Abstract
An uncommon disseminated Mycobacterium tuberculosis infection is described in a 12-year-old female dog presenting with fever, dyspnea, cough, weight loss, lymphadenopathy, melena, epistaxis, and emesis. The dog had a history of close contact with its owner, who died of pulmonary tuberculosis. Radiographic examination revealed diffuse radio-opaque images in both lung lobes, diffuse visible masses in abdominal organs, and hilar and mesenteric lymphadenopathy. Bronchial washing samples and feces were negative for acid-fast organisms. Polymerase chain reaction (PCR)-based species identification of bronchial washing samples, feces, and urine revealed M. tuberculosis using PCR-restriction enzyme pattern analysis-PRA. Because of public health concerns, which were worsened by the physical condition of the dog, euthanasia of the animal was recommended. Rough and tough colonies suggestive of M. tuberculosis were observed after microbiological culture of lung, liver, spleen, heart, and lymph node fragments in Löwenstein-Jensen and Stonebrink media. The PRA analysis enabled diagnosis of M. tuberculosis strains isolated from organs.
Case Report
In January 2011, a 12-year-old non-neutered cross-breed female dog was admitted to the Veterinary Hospital at the School of Veterinary Medicine and Animal Science, UNESP, Botucatu, State of Sao Paulo, Brazil. This animal presented with a history of progressive weight loss, anorexia, nonproductive cough, melena, hematemesis, epistaxis, mild diarrhea, and dyspnea. According to a member of the family, the dog had no contact with other domestic animals. For almost a year, the dog had a history of close contact with its owner, a family member who had died recently of pulmonary tuberculosis that was diagnosed by the standard protocol of the Brazilian Human Tuberculosis Control Program.1 The referenced man died at 46 years of age and had been diagnosed ∼1 year before death, with marked clinical worsening in the last 3 months of life. The man expectorated throughout the house in places to which the dog had access, and on several occasions, the dog had been observed licking the expectorated secretions. The owner had a history of alcohol abuse for 5 years and smoked for 34 years. During treatment, the owner continued to use alcohol and nicotine. This behavior worsened his body condition and contributed to his poor response to antimicrobial therapy, which was a combination of rifampicin, isoniazid, pyrazinamide, and ethambutol, as recommended by the Brazilian Human Tuberculosis Control Program1 and lead to his death.
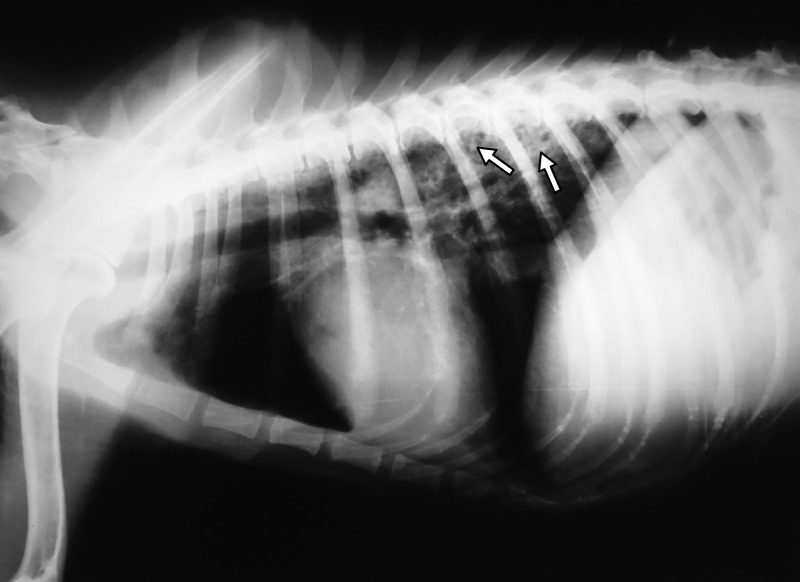
The clinical examination showed generalized musculo-skeletal weakness, fever (39.8°C), bloody nasal discharge, lymphadenopathy, respiratory distress, right-side heart failure, and enlargement of the liver and spleen. Radiographic examination revealed diffuse radio-opaque images in lung lobes (Figure 1), miliary lesions in the lungs and heart, and enlargement of the liver, spleen, hilar, and mesenteric lymph nodes.
Figure 1.
