Abstract
The analysis of promastigote excreted–secreted antigen (ESA) reactivity with 53 visceral leishmaniasis (VL) cases showed that each sample reacted regardless of the antigen or the Leishmania species used in enzyme-linked immunosorbent assay (ELISA) displayed 100% positivity with the L. (L.) chagasi ESA-blot recognizing bands of molecular weight ranging from 26.5 to 31.5 kDa. The analysis of 160 non-visceral cases showed that 5% of the samples cross-reacted with the L. (L.) chagasi ESA-ELISA and 9.4% reacted with the ESA isolated from L. (L.) amazonensis and L. (V.) braziliensis, whereas a high cross-reaction ranging from 24.4% to 25% was observed with total crude promastigote antigens (PRO-ELISA). The ESA-blot of L. (L.) chagasi tested with non-visceral sera samples showed a cross-reaction with 8.8% of cases; most of these cases represented tegumentary leishmaniasis and only one acute chagasic case. These data lead us to recommend the use of ESA as an alternative antigen in VL diagnosis.
Introduction
Leishmaniasis is caused by the protozoan Leishmania spp. The disease syndromes vary depending on the species of Leishmania.1,2 In the New World, L. (L.) chagasi, currently described as L. (L.) infantum,3 is the principal causative species of visceral leishmaniasis (VL). The VL is transmitted by Lutzomyia longipalpis and is characterized with a peridomestic cycle using the dog as the reservoir. Such parasite infections represent a public health problem in many countries worldwide and are associated with a variety of clinical syndromes with either visceral or tegumentary involvement, eventually leading to morbidity and mortality. The diagnosis of VL is usually based on clinical signs and laboratory tests, and the classic confirmatory test involves the microscopic examination of bone marrow or spleen aspirates to visualize the parasites. However, this method is very invasive, and the levels of specificity and sensitivity vary with this method.1,2
The search for non-invasive diagnostic methods has aided the development of new serological tests designed to identify specific anti-VL antibodies using recombinant or purified antigens.4–6 Nevertheless, even with the discovery of new antigens and the development of various methodologies, the sensitivity and specificity of serological tests still vary because although the levels of sensitivity depend on the technique, the specificity depends on the antigen rather than the serological procedure.1,2,4–6 More than four decades ago, excreted–secreted antigenic molecules found in Leishmania promastigotes were reported in culture media. Greemblat and Glaser7 were the first to draw attention to the production by Leishmania promastigotes of molecules that accumulated in the incubation media. Since this initial observation, several studies have shown that some of these molecules are immunogenic and antigenic,8 and several efforts have been made to identify Leishmania spp. excreted–secreted proteins.9,10 Because some of these proteins have been described to stimulate cellular and humoral immune responses, it is reasonable to expect that they may be applicable for vaccine, diagnostic test, and drug target development.8,11
Leishmania spp. conditioned medium has been used as a potential source of antigens for immunodiagnosis in the detection of cases of human VL,5,6,12,13 canine leishmaniasis,12,14 and cutaneous leishmaniasis (CL).13,15 Although serological tests, such as the direct agglutination test, immunofluorescence test, indirect hemagglutination assay, enzyme-linked immunosorbent assay (ELISA), and Western blotting have been found to display high levels of sensitivity, the specificity indices were not significant.1,2 The reason for this result may be because the antigens used in immunodiagnostic tests are usually derived from crude promastigotes of different Leishmania species, which express a variety of complex molecules that are common to other microorganisms, such as Trypanosoma, Plasmodium, and Mycobacterium,16–20 or proteins that can react non-specifically.21 However, tests based on recombinant antigens have also displayed varying levels of sensitivity and specificity depending on the geographical area from which the cases originated.4,22
In Brazil, the specificity of diagnostic tests is very important, as leishmaniases occur concomitantly with pathologies caused by the parasites mentioned previously. This study aims to demonstrate that ELISA or immunoblotting (blot) using excreted–secreted antigens (ESAs) isolated from L. (L.) chagasi promastigotes may represent an effective alternative for the detection of VL in humans, as the tests revealed high levels of sensitivity and no cross-reaction with non-visceral leishmaniasis cases (excluding tegumentary leishmaniasis).
Materials and Methods
Antigens.
All experiments were performed with promastigotes of L. (L.) amazonensis (MHOM/BR/73/M2269), L. (V.) braziliensis (MHOM/BR/75/M2903), and L. (L.) chagasi (MHOM/BR/72/LD) isolated in Brazil. The parasites were routinely grown at 26°C in liver infusion tryptose medium containing 5% (v/v) heat-inactivated fetal calf serum (Cultilab, Brazil) and 5% sterile human male urine23; the parasites were washed twice in serum-free RPMI 1640 medium. The ESAs from Leishmania promastigote forms were obtained from the RPMI 1640 medium after the incubation of 5 × 108 promastigotes/mL for 24 hours at 26°C without agitation. Different batches of ESA were obtained by centrifugation at 2,800 × g for 15 minutes at 4°C; the supernatants were recentrifugated (7,000 × g for 30 minutes at 4°C) to ensure the complete pelleting of possible cell debris and were filtered using a cellulose acetate membrane (pore size, 0.20 μm). The ESA was either used immediately without any further concentration or purification or stored at −70°C in small aliquots. The protein content of 30–40 μg protein/mL was quantified for different supernatants (Micro-bicinchoninic acid protein assay reagent kit; Pierce Co., Rockford, IL). Crude promastigote antigens (PRO) were prepared as previously described for Trypanosoma cruzi antigens.24 Briefly, L. (L.) amazonensis, L. (V.) braziliensis, and L. (L.) chagasi promastigotes were digested with 0.3 N NaOH for 18 hours at 4°C, neutralized with 0.3 N HCl to pH 7–8, centrifuged at 12,000 × g for 1 minute at 4°C and stored at −70°C after quantifying the protein concentration (Macro- bicinchoninic acid protein assay reagent kit; Pierce Co., Rockford, IL). A total of 3 × 106 whole promastigotes (WP)/lane were used for immunoblotting as a control for tubulin detection.25
ELISA.
