Abstract
We describe a case of hemophagocytic lymphohistiocytosis related to visceral leishmaniasis in late adulthood. Because clinical features of visceral leishmaniasis can mimic those of hemophagocytic lymphohistiocytosis, diagnosing leishmaniasis as the underlying etiology can be quite challenging. In our case, treatment with amphotericin B resulted in a dramatic resolution of clinical abnormalities.
Introduction
Visceral leishmaniasis (VL) is a chronic and frequently lethal disease caused by protozoan parasites of the Leishmania donovani complex. The clinical spectrum of VL is highly variable, with a rate of lethality directly related to the delay in diagnosing and treating this infection. Some reports of hemophagocytic lymphohistiocytosis (HLH) secondary to leishmaniasis have been published, most of which referring to young children.1 However, because many clinical and laboratory features of VL and HLH overlap, diagnosing leishmaniasis as the inciting etiology of this syndrome can be quite challenging.2
Herein, we describe a case of Leishmania-related HLH complicated by disseminated intravascular coagulation occurring in late adulthood.
Case Report
A 72-year-old man was admitted to the Hospital das Clínicas, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, on April 2012, with a 3-week history of fever, unilateral headache, fatigue, hematochezia, and progressive dyspnea. His previous medical history was unremarkable, except for a supposedly cured colorectal cancer, which had been diagnosed 7 years earlier.
On physical examination, the patient was febrile (38.5°C) and pale. There was moderate weight loss and failure to thrive. Additionally, the patient experienced tachydyspnoea and oxygen desaturation. He had hepatosplenomegaly (8 cm below the right costal margin and under the left costal margin, respectively). Hematological investigation revealed pancytopenia, with a hemoglobin of 5.1 g/dL, white blood cell count of 2,770/mm3 (89% neutrophils, 10% lymphocytes, and 1% eosinophils), and a platelet count of 55,000/mm3. C-reactive protein was 262 mg/dL. Liver function tests were as follows: alanine aminotransferase 114 U/L (normal: 13–69 U/L), aspartate aminotransferase 374 U/L (normal: 15–46 U/L); alkaline phosphatase 602 U/L (normal: 38–126 U/L); gamma-glutamyl transferase 813 U/L (normal: 15–73); and a total bilirubin of 3.9 mg/dL (normal: 0.2–1.3 mg/dL). Renal function was normal. A prothrombin time of 23 sec was recorded (international normalized ratio 1.65), with an activated prothrombin time of 84 sec. There was no serological evidence of infection with viral hepatitis A, B, or C, cytomegalovirus, Epstein-Barr virus, human immunodeficiency virus, or toxoplasmosis. Autoimmune studies, including Coombs' test, were negative.
A contrast-enhanced computed tomography (CT) of the chest displayed a small to moderate bilateral pleural effusion associated with polygonal arcades caused by interlobular septal thickening of the lung parenchyma. Both the abdominal ultrasound and the CT confirmed hepatosplenomegaly, with an additional finding of perihepatic small lymph nodes and cholelytiasis. Transthoracic echocardiography was unrevealing.
Obscure fever and tachydyspnea persisted over the following days, prompting the prescription of empiric piperacillin/tazobactam, which was discontinued after 7 days because of persistently negative microbiological studies and unresolved fever. The patient's clinical condition worsened during follow-up, and he evolved to dialytic renal failure and disseminated intravascular coagulation. Given the previous chest CT findings compatible with pulmonary lymphangitic carcinomatosis, the presumptive diagnosis of paraneoplastic syndrome was made, and the patient received dexamethasone, 4 mg every 6 hours. An alternative diagnosis of congestive pulmonary disease was considered, thus justifying intensification of the dialytic regimen. The increased risk of hemorrhage along with the respiratory dysfunction precluded the performance of a diagnostic colonoscopy to investigate relapsing or de novo colorectal neoplasia.