Lateral thoracic radiograph of a dog with disseminated Mycobacterium tuberculosis infection. Note diffuse radiopaque images (arrows) in dorsal and caudal lung lobes, suggesting lung consolidation and granuloma formation. Botucatu, SP, Brazil, 2011.
The leukogram showed leukocytosis (leukocyte count: 25.5 × 109/L), neutrophilia (88.0%) with toxic neutrophils, monocytosis (10.0%), and lymphopenia (3.0%). The erythrogram showed moderate anemia (erythrocyte count: 4.6 × 109/L). The blood biochemistry, including renal (blood urea and creatinine concentrations) and hepatic function (alanine aminotransferase [ALT] and aspartate aminotransferase) tests revealed elevated total protein (9.7 g/dL), globulin (8.10 g/dL), and alkaline phosphatase (545.0 UI/L), urea (85.0 mg/dL), and ALT (14.0 UI/L).
Bronchial washings and feces were subjected to Ziehl-Neelsen staining and were negative for mycobacteria or other acid-fast organisms. Bronchial washings were also used for microbiological culture in defibrinated blood agar, MacConkey agar, and Sabouraud agar,2 and no growth of pathogenic bacteria or fungi was observed in these media. Additionally, bronchial washings, urine, and feces were subjected to polymerase chain reaction (PCR) restriction enzyme pattern analysis (PRA)3,4 and these specimens were positive for a molecular diagnosis of Mycobacterium tuberculosis. Euthanasia was recommended because of the declining health of the dog, the public health concerns,5 the uncertain prognosis of tuberculosis (TB) in dogs6 and the fact that treatment of TB is generally not advised for this species.7
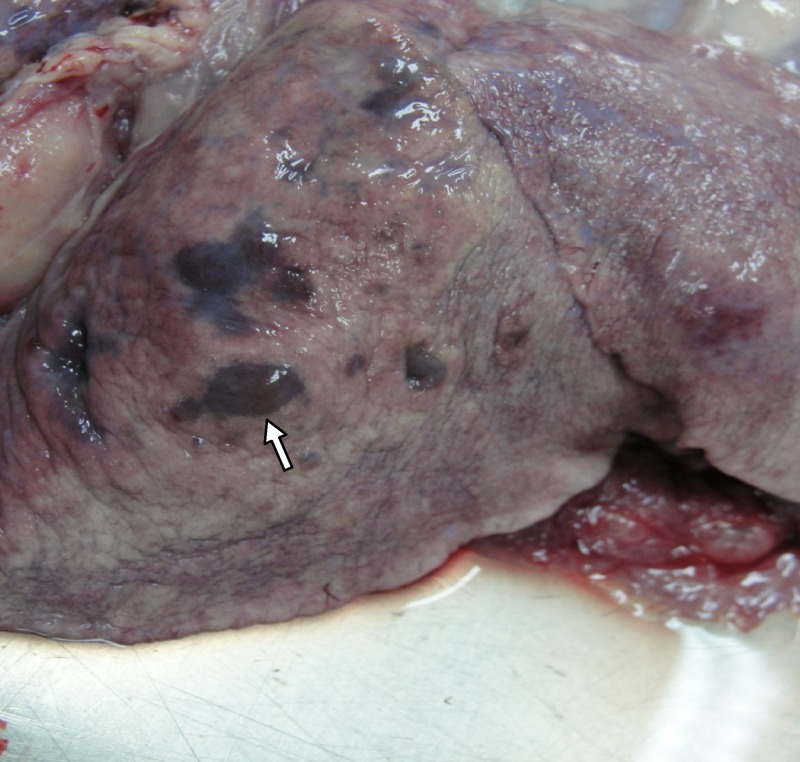
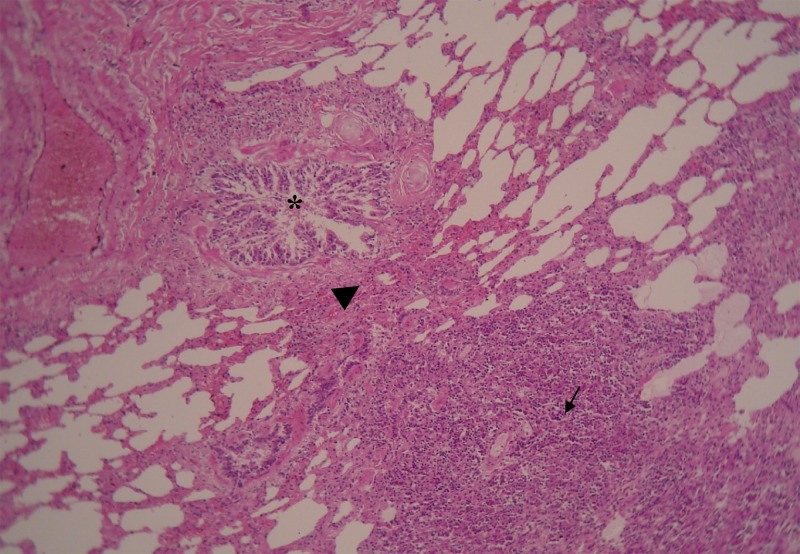
Generalized emaciation, granulomatous pneumonia, and lung consolidation particularly in the dorsal and caudal lung lobes (Figure 2), exudative pleuritis with a clear greenish aspect, pleural adhesion, a small amount of serous fluid in the pericardium, and circumscribed lesions (1–2 mm) disseminated on the diaphragm were observed in the post-mortem examination. Hemorrhagic enteritis, hepatitis, kidney congestion with marked striation of both cortical and medullar zones, bloody exudate in the abdominal cavity, enlargement of the spleen, hilar and mesenteric lymph nodes were