Abstract
Nano-sized hepatitis B virus core virus-like particles (HBc-VLP) are suitable for uptake by antigen-presenting cells. Mycobacterium tuberculosis antigen culture filtrate protein 10 (CFP-10) is an important vaccine candidate against tuberculosis. The purified antigen shows low immune response without adjuvant and tends to have low protective efficacy. The present study is based on the assumption that expression of these proteins on HBc nanoparticles would provide higher protection when compared to the native antigen alone. The cfp-10 gene was expressed as a fusion on the major immunodominant region of HBc-VLP, and the immune response in Balb/c mice was studied and compared to pure proteins, a mixture of antigens, and fusion protein-VLP, all without using any adjuvant. The humoral, cytokine, and splenocyte cell proliferation responses suggested that the HBc-VLP bearing CFP-10 generated an antigen-specific immune response in a Th1-dependent manner. By virtue of its self-adjuvant nature and ability to form nano-sized particles, HBc-VLPs are an excellent vaccine delivery system for use with subunit protein antigens identified in the course of recent vaccine research.
Keywords: Mycobacterium tuberculosis, VLP, hepatitis B virus core particle, CFP-10, self-adjuvant, vaccine delivery
The number of new cases of tuberculosis (TB) continues to increase and approached 10 million globally in 2010.1 Bacille Calmette–Guerin (BCG) vaccination has long been considered as the best known form of disease prevention for tuberculosis, but unfortunately its efficacy varies from 0% to 80%.2 Clinical trials of BCG have shown an almost total lack of protection in regions of the world where the disease is widespread, and thus BCG vaccination can be considered ineffective.3 In addition, BCG is a live vaccine and can cause disseminated disease in immunocompromised individuals.4 Therefore, there is an urgent need to develop more effective vaccines against TB.
Vaccines based on minimal pathogen components that are able to stimulate a protective response in a host are called subunit vaccines. Adjuvant-mediated vaccines are more stable and safer than classical vaccines, but without adjuvants these vaccines are less effective.5 Use of subunit vaccine with adjuvants is a promising approach for developing new vaccines against TB as well. In the case of TB, identification of Mycobacterium tuberculosis (Mtb) antigens recognized by both CD4 and CD8 T cells, which are capable of generating immune responses and blocking initial infection as well as reactivation, would be a good vaccine candidate.6 The proteins secreted by Mtb have been suggested for the protective immune response of live vaccines,7,8 so a vaccine based on these proteins is a promising strategy for an effective vaccine against TB.9 Subunit vaccination based on the secretory proteome of Mtb is a promising vaccine strategy against TB.10 Although a number of antigens with vaccine potential have been identified, the majority of vaccine studies have relied on a small number of immunodominant antigens from Mtb, of which one of the most studied is culture filtrate protein 10 (CFP-10). CFP-10 is strongly recognized as a T-cell antigen in the initial stage of infection and has well-characterized epitopes providing significant efficacy in animal models.11–13 CFP-10 induces Cytotoxic T lymphocyte (CTL) activity and interferon-gamma (IFN-γ) production in animal models and in humans infected with Mtb, making it an excellent anti-TB vaccine candidate.14,15
A particulate vaccine delivery system, such as nano-sized virus-like particles (VLPs), can act as adjuvant as well as antigen delivery through enhancing the antigen uptake by antigen-presenting cells.16 VLPs also have a special advantage over a conventional system in size, stability, and capacity to transport across biological barriers.17 Particles in the 20–200 nm range are also able to generate CD4, CD8, and Th1 responses.18 Hepatitis B virus core protein-VLPs (HBc-VLPs) are in this size range and are able to generate both cellular and humoral immune responses.19–21
Recent research has shown that in comparison to native antigens, the fusion of two or more proteins when administered with adjuvants generated protective immunity in animal models.22 However, purified proteins show low immune response without adjuvants and tend to have a protective efficacy that is lower than BCG. CFP-10 is a major secretory protein and is one of the most immunodominant and pathogen-specific antigens from Mtb.23 A few studies have shown that nanoparticle-mediated delivery of immunogens may result in improved protection against TB.24–26 In this study we synthesized nano-sized HBc-VLPs bearing mycobacterial antigen CFP-10 and compared its immune response in a mice model. We also demonstrated that HBc-VLP::CFP-10 fusion protein induces an increased immune response in Balb/c mice compared to mixtures of native antigen.
