Abstract
Despite the availability of many methods for rapid and early diagnosis of dengue, there is still a need to develop new approaches that not only combine low cost, specificity, and sensitivity, but also are capable of accurately detecting secondary infection in the early stages of the disease. We report the potential of the high mobility group box 1 protein as an auxiliary biomarker for early dengue diagnosis. We tested a 205-sample serum panel that included negative and positive samples from primary and secondary dengue cases, as well as samples from patients with dengue-like symptoms. We observed that high mobility group box 1 protein was generally detected only in dengue-positive samples for persons with primary and secondary infections. These results highlight the possibility of using this endogenous molecule as an auxiliary biomarker to aid in dengue detection and improve current methods for early diagnosis of dengue.
Dengue infection affects at least 50 million persons per year in tropical and subtropical regions. Severe cases of dengue are responsible for more than 500,000 hospitalizations and thousands of deaths, which occur principally in children.1 Dengue infection presents as a wide range of clinical symptoms that varies from an asymptomatic infection to a self-limiting, mild-fever disease (dengue fever [DF]) to a severe and potentially fatal hemorrhagic disorder (dengue hemorrhagic fever/dengue shock syndrome).2 A rapid and accurate diagnosis of dengue in the first days after the onset of symptoms is a critical step in dengue surveillance and outbreak control.
Detection of viral antigens is a simple and a reliable method that is commonly used. However, during a secondary infection, the sensitivity of this method may be significantly compromised because of pre-existing immunocomplexes. Precise detection of secondary infection is important because of the risks it represents for development of severe dengue. Early detection of severe disease has the potential to decrease morbidity and mortality, and new biomarkers that can reliably distinguish hemorrhagic cases are urgently needed.3 Although there are many commercial kits that are based on the early detection of dengue virus (DENV) infection, there is still a need for new approaches that combine specificity, sensitivity, rapid results, ease of use, and low cost for the diagnosis of primary or secondary DENV infection in the first few days after the appearance of symptoms.3
The high mobility group box 1 (HMGB1) was first described as a non-histone nuclear protein that binds and bends DNA and thus acts as a nuclear remodeling factor to facilitate the physical interactions between DNA and many others proteins.4 Although HMGB1 is usually found in the cell nucleus, it can be translocated to the cytoplasm or even be secreted into the extracellular milieu under some circumstances.5 The secretion of HMGB1 occurs through at least two pathways: passively from necrotic cells and/or actively by activated immune cells.6,7 Once outside the cell, it acts as a soluble mediator that plays an important role as a pro-inflammatory cytokine.8 The involvement of HMGB1 in DENV infection was first observed in DENV-infected epithelial cells undergoing necrosis, which passively released this molecule into the extracellular milieu.9 Another report showed that DENV-infected dendritic cells actively translocate HMGB1 to the cytoplasm or even secrete it.10 In our previous study, we demonstrated that circulating levels of HMGB1 in serum of DENV-infected patients were significantly increased, and the highest levels occurred during the first days after appearance of symptoms and in patients with a secondary infection.11 We investigated the potential of the HMGB1 protein as an auxiliary biomarker for early dengue diagnosis to detect either primary or secondary infection without the risk of immunocomplex assembly.
We used a 205-sample serum panel that included negative samples (healthy blood donors [HD]) that were obtained from the Cuban National Blood Bank and positive samples that were obtained from Dengue Serum Bank at the Pedro Kouri Tropical Medicine Institute of Havana, Cuba. All positive samples were obtained from adult patients with non-hemorrhagic cases of dengue who had been classified according to the type of infection (primary or secondary) and by the number of days after symptom onset during which samples were collected (day 0 was considered the first day of symptoms (Table 1).
Table 1.
Classification of the serum panel according to DENV and HMGB1 detection*
| Days post-symptoms | No. positive/total (%) HMGB1 positive | ||||||
|---|---|---|---|---|---|---|---|
| DENV negative | DENV positive | ||||||
| HD | DLF | Total | Primary | Secondary | Total | CC | |
| – | 1/34 (2.94) | – | 1/34 (2.94) | – | – | – | – |
| 0–3 | – | – | – | 12/30 (40) | 11/22 (50) | 23/52 (44.3) | 28/63 (44.5) |
| 4–7 | – | 3/24 (12.5) | 3/24 (12.5) | 3/11 (27.3) | 7/21 (33.3) | 10/32 (31.25) | – |
| Total | 1/34 (2.94) | 3/24 (12.5) | 4/58 (6.89) | 15/41 (36.6) | 18/43 (41.9) | 33/84 (39.3) | 28/63 (44.5) |
DENV = dengue virus; HGMB1 = high mobility group 1 box protein; HD = healthy donors; DLF = DENV-like fever; CC = clinical cases of fever from dengue-endemic region.
