Abstract
We studied a small rural community of 411 inhabitants localized in the state of Campeche in the Yucatan Peninsula, Mexico. In 44 collected triatomines captured inside the houses, human feeding source was revealed in 23 of 44 (52%) samples, and chicken feeding source was revealed in 16 of 44 (36%) samples. In a set of 29 triatomines, mouse was the feeding source in 13 (44%) samples, and dog was the feeding source in 7 (24%) samples. Infection index with Trypanosoma cruzi in collected triatomines was 38%, and all parasites belonged to discrete type unit I. Inhabitants referred high contact with triatomine's bite in 60 of 128 (47%) samples, but seroprevalence was 2.3% (3/128). Evidence of electrocardiographic alteration compatible with Chagas disease was observed only in one asymptomatic seropositive subject. In conclusion, Triatoma dimidiata in this region are preferentially infected with T. cruzi I and feed on human beings with relative high frequency, but seroprevalence and Chagas disease in humans is relatively low.
Introduction
Triatoma dimidiata is among the vector species of greatest epidemiological significance in Chagas disease, widely distributed from Meso-America down to Colombia, Venezuela, Ecuador, and northern Peru. T. dimidiata varies enormously in genetic traits, phenotypic traits, epidemiological importance, and behavior across its geographic range.1,2 In the Yucatan Peninsula of Mexico, cryptic species were found; specimens from Yucatan should be considered as separate species rather than T. dimidiata.2 Several studies have shown that the vector population in the domestic and peridomestic cycles in the Yucatan Peninsula comes from sylvan stocks and presents clear seasonality3–7. In this cycle of transmission, several reservoirs are involved: infected Didelphys virginiana and Peromyscus leucopus have been found in the sylvan cycle, and in the peridomestic and domestic cycles, Canis familiaris, Rattus rattus, Mus musculus, and humans have been found at different rates.7–9 The precise epidemiological role of T. dimidiata in human infection in the Yucatan peninsula should be addressed.
In this work, we studied a small rural community of 411 inhabitants located in the state of Campeche in the Yucatan Peninsula. Previous studies in this community indicated that intradomestic infestation in the months between April and June (months that correspond to hot and dry season) can reach up to 53%, and the infection index can reach 20%; however, the colonization index is below 5%.10
The above data suggest that inhabitants of this community could be at risk of Trypanosoma cruzi infection and Chagas disease.
The goal of this work was to identify feeding sources in triatomines vectors captured inside the houses, especially human blood, in addition to determining lineages of T. cruzi in infected triatomines. Furthermore, we aimed at determining the presence of anti-T. cruzi IgG antibody and electrocardiographic alterations compatible with Chagas disease in volunteer inhabitants.
Materials and Methods
Locality studied and triatomines collection.
San Juan Bautista Sakcabchen is a rural community surrounded by bush located in the Yucatan Peninsula in the state of Campeche (N 19°52′22″, W 89°55′41″) (Figure 1). Its population is 411 inhabitants,11 with very low migration. Houses are of various types, ranging from wooden sticks and adobe blocks (localized at the periphery of the village) to cement blocks and concrete houses, and all are surrounded by large yards with poultry (chickens and turkeys); dogs are the main pet (at least one per house). Insects were collected with community participation. For this purpose, we planned two workshops and individual visits to households to provide information on Chagas disease and the vector. Family members were instructed how to collect triatomines found inside their houses. The collected triatomines were placed in plastic vials and gathered during regular visits every 2 weeks from March to May of 2010.
Figure 1.
Localization of studied village in the Yucatan Peninsula of Mexico.
Identification of feeding sources in triatomines.
All triatomines were dissected, and their digestive tract content was obtained. DNA was extracted using proteinase K digestion and phenol-chloroform, and DNA was precipitated with sodium acetate and ethanol. The DNA was kept at −70°C until use. The identification of blood meal was done through two approaches: a simple polymerase chain reaction (PCR) with a set of specific primers and PCR-Heteroduplex (PCR-HDA).
We designed specific primers for cytochrome b gene for identification of human and chicken DNA. The specific primers for human DNA were 5′ CAAATATCATTCTGAGG 3′ (ICOM) and 5′ TGT-TGT-GAA-GTA-TAG-T 3′ (IMAN), which produces an amplicon of 600 bp. For chicken DNA, primers were ICOM and 5′ GTGAGTATGAGAGTTAA 3′ (IGAL), which produces an amplicon of 260 bp. The PCR consisted of 30 cycles: denaturalization at 94°C, alignment at 36°C, and extension at 72°C. The reaction was performed in a volume of 50 μL using 1 μL DNA sample. All assays included positive control consisting of human and chicken DNA, respectively. To check for specificity, each pair of primers was tested against mouse, rat, dog, opossum, cat, and rabbit DNA.