Materials and methods
Gene amplification and plasmid construction
The gene sequence required for the formation of HBc-VLP (149 amino acid coding region) was amplified from genomic DNA of hepatitis B virus by using primers CEFP2 and CEHsR (designed to support a 6 HIS- tag at the C terminal of the expressed protein). The sequence of the HBc gene encoding major immunodominant region (MIR) was modified using overlap extension polymerase chain reaction (OEPCR) with primers CMPRP and CMPFP (Table 1). The resulting MIR sequence formed restriction sites for Eco RI and Hind III, which were flanked by two linker sequences (G4SG4T and G4SG4) that replaced six nucleotides from the MIR region. The modified and unmodified HBc genes were separately cloned into pET32a by using Nde I and Xho I restriction sites, resulting in pETMHBc and pETHBc plasmids, respectively.
Table 1.
Primer sequences
| Sl no | Primer name | Sequence |
|---|---|---|
| 1 | CEFP2 | GGAATTCCATATGGACATTGACCCTTATAAAGA |
| 2 | CEHsR | CCGCTCGAGCTAATGGTGATGGTGATGGTGAACAACAGTAGTCTCCGGAAGTG |
| 3 | CMPRP | AAGCTTGGGCCGGAATTCGGTGCCACCGCCACCAGAGCCACCGCCACCATCTTCCAA |
| 4 | CMPFP | GAATTCCGGCCCAAGCTTGGTGGCGGTGGCTCTGGTGGCGGTGGCTCTAGGGAC |
| 5 | Mcfp10F | CCGGAATTCGCAGAGATGAAGACCGATG |
| 6 | Mcfp10R | CCCAAGCTTGAAGCCCATTTGCGAGGACAGC |
| 7 | CFP10ptF | GGAATTCCATATGCACCATCACCATCACCATGCAGAGATGAAGACCGATG |
| 8 | CFP10ptR | CCGCTCGAGTCAGAAGCCCATTTGCGAGGACAGC |
Notes: Sequences 1 and 2 are for amplification of the HBc gene for cloning into the pET vector. Sequences 3 and 4 are for modifying the MIR region (using overlap extension PCR). Sequences 5 and 6 are for cloning the cfp-10 gene into the modified MIR region of the HBc gene. Sequences 7 and 8 are for cloning the cfp-10 gene into the pET vector.
Abbreviations: HBc, Hepatitis B virus core; pET, PET-32a; MIR, major immunodominant region; PCR, polymerase chain reaction.
For cloning into pETMHBc, the CFP-10 coding sequence was amplified from Mtb H37Rv by using primers Mcfp10F and Mcfp10R, which had restriction sites Eco RI and Hind III. The complete CFP-10 gene was amplified from Mtb H37Rv by using primers CFP10ptF and CFP10ptR (designed to sport a 6 HIS- tag at the N terminal of the expressed protein) containing restriction sites Nde I and Xho I and was cloned into pET32a for expression and purification of CFP-10 (pETCFP).
Protein expression and purification
CFP-10 expression and purification
For CFP-10 expression, pETCFP was transformed into Escherichia coli BL21(DE3) (Novagen, Billerica, MA, USA). Protein expression was induced by adding isopropyl-1-thio-β-D-galactopyranoside (IPTG) at a final concentration of 0.5 mM at 28°C. The cell pellets were resuspended in lysis buffer (50 mM NaHPO4, 200 mM NaCl, pH 8), ultrasonicated, and centrifuged at 10,000 g for 20 minutes at 4°C. The clear supernatant was collected, and the protein was purified by immobilized metal affinity chromatography with Ni-silica resin (Promega, Madison, WI, USA). Protein-bound resin was washed with buffer containing 20 mM imidazole, and the bound protein was eluted with buffer containing 250 mM imidazole. The eluted protein was desalted and concentrated with a U-tube concentrator (Novagen). Protein concentration was estimated by using the BCA assay (Thermo Scientific, Rockford, IL, USA), analyzed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and confirmed by Western blot using anti-CFP-10 antibody (Thermo Scientific). Lipopolysaccharide (LPS) contamination was determined using the EndoLISA endotoxin detection assay (Hyglos GmbH, Bavaria, Germany).
