Abstract
A 22-month-old girl presented with neck pain and stiffness and magnetic resonance imaging showed an extradural mass extending from C2 through the C4 level with moderate to severe compression of the cord. A left unilateral C2–C4 laminectomy was performed revealing an extradural rubbery tumor; a small biopsy was obtained. Examination of stained tissue revealed the presence of a parasitic worm that was identified as a gravid female Onchocerca lupi. A magnetic resonance imaging at 7 weeks follow-up showed a significantly decreased size of the enhancing lesion and the patient's symptoms gradually resolved. This is the first report of zoonotic O. lupi in the United States. The parasite has been reported in dogs and cats in the western United States, and from people in four cases reported from Europe. A great deal more needs to be learned, including full host range and geographic distribution, before we fully understand O. lupi infections in animals and man.
Introduction
Zoonotic Onchocerca infections are uncommon with 20 cases being reported worldwide1–20; of those, three cases were infections of the anterior chamber of the eye,8,14,20 whereas the remaining cases most typically have been associated with dense connective tissues around bony structures or the conjunctiva. The exact species identification has been difficult in a number of cases, and several Onchocerca species that infect animals have been speculated to be the cause. However, for some cases species identification was possible based on morphologic features or a combination of morphology and molecular typing.
The vast majority of Onchocerca species, with the exception of Onchocerca volvulus in people, are parasites of ungulates. Onchocerca lupi, in contrast, has emerged over the past decade as a cause of unusual infection in dogs, more recently in people, and most recently in cats across Europe and in the United States. By parasitizing carnivores (canids and felids), O. lupi is unusual in its host preference. Furthermore, the presentation of discrete nodules associated with the eye in both domestic animals and humans is also highly unusual. We still do not know the natural definitive host, the arthropod vector involved in transmitting the infection, the geographic range of infection, and whether the preferred site of localization in the host is the conjunctiva and orbital ligaments.
This report describes the 21st case of zoonotic Onchocerca, the fifth from the United States, and the first in the United States to be attributed to O. lupi. It is atypical from all previous reported cases, including those of O. lupi, in that the infection was located in a cervical spinal mass.
Case Report
The patient was a 22-month-old Native American girl residing in Northern Arizona who presented with a 4-week history of gradually progressive neck pain associated with limitation of the neck's range of motion. Ten days before admission she developed fever that resolved after treatment with a course of amoxicillin as an outpatient. Because of progressive neck symptoms, she presented to an outside hospital and was transferred to a reference hospital. Her past history was otherwise unremarkable and her growth and development were appropriate for her age. She was born by cesarean section following an uneventful term pregnancy in a hospital in New Mexico and had otherwise spent all of her life with her nuclear family on a reservation in Northern Arizona. She was exposed to sheep, horses, and dogs. Her family history was non-contributory. Her immunizations were up to date.
Physical examination on presentation showed restricted range of motion of the neck; however, was otherwise within normal limits; there was no palpable mass or change in the overlying skin. A computed tomography scan of the neck (Figure 1A) showed a soft tissue mass in the cervical central canal extending from C2 through the C4 level, which narrowed the central canal by 75% at the C2–C3 level. A magnetic resonance imaging (MRI) scan of the cervical spine showed a 19 mm enhancing extradural mass at the level of C2–C4 with moderate to severe compression upon the cord resulting in mild cord edema. The MRI of the brain, thoracic, and lumbar spines were all normal. Bone scan and computed tomography of the chest, abdomen, and pelvis were all normal. Ophthalmologic evaluation was normal.
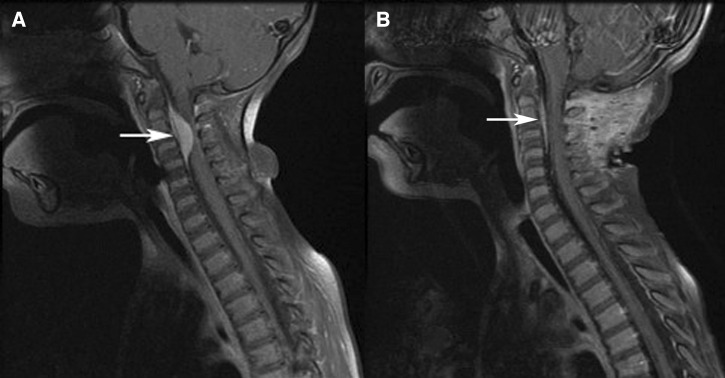
Figure 1.