The optimal concentration of antigens, sera, and peroxidase conjugate were pre-determined by checkerboard titration. Flat-bottom 96-well microtiter plates (Costar, Hybond polystyrene plates, Inc., New York, NY) were coated with 50 μL of L. (L.) amazonensis, L. (V.) braziliensis, or L. (L.) chagasi PRO antigens (3 μg/mL) or ESA (2–3 μg/mL) diluted in 0.05 M carbonate-bicarbonate buffer (pH 9.6) for 18 hours at 4°C. The plates were blocked with phosphate-buffered saline pH 7.2 (v/v) plus 0.05% Tween 20 (PBS-T) containing 5% fat-free milk (Molico, Nestlé, SP, Brazil) for 30 minutes at room temperature. The plates were incubated with 50 μL of diluted sera (1:200) for 1 hour at 37°C, washed, and incubated with peroxidase conjugate anti-human IgG (SIGMA Chemical Co., St. Louis, MO) for 1 hour at 37°C. After the addition of hydrogen peroxide and O-phenylenediamine dihydrochloride (Sigma Co.) for 30 minutes at 37°C in the dark, the reaction was stopped by adding 25 μL of 4 N HCl, and the absorbance (A492nm) was measured using a plate ELISA reader (Labsystems Multiskan MS, VA). 30 minutes later. All experiments were repeated at least twice on different days.
SDS-PAGE and immunoblotting.
Medium containing ESA released from L. (L.) amazonensis, L. (V.) braziliensis, and L. (L.) chagasi (30 μL/lane), PRO (1 μg/lane), and WP (3 × 106 promastigotes/lane) of L. (L.) chagasi were diluted in SDS-sample buffer (60 mM Tris-HCl [pH 6.8], 5% 2-mercaptoethanol, 10% glycerol, and 0.01% bromophenol blue), and boiled for 5 minutes at 100°C. The samples were loaded on a 12% polyacrylamide minigel (Mini-Protean II; Bio-Rad, Hercules, CA), and the separated proteins were electrophoretically transferred onto 0.45 μm pore nitrocellulose membranes (Millipore, SP, Brazil) as described previously.26 The nitrocellulose membranes (NTC) containing ESA, PRO, and WP antigens were blocked with 5% fat-free milk in PBS and incubated with either monoclonal anti-α-tubulin mouse ascite fluid clone B512 (Sigma Co.) or VL sera pool (1:100). The ESA blot strips (5 mm wide) were probed with human sera (1:100) diluted in 1% fat-free milk and incubated for 2 hours with mechanical agitation. After 5 washes, the blots were incubated with either peroxidase goat anti-human IgG (Sigma Co.) or anti-mouse conjugates (Sigma Co.). Hydrogen peroxide and 4-chloro-1-naphthol (Sigma Co.) were added as substrates, and the reaction was stopped with deionized water. The molecular mass protein standard used ranged from 14,200 to 66,000 Da (Sigma Co.).
Sera.
Serum samples were collected from 213 patients admitted to various clinics in Brazil; 53 sera were donated by C. Barbiéri, which were isolated from patients with VL living in Piauí, Brazil, whose clinical and true diagnostic data had been previously reported,27 and 160 non-visceral leishmaniasis cases. The latter consisted of 23 sera from tegumentary leishmaniasis cases (8 cutaneous leishmaniasis [CL] and 15 mucocutaneous leishmaniasis [MCL]) from the Instituto Emilio Ribas de São Paulo, Brazil; 25 from Brazilian patients with Chagas disease (5 patients in the acute phase and 20 patients in the chronic phase); 27 from patients with autoimmune disease (positive for anti-nuclear antibodies); 20 from malaria patients (10 infected by P. falciparum and 10 patients infected by P. vivax); 6 from paracoccidioidomycosis patients; 5 from schistosomiasis patients; 3 from toxocariasis patients; 5 from toxoplasmosis patients; 14 from tuberculosis patients; and 32 from healthy Brazilian blood donors, which were used as negative controls.
Data analysis.
The ESA-blot results were defined as positive by naked-eye observation of bands. For the ESA-blots corresponding to L. (L.) amazonensis and L. (V.) braziliensis, the blots were defined as positive when they exhibited bands of molecular weights ranging from 25.5 to 30.0 kDa. For the L. (L.) chagasi blots, they were regarded as positive when the bands ranged in size from 26.5 to 31.5 kDa. For ESA-ELISA and PRO-ELISA, the samples were recorded as positive or negative based on the cutoff value, which was calculated as the mean A492nm of the sera collected from blood donors plus 3 SD. Statistical analysis was performed using GraphPad Prism 3 (GrapHPad Software Inc., La Jolla, CA) with a non-parametric one-way analysis of variance test and Dunnett's multiple statistical analysis for significant differences in mean titers between the ESA and PRO antigens and among the species of Leishmania (significant level of P < 0.05, and 95% confidence interval).
