Abstract
The use of standardized tools for continuous quality improvement of laboratory services is crucial to identify service gaps, plan targeted interventions, and prove successes. Laboratory quality improvement tools (LQITs) were developed and applied for 18 months at five health centers and one faith-based hospital laboratories in Southwest Showa Zone in Ethiopia to assess and monitor the quality of malaria and acid-fast bacilli (AFB) microscopy total testing processes. For the six laboratories, baseline malaria microscopy scores were 55%, 42%, 52%, 55%, 54%, and 61%. Similarly, baseline AFB microscopy scores were 49%, 41%, 46%, 58%, 44%, and 70%. On the sixth quarter for the first four laboratories and the fourth quarter for the last two laboratories, malaria microscopy scores were 89%, 88%, 88%, 90%, 88%, and 89%, whereas AFB microscopy scores were 90%, 88%, 89%, 95%, 88%, and 90%. All laboratories scored above 85% for both services at the end of interventions.
Background
Many health programs in sub-Saharan African countries are often implemented using a vertical strategy to address disease-specific issues. On one hand, this silo approach has the benefit of reaching lower health facilities, where the majority of the population accesses the health system, and hence, a specific disease can be significantly controlled. On the other hand, by only tackling a single infection, a great opportunity for strengthening the laboratory as a whole is missed. The unprecedented investments in programs to fight human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) in many resource-limited settings have resulted in major developments in the diagnostic capacity of medical laboratories to support HIV care and treatment initiatives in many countries. Taking advantage of the improvements of diagnostic services related to HIV/AIDS, the Federal Ministry of Health (FMOH) in Ethiopia gave the mandate to the Ethiopian Health and Nutrition Research Institute (EHNRI) to enhance the quality of all services provided by medical laboratories, not only a specific program or disease. This approach is directed to the implementation of a Master Plan for the Public Health Laboratory System in Ethiopia1 for the period 2009–2013 by the EHNRI/FMOH and its national and international partner organizations.
At present, in Ethiopia, FMOH/EHNRI supports 39 medical laboratories from government hospitals, national and regional public health laboratories, and non-governmental faith-based hospital to accreditation through assessments, trainings, supportive supervision, and mentoring. This effort is the first large-scale effort for accrediting medical laboratories in Ethiopia, and it is one of the numerous initiatives to support the accreditation of secondary and tertiary medical laboratories in sub-Saharan Africa according to the five-star accreditation scheme of the World Health Organization, African Office (WHO-AFRO).2
Medical laboratories at secondary and tertiary levels of the health system play an important role for advancing diagnostic capacity, but they are also crucial for strengthening public health and surveillance systems.3 Additionally, they are founding the framework of centralized quality systems at the national level,4 with the overall goal of ensuring quality of testing at all laboratories levels. Thus, many countries in sub-Saharan Africa have allocated resources to expand national external quality assurance (EQA) schemes, mainly through panel testing for different analytes. However, very little effort is devoted to improving the quality of medical laboratory services at the lower level of the health system. Medical laboratories at the primary level play a key role in the decentralization of primary care,5–12 but they are also crucial in public health surveillance and action activities, such as malaria interventions and infectious diseases outbreaks.13,14 Improving the quality of laboratory operations at primary health facilities is, therefore, essential for strengthening the entire health system. A common area lacking quality at primary medical laboratories is microscopy, specifically for AFB and malaria diagnosis. This lack of quality is mainly because of the lack of continuous professional training and quality assurance programs.15–21 The situation can temporarily improve with refresher courses,22 donation of new equipment,23 and detailed assessment of sputum smear microscopy and malaria diagnostic capacity at the site level,24,25 but there is still a lack of systematic approach to integrating continuous quality improvement (CQI) activities at peripheral medical laboratories26–28 similar to those laboratories targeted by WHO-AFRO accreditation processes for upper-level laboratories. Improving the overall quality of microscopy-based services gives primary health facilities additional opportunities to function as research units for medical research in country29 and facilitate the introduction of new diagnostic tests,30 with the outcome of boosting the motivation of laboratory staff and thus, improving their retention.