T1 post-contrast magnetic resonance (MR) images of the neck with fat saturation of patient with zoonotic Onchocerca infection. (A) Image pre-biopsy showing a soft tissue mass (arrow) in the cervical central canal extending from C2 through the C4 level. (B) Image 7 weeks post biopsy showing significant reduction in the soft tissue mass (arrow) in the cervical central canal extending from C2 through the C4 level.
The patient underwent an excisional biopsy by left unilateral C2–C4 laminectomy during which an extradural rubbery avascular tumor was observed. Because of the consistency of the mass complete excision was not feasible, therefore only a very small biopsy was taken. The patient's symptoms improved after the surgery and she gradually returned to her baseline. An MRI taken immediately after the surgery did not show any changes in the size of the lesion, however an MRI performed 7 weeks later (Figure 1B) showed a significantly decreased size of the enhancing lesion in the anterior epidural space of the cervical spine from C2 to C4. It is not clear why the mass decreased in size so quickly, however the biopsy likely killed the female worm and the treatment with albendazole and ivermectin may have also hastened the process.
The biopsy tissue was fixed in formalin and submitted to a reference pathology laboratory, where it was sectioned and stained with hematoxylin and eosin stain. Three slides with several sections of worm were available for study. The specimen represented a mature, gravid female filarial worm, and the following morphologic features were evident that allowed an identification as O. lupi. In cross section (Figure 2A), the cuticle was relatively thick, the hypodermis was prominent, especially in the region of the lateral chords, which were both tall and broad, the muscle cells were observed to be few per quadrant, and the contractile portion was weak and, paired uteri containing microfilariae, and a small gut were all present. In more tangential sections (Figure 2B), these same features were present, and in addition, the nature of the distinctive cuticle was more clearly evident. Outer circular ridges were prominent, were relatively close together, and were distinctively dome-shaped. In the most longitudinal of cuts (Figure 3), the outer circular ridges and inner striae were apparent, again, there being one stria under each ridge and one between adjacent ridges (i.e., two striae per ridge). This combination of morphologic features allowed not only for a diagnosis of Onchocerca, but definitive identification as O. lupi. The small bit of human tissue containing an even smaller amount of parasite material that remained in the paraffin block was subjected to extraction and efforts to do molecular confirmation. Unfortunately, although human DNA was extracted, insufficient parasite DNA was available for evaluation.
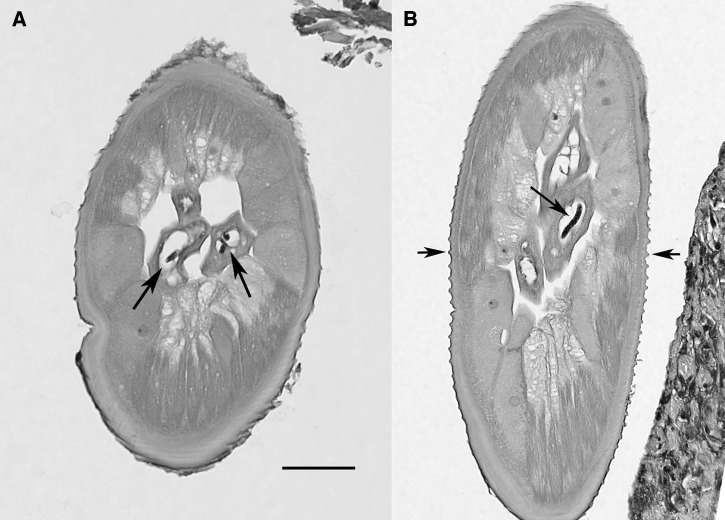
Figure 2.
Tissue sections of female Onchocerca lupi, hematoxylin and eosin stain. (A) Cross-section illustrating the morphology of the worm, including the detail of the muscle cells and large lateral chords, and the presence of microfilariae in utero (arrows). Scale bar = 50 μm. (B) Tangential section in which the same morphologic features are evident, including the presence of a microfilaria in utero (large arrow), and illustrating the distinctive cuticular ridges (small arrows). Scale bar same as in (A).