Study site.
This study was conducted at the Instituto de Medicina Tropical de São Paulo (IMTSP) da Universidade de São Paulo, São Paulo, Brazil, and the study protocol was approved by the Ethical Committee on Human Research of Instituto de Ciências Biomédicas da Universidade de São Paulo, São Paulo, Brazil, in 2008 (CEP/833).
Results
Reactivity analysis of VL cases for ESA released from L. (L.) amazonensis, L. (V.) braziliensis, and L. (L.) chagasi.
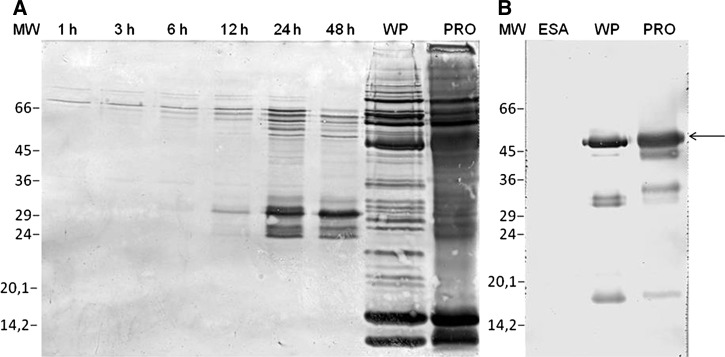
A time-course study with L. (L.) chagasi showed that the promastigote forms excrete and secrete several immunogenic molecules in a quantitatively modulated fashion during incubation. The VL pooled sera revealed less reactive bands ranging from 26.5 to 31.5 kDa for the blots corresponding to supernatants collected after 1, 3, 6, and 12 hours of parasite incubation (Figure 1A; lanes 1h–12h) and more reactive bands in the supernatants collected at 24 and 48 hours (Figure 1A; lanes 24h–48h). Immunoblots performed with WP (Figure 1A; WP) or PRO antigen (Figure 1A; lane PRO) incubated with VL pooled sera displayed a complex pattern of reactive bands. A minimum number of 5 × 108/mL promastigotes was required to generate quantitatively visible bands in the 25.5–31.5 kDa range, because the blot performed with ESAs obtained from a medium containing 5 × 107 promastigotes/mL reacted weakly with the VL pooled sera (data not shown). Based on these results, the subsequent experiments were performed with ESAs recovered after the incubation of 5 × 108 parasites/mL during 24 hours that were used without any further concentration or purification.
Figure 1.
Immunoblotting of L. (L.) chagasi ESA (excreted–secreted antigens), WP (whole promastigote), and PRO (crude promastigote antigen). (A) Visceral leishmaniasis (VL) sera pool IgG reactivity to ESA isolated from L. (L.) chagasi after 1, 3, 6, 12, 24, and 48 hours incubation; WP and PRO. (B) Monoclonal anti-α tubulin antibody reactivity to ESA, WP, and PRO. MW = low molecular weight marker.
After the incubation of the ESA-blot with monoclonal anti-α tubulin antibody, it was confirmed that the ESA fraction was free of tubulin, which displayed low levels of lysed parasites (Figure 1B; lane ESA). Conversely, the blot with WP (Figure 1B; lane WP) and PRO antigens (Figure 1B; lane PRO) displayed reactive bands. Several ESA batches that had been stored for 6 to 12 months at −70°C, heated at 100°C for 5 minutes, and treated with protease inhibitors displayed high reproducibility with the ELISA and immunoblots (data not shown).
Differences in the reactive bands were observed between the L. (L.) chagasi ESA-blot, which displayed reactive bands in the 26.5–31.5 kDa range (Figure 2; lane Lc), and L. (L.) amazonensis (Figure 2; lane La), or L. (V.) braziliensis (Figure 2; lane Lb), which displayed reactive bands ranging from 25.5 to 30.0 kDa, as detected when incubated with VL pooled sera.
Figure 2.
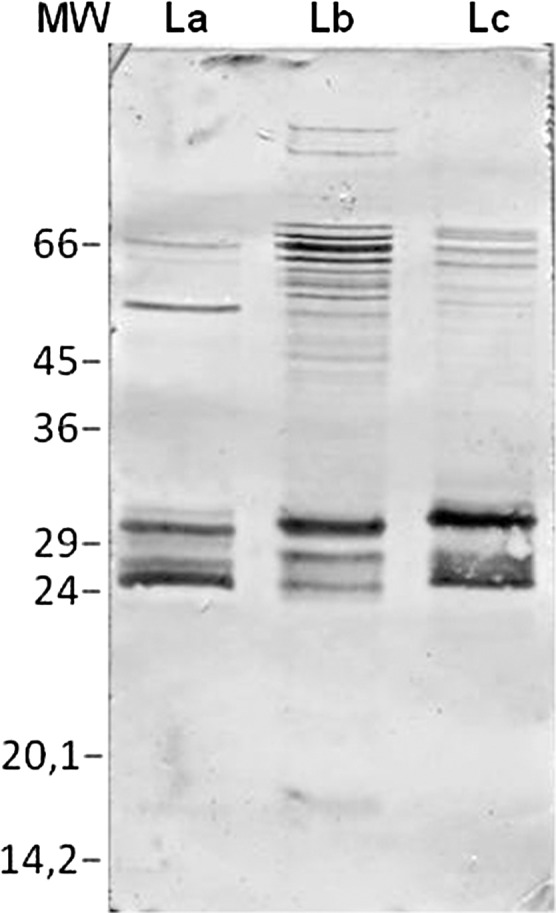
IgG reactivity of visceral leishmaniasis (VL) sera pool to excreted–secreted antigens (ESA) isolated from L. (L.) amazonensis (La), L. (V.) braziliensis (Lb), and L. (L.) chagasi (Lc) via immunoblotting. MW = low molecular weight marker.