Purification of VLPs
Plasmids pETHBc and pETMHBc::CFP-10 were transformed into E. coli BL21 (DE3), and protein expression was induced with 0.5 mM and 1 mM IPTG, respectively. Purification of HBc-VLPs and fusion protein VLPs (FVLP) was performed as noted in earlier reports27,28 with minor modifications. Briefly, C-terminal HIS-Tagged proteins were purified using Ni-silica resin, and reassembled VLPs were recovered by 10%–60% sucrose density gradient ultracentrifugation. Proteins were detected by Western blotting using anti-HBc and anti-CFP-10 antibodies.
Transmission electron microscopy
Sucrose density gradient fractions were diluted in phosphate-buffered saline (PBS), layered onto copper grids, and negatively stained using 2% uranyl acetate. Electron micrographs were obtained on a transmission electron microscope (Jeol, Tokyo, Japan).
Animals and immunization
Pathogen-free Balb/c female mice (6–8-weeks old) were used for this study, and all experiments were approved by the Institutional Animal Ethics Committee. We used five groups of eight mice each: G1, sham immunized; G2, CFP-10 immunized; G3, HBc-VLP immunized; G4, HBc-VLP and CFP-10 protein mixture (ratio similar to FVLPs) immunized; G5, FVLP immunized. Each group was immunized three times at 2-week intervals. One week after the final immunization, mice were sacrificed and blood and spleen were collected aseptically. The spleens were crushed and passed through a 70 μm cell strainer (BD Falcon, Bedford, MA, USA). The red blood cells were lysed by using Ammonium-chloride-potassium (ACK) lysis buffer (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.4) and then resuspended in warm RPMI (Sigma-Aldrich, St.Louis, MD, USA). The spleens were all processed separately.
Antibody assay
Direct enzyme-linked immunosorbent assay (ELISA) was used for detection of anti-CFP-10 antibodies in the sera of immunized animals. Briefly, ELISA plates (BD Falcon) coated with CFP-10 (5 μg/well) in PBS overnight at 4°C were blocked with 3% bovine serum albumin in PBS for 2 hours, washed with PBS containing 0.05% Tween 20, and incubated with 1:500 prediluted serum. The washed plates were incubated with horseradish peroxidase-conjugated anti-mouse immunoglobulin (Ig)G (Sigma-Aldrich) or anti-mouse IgG1 or anti-mouse IgG2a (Santa Cruz Biotechnology, CA, USA). The plates were developed with tetramethylbenzidine substrate solution (BD Biosciences, San Jose, CA, USA) and 1 M H3PO4. The optical density (OD) at 450 nm was read on a microplate reader (Bio-Rad, Laboratories, Hercules, CA, USA).
Cytokine analysis
Splenocytes (2 × 105 cells/well) were cultured in 96-well plates (BD Biosciences) in RPMI 1640 supplemented with 1% penicillin–streptomycin, 1 mM glutamine, and 10% Fetal borine serum (FBS) at 37°C/5% CO2. Triplicate wells were stimulated with buffer (control), concanavalin A (Con A ), CFP-10, and culture filtrate (CF) of Mtb (prepared as per an earlier report)29 at 5 μg/mL. Supernatants were collected after 72 hours and IFN-γ, Interleukin (IL)-2, tumor necrosis factor (TNF), IL-5, and IL-10 were quantified using cytokine ELISA (BD Biosciences).