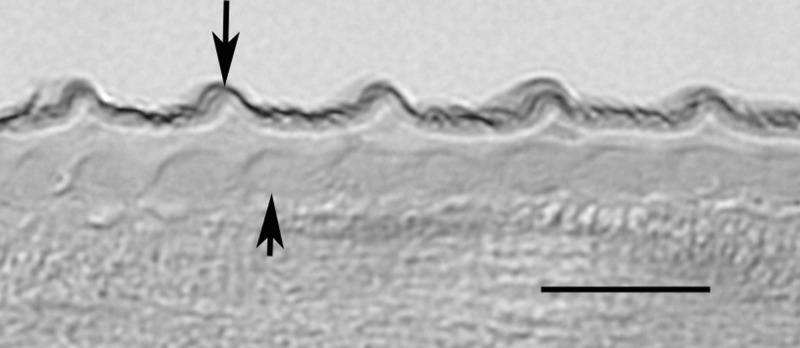
Figure 3.
Longitudinal section of female Onchocerca lupi at higher magnification illustrating the multilayered cuticle with external circular ridges (long arrow) and inner striae (short arrow). Scale bar = 10 μm.
Because the female worm was gravid and contained microfilariae, two skin snips were taken, one from the hip and one over the midline of the upper back, and examined for the presence of microfilariae. The snips were first incubated in saline for several hours and then alcohol added to the solution to preserve the skin snip and any microfilariae. The saline/alcohol fluid was centrifuged and the sediment examined for microfilariae in a wet preparation. The preparation was then allowed to dry and stained with Giemsa, and the slides read microscopically a second time. No microfilariae were observed in either the wet prep or stained material. An ophthalmologic exam was performed to exclude the presence of microfilariae in the eye.
Based on the preliminary results, the patient received albendazole (15 mg/Kg/day) for a total of 12 days. There is no proven treatment of O. lupi; however, because ivermectin is known to kill the larvae of O. volvulus and to accelerate the death of the adult worms when given 2–4 times a year,21,22 the regimen was switched to ivermectin (150 μg/Kg/dose, as a single dose to be repeated every 3 months for 5 years).
Discussion
This case is unusual in a number of regards, including the age of the patient, the location of the nodule, and the presence of microfilariae in the female worm (Table 1). It represents the youngest patient to have acquired a zoonotic Onchocerca infection, and one of the youngest for zoonotic filarial infections overall. Simmons and others23 reported a 9-month-old child from Oklahoma with a zoonotic Brugia infection and Beaver and others6 reported an Onchocerca infection in a 2-year-old child in Japan. Given the typically long prepatent period observed in Onchocerca, it is likely that the child in this case acquired the infection somewhere between 9 and 15 months of age. The vast majority of zoonotic filarial infections is constituted of a single worm, and hence rarely are gravid female worms observed. Somewhat unique to human and animal O. lupi infections, most have been gravid. In this case, only a female worm was present in the tissue sections available for study, but the presence of microfilariae in utero signaled that a male worm was present, and that both worms had matured and mated.
Table 1.
Summary of zoonotic onchocerciasis cases
| Case | Patient | Lesion | Parasite | Reference | ||
|---|---|---|---|---|---|---|
| Age/sex location | Sex species | |||||
| 1 | 15 F | USSR | Eye muscle tendon | F | O. gutturosa/cervicalis | 2 |
| 2 | 25 M | Switzerland | Knee | F | O. gutturosa/cervicalis | 3 |
| 3 | 48 F | Illinois, USA | Wrist | F | O. gutturosa/cervicalis | 4 |
| 4 | 43 F | Ontario, Canada | Wrist | F | O. gutturosa/cervicalis | 5 |
| 5 | 2 F | Japan | Sole of foot | F | O. dewittei japonica* | 6 |
| 6 | 57 F | Japan | Wrist | F | O. dewittei japonica* | 7 |
| 7 | 52 F | Colorado, USA | Ant. Chamber | F | O. gutturosa/cervicalis | 8 |
| 8 | 52 F | Japan | Head | M | O. dewittei japonica | 9 |
| 9 | 16 M | Albania | Subconjunctiva | F | O. lupi | 10 |
| 10 | 50 F | Minnesota, USA | Shoulder | F | O. gutturosa | 11 |
| 11 | 58 F | Japan | Hand | F | O. dewittei japonica | 12 |
| 12 | 69 F | Japan | Neck | F | O. dewittei japonica | 13 |
| 13 | 65 M | Hungary | Ant. Chamber | F | O. sp. (larva) | 14 |
| 14 | 59 F | Austria | Head | F | O. jakutensis | 15 |
| 15 | 12 F | Kuwait | Abdomen | F | O. sp. | 16 |
| 16 | 70 M | Japan | Knee | F | O. dewittei japonica | 17 |
| 17 | 18 F | Turkey | Subconjunctiva | F | O. lupi | 18 |
| 18 | 26 M | Turkey | Subconjunctiva | F | O. lupi | 19 |
| 19 | 8 | Tunisia | Subconjunctiva | F | O. lupi | 20 |
| 20 | 56 M | Oregon, USA | Ant. Chamber | F | O. sp. (larva) | 21 |
| 21 | 2 F | Arizona, USA | Neck | F | O. lupi | Present case |
Subsequent study of these cases has resulted in modification of the original species diagnosis.