The Institute of Human Virology of the University of Maryland School of Medicine (IHV-UMSOM) is among the US President's Emergency Plan for AIDS Relief (PEPFAR) University partners in Ethiopia providing HIV care and treatment services to primary- and secondary-level laboratories. In 2009, IHV-UMSOM, the technical lead of the AIDSRelief Consortium, began supporting six health facilities in the Southwest Showa Zone of Oromia Region. Among the supported sites, five health centers (HCs) and one faith-based hospital, only the medical laboratory at the faith-based hospital had CD4 + T lymphocytes, clinical chemistry, and hematology testing capacity.31,32
In this paper, we present the results the IHV-UMSOM obtained by using the CQI approach to malaria and AFB microscopy services at six health facilities in Ethiopia.
Methods
Laboratory quality improvement tools (LQITs) were developed to assess and monitor the quality of both malaria and AFB microscopy total testing processes. Each tool comprised 100 close-ended questions divided into 12 sections covering general and specific aspects consisting of human resources, safety, equipment, maintenance, standard operating procedures (SOPs), documentation, inventory management, sample preparation and processing, slides reading, results reporting, and quality controls. The total score was a sum of the single scores of the 12 sections, and their effectiveness was based on both questions and direct observations. The LQITs were used at five HCs and one faith-based hospital laboratories in Southwest Showa Zone of Oromia Region in Ethiopia for 18 months as part of the technical support provided by the IHV-UMSOM HIV care and treatment programs. The six health facilities are situated at 125.4, 104, 128, 116, 136, and 77 km away from Addis Ababa, the capital city of Ethiopia. The Zone is among places in Ethiopia that are highly affected by malaria epidemic. One-year data collected from December of 2009 to November of 2010 on malaria microscopy performed in five of six health facility laboratories showed that, of 6,016 malaria morbidity-confirmed positive samples, 4,680 (78%) were infected by P. vivax, whereas the rest (1,336; 22%) were infected by P. falciparum.
Data were collected at baseline and on quarterly basis for all six sites and analyzed and shared with laboratories for planning interventions. Monthly follow-up, onsite and offsite trainings, on-the-job mentoring, and documentation and quality assurance supports were provided to fill in tracked gaps and sustain successes. The performance of the laboratories was graded as unsatisfactory if total score was below 65%, satisfactory if between 65% and 74%, good if between 75% and 84%, and excellent if above 85%. The total scores using both LQITs for malaria and AFB microscopy were named MalScore and AFBScore, respectively.
Four health facilities' laboratories received six quarterly visits from December of 2009 to May of 2011, whereas two more health facilities' laboratories added later received four quarterly visits from June of 2010 to May of 2011.
At the baseline visit, the CQI approach and the purpose of the intervention were presented to all laboratory staff. As a second step, a baseline assessment of the laboratories was carried out using the LQITs. The gaps identified with the LQITs allow for prioritizing the topics to be covered during onsite visits and refresher trainings.
Results and Discussion
Baseline scores on malaria microscopy were 55%, 42%, 52%, 55%, 54%, and 61%. Similarly, baseline AFB microscopy scores were 49%, 41%, 46%, 58%, 44%, and 70%. Thus, the baseline MalScore was unsatisfactory (below 65%) for all six laboratories, whereas AFBScore was unsatisfactory for five and satisfactory (between 65% and 74%) for one HC laboratory. The baseline MalScore was almost similar to all laboratories assessed, unlike another study that assessed laboratory malaria diagnostic capacity within the same region and reported marked variability in laboratory diagnostic capacity among facilities assessed.25 The contrast between the two studies may be because of either the difference in number of health facilities included in the study or discrepancies in the level of health facilities considered.