Lymphocyte proliferation assay
Splenocytes (4 × 105 cells/well) were cultured in RPMI supplemented with 10% FBS and stimulated with CFP-10 (5 μg/mL) and Con A in triplicate. After 72 hours cell proliferation was assessed by using the CellTiter 96 kit (Promega) according to the manufacturer’s instruction. The proliferative responses were expressed as the stimulation index (SI),
ELISPOT assay
Enzyme-linked immunospot (ELISPOT) assays were performed with the ELISPOT assay kit (BD Biosciences). Briefly, plates were coated with anti-mouse IFN-γ antibody and blocked with RPMI, 10% FBS, and L-glutamine. Splenocytes taken from individual mice were cultured in triplicate (at 5 × 105 cells/well) in RPMI for 20–24 hours at 37°C and 5% CO2 with Con A or antigen (CFP-10). Cells were discarded post stimulation, and plates were developed with 3-amino-9-ethylcarbazole (AEC) substrate reagent (BD Biosciences). Spots were counted under a dissecting microscope (Olympus, Tokyo, Japan).
Statistical analysis
Statistical analysis was done by parametric testing of mean values via one-way analysis of variance (with Tukey’s post hoc testing to identify between-group significance).
Results
Expression of CFP-10, HBc, and FVLP
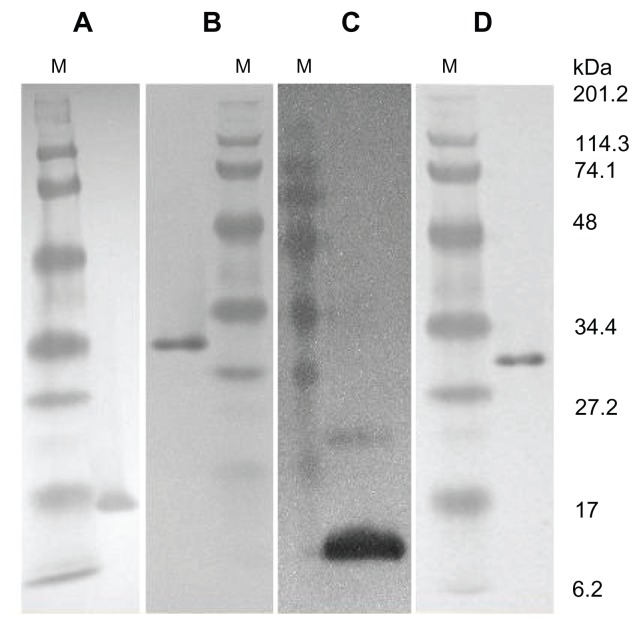
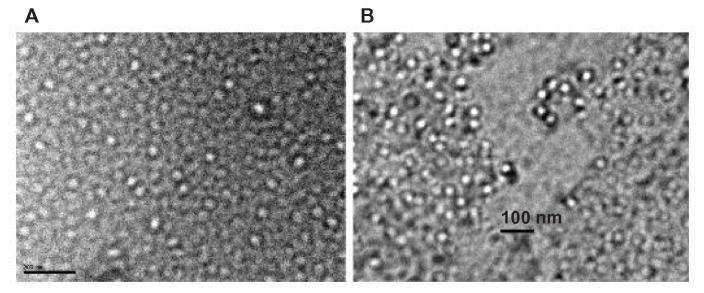
Western blot using anti-CFP-10 and anti-HBc antibodies showed that all proteins were expressed correctly (Figure 1). Electron microscopic studies revealed that both HBc and fusion protein formed VLPs (Figure 2). The LPS levels in the purified proteins were found to be low at ~1 ng/mg of antigen.
Figure 1.
Expression of CFP-10, HBc, and FVLP.
Notes: Western blot of purified proteins. A, purified HBc probed with anti-HBc antibody; B, purified FVLP with anti-HBc antibody; C, purified CFP-10 with anti-CFP- 10 antibody; D, FVLP with anti-CFP-10 antibody.
Abbreviations: M, protein molecular weight marker in kDa; CFP-10, Culture filtrate protein-10; HBc, Hepatitis B core protein.
