. [Google Scholar]
- 20.Chamberlain RW, Sudia WD. The effects of temperature upon the extrinsic incubation of eastern equine encephalitis in mosquitoes. Am J Hyg. 1955;62:295–305. doi: 10.1093/oxfordjournals.aje.a119780. [DOI] [PubMed] [Google Scholar]
- 21.Kay BH, Fanning ID, Mottram P. The vector competence of Culex annulirostris, Aedes sagax and Aedes alboannulatus for Murray Valley encephalitis virus at different temperatures. Med Vet Entomol. 1989;3:107–112. doi: 10.1111/j.1365-2915.1989.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 22.Kay BH, Fanning ID, Mottram P. Rearing temperature influences flavivirus vector competence of mosquitoes. Med Vet Entomol. 1989;3:415–422. doi: 10.1111/j.1365-2915.1989.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 23.Turell MJ. Effect of environmental temperature on the vector competence of Aedes taeniorhynchus for Rift Valley fever and Venezuelan equine encephalitis viruses. Am J Trop Med Hyg. 1993;49:672–676. doi: 10.4269/ajtmh.1993.49.672. [DOI] [PubMed] [Google Scholar]
- 24.Richards SL, Mores CN, Lord CC, Tabachnick WJ. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinqefasciatus (Diptera: Culicidae) for West Nile virus. Vector Borne Zoonotic Dis. 2007;7:629–636. doi: 10.1089/vbz.2007.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards SL, Lord CC, Pesko KA, Tabachnick WJ. Environmental and biological factors influencing Culex pipiens quinqefasciatus Say (Diptera: Culicidae) vector competence for Saint Louis encephalitis virus. Am J Trop Med Hyg. 2009;81:264–272. [PMC free article] [PubMed] [Google Scholar]
- 26.Kilpatrick MA, Meola MA, Moudy RM, Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008;4:e1000092. doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson SL, Richards SL, Smartt CT, Tabachnick WJ. The effects of West Nile virus dose on temporal progression of vector competence in Culex pipiens quinquefasciatus Say (Diptera: Culicidae) J Am Mosq Control Assoc. 2010;26:103–107. doi: 10.2987/09-5926.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kramer LD, Hardy JL, Presser SB. Effect of temperature of extrinsic incubation on the vector competence of Culex tarsalis for western equine encephalomyelitis virus. Am J Trop Med Hyg. 1983;32:1130–1139. doi: 10.4269/ajtmh.1983.32.1130. [DOI] [PubMed] [Google Scholar]
- 29.Hardy JL, Meyer RP, Presser SB, Milby MM. Temporal variations in the susceptibility of a semi-isolated population of Culex tarsalis to peroral infection with western equine encephalomyelitis and St. Louis encephalitis viruses. Am J Trop Med Hyg. 1990;42:500–511. doi: 10.4269/ajtmh.1990.42.500. [DOI] [PubMed] [Google Scholar]
- 30.Hess AD, Cherubin CE, LaMotte LC. Relation of temperature to activity of western and St. Louis encephalitis viruses. Am J Trop Med Hyg. 1963;12:657–667. [Google Scholar]
- 31.Hardy JL, Rosen L, Kramer LD, Presser SB, Shroyer DA, Turell MJ. Effect of rearing temperature on transovarial transmission of St. Louis encephalitits virus in mosquitoes. Am J Trop Med Hyg. 1980;29:963–968. doi: 10.4269/ajtmh.1980.29.963. [DOI] [PubMed] [Google Scholar]
- 32.Muturi EJ, Alto BW. Larval environmental temperature and insecticide exposure alters Aedes aegypti competence for arboviruses. Vector Borne Zoonotic Dis. 2011;11:1157–1163. doi: 10.1089/vbz.2010.0209. [DOI] [PubMed] [Google Scholar]
- 33.Muturi EJ, Costanzo KS, Kesavaraju B, Lampman R, Alto BW. Effect of temperature and insecticide stress on life-history traits of Culex restuans and Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2011;48:243–250. doi: 10.1603/me10017. [DOI] [PubMed] [Google Scholar]
- 34.Shang C-S, Fang C-T, Liu C-M, Wen T-H, Tsai K-H, King C-C. The role of imported cases and favorable meteorological conditions in the onset of dengue epidemics. PLoS Negl Trop Dis. 2010;4:e775. doi: 10.1371/journal.pntd.0000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johansson MA, Dominici F, Glass GE. Local and global effects of climate on dengue transmission in Puerto Rico. PLoS Negl Trop Dis. 2009;3:e382. doi: 10.1371/journal.pntd.0000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsuzuki A, Duoc VT, Higa Y, Yen NT, Takagi M. High potential risk of dengue transmission during the hot-dry season in Nha Trang City, Vietnam. Acta Trop. 2009;111:325–329. doi: 10.1016/j.actatropica.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Watts DM, Burke DS, Harrison BA, Whitmire RE, Nisalak A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am J Trop Med Hyg. 1987;36:143–152. doi: 10.4269/ajtmh.1987.36.143. [DOI] [PubMed] [Google Scholar]