found, which are suggestive of disseminated infection. Multifocal grayish-white, circumscribed lesions (1–2 mm) were found in the pericardium, pleura, liver, lung, spleen, intestine, and hilar and mesenteric lymph nodes. Histological examination of these lesions (H&E) revealed multifocal granulomas and areas of focal necrosis, and infiltrations with predominance of macrophages and epithelioid cells (Figure 3) surrounded by a thin connective capsule. Short-chained, pleomorphic, acid-fast bacilli were detected intracellularly in the periphery of necrotic lesions in all pulmonary parenchyma. Pleomorphic acid-fast micro-organisms in the bronchial tree were found indicating active TB infection.
Figure 2.
Pneumonia, congestion, and areas of focal necrosis (arrow) observed in caudal lung lobes at necropsy of a dog with disseminated Mycobacterium tuberculosis infection. Botucatu, SP, Brazil, 2011.
Figure 3.
Photomicrograph of a lung from a 12-year-old dog infected by Mycobacterium tuberculosis, showing granulomatous reaction with central necrosis, presence of epithelioid cells and fibroblasts in the middle zone (little arrow). Note proximity of granuloma (large arrow) with bronchial tree (*) indicating active tuberculosis (TB) infection (H&E stain, ×200).
After the post-mortem examination, lung, liver, spleen, heart, and lymph node fragments, tracheal secretions, feces, and urine were subjected to the Petroff decontamination method, using 4% NaOH and 4% H2SO4, for mycobacterial culture.8 Specimens were cultured on Löwenstein-Jensen and Stonebrink media, and incubated aerobically at 37°C for 90 days. Colonies suggestive of mycobacteria were subjected to Ziehl–Neelsen staining, classified by phenotypic conventional methods2,8 and subjected to PCR-based species identification using PRA. Rough, tough yellowish-orange colonies suggestive of M. tuberculosis were observed 27 days after microbiological culture of the samples in Löwenstein-Jensen and Stonebrink media. The PRA analysis3,4 of these specimens led to the molecular diagnosis of M. tuberculosis. After the post-mortem examination, the same samples were plated onto defibrinated blood agar, MacConkey agar, and Sabouraud agar,2 and no growth of bacteria or fungi was observed in these media.