Figure 2.
Transmission electron microscopic pictures of VLPs.
Notes: (A), HBc-VLP. Bar = 200 nm; (B), FVLP. Bar = 100 nm.
Abbreviations: VLPs, virus like particles; HBC-VLP, hepatitis B core protein VLP; FVLP, fusion protein VLP.
FVLP generated a higher CFP-10-specific humoral response
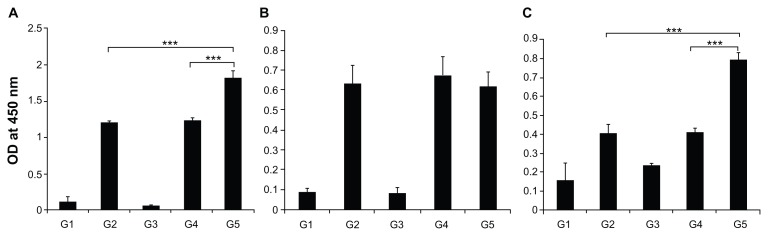
To analyze whether the FVLP could enhance immunogenicity when compared to CFP-10 protein alone, ELISA was performed to detect the levels of anti-CFP-10 antibodies in the sera collected from mice immunized with different proteins. The results showed that FVLP was more immunogenic and produced higher antibody responses than CFP-10 alone (P < 0.001) or CFP-10 mixed with HBc (P < 0.001) (Figure 3A). IgG1 production against FVLP was less than that of the other two groups (Figure 3B), although this was not statistically significant. However, significantly higher levels of IgG2a antibodies (P < 0.001) were produced against FVLP when compared to mixed proteins/CFP-10 (Figure 3C).
Figure 3.
CFP-10 specific humoral responses in different mice groups after immunization. Blood samples from Balb/c mice were collected after final immunization. (A) Antibody response against CFP-10 in all groups. (B) CFP-10-specific IgG1 responses in immunized mice groups. (C) CFP-10-specific IgG2a responses in immunized mice groups. Optical densities were read at 450 nm, and results are expressed as the arithmetic mean of the titer obtained.
Notes: The data are presented as mean ± standard deviation. Statistical significance between groups is indicated as ***P < 0.001.
Abbreviations: OD, optical density; CFP-10, culture filtrate protein-10; G1, buffer-immunized group; G2, CFP-10-immunized group; G3, HBc-VLP-immunized group; G4, HBc + CFP-10 mixture-immunized group; G5, FVLP-immunized group.
Cytokine response
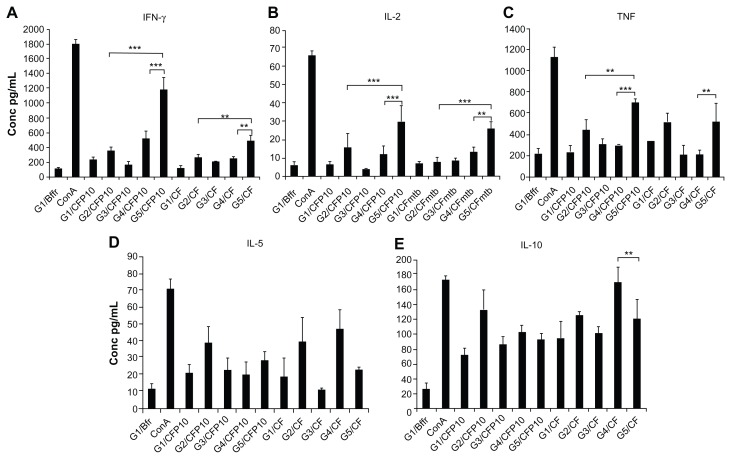
Splenocytes from different groups of mice were stimulated with CFP-10, and cells from FVLP-immunized mice showed significantly higher production of IFN-γ, IL-2, and TNF compared to splenocytes from CFP-10 or mixture-immunized mice (Figure 4A–C). Interestingly, except for TNF the same picture emerged when they were stimulated with CF. Stimulation of splenocytes from fusion protein-immunized mice with either CFP-10 or CF showed lower levels of both IL-5 and IL-10 (Figure 4D and E), although this was not statistically significant when compared to CFP-10 or mixture-immunized mouse cells.