The effort in analyzing the baseline data and sharing to health facility heads and laboratory staffs helped in tracking gaps and planning interventions. The monthly follow-up, onsite and offsite trainings, on-the-job mentoring, and documentation and quality assurance supports provided helped in improving laboratory services at the health facilities. In particular, the efforts invested to increase the numbers of appropriately trained laboratory staff—essential for efficient delivery of high-quality medical laboratory services—by using the onsite mentoring/coaching approach recommended by EHNRI for in-service training of laboratory professionals contributed to significant improvement. This approach allowed for sustaining new practices, strengthening routine job activities, and ensuring that appropriate technical skills were applied over time. A total of 20 laboratory professionals received on-the-job mentoring to address the gaps identified by using the LQIT.
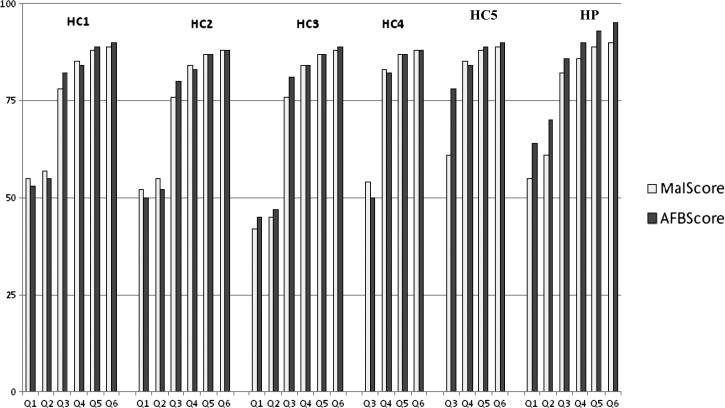
At the end of quarter six, malaria microscopy scores were 89%, 88%, 88%, 90%, 88%, and 89%, respectively, whereas AFB microscopy scores were 90%, 88%, 89%, 95%, 88%, and 90%, respectively. Thus, at the end of quarter 6, all six medical laboratories scored above 85% (excellent) for both AFB and malaria microscopy. Moreover, six of six (100%) laboratories sustained without decreasing their score over time. Quarterly scores of the six laboratories both for malaria and AFB microscopy are reported in Figure 1.
Figure 1.
Quarterly malaria and AFB scores for all six laboratories.
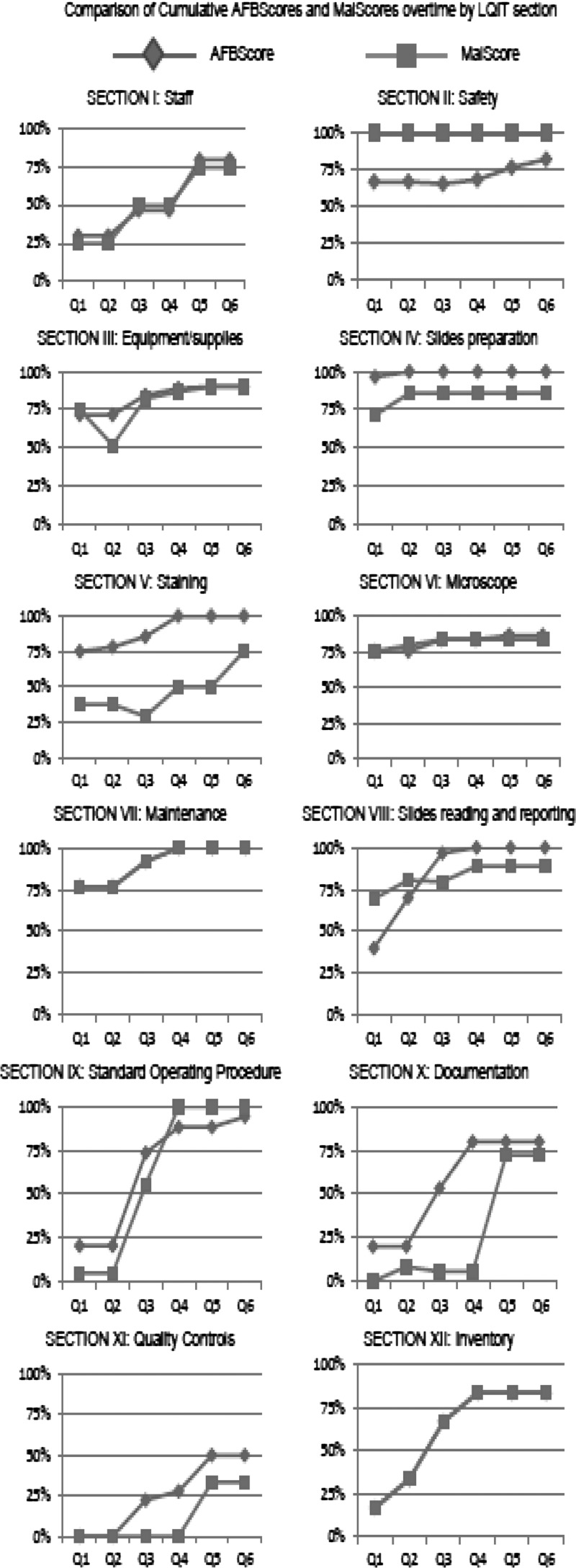
Figure 2 reports the comparison of cumulative scores for all six facilities per quarter for each section of the LQITs.