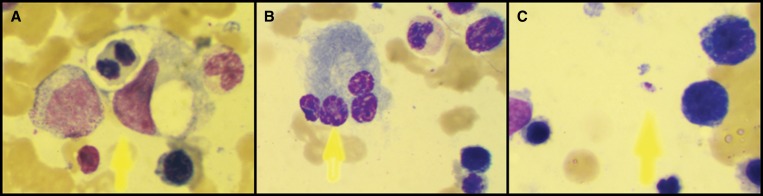
During investigation, a Wright-Giemsa stained bone marrow smear revealed a hypercellular marrow with normoblastic erythroid hyperplasia, phagocytosed erythrocytes, and increased/atypical lymphoplasmacyte cells (19.5% of bone marrow cells) (Figure 1A and 1B). No parasites were detected at this time. Immunoelectrophoresis showed polyclonal hypergammaglobulinemia. Furthermore, a low fibrinogen level was observed (79 mg/dL), with a strikingly elevated d-dimer (19,177 ng/mL; normal: < 500 ng/mL) and serum lactate dehydrogenase (3,514 U/L; normal: 313–618 U/L). Moreover, serum triglycerides were increased to 266 mg/dL, with normal cholesterol levels, and the ferritin level was higher than 1,000 ng/mL. These findings, together with the clinical picture and laboratory data presented previously, were compatible with a diagnosis of HLH.
Figure 1.
Wright-Giemsa stained bone marrow aspirate (1000×) showing: A, hemophagocytosis of a mitotic erythroblast; B, atypical tetranucleated plasmacyte; C, Amastigote form of Leishmania.
About 1 month after hospital admission, even though no parasites had been identified in the bone marrow aspirate, a rK39 rapid dipstick test (Kalazar detect, InBios International Inc., Seattle, WA) was performed, as part of a thorough investigation of HLH etiology, and was positive for Leishmaniasis. Positivity was further confirmed by an indirect immunofluorescence test with a titer of 1:320. Therefore, a 5-day therapeutic regimen of liposomal amphotericin B, 5 mg/kg/day, was administered. This was followed by the resolution of fever and gradual improvement of the organomegaly and lung injury. Dexamethasone was slowly tapered off over the course of 1 month. After 15 days of antileishmanial treatment, complete blood count revealed hemoglobin of 8.9 g/dL; a white blood cell count of 3,780/mm3; and a platelet count of 104,000/mm3. Liver enzymes, hyperferritinemia, and hypertriglyceridemia normalized after a month. Afterward, an extensive revision of bone marrow slides revealed few amastigote forms of Leishmania (Figure 1C). At discharge, physical examination was normal and the patient was no longer dependent on hemodialysis.
Discussion
A thorough search through the Medline, Embase, and Lilacs databases was performed, using the following terms: “leishmaniasis,” “visceral leishmaniasis.” “kala-azar,” “haemophagocytic syndrome,” hemophagocytic syndrome,” and “lymphohistiocytosis” to identify Leishmania-induced HLH cases occurring in elderly individuals. Articles in English, Spanish, and Portuguese were considered. As a whole, 14 cases of Leishmania-related HLH in adults were identified, none of which occurring in late adulthood.
The HLH was first described in 1939 by Scott3; this condition was subsequently classified into primary or genetic HLH and secondary or reactive HLH. Either in its primary or secondary form, HLH is characterized by activation and uncontrolled non-malignant proliferation of T-lymphocytes and macrophages. Secondary HLH has been shown to be associated with a myriad of viral, bacterial, fungal, and parasitic infections, as well as autoimmune diseases and malignant disorders.4 An increasing number of HLH secondary to tropical infections have been reported, including those associated with VL.1 Because the hematologic features observed in VL may considerably overlap with those of HLH, the diagnosis of leishmaniasis as the inciting etiology can be quite challenging, even in endemic areas.