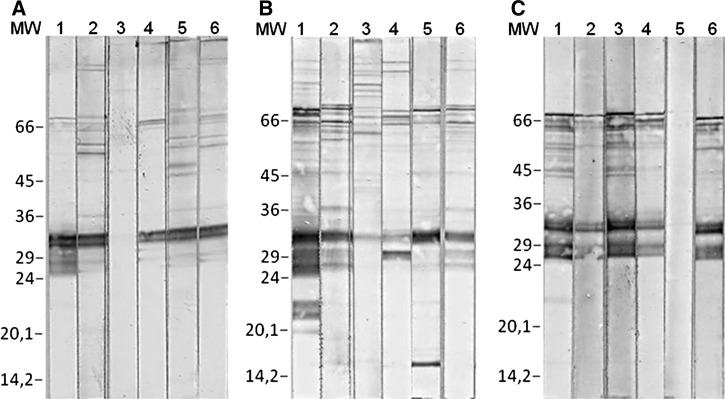
Analysis of the ESA-blots showed that the sera isolated from some VL patients recognized several high molecular weight bands (Figure 3A, B; lanes 1–3), whereas all sera samples recognized bands ranging from 26.5 to 31.5 kDa for the L. (L.) chagasi ESA-blot (Figure 3C; lanes 1–3). The diagnosis with the L. (L.) amazonensis and L. (V.) braziliensis ESA-blots alone appears to be unsuitable for use because they revealed primarily weak levels of reactivity and in some cases no reactivity to the 25.5-kDa polypeptides (Figure 3A and B; lane 3), although they were positive for the 30.0-kDa band in 88.7% (Figure 3A; lanes 1 and 2, Table 1) and 90.6% (Figure 3B; lanes 1–3; Table 1) of the VL cases, respectively.
Figure 3.
Immunoblotting of excreted–secreted antigens (ESA) (ESA-blot) with a panel of serum. IgG reactivity of visceral leishmaniasis (VL) sera samples to ESA from (A) L. (L.) amazonensis, (B) L. (V.) braziliensis, and (C) L. (L.) chagasi, (lanes 1–3); a cutaneous leishmaniasis (CL) case (lane 4); a chronic chagasic case (lane 5); and an acute chagasic case from a co-endemic region for leishmaniasis and Chagas disease (lane 6). MW = low molecular weight marker.
Table 1.
Number and percentage of positive cases assessed by PRO-ELISA, ESA-ELISA, and ESA-blot with L. (L.) m amazonensis, L. (V.) braziliensis, and L. (L.) chagasi
| Patients' clinical diagnosis | Total | Number (% of positive cases) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PRO-ELISA | ESA-ELISA | ESA-blot | ||||||||
| n | La | Lb | Lc | La | Lb | Lc | La* | Lb* | Lc† | |
| Visceral leishmaniasis | 53 | 53 (100) | 53 (100) | 53 (100) | 53 (100) | 53 (100) | 53 (100) | 47 (88.7) | 48 (90.6) | 53 (100) |
| Non-visceral leishmaniasis | 160 | 40 (25) | 40 (25) | 39 (24.4) | 15 (9.4) | 15 (9.4) | 8 (5) | 19 (11.9) | 18 (11.3) | 14 (8.8) |
| CL | 8 | 5 (62.5) | 5 (62.5) | 5 (62.5) | 3 (37.5) | 3 (37.5) | 3 (37.5) | 7 (87.5) | 7 (87.5) | 7 (87.5) |
| MCL | 15 | 11 (73.3) | 11 (73.3) | 11 (73.3) | 2 (13.3) | 2 (13.3) | 2 (13.3) | 5 (33.3) | 6 (40) | 6 (40) |
| Chagas disease-acute | 5 | 4 (80) | 4 (80) | 4 (80) | 4 (80) | 3 (60) | 1 (20) | 1 (20) | 1 (20) | 1 (20) |
| Chagas disease-chronic | 20 | 13 (65) | 13 (65) | 13 (65) | 5 (25) | 5 (25) | 1 (5) | 6 (30) | 4 (20) | 0 |
| Autoimmune disease | 27 | 2 (7.4) | 1 (3.7) | 1 (3.7) | 0 | 0 | 0 | 0 | 0 | 0 |
| Malaria (P. falciparum) | 10 | 3 (30) | 3 (30) | 3 (30) | 0 | 0 | 0 | 0 | 0 | 0 |
| Malaria (P. vivax) | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PBM | 6 | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 0 | 0 |
| Schistosomiasis | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Toxocariasis | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Toxoplasmosis | 5 | 1 (20) | 1 (20) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tuberculosis | 14 | 1 (7.1) | 2 (14.3) | 2 (14.3) | 1 (7.1) | 1 (7.1) | 1 (7.1) | 0 | 0 | 0 |
| Healthy | 32 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sensitivity (%) | 53 | 100 | 100 | 100 | 100 | 100 | 100 | 88.7 | 90.6 | 100 |
| Specificity (%) | 160 | 75 | 75 | 75.6 | 90.6 | 90.6 | 95 | 88.1 | 88.7 | 91.2 |
ESA-blot was defined as positive when they exhibited bands of molecular weight for the 30 kDa and not high proteins.