- 38.Wright RE, Knight KL. Effect of environmental factors on biting activity of Aedes vexans (Meigen) and Aedes trivittatus (Coquillett) Mosq News. 1966;26:565–578. [Google Scholar]
- 39.Rowley WA, Graham CL. The effect of temperature and relative humidity on the flight performance of female Aedes aegypti. J Insect Physiol. 1968;14:1251–1257. doi: 10.1016/0022-1910(68)90018-8. [DOI] [PubMed] [Google Scholar]
- 40.Meyer RP, Hardy JL, Reisen WK. Diel changes in adult mosquito microhabitat temperatures and their relationship to the extrinsic incubation of arbovirues in mosquitoes in Kern County, California. J Med Entomol. 1990;27:607–614. doi: 10.1093/jmedent/27.4.607. [DOI] [PubMed] [Google Scholar]
- 41.Gray KM, Burkett-Cadena ND, Eubanks MD, Unnasch TR. Crepuscular flight activity of Culex erraticus (Diptera: Culicidae) J Med Entomol. 2011;48:167–172. doi: 10.1603/me10176. [DOI] [PubMed] [Google Scholar]
- 42.Briegel H, Timmermann SE. Aedes albopictus (Diptera: Culicidae): physiological aspects of development and reproduction. J Med Entomol. 2001;38:566–571. doi: 10.1603/0022-2585-38.4.566. [DOI] [PubMed] [Google Scholar]
- 43.Briegel H, Waltert A, Kuhn R. Reproductive physiology of Aedes (Aedimorphus) vexans (Diptera: Culicidae) in relation to flight potential. J Med Entomol. 2001a;38:557–565. doi: 10.1603/0022-2585-38.4.557. [DOI] [PubMed] [Google Scholar]
- 44.Briegel H, Knusel I, Timmermann SE. Aedes aegypti: size, reserves, survival, and flight potential. J Vector Ecol. 2001b;26:21–31. [PubMed] [Google Scholar]
- 45.O'Meara GF, Vose FE, Carlson DB. Environmental factors influencing oviposition by Culex (Culex) (Diptera: Culicidae) in two types of traps. J Med Entomol. 1989;26:528–534. doi: 10.1093/jmedent/26.6.528. [DOI] [PubMed] [Google Scholar]
- 46.National Oceanic and Atmospheric Administration (NOAA) http://www.cpc.ncep.noaa.gov/products/analysis_monitoring/regional_monitoring/ Available at. Accessed July 1, 2012.
- 47.Callahan JD, Wu S-JL, Dion-Schultz A, Mangold BV, Peruski LF, Watts DM, Porter KR, Murphy GR, Suharyono W, King C-C, Hayes CG, Temenak JJ. Development and evaluation of serotype- and group-specific fluorogenic reverse transcriptase PCR (TaqMan) assays for dengue virus. J Clin Microbiol. 2001;39:4119–4124. doi: 10.1128/JCM.39.11.4119-4124.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alto BW, Lounibos LP, Mores CN, Reiskind MH. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proc Biol Sci. 2008;275:463–471. doi: 10.1098/rspb.2007.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 50.Lounibos LP, Suárez S, Menéndez A, Nishimura N, Escher RL, O'Connell SM, Rey JR. Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? J Vector Ecol. 2002;27:86–95. [PubMed] [Google Scholar]
- 51.Delatte H, Gimonneau G, Triboire A, Fontenille D. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J Med Entomol. 2009;46:33–41. doi: 10.1603/033.046.0105. [DOI] [PubMed] [Google Scholar]
- 52.Westbrook CJ, Reiskind MH, Pesko KN, Green KE, Lounibos LP. Larval environmental temperature and the susceptibility of Aedes albopictus Skuse (Diptera: Culicidae) to chikungunya virus. Vector Borne Zoonotic Dis. 2010;10:241–247. doi: 10.1089/vbz.2009.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reiskind MH, Zarrabi AA. Is bigger really bigger? Differential responses to temperature in measures of body size of the mosquito, Aedes albopictus. J Insect Physiol. 2012;58:911–917. doi: 10.1016/j.jinsphys.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 54.Nasci RC. The size of emerging and host-seeking Aedes aegypti and the relation of size to blood-feeding success in the field. J Am Mosq Control Assoc. 1986;2:61–62. [PubMed] [Google Scholar]