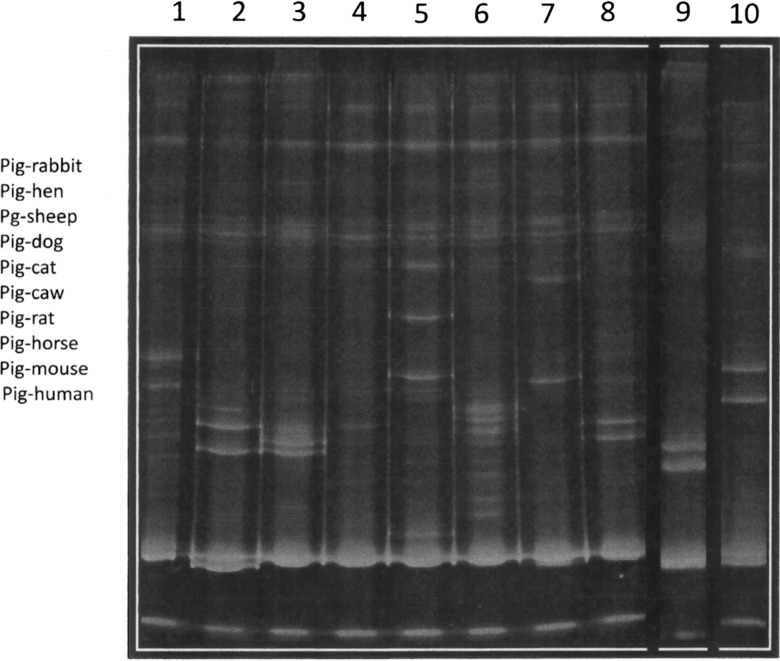
PCR-HDA was carried out as previously reported.12 Before carrying out the HDA, we performed a PCR with DNA from bug abdomen samples using primers specific for vertebrate cytochrome b, which generated a 383-bp form. Only positive samples proceeded to HDA assay. Heteroduplex profiles were generated with PCR products from Sus scrofa domesticus (pig) cytochrome b as hybridization driver. In brief, 5-μL DNA samples were used in the PCR for cytochrome b. In the heteroduplex assay, 12 μL driver DNA amplification product and 12 μL sample amplification product were heated at 95°C for 2.5 minutes and immediately chilled at 4°C for 15 minutes. The HDA patterns were analyzed in 10% acrylamide gels in Tris-borate-ethilen diamino tetra acetic (EDTA) buffer and compared with standards obtained with different DNAs from known species (Figure 2.
Figure 2.
PCR-HDA patterns for identification of meal source. Heteroduplex profiles were generated with PCR products from S. scrofa domesticus (pig) cytochrome b as hybridization driver and analyzed in 10% acrylamide gels. Control heteroduplex patterns from known species. Lanes 1–10: rabbit, hen, sheep, dog, cat, cow, rat, horse, mouse, and human, respectively.
Identification of T. cruzi infection and lineages in triatomines.
The digestive contents of captured triatomes were analyzed by direct observation under the microscope searching for flagellates and by PCR. The DNA was obtained as mentioned above. To identify T. cruzi DNA, we used kinetoplast target as previously reported.13 The amplification reaction was performed in a 50-μL volume containing 1- to 2-μL DNA samples and 10 μM each primer: 121 (5′ AAATAATGTACGGGTGAG ATGCATGA 3) and 122 (5′ GGTTCGATTGGGGTTGGTGTAATATA 3), which generated an amplicon of 330 bp.
For T. cruzi typing, we used the miniexon gene to define discrete type unit (DTU) as reported.14 In brief, amplification reaction was performed in a 50-μL volume containing 1- to 3-μL DNA samples and 10 μM each primer: 5′ GTGTCCGCCACCTCCTTCGGGCC 3′ (DTU1-), 5′ CCTGCAGGCACACGTGTGTGTG 3′ (DTU 2), and 5′ CCCCCCTCCCAGGCCACACTG 3′ (common to both DTU)—30 cycles at 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. Amplicon of 350 bp identifies lineage I.
Identification of immunoglobulin G anti-T. cruzi in humans.
In 128 volunteers that accepted to enroll in this study, we applied a risk questionnaire, took a blood sample, and performed an electrocardiogram.
A homemade enzyme-linked immunosorbent assay (ELISA) standardized technique was used.8 In brief, a polystyrene plate (Nunc polysorb plates, Thermo Fisher Scientific Inc., Waltham, MA) was coated with 10 μg/mL protein of Mexican T. cruzi crude extract in alkaline-buffered solution. Human serum was diluted at 1:400, and anti-human IgG-peroxidase conjugate was used at 1:15,000 dilutions (Zymed Laboratory, Santa Cruz, CA). The reaction was revealed by addition of O-phenylendiamine and read at 490 nm in an automatic ELISA reader (Biorad, Palo Alto, CA). The cutoff value was set as follows: sera from 30 healthy volunteers previously tested as negatives for anti-T. cruzi antibodies were analyzed for their distribution. The mean of optical density (OD) of seronegative healthy individuals plus 3 SDs was fixed to set the cutoff. All healthy seronegative individuals had values classified as negative after the cutoff value was set (mean + 3 SD).