For PCR-based species identification using PRA, organ fragments, and other bodily fluids were suspended in 100 mL of TE buffer at pH 8.0, boiled for 10 minutes (thermolysis) and frozen (−20°C) three times. Two microliters of DNA-containing supernatant were subjected to PCR amplification of a 441-bp fragment of the hsp65 gene using primers Tb11 (5′–ACCAACGATGGTGTGTCCAT–3′) and Tb12 (5′–CTTGTCGAACCGCATACCCT–3′)3,4 with 25 pmol of each primer and 26 μL of PCR Master Mix 2× (Fermentas, Thermo Fisher Scientific, Vilnius, Lithuania). The samples were submitted to denaturation at 95°C for 10 min, 45 cycles at 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min and extension at 72°C for 5 min (Thermocycler MJ Research PTC–100, Basel, Switzerland). Electrophoresis in agarose gel (1%) with addition of a 100-bp molecular weight marker (Invitrogen, Carlsbad, CA) was used to confirm the amplification of the 441-bp fragment. Aliquots of the PCR product were digested with BstEII and HaeIII (Fermentas). The resulting restriction fragments were separated by electrophoresis in agarose gel (4%) with the addition of two molecular weight markers of 25 bp and 50 bp (Invitrogen), and fragment sizes were estimated using Alpha Ease–Alpha Innotech software (version 6.0; San Leandro, CA). The results were compared with patterns reported online9 and were compatible with M. tuberculosis complex.3
Species determination was performed using gyrB-restriction fragment length polymorphism (gyrB-RFLP),10 in which 3 μL of the mycobacterial DNA was added to 44 μL of PCR Master Mix 2× (Fermentas). This mixture was subjected to PCR amplification of a 1,020-bp fragment of the gyrB gene using 100 pmol of each primer MTUBf (5′–TCGGACGCGTATGCGATATC–3′) and MTUBr (5′–ACATACAGTTCGGACTTGCG–3′). The PCR reaction occurred under an initial denaturation for 10 min at 95°C, 35 amplification cycles of 1 min at 94°C, 1 min at 65°C, and 1.5 min at 72°C and a final extension of 10 min at 72°C. Electrophoresis in agarose gel (1%) confirmed the fragment amplification. The amplicon was further digested with RsaI, TaqI, and SacII enzymes (Fermentas). The restriction digests were separated in agarose gel (2%) using 50-bp and 100-bp DNA molecular weight markers (Invitrogen). The RFLP values of the 1,020-bp gyrB fragments were estimated using Alpha Ease–Alpha Innotech software (version 6.0) and analyzed according to the previously described patterns.10 The fragment sizes were compatible with M. tuberculosis.
Discussion
Tuberculosis caused by the M. tuberculosis complex is recognized as an ancient disease of humans and animals. The pathogenic mycobacteria species that affect humans and/or animals include M. tuberculosis, Mycobacterium bovis, Mycobacterium africanum, Mycobacterium pinnipedii, Mycobacterium bovis subsp. caprae, and Mycobacterium microti.6,11
In recent decades, despite significant progress toward the control and/or eradication of TB in industrialized countries, this disease remains highly prevalent in humans and domestic animals in several countries of the world.5,11 Approximately one-third of the world's human population is infected by TB bacillus, and 1.5 to 2 million of people die victims of the disease each year, with a majority of deaths among human immunodeficiency virus (HIV)-positive patients.12 In various countries, including Brazil, there is little epidemiological and clinical information regarding canine TB. The PCR-based species identification has been used recently in the diagnosis of canine Mycobacterium infections. However, canine TB cases remain largely misdiagnosed or undetected in these countries.13–15
Tuberculosis in naturally infected companion animals is most commonly a subclinical disease. In dogs that develop clinical signs, the most common primary sites of TB infections are the lungs and the pulmonary lymph tract.6,16 In this report, the dog presented with many clinical manifestations, including signs of pulmonary, intestinal, hepatic, and renal involvement, which are suggestive of disseminated infection. The disseminated form of M. tuberculosis infection in dogs is considered rare.6 Systemic infection with Mycobacterium avium in dogs14,16 and M. bovis in companion animals has been described elsewhere.6
Different approaches have been used in the diagnosis of natural and experimental mycobacterial infections in companion animals, including clinical and epidemiological data; pathological, microbiological, histological, and cytological findings; intradermal (tuberculin) and serological tests; and currently PCR assays.6,13,17 Mycobacterial culture is considered the reference standard for TB diagnosis, although PCR techniques are becoming very valuable.12 Cytological findings can be obtained from tissue aspirates or impression smears by biopsy or necropsy, in which an acid-fast stain, such as the Ziehl-Neelsen stain, which is the most common method for presumptive TB diagnosis, is used. Intradermal tuberculin and serological tests in dogs are considered inconsistent and unreliable.6 The PCR assays for canine TB diagnosis have been developed in the last two decades to facilitate faster diagnosis and treatment decision.3,4 No single test or approach is exclusively indicated for the diagnosis of TB in companion animals because of the lack of clinical signs in the early stages of infection and nonspecific reactions observed in some of diagnostic methods.6,17
Clinical laboratory findings are nonspecific, and eventually, mycobacteria can be visualized in leukocytes of blood and bone marrow smears. Because the lipid-containing cell wall cannot be stained using Romanowsky's stains, the bacilli will appear as unstained areas within the leukocytes. The most common hematological findings in canine TB include leukocytosis and anemia.6 In our dog, leukocytosis, neutrophilia, monocytosis, and moderate anemia were also observed. Leukocytosis and monocytosis may be indicative of systemic infection and chronic disease, respectively. Increased alkaline phosphatase, urea, and ALT in our dog were most likely caused by hepatic and renal involvement by the M. tuberculosis infection. Radiographic and ultrasonographic abnormalities may be visible in companion animals with TB.6,18 In our dog, lesions resembling granulomas in the lungs; enlargement of the liver, spleen and lymph nodes; and altered images of other organs were present in the radiographic images, suggesting disseminated M. tuberculosis infection.