Our patient presented to us with pancytopenia, hepatosplenomegaly, fever, and dyspnea. At first, the chest CT images suggestive of lymphangitic carcinomatosis occurring in an elderly patient misled us to the diagnosis of a metastatic cancer. The presence of a more pronounced hepatomegaly than splenomegaly, the absence of parasite identification, and the excessive amount of lymphoplasmacytic cells in bone marrow smears may also have acted as confounding factors, mimicking a hematological neoplasm, such as lymphoma or myeloma. In fact, in this case, dexamethasone was first introduced as a form of palliative care toward lymphangitic carcinomatosis of the lungs. Nonetheless, the identification of hemophagocytosis after reevaluation of the bone marrow aspirate led to the suspicion of HLH. Hyperferritinemia, hypertriglyceridemia, and coagulopathy with hypofibrinogenemia and liver dysfunction, further fulfilled the diagnostic criteria for this syndrome.5 A restless search for the cause of hemophagocytosis guided us to the final diagnosis of VL before the patient developed irreversible multiple organ failure. In a retrospective point of view, the initial corticosteroid-induced immunosuppression without a specific antimicrobial therapy against Leishmania could have had both life-saving and disastrous consequences.1 Earlier reports have shown that immunosuppressive therapy usually increases the number of amastigotes present on a bone marrow smear.6–8
Despite the fact that our patient had completed a 15-day course of steroids, we detected very few amastigotes in his bone marrow. Indeed, previous reports have shown that the first bone marrow aspirate often fails to establish the presence of Leishman-Donovan bodies in 36.3% of cases.1 Given the difficulties in making a parasitological diagnosis at the onset of the disease and the severe consequences of an already delayed diagnosis, serology was crucial for diagnosis in this case. In fact, the rK39 rapid test has been previously validated in Brazil and has presented a sensitivity of 90% and a specificity of 100%.9 Based on this data, rK39 test positivity allowed us to promptly initiate the antiprotozoal therapy. Previous studies have shown that the mean delay in diagnosis of HLH associated with VL is of 9 weeks (range: 1 week–8.5 months).1 In the present case, a 30-day extensive investigation was necessary to reach a final diagnosis and start specific treatment.
Several therapeutic protocols have been proposed in HLH and depend on the type of hemophagocytic syndrome. In reactive HLH secondary to infection, supportive care and specific treatment of the underlying infection are associated with recovery in 60–70% of cases.10 Therefore, the specific therapy of Leishmania-related HLH is based on the use of pentavalent antimonials or amphotericin B, plus corticosteroids. Liposomal amphotericin B seems most suitable for VL-related HLH, because lipid-associated amphotericin B is selectively uptaken by reticuloendothelial cells, with less toxicity and better efficacy. In the current case, the patient received liposomal amphotericin B at a dose of 1mg/kg per day for 5 days based on previous positive reports. Clinical and laboratory response was observed within the first week.
It is well known that Leishmania bind to complement receptor type 3 (CR3), and are then phagocytized by macrophages 6. Amastigote sequestration and chronic intracellular infection of macrophages could prompt uncontrolled macrophage activation, with secretion of proinflammatory cytokines and subsequent HLH development. On the other hand, amphotericin B may inhibit several cellular functions of the immune system, including macrophage function, cytokine expression, mitogen, and antigen-induced proliferation of T and B cells in vitro, as well as the cytolytic function of cytotoxic T cells.11–14 Accordingly, it is reasonable to conceive that, in the present case, amphotericin may have exerted a dual effect on both HLH and leishmaniasis outcomes.
In conclusion, Leishmania infection must be considered as part of the differential diagnosis in patients presenting with HLH, regardless of their age. Clinicians must be aware of this possibility, especially in patients living in or having previously traveled to endemic areas. Amastigotes should be intensively sought on bone marrow smears, with repeated sampling and use of modern diagnostic methods, further preventing prolonged hospitalization, potentially harmful diagnostic procedures and treatments, and even death.
ACKNOWLEDGMENTS
We are greatly indebted to Gustavo Henrique Romani Magalhães for help in patient assistance and for providing the bone marrow examination photographs.