Indirect immunofluorescence was carried out as follows: a drop of T. cruzi epimastigote suspension was air-dried on a slide. Human experimental and control sera were then diluted at 1:32 (cutoff dilution) in phosphate saline buffer (PBS; 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4·7H2O, and 1.4 mM KH2PO4, pH 7.4) and incubated in a humidified chamber for 30 minutes. The slides were then washed and incubated with the conjugate anti-human immunoglobulin G (IgG) fluorescein for 30 minutes. After incubation, the slides were observed by epifluorescent microscopy. If fluorescence of the parasites was observed, the sample was scored as positive. In all assays, positive and negative controls were included.
Identification of electrocardiographic abnormalities in humans.
As mentioned above, 128 volunteers were subjected to an electrocardiogram study. A portable electrocardiograph (Fukuda Denshi FX-2111) device was used. The electrocardiograms were analyzed by a cardiologist; special care was placed on rhythm, conduction, and isquemia findings.
Ethical considerations.
The study is considered as low risk according to the “Reglamento de la Ley General de Salud; National Secretariat of Health Mexico.” A written consent was obtained from all participants. The study was approved by the Research Committee of the Laboratorio Estatal Salud Publica Campeche (Public Health State Laboratory).
Results
In San Juan Bautista Sakcabchen, two kinds of species were found to be the most important peridomestic animals: chicken and dogs. Insects were collected with community participation; the interest for the collection varied in the community, but women were the most interested in the project for both the bug collecting and also, the clinical and serological study. A total of 44 bugs were collected intradomiciliary in the period of 3 months, and 128 human volunteers were engaged in the clinical and serological study. The bugs were identified as T. dimidiata according to Lent and Wygodzinsky.15
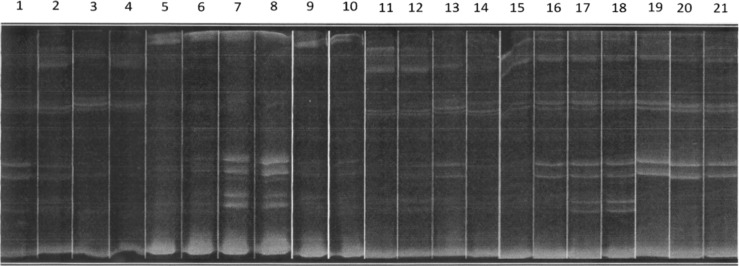
Using the simple PCR with specific primers for human cyt b in the 44 collected bugs, we identified that 15 of 44 (34%) triatomines fed on humans, and using a second approach, such as PCR-HDA assay, in 15 samples, the DNA quality was insufficient to perform heteroduplex hybridization; therefore, only a set of 29 bugs gave a positive results. In the HDA in this set of 29 samples, we identify human feeding source in 23 of 29 (79%) samples, which represents 8 more samples in addition to the 15 samples detected using the simple PCR. The overall triatomines fed on human were 23 of 44 (52%) (Figure 3 and Table 1). In the case of simple PCR with specific primers for chicken, we were able to detect 16 of 44 (36%) triatomines fed on chickens, and using the PCR-HDA in the set of 29 samples, 12 of 29 (41%) samples were positive, which represents 4 samples less than the 16 samples detected with simple PCR. The HDA let us also to identify mouse and dog feeding source in 13 of 29 (44%) and 7 of 29 (24%) samples, respectively. We only took into account those HDA patterns that correctly matched with standards. The majority of bugs that had fed on mouse (11/13), dog (6/7), and chicken (11/16) fed also on human (Figure 3 and Table 1).
Figure 3.
Representative image of heteroduplex patters in bug sample collected inside domicile. Lanes 1–21, with exceptions of lanes 4 and 15, show human pattern. Lanes 1–18, with exceptions of lanes 3, 4, 14, 15, and 16, are positive for mouse. Lanes 7, 8, 12, 16, and 17 are positive for hen.
Table 1.