F = female; M = male. For case 18, the sex was not given.
The location of the nodule close to the cervical spine and with protrusion into the spinal canal stands in distinct contrast to the previously reported human and animal cases of O. lupi, where the infection localized in nodules in the orbit of the eye.18,19,24–27 The anatomic location is also unusual and unique in regard to other zoonotic filaria infections caused by other species. Clinical management of this case was conservative, given the location, invasion into the spine with compression of the cord, and inability to remove the entire mass. As noted previously, the vast majority of zoonotic filariasis cases are constituted of a single worm, and biopsy or surgical resection is therefore curative. Because multiple worms, including a gravid female were present in this case, additional treatment seemed warranted. Treatment with doxycycline was considered as it is recognized that Onchocerca harbors Wolbachia symbionts, which are amenable to antibiotic treatment resulting in death of the adult worms over time.28 However, because of the patient's age and the clinical improvement following biopsy, it was felt to not be warranted. Because microfilariae were present in utero and possibly being released into the skin where they could migrate to the eye, a regimen of ivermectin treatment was prescribed. If on subsequent follow-ups, the mass completely resolves and the eye exam remains negative for the presence of microfilariae, it may be reasonable to stop the ivermectin. If the trend continues for zoonotic O. lupi infections to be constituted of multiple worms, including gravid females, it may be beneficial to develop a more specific treatment algorithm.
Very little outside of the reported clinical presentations is known about O. lupi infections, including its natural definitive host, host range, preferred location in the host, geographic distribution, or the arthropod vector. Originally described from a wolf in the Caucasus region of the Republic of Georgia, it appears to be common in dogs in Europe24,26 and recently reported in the United States27,29; it is not clear, however, whether dogs or wild canids are the natural host. Earlier reports of unusual Onchocerca infections in dogs in the United States30–32 that were not originally diagnosed as O. lupi can now, upon review of described morphologic features, be identified as O. lupi. The recent report of ocular infection in cats with O. lupi in the United States25 further confuses the question of the natural host. Interestingly, other than the one original description of the parasite from wolves, the parasite has not been reported in wildlife, including wild canids. In dogs and cats in Europe and the United States, the worm has always been associated with nodules in the connective tissue of the subconjunctiva, as was the case in the three human cases recently described.18,19 Zoonotic Onchocerca extracted from the anterior chamber in people have represented young, immature worms in which the cuticular morphology used as an aid to distinguishing species was not fully developed and hence no species determination. It is not clear that the worms in the anterior chamber would necessarily be the same species as those causing nodule formations in the subconjunctiva. In fact, the worm recovered from the patient in Colorado8 was sufficiently developed to see multiple striae between ridges, very distinct from that of O. lupi, where there is one stria between each ridge. For the time being, it seems reasonable that those worms removed from the anterior chamber not be ascribed to O. lupi. Similarly, the morphology of the worm in the first reported case of zoonotic Onchocerca in the United States4 was consistent with Onchocerca gutturosa of cattle or Onchocerca cervicalis of horses as there were widely spaced external circular ridges, with four striae per ridge (one under and three between).