Mycobacterial culture is the reference standard method for the diagnosis of human12 and canine TB.5,6 Despite the very slow growth of some mycobacterial species and the necessity of selective media, assessment of the growth rates and colony pigmentation are practical tools in Mycobacterium genus classification. These phenotypic characteristics are often related to the virulence of the strains, and to the clinical presentation of TB in companion animals6; in the current case, the microorganism was isolated from different organs and bodily fluids after 27 days of microbiological culture in both Stonebrink and Löwestein-Jensen media, which are two egg-enriched media used for mycobacteria culture. This result reinforces the importance of these selective media types for the primary culture of M. tuberculosis.6,17
The use of Ziehl-Neelsen staining in microscopic examination, cytology, and/or histology samples for the identification of acid-fast organisms is a valuable method for routine mycobacterial diagnosis.18 However, it is a relatively labor-intensive test and has not been recommended for identifying paucibacillary or extra-pulmonary TB.12 These false-negative results for acid-fast organisms have been previously described in companion animals6 and show the need for combining methods in the diagnosis of canine TB.17
Previous examinations of dogs with M. tuberculosis pulmonary infection or other clinical manifestations reflect the development of granulomatous inflammation, which is characterized by the presence of the bacteria in the center of the lesion and is involved with macrophages, epithelioid and plasma cells, and fibroblasts, all surrounded by a fibrous capsule.6,19 In the dog we examined, a typical granuloma was identified in some organs, which is further evidence for the presence of a disseminated M. tuberculosis infection. The M. tuberculosis production of granulomatous lesions in domestic animals is closely related to the resistance of the microorganism to humoral and cellular immune responses, and to therapy with conventional drugs.6
Currently, PCR-based species identification has become useful in taxonomic classification and molecular epidemiology of mycobacterial infections in both humans and domestic animals.5,13 In companion animals, PCR-based species identification has been used to detect both experimental17 and natural20 M. tuberculosis infections. Furthermore, PCR-based species identification can be used in several body fluids and tissue specimens for the identification of mycobacteria strains that grow slowly in conventional culture media.6,17 In the current case, despite the initial negative result produced by the acid-fast staining of bronchial washings and feces upon the arrival of the animal in the hospital, PRA detected M. tuberculosis in both these samples and enabled the diagnosis of the microorganism in various body organs and fluids (nasal secretions, urine, and feces) of the animal after the post-mortem examination. Application of gyrB-RFLP cannot distinguish M. tuberculosis from M. africanum subtype II and “Mycobacterium canetti,” but epidemiological studies indicate that M. africanum subtype II and “M. canetti” are restricted to African territories.21,22 Our findings indicate that PCR-based species identification is valuable for M. tuberculosis detection in dogs17,20 and highlight the importance of using a combination of different methods to increase the success of the diagnosis of canine TB.