Feeding sources and T. cruzi infection of intradomiciliated T. dimidiata in a rural community of Campeche, Mexico
| Triatomine number | T. cruzi infection* | Human blood | Mouse blood | Chicken blood | Dog blood |
|---|---|---|---|---|---|
| 1 | − | + | − | − | − |
| 2 | − | + | + | − | − |
| 3 | − | + | − | − | − |
| 4 | − | + | − | − | − |
| 5 | − | + | − | − | + |
| 6 | + | + | − | − | + |
| 7 | − | − | ND | − | ND |
| 8 | − | + | − | − | + |
| 9 | − | + | + | − | + |
| 10 | − | + | − | − | − |
| 11 | − | − | ND | − | ND |
| 12 | + | + | − | − | − |
| 13 | − | + | + | − | − |
| 14 | − | + | + | − | − |
| 15 | + | + | − | + | − |
| 16 | + | + | − | + | − |
| 17 | − | + | − | + | − |
| 18 | − | - | ND | − | ND |
| 19 | + | - | ND | + | ND |
| 20 | − | − | ND | − | ND |
| 21 | + | - | ND | + | ND |
| 22 | + | + | − | + | + |
| 23 | − | + | + | + | − |
| 24 | + | − | ND | − | ND |
| 25 | − | + | + | + | − |
| 26 | − | − | ND | + | ND |
| 27 | + | − | + | − | − |
| 28 | − | − | + | + | − |
| 29 | + | + | + | − | − |
| 30 | − | + | + | + | − |
| 31 | + | + | + | − | |
| 32 | + | − | ND | + | ND |
| 33 | − | − | ND | − | ND |
| 34 | + | − | ND | − | ND |
| 35 | − | − | ND | − | ND |
| 36 | − | − | ND | − | ND |
| 37 | − | − | − | − | − |
| 38 | − | − | ND | − | ND |
| 39 | − | + | − | + | − |
| 40 | + | − | − | − | − |
| 41 | + | + | − | + | − |
| 42 | + | + | − | + | − |
| 43 | + | + | + | − | − |
| 44 | − | − | ND | − | ND |
Bugs were collected inside domicile; simple end-point PCR was applied to all 44 samples collected using specific primers for human and chicken, whereas the PCR-HDA was applied only to a set of 29 bugs in which vertebrate cytochrome b PCR gave positive results, as mentioned in Materials and Methods. ND = not determined (vertebrate cytochrome b PCR gave negative result).
All T. cruzi were classified as DTU 1.
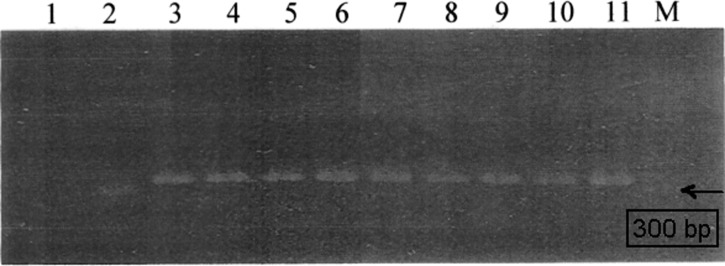
We found that 18 of 44 (38%) collected T. dimidiata were infected with T. cruzi and 100% of parasites belong to discrete type unit I using the miniexon gene (Figure 4 and Table 1).
Figure 4.
Lineage identification of Trypanosoma cruzi found in the intestine of Triatoma dimidiata. Representative image of PCR products using specific primers for T. cruzi miniexon gen: lane 1, blank; lane 2, CL-Brener strain; lanes 3–10, triatomine's faces 3–10; lane 11, DTU 1 Ninoa mexican strain.
Although humans were a frequent feeding source and T. dimidiata showed high rate of infection with T. cruzi (38%), the seroprevalence in human was lower than expected; only 3 of 128 (2.3%) volunteers were identified as carriers of antibodies against T. cruzi. Our sample consisted of 128 subjects with a mean age of 47 years, and the most frequent findings were ventricular hypertrophy (26/128; 28%) and conduction or rhythm abnormalities (9/128; 7%); however, only one seropositive subject presented electrocardiographic alteration compatible with Chagas disease, and one more subject presented ventricular hypertrophy, which is not compatible with Chagas disease. The other showed not alterations (Table 2).
Table 2.
Electrocardiogram findings and serology anti-T. cruzi in 128 volunteers in San Juan Bautista Sakcabchen, Campeche, Mexico
| Ventricular hypertrophy (%) | Conduction alteration (%) | Rhythm alteration (%) | Normal ECG | Some ECG abnormality | |
|---|---|---|---|---|---|
| 36/128 (28%) | 9*/128 (7%) | 4†/128 (3%) | 87/128 (68%) | 42/128 (32%) | |
| Positive serology | 1/36 (6.2%) | 0/9 (0%) | 1/4 (25%) | 1/87 (1.1%) | 3/128 (2.3%) |
| Triatomine's bite‡ | 5/36 (14%) | 0/9 (0%) | 1/4 (25%) | 25/87 (28%) | 60/128 (47%) |
The sample consisted of 110 females and 18 males. The mean age was 45.8 years. There were subjects with more than one electrocardiogram (ECG) alteration. The age in seropositive cases was over 50 years. ECG.
The conduction alterations recorded were incomplete right and left bundle branch blockage and auricule-ventricule blockage (A-V block).
Rhythm alterations included sinusal bradycardia, ventricular extrasystoles, and sinusal tachycardia.
Volunteers referred triatomine's bite by anamnesis.
In addition, 60 of 128 (47%) participants had experienced contact with triatomines's bite, called chinchoma, characterized by erythema, itching, and induration that could last 1 week, which means that residents are very familiar with the vector because of this nuisance.