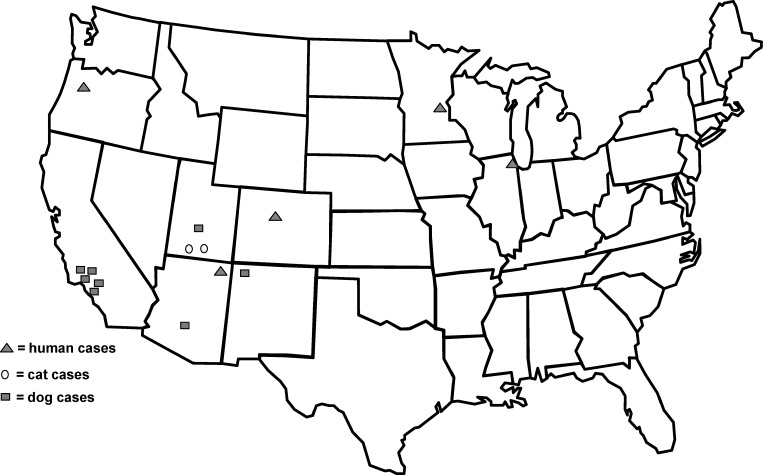
In the United States, there have been 15 reports of unusual infection with Onchocerca; five in people, two in cats, and eight in dogs (Figure 4). All of the infections in cats and dogs are now attributed to O. lupi; there has been recent molecular confirmation of the species identification for several of these cases.33 In two of the previous cases reported in people, O. lupi can be excluded because of the multiple striae per ridge,4,8 whereas in one case20 because of the immature nature of the worm, species identification was not possible. This case in a young child represents the first definitively identified case of zoonotic O. lupi in the United States. Clinicians should be aware that nodular masses may be a result of a variety of zoonotic filarial infections and that biopsy and accurate identification of the parasite is indicated. The case also highlights the need for more data to clarify epidemiologic risk factors and features of the clinical manifestations of O. lupi infections in humans.
Figure 4.
Map of the United States illustrating the approximate location where the 15 unusual cases of onchocerciasis have been reported in humans, dogs, and cats.
Footnotes
Authors' addresses: Mark L. Eberhard, Blaine A. Mathison, Henry S. Bishop, and Paul T. Cantey, Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: mle1@cdc.gov, gqa4@cdc.gov, hsb2@cdc.gov, and gdn9@cdc.gov. Gholamabbas Amin Ostovar and Kote Chundu, Department of Pediatrics, Maricopa Medical Center, Phoenix, AZ. Dan Hobohm, Department of Pathology, Maricopa Medical Center, Phoenix, AZ. Iman Feiz-Erfan, Division of Neurosurgery, Maricopa Medical Center, Phoenix, AZ, E-mails: Amin_Ostovar@dmgaz.org, Kote_Chundu@dmgaz.org, Dan_Hobohm@dmgaz.org, and Iman_Feiz-Erfan@dmgaz.org.
References
- 1.Eberhard ML. Zoonotic filariasis. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical Infectious Diseases: Principles, Pathogens, and Practices. Third edition. New York, NY: Elsevier; 2011. pp. 750–758. [Google Scholar]
- 2.Azarova NS, Miretskii OI, Sonin MD. The first case of human infection by the nematode Onchocerca, Diesing, 1841 in the USSR. Medizinskaia Parazitologyi. 1965;34:156–158. [PubMed] [Google Scholar]
- 3.Siegenthaler R, Gubler R. Paraarticulares nematodengranulom (einheimische Onchocerca) Schweiz Med Wochenschr. 1965;95:1102–1104. [PubMed] [Google Scholar]
- 4.Beaver PC, Horner GS, Bilos JZ. Zoonotic onchocerciasis in a resident of Illinois and observations on the identification of Onchocerca species. Am J Trop Med Hyg. 1974;23:595–607. doi: 10.4269/ajtmh.1974.23.595. [DOI] [PubMed] [Google Scholar]
- 5.Ali-Khan Z. Tissue pathology and comparative microanatomy of Onchocerca from a resident of Ontario and other Onchocerca species from Canada and USA. Ann Trop Med Parasitol. 1977;71:469–482. doi: 10.1080/00034983.1977.11687213. [DOI] [PubMed] [Google Scholar]
- 6.Beaver PC, Yoshimura H, Takayasu S, Hashimoto H, Little MD. Zoonotic Onchocerca in a Japanese child. Am J Trop Med Hyg. 1989;40:298–300. doi: 10.4269/ajtmh.1989.40.298. [DOI] [PubMed] [Google Scholar]
- 7.Takaoka H, Bain O, Tajimi S, Kashima K, Nakayama I, Korenaga M, Aoki C, Otsuka Y. Second case of zoonotic Onchocerca infection in a resident of Oita in Japan. Parasite. 1996;3:179–182. doi: 10.1051/parasite/1996032179. [DOI] [PubMed] [Google Scholar]