Dengue diagnosis was carried out using at least one of the following methods: isolation and virus identification by immunofluorescent assays using specific monoclonal antibodies, polymerase chain reaction, and/or IgM/IgG detection. Samples were similar to those used in our previous study.11 Because DF symptoms are similar to symptoms of other febrile tropical illnesses, we tested a group of samples from patients who had DF-like symptoms but who were negative for DENV infection (dengue-like fever [DLF]). In addition, to corroborate the usefulness of HMGB1 as a biomarker for dengue detection, we also tested a group of samples from patients with DF-like symptoms who lived in a dengue outbreak area but in whom DENV infection was not confirmed by using the classical methods; these samples were named clinical cases (CC).
To detect HMGB1 protein in serum, we performed a capture enzyme-linked immunosorbent assay. Wells of a 96-well microtiter plate (Greiner Bio-One, Kremsmünster, Austria) were coated with mouse monoclonal antibody against HMGB1 (Sigma Aldrich, St. Louis, MO) in phosphate-buffered saline (PBS) buffer. Plates were blocked with 1% bovine serum albumin in 0.05% Tween-20 in PBS and washed five times with 0.05% Tween-20 in PBS. This step was performed after each period of incubation. Wells were then incubated with patient serum samples that had been diluted 1:2 in PBS. Subsequently, wells were incubated with polyclonal antibody against HMGB1 (Abcam, Cambridge, MA) that was diluted in PBS containing 2% skim milk and then incubated with anti-IgG rabbit antibody that was conjugated to horseradish peroxidase (Promega, Madison, WI). Reactions were visualized with o-phenylenediamine dihydrochloride (Sigma Aldrich) and H2O2 as substrates and 12.5% H2SO4 as the quencher and monitored by measuring the optical density at 490 nm (OD490nm). The cutoff value was calculated as the mean OD490nm of the HD group plus 2 SD, and samples were considered positive for HMGB1 when the measured OD490nm was > 0.198. Statistical analyses were performed by using the non-parametric Mann-Whitney U test on GraphPad Prism software version 5.00 for Windows (GraphPad Software, San Diego, CA).
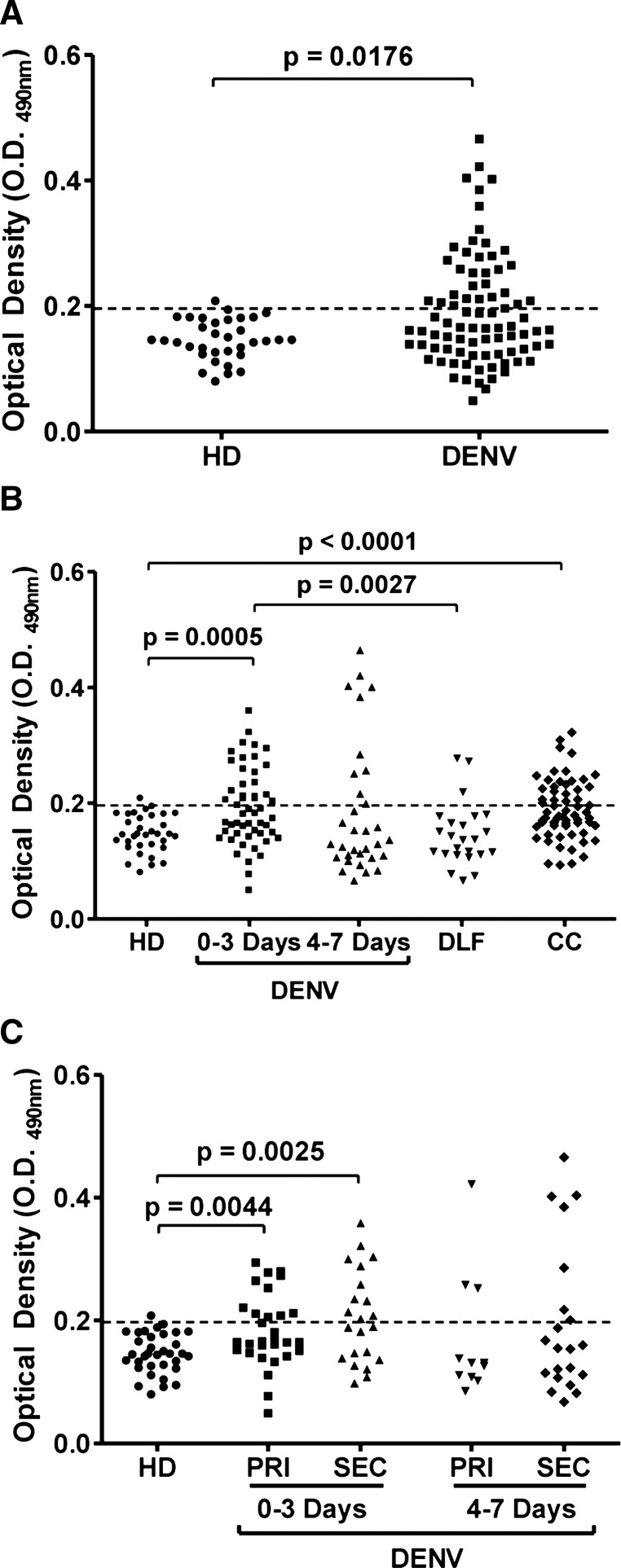
As expected, only 2.94% (1 of 34) of the negative samples (HD) were above the cutoff value, whereas 39.3% (33 of 84) of the DENV-positive samples were also positive for HMGB1 protein (Figure 1A and Table 1). In agreement with our previous work,11 44.3% (23 of 52) HMGB1-positive samples were found within the first three days after the appearance of symptoms; this value was significantly different (P = 0.0005) from the value obtained for the HD cases. As the infection progressed, the number of HMGB1-positive samples decreased to 31.2% (10 of 32) during the period 4–7 days after the onset of symptoms. Statistical analysis of this group in comparison with the HD group showed no significant differences (Figure 1B and Table 1). Interestingly, similar to the observations in the HD group, only 12.5% of the DLF samples were positive for HMGB1 protein (3 of 24) (Figure 1B and Table 1), and comparison of DLF samples with 0–3-day DENV-positive samples showed a significant difference (P = 0.0027). These results suggest that the HMGB1 protein can be used as a potential biomarker for the early detection of DENV infection.
Figure 1.