ESA-blot was defined as positive when they exhibit bands ranging from 26.5 to 31.5 kDa.
PRO = promastigote antigens; ESA = excreted–secreted antigens; CL = cutaneous leishmaniasis; MCL = mucocutaneous leishmaniasis; PBM = paracoccidioidomycosis.
However, all 53 VL cases reacted with the PRO-ELISA and ESA-ELISA without any specie-specific discrimination between the tested Leishmania and lacked statistically significant differences (P > 0.05) (Table 1).
Non-visceral leishmaniasis: tegumentary CL and MCL.
Tegumentary leishmaniasis cases showed high cross-reaction with PRO-ELISA at 62.5% with CL cases and 73.3% with MCL cases (Table 1); no species-specific differentiation was detected. However, the ESA-ELISA displayed lower levels of cross-reactivity with CL cases (37.5%) and MCL cases (13.3%) regardless of the Leishmania species used. Statistical differences were observed between ESA and PRO (P < 0.05) but not between the Leishmania species (P > 0.05). In contrast, 87.5% (7 of 8) of the CL cases (Figure 3A–C; lane 4) reacted with the ESA-blots independent of the Leishmania species, whereas 33.3% of the MCL cases reacted with the ESA isolated from L. (L.) amazonensis and 40% reacted with L. (V.) braziliensis and L. (L.) chagasi (Table 1).
Non-visceral leishmaniasis: Chagas disease.
Although high positivity rates were observed for the chronic chagasic patients tested with the PRO-ELISA (65%) with Leishmania species, low levels of cross-reaction were observed for the ESA-ELISA (5%) with L. (L.) chagasi ESA, and 25% was observed with L. (L.) amazonensis and L. (V.) braziliensis (Table 1). When the ESA isolated from L. (L.) chagasi was used for immunoblotting, no cross-reaction was observed with the chronic chagasic cases, although 30% of the cases tested positive for L. (L.) amazonensis and 20% tested positive for L. (V.) braziliensis (Table 1). Figure 3A and B, lane 5, show the reactivity pattern observed with the chronic chagasic cases that were negative for L. (L.) chagasi (Figure 3C; lane 5).
The PRO-ELISA analysis detected four acute chagasic cases (80%) independent of the Leishmania species, whereas the ESA-ELISA detected only one case (20%) with L. (L.) chagasi, four cases (80%) with L. (L.) amazonensis, and three cases (60%) with L. (V.) braziliensis (Table 1). Analysis of the ESA-blots showed that only one serum sample isolated from an acute chagasic patient (20%) reacted to L. (L.) amazonensis, L. (V.) braziliensis, and L. (L.) chagasi (Figure 3A–C; lane 6); this result requires verification as this serum was isolated from a person who lives in Goias, which is a state that is co-endemic for both leishmaniasis and Chagas disease. The same serum sample also reacted with the PRO-ELISA and the ESA-ELISA with the three Leishmania species.
Non-visceral leishmaniasis: other diseases and healthy blood donors.
The PRO-ELISA and ESA-ELISA were tested with 32 healthy blood donors and 80 cases of parasitic or other infectious diseases from endemic areas overlapping with those of the VL cases. These cases displayed low levels of cross-reactivity with the PRO-ELISA using L. (L.) amazonensis and L. (V.) braziliensis (7 of 80), L. (L.) chagasi (6 of 80), and with the ESA-ELISA using L. (V.) braziliensis (2 of 80), L. (L.) amazonensis, and L. (L.) chagasi (1 of 80) (Table 1). This group showed no cross-reaction with the ESA-blots (Table 1). As some antibodies cross-react with parasite tubulin, we attempted to identify this protein in the ESA preparations. The incubation of the ESA-blots with monoclonal anti-α-tubulin revealed no reactive bands (Figure 1B; lane ESA).
These data confirm that ESA extracted from the supernatants of promastigote cultures of all Leishmania species, particularly L. (L.) chagasi, display higher levels of specificity than crude PRO antigen.
Discussion
Commercial tests designed for the serological diagnosis of VL usually use total antigens of promastigote forms of different Leishmania species.2,19 However, the sensitivity and specificity levels of these tests vary depending on the origin and species of Leishmania,2,4,22 and cross-reactivity with other diseases frequently leads to false positive results.2,28 An additional drawback of these tests is that immunosuppressed individuals may display false negative results.2 Several studies have shown that Leishmania and other protozoa, such as T. cruzi, Plasmodium, and Toxoplasma, express similar molecules, which may partially account for the cross-reactivity. Such common molecules include two rare carbohydrates: gal (α 1, 3), gal (2), and β-galactofuranose.16,17
Several reports have indicated that such cross-reactions can be minimized in tests using purified molecules, such as fucose-mannose ligand,29 recombinant proteins,1,2,4,27 or molecules secreted by Leishmania spp. promastigotes.5,6 However, the levels of antibodies detected, even with recombinant proteins such as rK39, may vary depending on the geographical region from which the samples originate, thereby leading to discordant results.4,19,30
It has been suggested that immunodiagnostic tests using excreted–secreted antigens for the diagnosis of protozoan diseases, such as Toxoplasma gondii31 and T. cruzi,24,26 should be considered to be reference tests because of the high levels of sensitivity and specificity. Although the molecules excreted and secreted by Leishmania spp. have been a target of interest for decades and have been identified as potential candidates for vaccines,32 they have not been extensively explored for the diagnosis of leishmaniasis.5,6,12,13,15
In this report, we present new data suggesting that ESAs isolated from three different species of Leishmania (from the most prevalent species in the Americas) L. (L.) chagasi, L. (L.) amazonensis, and L. (V.) braziliensis can be used in the immunodiagnosis of VL. We showed that VL samples reacted with all Leishmania spp. antigens (ESA or PRO) by ELISA, as confirmed by other works.5,20 Indeed, 100% positivity of the VL cases was observed exclusively when L. (L.) chagasi ESA was used for immunoblotting (Table 1).