- 8.Burr WE, Brown MF, Eberhard ML. Zoonotic Onchocerca (Nematoda: Filarioidea) in the cornea of a Colorado resident. Ophthalmology. 1998;105:1494–1497. doi: 10.1016/S0161-6420(98)98035-6. [DOI] [PubMed] [Google Scholar]
- 9.Takaoka H, Bain O, Uni S, Korenaga M, Tada K, Ichikawa H, Otsuka Y, Eshita Y. Human infection with Onchocerca dewittei japonica, a parasite from wild boar in Oita, Japan. Parasite. 2001;8:261–263. [PubMed] [Google Scholar]
- 10.Pampiglione S, Vakalis N, Lyssimachou A, Kouppari G, Orihel TC. Subconjunctival zoonotic Onchocerca in an Albanian man. Ann Trop Med Parasitol. 2001;95:827–832. doi: 10.1080/00034980120111163. [DOI] [PubMed] [Google Scholar]
- 11.Wright RW, Neafie RC, McLean M, Markman AW. Zoonotic onchocerciasis of the shoulder. J Bone Joint Surg. 2002;84:627–629. doi: 10.2106/00004623-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Takaoka H, Bain O, Uni S, Korenaga M, Kozek WJ, Shirasaka C, Aoki C, Otsuka Y, Fukuda M, Eshita Y, Daa T. Zoonotic onchocerciasis caused by a parasite from wild boar in Oita, Japan. A comprehensive analysis of morphological characteristics of the worms for its diagnosis. Parasite. 2004;11:285–292. doi: 10.1051/parasite/2004113285. [DOI] [PubMed] [Google Scholar]
- 13.Takaoka H, Yanagi T, Daa T, Anzai S, Aoki C, Fukuda M, Uni S, Bain O. An Onchocerca species of wild boar found in the subcutaneous node of a resident of Oita, Japan. Parasitol Int. 2005;54:91–93. doi: 10.1016/j.parint.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Sallo F, Eberhard ML, Fok E, Baska F, Hatvani I. Zoonotic intravitreal Onchocerca in Hungary. Am Acad Ophthalmol. 2005;112:502–504. doi: 10.1016/j.ophtha.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 15.Koehsler M, Soleiman A, Aspock H, Auer H, Walochnik J. Onchocerca jakutensis filariasis in humans. Emerg Infect Dis. 2007;13:1749–1752. doi: 10.3201/eid1311.070017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hira PR, Al-Buloushi A, Khalid N, Iqbal J, Bain O, Eberhard ML. Zoonotic filariasis in the Arabian Peninsula: authochthonous onchocerciasis and dirofilariasis. Am J Trop Med Hyg. 2008;79:739–741. [PubMed] [Google Scholar]
- 17.Uni S, Boda T, Daisaku K, Ikura Y, Maruyama H, Hasegawa H, Fukuda M, Takaoka H, Bain O. Zoonotic filariasis caused by Onchocerca dewittei japonica in a resident of Hiroshima Prefecture, Honshu, Japan. Parasitol Int. 2010;59:477–480. doi: 10.1016/j.parint.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Otranto D, Sakru N, Testini G, Gurlu VP, Yakar K, Lia RP, Dantas-Torres F, Bain O. Case report: first evidence of human zoonotic infection by Onchocerca lupi (Spirurida, Onchocercidae) Am J Trop Med Hyg. 2011;84:55–58. doi: 10.4269/ajtmh.2011.10-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otranto D, Dantas-Torres F, Cebeci Z, Yeniad B, Buyukbabani N, Boral OB, Gustinelli A, Mounier T, Mutafciev Y, Bain O. Human ocular onchocerciasis: further evidence on the zoonotic role of Onchocerca lupi. Parasit Vectors. 2012;5:84. doi: 10.1186/1756-3305-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eberhard ML, Simms AC, Bishop HB, Mathison BA, Hoffman RA. Ocular zoonotic Onchocerca infection in a resident of Oregon. Am J Trop Med Hyg. 2012;87:1073–1075. doi: 10.4269/ajtmh.2012.12-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cupp EW, Cupp MS. Short report: impact of ivermectin community-level treatments of elimination of adult Onchocerca volvulus when individuals receive multiple treatments per year. Am J Trop Med Hyg. 2005;73:1159–1161. [PubMed] [Google Scholar]