Figure 4.
Cytokine production by the splenocytes stimulated with CFP-10. Splenocytes collected from each group of mice 1 week after the final immunization were stimulated with CFP-10 (5 μg/mL). Con A (5 μg/mL) and buffer were used as controls. (A) IFN-γ; (B) IL-2; (C) TNF; (D) IL-5; (E) IL-10.
Notes: The concentrations of cytokines in the splenocyte culture supernatant were determined by ELISAs. The data are presented as mean ± standard deviation. Statistical significance between groups is indicated as ***P < 0.001, **P < 0.05.
Abbreviations: IFN-V, interferon-gamma; IL-2, interleukin-2; TNF, tumor necrosis factor; IL-5, interleukin-5; IL-10, interleukin-10; CF, culture filtrate of Mtb; G1, buffer-immunized group; G2, CFP-10-immunized group; G3, HBc-VLP-immunized group; G4, HBc + CFP-10-immunized group; G5, FVLP-immunized group; Con A, concanavalin A; CF, culture filtrate of Mtb.
FVLP-generated antigen-specific splenocyte proliferation
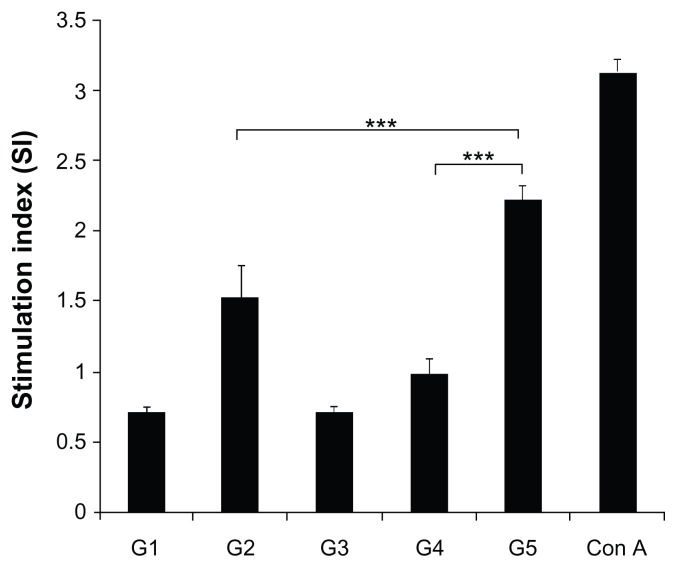
Splenocytes from animals immunized with CFP-10 (G2) and FVLP (G5) produced significantly higher proliferative responses on stimulation with CFP-10 in vitro when compared to cells from the other three groups. Mice that were immunized with FVLPs demonstrated maximum proliferation of splenocytes against CFP-10 (P < 0.001) (Figure 5).
Figure 5.
Effect of FVLP immunization on splenocyte proliferation.
Notes: Lymphocyte proliferation in response to CFP-10 (5 μg/mL) stimulation was evaluated using the CellTiter Cell Proliferation assay. Absorbance was measured at 490 nm, and the proliferative responses were expressed as the stimulation index (SI). Con A (5 μg/mL) was used as control. The data are presented as mean ± standard deviation. Statistical significance between groups is indicated as ***P < 0.001.
Abbreviations: CFP-10, culture filtrate protein-10; G1, buffer-immunized group; G2, CFP-10-immunized group; G3, HBc-VLP-immunized group; G4, HBc + CFP-10- immunized group; G5, FVLP-immunized group; Con A, concanavalin A.