Detection of high mobility group box 1 protein (HMGB1) in serum samples from dengue virus (DENV)–infected patients. A, DENV-positive samples were plotted with those from healthy blood donors (HD). B, DENV-positive samples were stratified according to the number of days post-symptom onset and plotted with HD samples, samples from patients exhibiting dengue fever–like symptoms but who were negative for DENV infection (DLF), and samples from patients with dengue fever–like symptoms who lived in an outbreak area but in whom DENV infection was not confirmed by using the classical methods (CC). C, DENV-positive samples were stratified according to the number of days post-symptom onset and the type of infection. PRI = primary cases; SEC secondary cases. The cut-off value was calculated as the mean optical density at 490 nm (OD490nm) of the HD samples plus 2 SD. Samples with OD490nm values > 0.198 were considered positive for the HMGB1 protein.
To evaluate whether HMGB1 detection was increased in patients with primary or secondary infections, we stratified the DENV-positive samples according to these two types of infection. We observed that 41.9% (18 of 43) of HMGB1-positive samples were found during secondary infection, 50% (11 of 22) of these cases were found during 0–3 days post-symptom onset, and 33.3% (7 of 21) were found during 4–7 days post-symptom onset. During primary infection, HMGB1 was detected in 36.6% (15 of 41) of the cases, and 40% (12 of 30) and 27.3% (3 of 11) were found during 0–3 days and 4–7 days post-symptom onset, respectively (Figure 1C and Table 1). Overall, the maximum rate of HMGB1 detection (44.3% [23 of 52]) was found during 0–3 days post-symptom onset in primary (P = 0.0044) or secondary cases (P = 0.0025) (Table 1). These results illustrate the potential of HMGB1 protein in detection of DENV infection in primary and secondary cases.
We then evaluated the usefulness of HMGB1 for diagnosing DENV infection in samples from patients living in a dengue outbreak area who had symptoms similar to those of persons with DF. These samples were collected 0–3 days post-symptom onset (Table 1). Interestingly, the HMGB1 recognition profile of CC samples was similar to the profile in DENV-positive cases with HMGB1 detected in 44.5% (28 of 63) of the samples. For the DENV-positive samples, HMGB1 positivity reached 44.3% (Figure 1B and Table 1). Statistical analyses showed a significant difference between the CC and HD groups (P < 0.0001). These data corroborate the usefulness of HMGB1 as a biomarker for early dengue diagnosis.
Current dengue diagnosis is conducted by detection of virus particles, viral RNA, or viral antigens, as well as by titration of specific immunoglobulins against DENV. The quantitation of cytokines or endogenous proteins is uncommon because they are not specific for DENV infection, they may vary significantly in each patient, and their highest levels occur early in the infection, which makes them difficult to measure. It was observed that the pro-inflammatory cytokine HMGB1 is involved in DENV infection9,10 and is detected at increased levels in serum samples from DENV-infected patients.11 In contrast to other cytokines, HMGB1 is described as a late mediator of sepsis in comparison with the classical mediators, including tumor necrosis factor and interleukin 1.12
On the basis of these observations, we explored the potential of HMGB1 as a biomarker for early diagnosis of dengue. We observed that a large number of DENV-positive samples was also positive for HMGB1. In contrast, few HD and DLF patient samples were positive for HMGB1. These results indicate that HMGB1 is not involved in other dengue-like illnesses, despite its participation in the physiologic process of sterile inflammation13 and its role in other viral diseases.14,15 In addition, HMGB1 was detected in primary and secondary cases during the first day after symptom onset. Analysis of CC samples confirmed the potential of HMGB1 as an early dengue biomarker. However, dengue diagnosis based only on an endogenous molecule is not recommended because of the lack of evidence of viral infection.