Discussion
T. dimidiata vector is widely distributed in Mexico and expanding to Central and South America; it varies enormously in genetic traits, phenotypic traits, epidemiological importance, and behavior across its geographic range.1,2
There are several epidemiological factors that may influence the risk of transmission to humans in which the vector is directly involved. Natural infection rate is one important factor; several studies have reported a wide range of natural infection in T. dimidiata that varies from very low, such as 4%, to high natural infection, such as up to 34%.3,4,16 For example, low natural infection of 4% has been found in sylvan populations of T. dimidiata in the Yucatan state, Mexico, whereas in the Peten, Guatemala, it can reach up to 25% in sylvan populations.4,17 In this work, we found natural infection of 38%; these data are similar to those results in the work by Ramirez-Sierra and others,3 which reported 34% natural infection in a rural community in the Yucatan state, Mexico.
From the literature, we can see that natural infection index varies dramatically from one place to the other as well as among triatomine species; for example, in Mexico, T. barberi can be found naturally infected from 15% to 72%,18 and T. phyllosoma can be found naturally infected from 2% to 40%.19 Additionally, T. sordida in Matto Grosso Do Sul, Brazil can be found naturally infected as low as 0.1%.20 However, there is no conclusive explanation for this behavior, but there is a common idea that suggests that higher natural infection increases the risk of T. cruzi infection to human.
If the above assumption is reliable, then residents of San Juan Bautista Sakcabchen would be at high risk to acquire T. cruzi infection and Chagas disease, because in a previous work in this community, it was reported that, in the months between March and June, infestation index reached up to 53%, with infection index of 20%.10 However, colonization was very low (less than 5%). These data may support the notion that T. dimidiata is rather a visiting vector than a domiciliated vector.
In this study, we found that 47% of human participants referred contact with triatomine's bite. They describe the inflammatory reaction to triatomine's saliva (chinchoma) as itchy rash that may last 1 or 2 weeks. If we consider the mean age of participants to be 47 year old, and it is likely that they were exposed several times to triatomine's bite in their life. Determining the feeding sources in triatomines trapped inside houses may help us to infer the potential risk to acquire T. cruzi infection by humans. We found that humans were one of the principle feeding sources in collected triatomines (52%; 23/44) (Table 1) followed by mice (44%), chickens (36%), and dogs (24%) (Table 1). Notably, we observed that, when mouse, dog, and chicken blood was identified in T. dimidiata, human blood was also identified, suggesting that the vector is able to disperse between peridomicile and domicile sites to search for better host availability and accessibility as previously reported.21
The above data, in addition to the infection index of triatomines trapped inside houses of 38%, may suggest that high risk of T. cruzi infection to humans could exist in this community, but seroprevalence against T. cruzi in the population revealed only 2.3% (3/128). This seroprevalence is low compared with other endemic regions, such as the states of Chiapas and Oaxaca in Mexico (their seroprevalence is above 10%),22 and exceptionally high 67% and 32% in some communities of Oaxaca and Chiapas, respectively.23,24 The seroprevalance of 2.3% was similar to the seroprevalance found in those regions in which T. dimidiata is reported as the main vector; for example, in Veracruz and San Luis Potosi, Mexico, the seroprevalence is 5.2% and 6.5%, respectively.16,25 Exceptionally high seroprevalence is observed in some areas of Veracruz, where it can reach up to 16.8%.26 For the Yucatan peninsula, there is no available update on the seroprevalence surveys in rural communities, although seroprevalence in urban blood donors indicates low human infection between 0.7% in the Yucatan State and 0.5% in Campeche State.27,28
There are several possible explanations for this low transmission of T. cruzi despite the high infestation index reached in some months of the year, high contact with triatomines's bite, and relatively moderate natural infection of triatomines. On one hand, it has been reported that T. dimidiata is a poor vector, because their defecation time after taking a meal is 30 minutes.16 In our experience, we observed even longer times. Another additional possibility is the amount of metacyclic forms in feces. We observed experimentally that metacyclogenesis in T. dimidiata is moderate, reaching up to 30% and clearly influenced by the strain of T. cruzi, because in some cases, metacyclogenesis is only 7%.29
On the other hand, the high frequency of poultry (chickens and turkeys) in this community may contribute to reduce the risk of transmission of T. cruzi infection, because the avian complement lyses trypomastigotes in an antibody-independent manner.30 Some findings supporting this idea was reported by Cecere and others31; they found that, in areas where insects fed on chickens, there is a reduction in the prevalence of T. cruzi in humans. In addition, vectors that feed on mammals have higher rate of T. cruzi infection than when they feed on birds.19,32
The low metacyclogenesis, long-delayed defecation time, and relatively moderate infection index may explain, in part, the low effective transmission to human caused by the need of several exposures to get infected.