Human TB remains a serious health problem in Brazil. A Program operated by the Brazilian Ministry of Health estimated that ∼50 million people are infected, and TB is the first cause of death in HIV-positive patients in this country.1 Despite the implementation of control strategies and treatment protocols for the disease in Brazil, there are limited data regarding canine TB in this country. In one Brazilian study, canine TB was diagnosed by radiographic lesions and the presence of acid-fast organisms compatible with mycobacteria in histological examination. The dog presented with a history of close contact with 14 people with a positive intradermic TB test, from the same family.14 In another study, phenotypic methods were used to identify M. bovis in a farm dog that did not have apparent clinical signs, but did have a history of co-habitation with tuberculin-positive buffaloes.15
In companion animals, TB is considered an anthropozoonosis. The inhalation and consumption of sputum or contaminated food appears to be the major sources of transmission of M. tuberculosis from infected humans to dogs.5,6 Evidence indicates that many companion animals infected with M. tuberculosis had a history of living in an environment with humans infected by TB, or that the animals had intimate contact with owners infected with TB.5,7,17,18 Genotyping has confirmed that the same strain of M. tuberculosis infects the owner and the dog in the household.23 In fact, our dog had a history of close contact with its owner, who died of confirmed M. tuberculosis pneumonia. The proximity of the dog and the sick owner may have facilitated the inhalation of aerosolized droplets, contact with sputum, and/or ingestion of food contaminated with M. tuberculosis.5,6
Recently, experimental infection results led to the assumption that the transmission of M. tuberculosis between infected and healthy dogs kept in close contact is possible, suggesting that naturally infected dogs may be continuous sources of infection for humans and other animals.17 The PRA detection of M. tuberculosis in bronchial washing samples, urine, and feces from our dog suggests a hypothetical elimination of M. tuberculosis into the home environment. Thus, dogs are potential sources of infection for humans and other animals. This result emphasizes the epidemiological significance of the dissemination of this pathogen. Our study describes an unusual disseminated form of M. tuberculosis infection in a dog and is the first report of canine TB diagnosed by PCR-based species identification in Brazil.
Footnotes
Authors' addresses: Anna Paula Vitirito Martinho, Marília Masello Junqueira Franco, Márcio Garcia Ribeiro, Isabella Belletti Mutt Perrotti, Simone Henriques Mangia, Jane Megid, Luiz Carlos Vulcano, Gustavo Henrique Batista Lara, and Antonio Carlos Paes, Universidade Estadual Paulista-UNESP “Júlio de Mesquita Filho,” Faculdade de Medicina Veterinária e Zootecnia, Departamento de Higiene Veterinária e Saúde Pública, Botucatu, SP, Brasil, E-mails: aninhavitty@uol.com.br, marilia_gu@uol.com.br, mgribeiro@fmvz.unesp.br, isinhabmp@yahoo.com.br, simangia@hotmail.com, jane@fmvz.unes.br, vulcano@fmvz.unesp.br, gunnys7@gmail.com, and paesacmi@fmvz.unesp.br. Adolfo Carlos Barreto Santos and Clarice Queico Fujimura Leite, Laboratório de Micobacteriologia, Faculdade de Farmácia, UNESP, Araraquara, SP, Brasil, E-mails: adolfo.barreto@terra.com.br and leitecqf@fcfar.unesp.br. Osimar de Carvalho Sanches, Laboratório de Patologia Veterinária, Hospital Veterinário, Universidade do Oeste Paulista - UNOESTE, Presidente Prudente, São Paulo, Brazil, E-mail: osimarsanches@yahoo.com.br.
References
- 1.Ministério da Saúde . Manual de Recomendações para o Controle da Tuberculose no Brasil. Brasil: Ministério da Saúde; 2011. Programa Nacional de Controle da Tuberculose. Secretaria de Vigilância em Saúde. [Google Scholar]
- 2.Quinn PJ, Carter ME, Markey B, Carter GR. Clinical Veterinary Microbiology. London: Wolfe; 1994. pp. 137–143. [Google Scholar]
- 3.Telenti A, Marchesi F, Balz M, Bally F, Botrger EC, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31:175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parish T, Staker NG. Mycobacterium protocols. Methods in Molecular Biology. Totowa: Humana Press; 1998. pp. 101pp. 340–341. [Google Scholar]
- 5.Acha PN, Szyfres B. Tuberculose zoonótica. In: Acha PN, Szyfres B, editors. Zoonosis y Enfermidades Transmisibles Communes al Hombre y a los Animals. I. Washington: Organización Panamericana de la Salud; 2003. pp. 266–283. [Google Scholar]
- 6.Greene CE, Prescott JF. Mycobacterial infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. Third edition. St. Louis, MO: Elsevier; 2012. pp. 334–335. [Google Scholar]
- 7.Parsons SDC, Gous TA, Warren RM, van Helden PD. Pulmonary Mycobacterium tuberculosis (Beijing strain) infection in a stray dog. J S Afr Vet Assoc. 2008;79:95–98. doi: 10.4102/jsava.v79i2.252. [DOI] [PubMed] [Google Scholar]
- 8.Ministério da Saúde . Manual de Bacteriologia da Tuberculose. Third edition. Brasil: Ministério da Saúde; 2005. Centro de Referência Professor Hélio Fraga. [Google Scholar]
- 9.Prasite Identification of Mycobacteria. 2007. Http://app.chuv.ch/prasite/index.html Available at.