Figure 2.
Comparison of cumulative scores for all six facilities per quarter for each section of the LQITs.
Section I, related to human resources, showed constant increase over time, with the two major interventions being the identification of malaria and AFB microscopy focal persons and the provision of refresher training on both malaria and AFB microscopy. The rationale behind these interventions was based on staff being given a sense of responsibility and ownership that would increase their motivation. Although refresher training was provided to all laboratory workers, each focal person was the specific target of interventions related to each disease by making him/her fully accountable for the quality in that specific testing area. All HCs had two laboratory workers to share responsibilities, which facilitated with minimizing interpersonal issues within the same laboratory.
In terms of safety (section II), although MalScore was 100% at baseline assessment, AFBScore improved from satisfactory (67%) to good (82%). The major output was to establish a tuberculosis (TB) infectious waste disposal protocol that is understood and followed over time by laboratory staff.
Sections IV–VIII, strictly related to slide preparation, staining, microscope, maintenance, and slide reading/reporting, respectively, showed constant increase with the AFBScore for all three sections over time. The same procedure for smears preparation and staining at all sites was observed for AFB microscopy, unlike malaria microscopy, which was subjected to site-specific variations, such as lack of refresher trainings. These issues were addressed by introducing and implementing SOPs between quarters 3 and 4 and providing and displaying the WHO malaria staining procedure poster in the staining area in quarter 5. The fact that minimal issues related to microscope functionality were found was very positive. This result minimizes the risk of instrument-related errors for other microscope-based tests, such as parasitological examinations, blood smears, gram stain, and urine analysis, available at the laboratories.
The other major areas of improvement were related to the integration of properly documented quality procedures into the routine daily activities of the laboratory. This section showed discordant outcomes between the two procedures, with an overall poorer performance compared with other sections. The increase of MalScore between quarters 4 and 5 was because of the introduction of laboratory forms for reagent shelf life and quality. A similar increase was obtained for AFBScore in quarter 2 because of the introduction of laboratory forms for reagents preparation/troubleshooting staining solutions problems, troubleshooting microscope problems, and reagent shelf life and quality.
The scores for section XI (quality control for both malaria and AFB microscopy) were the lowest but showed a significant improvement at the end of quarter 4 because of the introduction of a standardized procedure for malaria and AFB slides staining and AFB quantification. The major reasons for the lower performance of the quality control section were lack of EQA programs for malaria and AFB microscopy at HCs level and the high turnaround time for feedback from the EQA provider. The only site participating in and receiving feedback on AFB and malaria microscopy EQA schemes was the laboratory at the hospital level. This aspect was tackled by EHNRI by building the capacity of Regional Laboratories (Strategic Objective 3 of the National Master Plan) and working closely with President's Malaria Initiative (PMI) and university partners.
Conclusions
The LQITs used for CQI in the approach described above targeted light microscopy-based tests that, at the level of health facilities supported by this intervention, still remain the gold standard for both malaria and TB diagnosis as per FMOH guidelines. The questions were designed using international guidelines and laboratory standards to be used across the seven different countries where IHV-UMSOM currently supports HIV care and treatment programs. The adoption of these standards for CQI in the context of the Ethiopian laboratory settings proved to be efficient, with an overall improvement over time in all 12 sections for both total testing processes.