The above hypothesis could help us to explain the low human infection found in this community, because there were only three seropositive individuals; only one case presented electrocardiographic findings compatible with Chagas disease, but he was considered asymptomatic. However, we do not discard the presence of Chagas disease in the region, because in a recently published paper, it was reported that chronic cardiomyopathy Chagas disease represents 14% of the dilated cardiopathy patients cared for at the General Hospital of the state of Campeche33; however, its frequency is lower compared with other regions of Mexico.34,35
In regard to the differences in sensitivity between simple PCR and PCR-HDA, this difference might be because PCR-HDA was carried out using five times more DNA sample than the simple PCR, especially to detect human DNA. The low detection of chicken blood using HDA compared with simple PCR is probably because the driver primer used in the heteroduplex assay was based on the pig, a mammal leading to less successful heteroduplex annealing; another possibility may exist that some chicken positive results could be, in fact, from turkey, because we did not check specificity between these species.
In conclusion, T. dimidiata seems to be a low competency vector in the Yucatan region, but it shows a wide range of feeding preferences, including humans; however, low transmission of T. cruzi to humans does exist.
Footnotes
Authors' addresses: Victor Monteon, Centro Investigaciones Biomédicas, Universidad Autónoma Campeche, Campeche, Mexico, E-mail: victormonteon@yahoo.com.mx. César Alducin, Hospital Especialidades—Cardiologia, Campeche, Mexico, E-mail: cesaralducin@yahoo.com. Jorge Hernández, Laboratorio Estatal de Salud Pública—Vectores, Campeche, Campeche, Mexico, E-mail: jordijorg@yahoo.com.mx. Angel Ramos-Ligonio, Universidad Veracruzana-Orizaba, Laboratorio Investigación y Servicios (LADISER), Inmunologìa, Orizaba, Veracruz, Mexico, E-mail: angramos@uv.mx. Ruth Lopez, Centro Investigaciones Biomedicas, Universidad Autonoma Campeche, Campeche, Mexico, E-mail: dzinup@hotmail.com.
References
- 1.Panzera F, Ferrandis I, Ramsey J, Ordoñez R, Salazar-Schettino PM, Cabrera M, Monroy MC, Bargues MD, Mas-Coma S, O'Connor JE, Angulo VM, Jaramillo N, Cordón-Rosales C, Gómez D, Pérez R. Chromosomal variation and genome size support existence of cryptic species of Triatoma dimidiata with different epidemiological importance as Chagas disease vectors. Trop Med Int Health. 2008;11:1092–1103. doi: 10.1111/j.1365-3156.2006.01656.x. [DOI] [PubMed] [Google Scholar]
- 2.Dorn P, Calderon C, Melgar S, Moguel B, Solorzano E, Dumonteil E, Rodas A, de la Rua N, Garnica R, Monroy C. Two distinct Triatoma dimidiata (Latreulle, 1811) Taxa are found in sympatry in Guatemala and Mexico. Plos NeglectTrop Dis. 2009;3:393–408. doi: 10.1371/journal.pntd.0000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramirez-Sierra MJ, Herrera-Aguilar M, Gourbiere S, Dumonteil R. Patterns of house infestation dynamics by non-domiciliated Triatoma dimidiata reveal a spatial gradient of infestation in rural villages and potential insect manipulation by Trypanosoma cruzi. Trop Med Int Health. 2010;15:77–86. doi: 10.1111/j.1365-3156.2009.02422.x. [DOI] [PubMed] [Google Scholar]
- 4.Rebollar-Tellez EA, Reyes-Villanueva F, Escobedo-Ortegon J, Balam-Briceño P, May-Concha I. Abundance and nigthtly activity bahaviour of a sylvan populations of Triatoma dimidiata (Hemiptera:Reduviidae:Triatominae) from the Yucatan, Mexico. J Vector Ecol. 2009;34:304–310. doi: 10.1111/j.1948-7134.2009.00038.x. [DOI] [PubMed] [Google Scholar]
- 5.Dumonteil E, Gourbière S, Barrera-Pérez M, Rodriguez-Félix E, Ruiz-Piña H, Baños-Lopez O, Ramirez-Sierra MJ, Menu F, Rabinovich JE. Geographic distribution of Triatoma dimidiata and transmission dynamics in the Yucatan Peninsula of Mexico. Am J Trop Med Hyg. 2002;67:183–189. doi: 10.4269/ajtmh.2002.67.176. [DOI] [PubMed] [Google Scholar]
- 6.Dumonteil E, Ruiz-Piña H, Rodriguez-Félix E, Barrera-Pérez M, Ramirez-Sierra MJ, Rabinovich JE, Menu F. Re-infestastion of houses by Triatoma dimidiata after intra-domicile insecticide application in the Yucatan peninsula, Mexico. Mem Inst Oswaldo Cruz. 2004;99:253–256. doi: 10.1590/s0074-02762004000300002. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Piña H, Cruz-Reyes A. The opossum Dideplphis virginiana as a synanthropic reservoir of Trypanosoma cruzi in Dzidzilché Yucatán México. Mem Inst Oswaldo Cruz. 2002;97:613–620. doi: 10.1590/s0074-02762002000500003. [DOI] [PubMed] [Google Scholar]
- 8.Balan LU, Yerbes IM, Piña MA, Balmes J, Pascual A, Hernández O, Lopez R, Monteón V. Higher seroprevalence of Trypanosoma cruzi infection in dogs than in humans in an urban area of Campeche, Mexico. Vector Borne Zoonotic Dis. 2011;11:843–844. doi: 10.1089/vbz.2010.0039. [DOI] [PubMed] [Google Scholar]
- 9.Zavala-Velázquez J, Barrera-Pérez M, Rodríguez-Félix ME, Guzmán-Marín E, Ruiz-Pina H. Infection by Trypanosoma cruzi in mammals in Yucatán, Mexico. A serological and parasitological study. Rev Inst Med Trop Sao Paulo. 1996;38:289–292. doi: 10.1590/s0036-46651996000400009. [DOI] [PubMed] [Google Scholar]
- 10.Hernández JL, Rebollar-Téllez EA, Infante F, Morón A, Castillo A. Indicadores de infestación e infección de Triatoma dimidiata (Latreille) (Hemiptera:Reduviidae) en Campeche, Mexico. Public Health. 2010;39:1024–1031. doi: 10.1590/s1519-566x2010000600027. [DOI] [PubMed] [Google Scholar]
- 11.http://www.inegi.org.mx/sistemas/TabuladosBasicos/preliminares2010.aspx Available at.