The primary difficulty in the immunodiagnosis of VL is not the sensitivity but the levels of specificity. Cross-reactivity with diseases such as toxoplasmosis, Chagas disease, malaria, and tuberculosis can occur when using crude parasite antigen.16,18,19,28,33 These findings were corroborated in this report, which showed high cross-reactivity, 62.5% with CL cases, 73.3% with MCL cases, 80% with acute Chagas disease, and 65% with chronic Chagas disease, when PRO was used regardless of the Leishmania species used in the ELISA (Table 1).Although sera from isolated acute and chronic Chagas patients showed significantly lower levels of reactivity to L. (L.) chagasi ESA compared with L. (L.) chagasi PRO, a high level of cross-reactivity to ESA isolated from L. (L.) amazonensis and L. (V.) braziliensis was still observed (Table 1).
A significant reduction in the cross-reactivity with other diseases, such as MCL, Chagas disease, and malaria (Plasmodium falciparum), was also observed with ESA isolated from the three Leishmania species used for the ELISA. Indeed, our findings of a 37.5% positivity of ESA with CL and 13.3% with the MCL cases (Table 1) do not agree with those of other authors who have reported 80–92.3% and 71% positive rates when CL samples were assayed with ESA isolated from L. (L.) mexicana and L. (V.) braziliensis, respectively.13,15 In contrast, similar positivity rates (62.5% for CL and 73.3% for MCL) were observed in our study when Leishmania spp. PRO was used (Table 1). This discrepancy may be caused by the low and heterogeneous production of antibodies during the course of CL and MCL development, which limits the use of some serological tests in the diagnosis of these conditions.
Our data show, for the first time, that sera isolated from Chagas disease patients do not react to ESA from L. (L.) chagasi (Table 1, Figure 3C; lane 5) however do react to ESA isolated from L. (L.) amazonensis and L. (V.) braziliensis (Table 1, Figure 3A and B; lane 5).
The superiority of ESA over PRO antigen was primarily observed when sera isolated from healthy subjects and subjects with other diseases were analyzed (Table 1).
Overall, significantly lower levels of cross-reaction (5%) were observed with ESA isolated from L. (L.) chagasi compared with promastigote extracts (24.4%). These levels of cross-reaction are comparable to those reported with Leishmania recombinant antigens used for the diagnosis of VL.4,22
Antibodies from different species (man and dog) with VL recognize many of these molecules12,13; currently, there is no agreement among the authors as to which molecules of the Leishmania ESA are specifically involved in the diagnosis of VL. In this report, the patients with VL recognized several molecules with high molecular weight in the ESA-blot regardless of the Leishmania species, but these molecules were not simultaneously recognized by all VL sera. The bands ranging in size from 25.5 to 30.0 kDa were recognized simultaneously by 88.7% and 90.6% of VL samples, respectively, with L. (L.) amazonensis and L. (V.) braziliensis (Figure 3A and B; lanes 1–3) but failed to permit easy diagnosis because in most cases, the reactivity to the 25.5-kDa polypeptide was weak (Figure 3B; lane 3) and sometimes absent (Figure 3A; lane 3); although, the VL sera always tested positive for the 30.0-kDa band (Figure 3B; lanes 1–3). A similar pattern of bands corresponding to molecular weights in the 26.5- to 31.5-kDa range was significantly recognized by all VL samples with the L. (L.) chagasi ESA-blot (Figure 3C; lanes 1–3).
We conclude that the lowest levels of cross-reaction and the best positivity rates were observed with the L. (L.) chagasi ESA-blot, which was the only antigen technique that did not display cross-reactivity with Chagas disease and was able to confirm the disease for all VL cases. A patient with acute-phase Chagas disease that was reactive for all ELISAs displayed positive results for the ESA-blot and was considered to be a case of concomitant T. cruzi and Leishmania spp. infection (Figure 3A–C; lane 6). The chronic Chagas disease case that displayed cross-reaction in all ESA-ELISAs was negative for the L. (L.) chagasi ESA-blot (Figure 3C; lane 5).
Despite the high level of sensitivity and the lack of cross-reactivity with sera isolated from Chagas disease patients, the ESA-blot with L. (L.) chagasi showed cross-reactivity with the CL and mucosal leishmaniasis cases, and the 25.5- to 31.5-kDa polypeptides displayed lower levels of specificity (Table 1). However, the ESA-blot represents additional support for use with L. (L.) chagasi ESA-ELISA in the diagnosis of VL and leads us to believe that the ESA-blot may be used as a reference test in the diagnosis of VL.
In summary, our findings are consistent with data in the literature that show the advantages of using ESA to diagnose VL.
Footnotes
Financial support: This work represents a portion of the Master's thesis produced by V. Pinedo-Cancino, who is a recipient of a fellowship from CNPq/Brazil. Partial funding was provided by LIM49-FMUSP. The authors thank S. Uliana for encouraging this study and for providing the Leishmania spp. parasites and M. P. Leal for the technical assistance.