This study was the first study where a CQI approach was used to strengthen the quality of malaria and AFB microscopy services at primary-level laboratories in Ethiopia. All the health facilities where the CQI approach was applied had shown commitment to improve the quality of their operations over time. None of the laboratories decreased their score over time for the duration of the project, resulting in maintenance of quality standards at primary-level medical laboratories. The use of the LQITs represented a valid approach for integrating CQI activities in the laboratory system, with the overall goal of reaching and maintaining high-quality total testing process for malaria and acid-fast AFB microscopy at primary-level health facilities in Ethiopia. Future interventions, aimed at evaluating long-term impact and the frequency that LQITs have to be used to maintain quality TB and malaria diagnostic services, are highly needed. A similar approach to malaria rapid diagnostic test is recommended to improve quality at health posts level, where the majority of malaria laboratory diagnoses is performed. Also, regional laboratories in the country would sustain the work and evaluate the CQI approach at a larger health facility level in different settings.
ACKNOWLEDGMENTS
This study was part of the activities that the Institute of Human Virology, AIDSRelief Program, currently carries out in Ethiopia in the context of the Presidential Emergency Plan for AIDSRelief to enhance the integration of quality interventions with the broader health and development programs of the US Government, country partners, multilateral organizations, and other donors.
Footnotes
Authors' addresses: Francesco Marinucci, Antonio D. Paterniti, Sandra Medina-Moreno, Matthew Wattleworth, Juliana Hagembe, and Robert R. Redfield, Institute of Human Virology, University of Maryland School of Medicine, Baltimore, MD, E-mails: fmarinucci@ihv.umaryland.edu, apaterniti@ihv.umaryland.edu, smmoreno@ihv.umaryland.edu, mwattleworth@ihv.umaryland.edu, jhagembe@ihv.umaryland.edu, and rredfield@ihv.umaryland.edu. Tsegahun Manyazewal, Institute of Human Virology, University of Maryland, US President's Emergency Plan for AIDS Relief, Addis Ababa, Ethiopia, E-mail: tsegahunm@gmail.com.
References
- 1.Federal Democratic Republic of Ethiopia Ministry of Health [FMoH] Master Plan for the Public Health Laboratory System in Ethiopia, 2009–2013. 2nd ed. Addis Ababa, Ethiopia: Ethiopian Health and Nutrition Research Institute (EHNRI), Federal Ministry of Health; 2009. [Google Scholar]
- 2.Gershy-Damet GM, Rotz P, Cross D, Belabbes el H, Cham F, Ndihokubwayo JB, Fine G, Zeh C, Njukeng PA, Mboup S, Sesse DE, Messele T, Birx DL, Nkengasong JN. The World Health Organization African region laboratory accreditation process: improving the quality of laboratory systems in the African region. Am J Clin Pathol. 2010;134:393–400. doi: 10.1309/AJCPTUUC2V1WJQBM. [DOI] [PubMed] [Google Scholar]
- 3.Nsubuga P, Nwanyanwu O, Nkengasong JN, Mukanga D, Trostle M. Strengthening public health surveillance and response using the health systems strengthening agenda in developing countries. BMC Public Health. 2010;10:S5. doi: 10.1186/1471-2458-10-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nkengasong JN, Nsubuga P, Nwanyanwu O, Gershy-Damet GM, Roscigno G, Bulterys M, Schoub B, DeCock KM, Birx D. Laboratory systems and services are critical in global health: time to end the neglect? Am J Clin Pathol. 2010;134:368–373. doi: 10.1309/AJCPMPSINQ9BRMU6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Floyd K, Skeva J, Nyirenda T, Gausi F, Salaniponi F. Cost and cost-effectiveness of increased community and primary care facility involvement in tuberculosis care in Lilongwe District, Malawi. Int J Tuberc Lung Dis. 2003;9:S29–S37. [PubMed] [Google Scholar]