- 12.Bosseno M, Santos L, Baunaure F, Magallon-Gastelum E, Soto M, Lozano-Kasten F, Dumonteil E, Breniere S. Short report: identification in Triatomine vectors of feeding sources and Trypanosoma cruzi variants by heteroduplex assay and miniexon polymerase chain reaction. Am J Trop Med Hyg. 2006;74:305–306. [PubMed] [Google Scholar]
- 13.Degrave W, Fragoso S, Britto C, Van Heuverswyn H, Kidane GZ, Cardoso MA, Mueller RU, Simpson L, Morel CM. Peculiar sequence organization of kinetoplast DNA minicircles from Trypanosoma cruzi. Mol Biochem Parasitol. 1988;27:63–70. doi: 10.1016/0166-6851(88)90025-4. [DOI] [PubMed] [Google Scholar]
- 14.Souto R, Fernandes O, Macedo A, Campbell D, Zingales B. DNA markers define two major phylogenetic lieages of Trypanosoma cruzi. Mol Biochem Parasitol. 1996;83:141–152. doi: 10.1016/s0166-6851(96)02755-7. [DOI] [PubMed] [Google Scholar]
- 15.Lent H, Wygodzinsky P. Revision of the triatominae (Hemiptera: Reduviidae), and their significance as vectors of Chagas' disease. Bull Am Mus Nat Hist. 1979;163:123–520. [Google Scholar]
- 16.Salazar-Schettino P, Rojas G, Buccio M, Cabrera M, Garcia G, Guevara Y, Tapia R. Seroprevalence of Trypanosoma cruzi antibodies and associated risk factors among the population under 18 years of age in Veracruz, Mexico. Rev Panam Salud Publica. 2007;22:75–82. doi: 10.1590/s1020-49892007000700001. [DOI] [PubMed] [Google Scholar]
- 17.Monroy MC, Bustamante DM, Rodas AG, Enriquez ME, Rosales RG. Habitats, dispersion and invasion of sylvatic Triatoma dimidiata (Hemiptera: Reduviidae: Triatominae) in Petén, Guatemala. J Med Entomol. 2003;40:800–806. doi: 10.1603/0022-2585-40.6.800. [DOI] [PubMed] [Google Scholar]
- 18.Salazar-Schettino P, Rojas-Wastavino G, Cabrera M, Buccio M, Martinez-Ibarra J, Monroy-Escobar M, Rodas-Retana A, Guevara-Gomez Y, Vences-Blanco M, Ruiz-Hernadez A, Torres-Guitierrez E. A revision of thirteen species of Triatominae (Hemiptera:Rediviidae) vectors of Chagas disease in Mexico. J Selva Andina Res Soc. 2010;1:57–80. [Google Scholar]
- 19.Villalobos G, Martínez-Hernández F, de la Torre P, Laclette JP, Espinoza B. Entomological indices, feeding sources and molecular identification of Triatoma phyllosoma (Hemiptera:Rediviidae) one of the main vector of Chagas disease in the Istmo de Tehuantepec, Oaxaca, Mexico. Am J Trop Med Hyg. 2011;85:490–497. doi: 10.4269/ajtmh.2011.10-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almeida PS, Ceretti Júnior W, Obara MT, Santos HR, Barata JM, Faccenda O. Survey of Triatominae (Hemiptera: Reduviidae) fauna in domestic environments and natural infection by Trypanosomatidae in the State of Mato Grosso do Sul. Rev Soc Bras Med Trop. 2008;41:374–380. doi: 10.1590/s0037-86822008000400010. [DOI] [PubMed] [Google Scholar]
- 21.Gürtler RE, Ceballos LA, Ordoñez-Krasnowski P, Lanati LA, Stariolo R, Kitron U. Strong host-feeding preferences of the vector Triatoma infestans modified by vector density: implications for the epidemiology of Chagas disease. PLoS Negl Trop Med. 2009;3:447. doi: 10.1371/journal.pntd.0000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruz-Reyes A, Pickering-López JM. Chagas disease in Mexico: an analysis of geographical distribution during the past 76 years—a review. Mem Inst Oswaldo Cruz. 2006;101:345–354. doi: 10.1590/s0074-02762006000400001. [DOI] [PubMed] [Google Scholar]