- 22.Basanez G, Pion SDS, Bookes E, Filipe JAN, Churcher TS, Boussinesq M. Effect of single-dose ivermectin on Onchocerca volvulus: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:310–322. doi: 10.1016/S1473-3099(08)70099-9. [DOI] [PubMed] [Google Scholar]
- 23.Simmons CF, Jr, Winter HS, Berde C, Schrater F, Humphrey GB, Rosen FS, Beaver PC, Weller PF. Zoonotic filariasis with lymphedema in an immunodeficient infant. N Engl J Med. 1984;310:1243–1245. doi: 10.1056/NEJM198405103101908. [DOI] [PubMed] [Google Scholar]
- 24.Komnenou A, Eberhard ML, Kaldrymidou E, Tsalie E, Dessiris A. Subconjunctival filariasis due to Onchocerca sp. in dogs: report of 23 cases in Greece. Vet Ophthalmol. 2002;5:119–126. doi: 10.1046/j.1463-5224.2002.00235.x. [DOI] [PubMed] [Google Scholar]
- 25.Labelle AL, Daniels JB, Dix M, Labelle P. Onchocerca lupi causing ocular disease in two cats. Vet Ophthalmol. 2011;14:105–110. doi: 10.1111/j.1463-5224.2011.00911.x. [DOI] [PubMed] [Google Scholar]
- 26.Sreter T, Szell Z. Onchocerciasis: a newly recognized disease in dogs. Vet Parasitol. 2008;151:1–13. doi: 10.1016/j.vetpar.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Zarfoss MK, Dubeilzig RR, Eberhard ML, Schmidt KS. Canine ocular onchocerciasis in the United States: two new cases and a review of the literature. Vet Ophthalmol. 2005;8:51–57. doi: 10.1111/j.1463-5224.2005.00348.x. [DOI] [PubMed] [Google Scholar]
- 28.Hoerauf A, Specht S, Buttner M, Pfarr K, Mand S, Fimmers R, Marfo-Debrekyei Y, Konadu P, Debrah AY, Bandi C, Brattig N, Albers A, Larbi J, Batsa L, Taylor MJ, Adjei O, Buttner DW. Wolbachia endobacteria depletion by doxycycline as antifilarial therapy has macrofilaricidal activity in onchocerciasis: a randomized placebo-controlled study. Med Microbiol Immunol (Berl) 2008;197:295–311. doi: 10.1007/s00430-007-0062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez MD, Orita VM, Nolan TJ. Pathology in practice. JAVMA. 2012;240:385–387. doi: 10.2460/javma.240.4.385. [DOI] [PubMed] [Google Scholar]
- 30.Orihel TC, Ash LR, Holshuh HJ, Santenelli S. Onchocerciasis in a California dog. Am J Trop Med Hyg. 1991;44:513–517. doi: 10.4269/ajtmh.1991.44.513. [DOI] [PubMed] [Google Scholar]
- 31.Gardiner CH, Dick EJ, Jr, Meininger AC, Lozano-Alarcon F, Jackson P. Onchocerciasis in two dogs. J Am Vet Med Assoc. 1993;203:828–830. [PubMed] [Google Scholar]
- 32.Eberhard ML, Ortega Y, Dial S, Schiller CA, Sears AW, Greiner E. Ocular Onchocerca infection in two dogs in western United States. Vet Parasitol. 2000;90:333–338. doi: 10.1016/s0304-4017(00)00252-1. [DOI] [PubMed] [Google Scholar]
- 33.Labelle AL, Maddox CW, Daniels JB, Lanka S, Eggett TE, Dubielzig RR, Labelle P. Canine ocular onchocerciasis in the United States is associated with Onchocerca lupi. Vet Parasitol. 2012 doi: 10.1016/j.vetpar.2012.12.002. DOI:10.1016/j.vetpar.2012.12.002. [DOI] [PubMed] [Google Scholar]