Footnotes
Authors' addresses: Guilherme Grossi Lopes Cançado, Guilherme Gomes Freitas, Flavia Helena Fidelis Faria, Antonio Vaz de Macedo, Hospital das Clínicas da Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil, E-mails: guilhermegrossi@terra.com.br, guigomesmed@gmail.com, flahelena@yahoo.com.br, and antoniovmac@hotmail.com. Vandack Nobre, Hospital das Clínicas da Universidade Federal de Minas Gerais, and Graduate Program in Infectious Diseases and Tropical Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil, E-mail: vandack@gmail.com
References
- 1.Rajagopala S, Dutta U, Chandra KS, Bhatia P, Varma N, Kochhar R. Visceral leishmaniasis associated hemophagocytic lymphohistiocytosis–case report and systematic review. J Infect. 2008;56:381–388. doi: 10.1016/j.jinf.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Tapisiz A, Belet N, Ciftci E, Ince E, Dogru U. Hemophagocytic lymphohistiocytosis associated with visceral leishmaniasis. J Trop Pediatr. 2007;53:359–361. doi: 10.1093/tropej/fmm024. [DOI] [PubMed] [Google Scholar]
- 3.Scott RB. Leukopenic myelosis: (section of medicine) Proc R Soc Med. 1939;32:1429–1434. [PMC free article] [PubMed] [Google Scholar]
- 4.Freeman HR, Ramanan AV. Review of haemophagocytic lymphohistiocytosis. Arch Dis Child. 2011;96:688–693. doi: 10.1136/adc.2009.176610. [DOI] [PubMed] [Google Scholar]
- 5.Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 6.Gagnaire MH, Galambrun C, Stephan JL. Hemophagocytic syndrome: a misleading complication of visceral leishmaniasis in children–a series of 12 cases. Pediatrics. 2000;106:E58. doi: 10.1542/peds.106.4.e58. [DOI] [PubMed] [Google Scholar]
- 7.Marom D, Offer I, Tamary H, Jaffe CL, Garty BZ. Hemophagocytic lymphohistiocytosis associated with visceral leishmaniasis. Pediatr Hematol Oncol. 2001;18:65–70. doi: 10.1080/088800101750059873. [DOI] [PubMed] [Google Scholar]
- 8.Tunc B, Ayata A. Hemophagocytic syndrome: a rare life-threatening complication of visceral leishmaniasis in a young boy. Pediatr Hematol Oncol. 2001;18:531–536. doi: 10.1080/088800101753328501. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho SF, Lemos EM, Corey R, Dietze R. Performance of recombinant K39 antigen in the diagnosis of Brazilian visceral leishmaniasis. Am J Trop Med Hyg. 2003;68:321–324. [PubMed] [Google Scholar]
- 10.Larroche C, Bruneel F, Andre MH, Bader-Meunier B, Baruchel A, Tribout B, Genereau T, Zunic P. Intravenously administered gamma-globulins in reactive hemaphagocytic syndrome. Multicenter study to assess their importance, by the immunoglobulins group of experts of CEDIT of the AP-HP. Ann Med Interne (Paris) 2000;151:533–539. [PubMed] [Google Scholar]
- 11.Boggs JM, Chang NH, Goundalkar A. Liposomal amphotericin B inhibits in vitro T-lymphocyte response to antigen. Antimicrob Agents Chemother. 1991;35:879–885. doi: 10.1128/aac.35.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauser WE, Jr, Remington JS. Effect of amphotericin B on natural killer cell activity in vitro. J Antimicrob Chemother. 1983;11:257–262. doi: 10.1093/jac/11.3.257. [DOI] [PubMed] [Google Scholar]
- 13.Kretschmar M, Geginat G, Bertsch T, Walter S, Hof H, Nichterlein T. Influence of liposomal amphotericin B on CD8 T-cell function. Antimicrob Agents Chemother. 2001;45:2383–2385. doi: 10.1128/AAC.45.8.2383-2385.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta RT, Mehta K, Lopez-Berestein G, Juliano RL. Effect of liposomal amphotericin B on murine macrophages and lymphocytes. Infect Immun. 1985;47:429–433. doi: 10.1128/iai.47.2.429-433.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]