- 6.Nganda B, Wang'ombe J, Floyd K, Kangangi J. Cost and cost-effectiveness of increased community and primary care facility involvement in tuberculosis care in Machakos District, Kenya. Int J Tuberc Lung Dis. 2003;9:S14–S20. [PubMed] [Google Scholar]
- 7.Okello D, Floyd K, Adatu F, Odeke R, Gargioni G. Cost and cost-effectiveness of community-based care for tuberculosis patients in rural Uganda. Int J Tuberc Lung Dis. 2003;9:S72–S79. [PubMed] [Google Scholar]
- 8.Sinanovic E, Floyd K, Dudley L, Azevedo V, Grant R, Maher D. Cost and cost-effectiveness of community-based care for tuberculosis in Cape Town, South Africa. Int J Tuberc Lung Dis. 2003;9:S56–S62. [PubMed] [Google Scholar]
- 9.Shargie EB, Mørkve O, Lindtjørn B. Tuberculosis case-finding through a village outreach programme in a rural setting in southern Ethiopia: community randomized trial. Bull World Health Organ. 2006;84:112–119. doi: 10.2471/blt.05.024489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Getahun H, Maher D. Contribution of ‘TB clubs’ to tuberculosis control in a rural district in Ethiopia. Int J Tuberc Lung Dis. 2000;4:174–178. [PubMed] [Google Scholar]
- 11.Hadley M, Maher D. Community involvement in tuberculosis control: lessons from other health care programmes. Int J Tuberc Lung Dis. 2000;4:401–408. [PubMed] [Google Scholar]
- 12.Yimer S, Holm-Hansen C, Yimaldu T, Bjune G. Health care seeking among pulmonary tuberculosis suspects and patients in rural Ethiopia: a community-based study. BMC Public Health. 2009;9:454. doi: 10.1186/1471-2458-9-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNabb SJ, Chungong S, Ryan M, Wuhib T, Nsubuga P, Alemu W, Carande-Kulis V, Rodier G. Conceptual framework of public health surveillance and action and its application in health sector reform. BMC Public Health. 2002;2:2. doi: 10.1186/1471-2458-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breman JG, Holloway CN. Malaria surveillance counts. Am J Trop Med Hyg. 2007;77:36–47. [PubMed] [Google Scholar]
- 15.Harries AD, Nyirenda TE, Banerjee A, Mundy C, Salaniponi FM. District sputum smear microscopy services in Malawi. Int J Tuberc Lung Dis. 1998;2:914–918. [PubMed] [Google Scholar]
- 16.Yimer S, Bjune G, Alene G. Diagnostic and treatment delay among pulmonary tuberculosis patients in Ethiopia: a cross sectional study. BMC Infect Dis. 2005;5:112. doi: 10.1186/1471-2334-5-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mfinanga SG, Mutayoba BK, Kahwa A, Kimaro G, Mtandu R, Ngadaya E, Egwaga S, Kitua AY. The magnitude and factors associated with delays in management of smear positive tuberculosis in Dar es Salaam, Tanzania. BMC Health Serv Res. 2008;8:158. doi: 10.1186/1472-6963-8-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Addo KK, Owusu-Darko K, Dan-Dzide M, Yeboah-Manu D, Ablordey A, Caulley P, Minamikawa M, Bonsu F, Lienhardt C, Akpedonu P, Ofori-Adjei D. Situation analysis of TB microscopy centres in Ghana. Int J Tuberc Lung Dis. 2006;10:870–875. [PubMed] [Google Scholar]
- 19.Mfinanga GS, Ngadaya E, Mtandu R, Mutayoba B, Basra D, Kimaro G, Chonde TM, Ngowi P, Mfaume S, Kilale AM, Egwaga S, Kitua AY. The qualities of sputum smear microscopy diagnosis of pulmonary tuberculosis in Dar es Salaam, Tanzania. Tanzan Health Res Bull. 2007;3:164–168. doi: 10.4314/thrb.v9i3.14323. [DOI] [PubMed] [Google Scholar]