- 23.Goldsmith RS, Zárate RJ, Zárate LG, Morales G, Kagan I, Drickey R, Jacobson LB. Clinical and epidemiologic studies of Chagas disease in rural communities of Oaxaca, Mexico and an eight-year follow up: II. Bull Pan Am Health Organ. 1992;26:47–59. [PubMed] [Google Scholar]
- 24.Mazariego-Arana MA, Monteón VM, Ballinas-Verdugo MA, Hernández-Becerril N, Alejandre-Aguilar R, Reyes PA. Seroprevalence of human Trypanosoma cruzi infection in different geographic zones of Chiapas, Mexico. Rev Soc Bras Med Trop. 2001;34:453–458. doi: 10.1590/s0037-86822001000500008. [DOI] [PubMed] [Google Scholar]
- 25.Juarez-Tobias S, Vaughan G, Torres-Montoya A, Escobar-Guitierrez A. Short report: seroprevalence of Trypanosoma cruzi among Teenek Ameridian residents of the Huasteca region in San Luis Potosi, Mexico. Am J Trop Med Hyg. 2009;81:219–222. [PubMed] [Google Scholar]
- 26.Ramos-Ligonio A, López-Monteon A, Guzmán-Gómez D, Rosales-Encina JL, Limón-Flores Y, Dumonteil E. Identification of a hyperendemic area for Trypanosoma cruzi infection in central Veracruz, Mexico. Am J Trop Med Hyg. 2010;83:164–170. doi: 10.4269/ajtmh.2010.09-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Montalvo B. Trypanosoma cruzi antibodies in blood donors in Yucatan state, Mexico. Rev Med Inst Mex Seguro Soc. 2011;49:367–372. [PubMed] [Google Scholar]
- 28.Dzib-Salazar D, Peña-Hernández V, Ake-Canche B, Lopez R, Monteon V. Leukoreduction by centrifugation does not eliminate Trypanosma cruzi from infected blood units. Vector Borne Zoonotic Dis. 2009;9:235–241. doi: 10.1089/vbz.2007.0278. [DOI] [PubMed] [Google Scholar]
- 29.Monteon V, Godinez S, Cruz-Zetina G, Balmes J, Lopez R, Hernandez O. Biological characterization of mexican Trypanosoma cruzi isolates: metacyclogenesis, parasitemia and beznidazol resistance. Rev Biomed. 2009;20:206–214. [Google Scholar]
- 30.Kierszenbaum F, Ivanyi J, Budzko B. Mechanism of natural resistance to Trypanosma cruzi infection. Role of complement in avian resistance to Trypanosoma cruzi infection. Immunology. 1976;30:1–6. [PMC free article] [PubMed] [Google Scholar]
- 31.Cecere MC, Gütler RE, Canale D, Chuit R, Cohen JE. The role of peridomiciliary area in the elimination of Triatoma infestans fron rural Argentine communities. Rev Panam Salud Publica. 1997;1:273–279. doi: 10.1590/s1020-49891997000400003. [DOI] [PubMed] [Google Scholar]
- 32.Pizarro J, Stevens L. A new method for forensic DNA analysis of the blood meal in Chagas disease vectors demonstrated using Triatoma infestans from Chuquisaca, Bolivia. PLoS One. 2008;3:e3585. doi: 10.1371/journal.pone.0003585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alducin-Téllez C, Rueda-Villegas E, Medina-Yerbes I, Hernández O, Lopez R, Peña-Hernandez V, Monteon V. Prevalence of positive serology to Trypanosoma cruzi in patients with clinical diagnosis of dilated myocardiopathy in the state of Campeche. Arch Cardiol. 2011;81:204–207. [PubMed] [Google Scholar]
- 34.Cordero-Pérez L, Zárate-Casteñada R, Ramos-Corrales MA, Cordero J. Dilated chagasic miocardiopathy in Chiapas State, Mexico. Rev Mex Cardiol. 2002;13:153–157. [Google Scholar]
- 35.Moreno-López R, Sánchez-Paredes L, Muñoz-Jiménez L, Monteon V, Reyes PA. Chronic chagasic cardiopathy in Tehuantepec. Preliminary report. Arch Inst Cardiol Mex. 2001;71:43–49. [PubMed] [Google Scholar]