- 55.Hawley WA. The effect of larval density on adult longevity of a mosquito, Aedes sierrensis: epidemiological consequences. J Anim Ecol. 1985;54:955–964. [Google Scholar]
- 56.Haramis LD. Larval nutrition, adult body size, and the biology of Aedes triseriatus. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of Mosquitoes: Proceedings of a Workshop. Vero Beach, FL: Florida Medical Entomology Laboratory; 1985. pp. 431–437. [Google Scholar]
- 57.Blanc G, Caminopetros J. Recherches experimentales sur la dengue. Ann Inst Pasteur (Paris) 1930;44:392–395. [Google Scholar]
- 58.Rohani A, Wong YC, Zamre I, Lee HL, Zurainee MN. The effect of extrinsic incubation temperature on development of dengue serotype 2 and 4 viruses in Aedes aegypti (L.) Southeast Asian J Trop Med Public Health. 2009;40:942–950. [PubMed] [Google Scholar]
- 59.Fang-zhen X, Yi Z, Han-guo X, Wen-qi S, Si H, Yan-qin D, Xiao-nong Z, Yan-sheng Y. Effect of temperatures on extrinsic incubation period of dengue virus type 2 in Aedes albopictus. Chin J Zoonoses. 2012;28:108–110. [Google Scholar]
- 60.Grimstad PR, Walker ED. Aedes triseriatus (Dipera: Culicidae) and La Crosse virus. IV. Nutritional deprivation of larvae affects the adult barriers to infection and transmission. J Med Entomol. 1991;28:378–386. doi: 10.1093/jmedent/28.3.378. [DOI] [PubMed] [Google Scholar]
- 61.Yadav P, Barde PV, Gokhale MD, Vipat V, Mishra AC, Pal JK, Mourya DT. Effect of temperature and insecticide stresses on Aedes aegypti larvae and their influence on the susceptibility of mosquitoes to dengue-2 virus. Southeast Asian J Trop Med Public Health. 2005;36:1139–1144. [PubMed] [Google Scholar]
- 62.Muturi EJ, Kim C-H, Alto BW, Berenbaum M, Schuler M. Larval environmental stress alters Aedes aegypti competence for Sindbis virus. Trop Med Int Health. 2011b;16:955–964. doi: 10.1111/j.1365-3156.2011.02796.x. [DOI] [PubMed] [Google Scholar]
- 63.Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB, Scott TW. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc Natl Acad Sci USA. 2011;108:7460–7465. doi: 10.1073/pnas.1101377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paaijmans KP, Read AF, Thomas MB. Understanding the link between malaria risk and climate. Proc Natl Acad Sci USA. 2009;106:13844–13849. doi: 10.1073/pnas.0903423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paaijmans KP, Blanford S, Bell AS, Blanford JI, Read AF, Thomas MB. Influence of climate on malaria transmission depends on daily temperature variation. Proc Natl Acad Sci USA. 2010;107:15135–15139. doi: 10.1073/pnas.1006422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Focks DA, Daniels E, Haile DG, Keesling JE. A simulation model of the epidemiology of urban dengue fever: literature analysis, model development, preliminary validation, and samples of simulation results. Am J Trop Med Hyg. 1995;53:489–506. doi: 10.4269/ajtmh.1995.53.489. [DOI] [PubMed] [Google Scholar]
- 67.Lu L, Lin H, Tian L, Yang W, Sun J, Liu Q. Time series analysis of dengue fever and weather in Guangzhou, China. BMC Public Health. 2009;9:395. doi: 10.1186/1471-2458-9-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Richards SL, Anderson SL, Lord CC, Smart CT, Tabachnick WJ. Relationships between infection, dissemination, and transmission of West Nile virus RNA in Culex pipiens quinquefasciatus (Diptera: Culicidae) J Med Entomol. 2012;49:132–142. doi: 10.1603/me10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murdock CC, Paaijman KP, Bell AS, King JG, Hillyer JF, Read AF, Thomas MB. Complex effects of temperature on mosquito immune function. Proc Biol Sci. 2012;279:3357–3366. doi: 10.1098/rspb.2012.0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adams B, Boots M. How important is vertical transmission in mosquitoes for the persistence of dengue? Insights from a mathematical model. Epidemics. 2010;2:1–10. doi: 10.1016/j.epidem.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Thongrungkiat S, Maneekan P, Wasinpiyamongkol L, Prummongkol S. Prospective field study of transovarial dengue-virus transmission by two different forms of Aedes aegypti in an urban area of Bangkok, Thailand. J Vector Ecol. 2010;36:147–152. doi: 10.1111/j.1948-7134.2011.00151.x. [DOI] [PubMed] [Google Scholar]