Authors' addresses: Viviana Pinedo-Cancino, Norival Kesper, and Eufrosina Setsu Umezawa, Instituto de Medicina Tropical de São Paulo, Universidade de São Paulo, Brazil, E-mails: vivicancino@usp.br, nkesper@usp.br, and eumezawa@usp.br. Clara Lúcia Barbiéri, Departamento de Microbiologia, Immunologia e Parasitologia, Universidade Federal de São Paulo, Brazil, E-mail: barbieri.clara@unifesp.br. José Angelo Lauletta Lindoso, Instituto Emilio Ribas de São Paulo, Brazil, E-mail: jlindoso@usp.br.
References
- 1.Srividya G, Kulshrestha A, Singh R, Salotra P. Diagnosis of visceral leishmaniasis: developments over the last decade. Parasitol Res. 2012;110:1065–1078. doi: 10.1007/s00436-011-2680-1. [DOI] [PubMed] [Google Scholar]
- 2.Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol. 2002;9:951–958. doi: 10.1128/CDLI.9.5.951-958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Momen H, Grimaldi Júnior G, Deane LM. Leishmania infantum, the etiological agent of American visceral leishmaniasis (AVL)? Mem Inst Oswaldo Cruz. 1987;82:447–448. doi: 10.1590/s0074-02761987000300022. [DOI] [PubMed] [Google Scholar]
- 4.Maia Z, Lírio M, Mistro S, Mendes CM, Mehta SR, Badaro R. Comparative study of rK39 Leishmania antigen for serodiagnosis of visceral leishmaniasis: systematic review with meta-analysis. PLoS Negl Trop Dis. 2012;6:e1484. doi: 10.1371/journal.pntd.0001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin SK, Thuita-Harun L, Adoyo-Adoyo M, Wasunna KM. A diagnostic ELISA for visceral leishmaniasis, based on antigen from media conditioned by Leishmania donovani promastigotes. Ann Trop Med Parasitol. 1998;92:571–577. doi: 10.1080/00034989859267. [DOI] [PubMed] [Google Scholar]
- 6.Rajasekariah GH, Ryan JR, Hillier SR, Yi LP, Stiteler JM, Cui L, Smithyman AM, Martin SK. Optimization of an ELISA for the serodiagnosis of visceral leishmaniasis using in vitro derived promastigote antigens. J Immunol Methods. 2001;252:105–119. doi: 10.1016/s0022-1759(01)00341-6. [DOI] [PubMed] [Google Scholar]
- 7.Greenblatt CL, Glaser P. Temperature effect on Leishmania enriettii in vitro. Exp Parasitol. 1965;16:36–52. doi: 10.1016/0014-4894(65)90031-7. [DOI] [PubMed] [Google Scholar]
- 8.Lemesre JL, Holzmuller P, Cavaleyra M, Goncalves RB, Hottin G, Papierok G. Protection against experimental visceral leishmaniasis infection in dogs immunized with purified excreted secreted antigens of Leishmania infantum promastigotes. Vaccine. 2005;23:2825–2840. doi: 10.1016/j.vaccine.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 9.DebRoy S, Keenan AB, Ueno N, Jeronimo SM, Donelson JE, Wilson ME. Leishmania infantum chagasi: a genome-based approach to identification of excreted/secreted proteins. Exp Parasitol. 2010;126:582–591. doi: 10.1016/j.exppara.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverman JM, Chan SK, Robinson DP, Dwyer DM, Nandan D, Foster LJ, Reiner NE. Proteomic analysis of the secretome of Leishmania donovani. Genome Biol. 2008;9:R35. doi: 10.1186/gb-2008-9-2-r35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosa R, Rodrigues OR, Marques C, Santos-Gomes GM. Leishmania infantum: soluble proteins released by the parasite exert differential effects on host immune response. Exp Parasitol. 2005;109:106–114. doi: 10.1016/j.exppara.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Cibrelus P, Précigout E, Sereno D, Carcy B, Lemesre JL, Gorenflot A. Secreted antigens of the amastigote and promastigote forms of Leishmania infantum inducing a humoral response in humans and dogs. Parasite. 1999;6:121–129. doi: 10.1051/parasite/1999062121. [DOI] [PubMed] [Google Scholar]
- 13.Ryan JR, Smithymam MA, Rajasekariah GH, Hochberg L, Stiteler MJ, Martin SK. Enzyme-linked immunosorbent assay based on soluble promastigote antigen detects immunoglobulin M (IgM) and IgG antibodies in sera from cases of visceral and cutaneous leishmaniasis. J Clin Microbiol. 2002;40:1037–1043. doi: 10.1128/JCM.40.3.1037-1043.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajasekariah GH, Cardoso L, Dogcio DA, Martin SK, Smithyman AM. A novel exo-antigen-based ELISA for the detection of canine leishmaniasis. Am J Trop Med Hyg. 2008;78:616–623. [PubMed] [Google Scholar]