- 10.Chimara E, Ferrazoli L, Leão SC. Mycobacterium tuberculosis complex differentiation using gyrB restriction fragment length polymorphism analysis. Mem Inst Oswaldo Cruz. 2004;99:745–748. doi: 10.1590/s0074-02762004000700014. [DOI] [PubMed] [Google Scholar]
- 11.LoBue PA, Enarson DA, Thoen CO. Tuberculosis in humans and animals: an overview. Int J Tuberc Lung Dis. 2010;14:1075–1078. [PubMed] [Google Scholar]
- 12.World Health Organization-WHO . Global Tuberculosis Control 2011. Geneva: WHO; 2011. WHO/HTM/TB/2011. [Google Scholar]
- 13.Parsons SD, Warren RM, Ottenhoff TH, Gey van Pittius NC, van Helden PD. Detection of Mycobacterium tuberculosis infection in dogs in a high-risk setting. Res Vet Sci. 2011;92:414–419. doi: 10.1016/j.rvsc.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Megid J, Bracarense AP, Reis AC, Sturion DJ, Martin LM, Pinheiro SR. Tuberculose canina e sua importância em saúde pública. Rev Saude Publica. 1994;28:309–310. doi: 10.1590/s0034-89101994000400011. [DOI] [PubMed] [Google Scholar]
- 15.Mota PM, Lobato FC, Assis RA, Lage AP, Parreiras PM. Isolamento de Mycobacterium bovis de cão. Arq Bras Med Vet Zootec. 2001;53:410–412. [Google Scholar]
- 16.Horn B, Forshaw D, Cousins D, Irwin PJ. Disseminated Mycobacterium avium infection in a dog with chronic diarrhea. Aust Vet J. 2000;78:320–325. doi: 10.1111/j.1751-0813.2000.tb11781.x. [DOI] [PubMed] [Google Scholar]
- 17.Bonovska M, Tzvetkov Y, Najdenski H, Bachvarova Y. PCR for detection of Mycobacterium tuberculosis in experimentally infected dogs. J Vet Med B. 2005;52:165–170. doi: 10.1111/j.1439-0450.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- 18.Turinelli V, Ledieu D, Guilbaud L, Marchal T, Magnol JP, Fournel-Fleury C. Mycobacterium tuberculosis infection in a dog from Africa. Vet Clin Pathol. 2004;33:177–181. doi: 10.1111/j.1939-165x.2004.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 19.Ma YH, Mo ZS, Pan H, Ban W, Hu Y, Yan ST. Investigation of tuberculosis in army and police dogs in South China. J Vet Sci. 1998;28:12–15. [Google Scholar]
- 20.Posthaus H, Bodmer T, Alves L, Oevermann A, Schiller I, Rhodes SG, Zimmerli S. Accidental infection of veterinary personnel with Mycobacterium tuberculosis at necropsy: a case study. Vet Microbiol. 2011;149:374–380. doi: 10.1016/j.vetmic.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 21.Fabre M, Koeck J, Le Flèche F, Simon F, Hervé V, Vergnaud G, Pourcel C. High genetic diversity revealed by variable-number tandem repeat genotyping and analysis of hsp65 gene polymorphism in a large collection of Mycobacterium canetti strains indicates that the M. tuberculosis complex is a recently emerged clone of M. canetti. J Clin Microbiol. 2004;42:3248–3255. doi: 10.1128/JCM.42.7.3248-3255.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Jong BC, Antonio M, Gagneux S. Mycobacterium africanum—review of an important cause of human tuberculosis in West Africa. PLoS Negl Trop Dis. 2010;4:e744. doi: 10.1371/journal.pntd.0000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erwin PC, Bemis DA, McCombs SB, Sheeler LL, Himelright IM, Halford SK, Diem L, Metchock B, Jones TF, Schilling MG, Thomsen BV. Mycobacterium tuberculosis transmission from human to canine. Emerg Infect Dis. 2004;12:2258–2260. doi: 10.3201/eid1012.040094. [DOI] [PMC free article] [PubMed] [Google Scholar]