Notwithstanding, the combined detection of virus-specific antigens and host molecules that are specifically involved in DENV infection will improve current diagnostic methods. The HMGB1 protein is a promising host biomarker for this purpose. For example, we believe that the detection of the nonstructureal protein 1 and HMGB1 protein together will principally favor the diagnosis of secondary infection during the early stages of clinical symptoms. Nonetheless, further studies that address the correlation between nonstructural protein 1 and HMGB1 should be performed. In summary, the present work is a preliminary study that highlights the usefulness of the HMGB1 protein as an auxiliary biomarker that will improve the current methods for early diagnosis of dengue.
ACKNOWLEDGMENTS
We thank Naifi Calzada for technical assistance.
Footnotes
Financial support: This work was supported by CAPES, CNPq, FAPERJ, IMBEBB, and the National Institutes of Science and Technology in Structural Biology and Bioimaging (INCT-INBEB).
Authors' addresses: Diego Allonso and Ronaldo Mohana-Borges, Laboratory of Structural Genomics, Biophysics Institute Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Brazil, E-mails: diegoars@biof.ufrj.br and mohana@biof.ufrj.br. Susana Vázquez and Maria G. Guzmán, Department of Virology, Pan American Health Organization/World Health Organization Collaborating Center for the Study of Dengue and its Vector, Pedro Kouri Tropical Medicine Institute, Havana, Cuba, E-mails: svazquez@ipk.sld.cu and lupe@ipk.sld.cu.
References
- 1.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martínez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8:S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simmons CP, Farrar JJ, Nguyen vV, Wills B. Dengue. N Engl J Med. 2012;366:1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 3.Peeling RW, Artsob H, Pelegrino JL, Buchy P, Cardosa MJ, Devi S, Enria DA, Farrar J, Gubler DJ, Guzman MG, Halstead SB, Hunsperger E, Kliks S, Margolis HS, Nathanson CM, Nguyen VC, Rizzo N, Vázquez S, Yoksan S. Evaluation of diagnostic tests: dengue. Nat Rev Microbiol. 2010;8:S30–S38. doi: 10.1038/nrmicro2459. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;20:35–36. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 5.Muller S, Scaffidi P, Degryse B, Bonaldi T, Ronfani L, Agresti A, Beltrame M, Bianchi ME. The double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J. 2001;20:4337–4340. doi: 10.1093/emboj/20.16.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 7.Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174:7506–7515. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 8.Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51:119–126. doi: 10.1016/j.cyto.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Chen LC, Yeh TM, Wu HM, Lin YY, Shyu HW. Dengue virus infection induces passive release of high mobility group box 1 protein by epithelial cells. J Infect. 2008;56:143–150. doi: 10.1016/j.jinf.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Kamau E, Takhampunya R, Li T, Kelly E, Peachman KK, Lynch JA, Sun P, Palmer DR. Dengue virus infection promotes translocation of high mobility group box 1 protein from the nucleus to the cytosol in dendritic cells, upregulates cytokine production and modulates virus replication. J Gen Virol. 2009;90:1827–1835. doi: 10.1099/vir.0.009027-0. [DOI] [PubMed] [Google Scholar]
- 11.Allonso D, Belgrano FS, Calzada N, Guzman MG, Vazquez S, Mohana-Borges R. Elevated serum levels of high mobility group box 1 (HMGB1) protein in dengue-infected patients are associated with disease symptoms and secondary infection. J Clin Virol. 2012;55:214–219. doi: 10.1016/j.jcv.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 13.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nowak P, Barqasho B, Sonnerberg A. Elevated plasma levels of high mobility group box protein 1 in patients with HIV-1 infection. AIDS. 2007;21:869–871. doi: 10.1097/QAD.0b013e3280b079b6. [DOI] [PubMed] [Google Scholar]
- 15.Torii Y, Ohta R, Imai M, Hara S, Kawano Y, Matsubayashi T, Inui A, Yoshikawa T, Nishimura N, Ozaki T, Morishima T, Kimura H. Increased levels of cytokines and high-mobility group box 1 are associated with the development of severe pneumonia, but not acute encephalopathy, in 2009 H1N1 influenza-infected children. Cytokine. 2011;56:180–187. doi: 10.1016/j.cyto.2011.07.016. [DOI] [PubMed] [Google Scholar]