- 15.Romero LI, Paz HM, Ortega-Barría E, Bayard V, Hochberg LP, Collins KM, Chan AS, Ryan JR. Evaluation of serological assays based on a novel excreted antigen preparation for the diagnosis of cutaneous leishmaniasis in Panama. J Microbiol Methods. 2004;37:391–397. doi: 10.1016/j.mimet.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Avila JL, Rojas M, Acosta A. Glycoinositol phospholipids from American Leishmania and Trypanosoma spp.: partial characterization of the glycan cores and the human humoral immune response to them. J Clin Microbiol. 1991;29:2305–2312. doi: 10.1128/jcm.29.10.2305-2312.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oppenheimer M, Valenciano AL, Sobrado P. Biosynthesis of galactofuranose in kinetoplastids: novel therapeutic targets for treating leishmaniasis and Chagas' disease. Enzyme Res. 2011;2011(415976) doi: 10.4061/2011/415976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed SG, Badaró R, Lloyd RM. Identification of specific and cross-reactive antigens of Leishmania donovani chagasi by human infection sera. J Immunol. 1987;138:1596–1601. [PubMed] [Google Scholar]
- 19.Romero HD, Silva LA, Silva-Vergara ML, Rodrigues V, Costa RT, Guimarães SF, Alecrim W, Moraes-Souza H, Prata A. Comparative study of serologic tests for the diagnosis of asymptomatic visceral leishmaniasis in an endemic area. Am J Trop Med Hyg. 2009;81:27–33. [PubMed] [Google Scholar]
- 20.Silvestre R, Santarém N, Teixeira L, Cunha J, Schalig H, Cordeiro-da-Silva A. Evaluation of Leishmania species reactivity in human serologic diagnosis of leishmaniasis. Am J Trop Med Hyg. 2009;81:202–208. [PubMed] [Google Scholar]
- 21.Howard MK, Gull K, Miles MA. Antibodies to tubulin in patients with parasitic infections. Clin Exp Immunol. 1987;68:78–85. [PMC free article] [PubMed] [Google Scholar]
- 22.Badaró R, Benson D, Eulálio MC, Freire M, Cunha S, Netto EM, Pedral-Sampaio D, Madureira C, Burns JM, Houghton RL, David JR, Reed SG. rK39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. J Infect Dis. 1996;173:758–761. doi: 10.1093/infdis/173.3.758. [DOI] [PubMed] [Google Scholar]
- 23.Howard MK, Pharoah MM, Ashall F, Miles MA. Human urine stimulates growth of Leishmania in vitro. Trans R Soc Trop Med Hyg. 1991;85:477–479. doi: 10.1016/0035-9203(91)90226-o. [DOI] [PubMed] [Google Scholar]
- 24.Umezawa ES, Nascimento MS, Stolf AM. Enzyme-linked immunosorbent assay with Trypanosoma cruzi excreted–secreted antigens (TESA-ELISA) for serodiagnosis of acute and chronic Chagas' disease. Diagn Microbiol Infect Dis. 2001;39:169–176. doi: 10.1016/s0732-8893(01)00216-4. [DOI] [PubMed] [Google Scholar]
- 25.Alcolea PJ, Alonso A, Larraga V. Proteome profiling of Leishmania infantum promastigotes. J Eukaryot Microbiol. 2011;58:352–358. doi: 10.1111/j.1550-7408.2011.00549.x. [DOI] [PubMed] [Google Scholar]
- 26.Umezawa ES, Nascimento MS, Kesper JN, Coura JR, Borges-Pereira J, Junqueira CV, Camargo ME. Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute, and chronic Chagas disease. J Clin Microbiol. 1996;34:2143–2147. doi: 10.1128/jcm.34.9.2143-2147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Souza SD, Pinheiro PH, Katz S, dos Santos MR, Barbiéri CL. A recombinant cysteine proteinase from Leishmania (Leishmania) chagasi suitable for serodiagnosis of American visceral leishmaniasis. Am J Trop Med Hyg. 2005;72:126–132. [PubMed] [Google Scholar]
- 28.Caballero ZC, Sousa OE, Marques WP, Saez-Alquezar A, Umezawa ES. Evaluation of serological tests to identify Trypanosoma cruzi infection in humans and determine cross-reactivity with Trypanosoma rangeli and Leishmania spp. Clin Vaccine Immunol. 2007;14:1045–1049. doi: 10.1128/CVI.00127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palatinik-de-Sousa CB, Dutra HS, Borojevic R. Leishmania donovani surface glycoconjugate GP36 is the major immunogen component of the fucose mannose ligand (FML) Acta Trop. 1993;53:59–72. doi: 10.1016/0001-706x(93)90006-w. [DOI] [PubMed] [Google Scholar]
- 30.Gontijo CMF, Melo NM. Visceral leishmaniasis in Brazil: current status, challenges and prospects. Rev Brasil Epidemiol. 2004;7:338–346. [Google Scholar]
- 31.Meira CS, Vidal JE, Costa-Silva TA, Frazatti-Gallina N, Pereira-Chioccola VL. Immunodiagnosis in cerebrospinal fluid of cerebral toxoplasmosis and HIV-infected patients using Toxoplasma gondii excreted/secreted antigens. Diagn Microbiol Infect Dis. 2011;71:279–285. doi: 10.1016/j.diagmicrobio.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Paape D, Aebischer T. Contribution of Leishmania spp. to the understanding of differentiation, drug resistance mechanisms, vaccine and drug development. J Proteomics. 2011;74:1614–1624. doi: 10.1016/j.jprot.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Abramo C, Fontes CJ, Krettli AU. Cross-reactivity between antibodies in the sera of individuals with leishmaniasis, toxoplasmosis, and Chagas' disease and antigens of the blood-stage forms of Plasmodium falciparum determined by indirect immunofluorescence. Am J Trop Med Hyg. 1995;53:202–205. doi: 10.4269/ajtmh.1995.53.202. [DOI] [PubMed] [Google Scholar]