- 20.Dini L, Frean J. Quality assessment of malaria laboratory diagnosis in South Africa. Trans R Soc Trop Med Hyg. 2003;97:675–677. doi: 10.1016/s0035-9203(03)80101-3. [DOI] [PubMed] [Google Scholar]
- 21.Durrhelm DN, Becker PJ, Billinghurst K, Brink A. Diagnostic disagreement–the lessons learnt from malaria diagnosis in Mpumalanga. S Afr Med J. 1997;87:609–611. [PubMed] [Google Scholar]
- 22.Kiggundu M, Nsobya SL, Kamya MR, Filler S, Nasr S, Dorsey G, Yeka A. Evaluation of a comprehensive refresher training program in malaria microscopy covering four districts of Uganda. Am J Trop Med Hyg. 2011;84:820–824. doi: 10.4269/ajtmh.2011.10-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Rie A, Fitzgerald D, Kabuya G, Van Deun A, Tabala M, Jarret N, Behets F, Bahati E. Sputum smear microscopy: evaluation of impact of training, microscope distribution, and use of external quality assessment guidelines for resource-poor settings. J Clin Microbiol. 2008;46:897–901. doi: 10.1128/JCM.01553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aziz M, Bretzel G. Use of a standardised checklist to assess peripheral sputum smear microscopy laboratories for tuberculosis diagnosis in Uganda. Int J Tuberc Lung Dis. 2002;6:340–349. [PubMed] [Google Scholar]
- 25.Hailegiorgis B, Girma S, Melaku Z, Teshi T, Demeke L, Gebresellasie S, Yadeta D, Tibesso G, Whitehurst N, Yamo E, Carter J, Reithinger R. Laboratory malaria diagnostic capacity in health facilities in five administrative zones of Oromia Regional State, Ethiopia. Trop Med Int Health. 2010;15:1449–1457. doi: 10.1111/j.1365-3156.2010.02646.x. [DOI] [PubMed] [Google Scholar]
- 26.Sarkinfada F, Aliyu Y, Chavasse C, Bates I. Impact of introducing integrated quality assessment for tuberculosis and malaria microscopy in Kano, Nigeria. J Infect Dev Ctrie. 2009;3:20–27. doi: 10.3855/jidc.101. [DOI] [PubMed] [Google Scholar]
- 27.Addo KK, Yeboah-Manu D, Dan-Dzide M, Owusu-Darko K, Caulley P, Mensah GI, Minamikawa M, Lienhardt C, Bonsu FA, Ofori-Adjei D. Diagnosis of tuberculosis in Ghana: the role of laboratory training. Ghana Med J. 2010;44:31–36. doi: 10.4314/gmj.v44i1.68854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girma A, H/Mariam D, Deribe K. Quality of tuberculosis care in six health facilities of Afar Region, Ethiopia. Ethiop Med J. 2010;48:195–202. [PubMed] [Google Scholar]
- 29.Chinery WA. Diagnostic laboratory parasitology—a stepping stone to medical research in tropical Africa. J Hyg Epidemiol Microbiol Immunol. 1992;36:356–361. [PubMed] [Google Scholar]
- 30.Boehme CC, Nabeta P, Henostroza G, Raqib R, Rahim Z, Gerhardt M, Sanga E, Hoelscher M, Notomi T, Hase T, Perkins MD. Operational feasibility of using loop-mediated isothermal amplification for diagnosis of pulmonary tuberculosis in microscopy centers of developing countries. J Clin Microbiol. 2007;45:1936–1940. doi: 10.1128/JCM.02352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marinucci F, Medina-Moreno S, Paterniti AD, Wattleworth M, Redfield RR. Decentralization of CD4 testing in resource limited settings: 7 years of experience in 6 African countries. Cytometry A. 2010;79:368–374. doi: 10.1002/cyto.a.21064. [DOI] [PubMed] [Google Scholar]
- 32.St Luke Catholic Hospital and College of Nursing and Midwifery [SLCH] Annual Report, 2010. Wolisso, Ethiopia: St Luke Catholic and College of Nursing and Midwifery; 2010. [Google Scholar]