FVLP-generated antigen-specific IFN-γ secretion
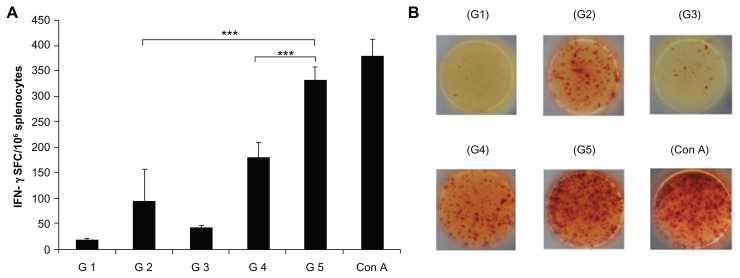
The number of IFN-γ-secreting cells was enumerated by ELISPOT assay. The cells from different groups of mice were stimulated with CFP-10. The FVLP group showed significantly (P < 0.001) higher numbers of IFN-γ-secreting cells compared to cells from CFP-10 alone or the protein mixture-immunized group of mice (Figure 6).
Figure 6.
CFP-10 specific IFN-γ response to VLP immunization. Splenocytes from different groups of mice were stimulated with CFP-10 (5 μg/mL). Con A (5 μg/mL) was used as control. (A) IFN-γ-secreting cells detected by ELISPOT assay. (B) Images represent splenic ELISPOT responses.
Notes: The data are expressed as the mean ± standard deviation. Statistical significance between groups is indicated as ***P < 0.001.
Abbreviations: CFP-10, culture filtrate protein-10; ELISPOT, enzyme-linked immunospot; IFN-γ, interferon-gamma; G1, buffer-immunized group; G2, CFP-10-immunized group; G3, HBc-VLP-immunized group; G4, HBc + CFP-10-immunized group; G5, fusion protein-immunized group; SFC, spot forming units; Con A, concanavalin A.
Discussion
CFP-10 is one of the ideal candidates for a subunit vaccine, either by itself or in combination with other relevant antigens of Mtb against TB.30 However, the specific immune response depends on the method of antigen delivery to the immune system.31 The critical component of protective immunity against TB is the T-cell-mediated response characterized by the secretion of IFN-γ and other cytokines.32 Some recent studies have also suggested that antibodies also enhance the protective effect against TB.33,34 These reports strengthen the emerging view that the most effective immune response is the one that combines both humoral and cellular components.33
In this study, we expressed the full-length CFP-10 protein on HBc-VLP. Results from electron microscopy coupled with Western blots confirmed that the fusion protein was correctly expressed, was able to form VLPs, and that the presence of CFP-10 antigen did not affect VLP assembly. The immune responses generated by FVLP were analyzed after immunizing Balb/c mice with mixtures of separate antigens and as fusion VLPs. This also helped to analyze the self-adjuvant nature of HBc-VLPs. Our results showed that except for FVLP-immunized mice, other groups were not able to confer the same level of immune response. This strongly suggests that VLPs are an excellent carrier of Mtb antigens and could act as a self-adjuvant, an important factor for vaccination. The high antibody response to the FVLP-immunized group contrasts with the fact that simply mixing the CFP-10 protein in its native form with HBc-VLP was not able to generate similar antibody responses. Thus, the mere presence of CFP-10 protein and native HBc-VLP was not sufficient to generate strong immune responses, emphasizing that a proper display of foreign antigen on VLPs is essential. The high level of IgG2a response to FVLP was not achieved when the mice were immunized with mixtures of antigen. The significant elevation of anti-CFP-10 IgG2a in G5 mice indicates that a fusion protein carrier induces preferentially Th1-type priming and interaction, even though IgG1 (a marker of Th2 immune response) is usually the predominant isotype in Balb/c mice.35,36
IFN-γ has been shown to be indispensable for resistance to mycobacterial and other intracellular infections and is often used as a single readout for Th1 responses.22,37 The secretion of comparatively high amounts of IFN-γ in response to CFP-10 stimulation in splenocytes of mice immunized with FVLP is a further indication of proper antigen delivery by VLPs. In addition, these splenocytes also showed significant induction of IFN-γ on stimulation with CF of Mtb. This was interesting because the secretion of CF proteins in experimental conditions resembles an environment that Mtb encounters in the host cell. In the absence of an infection model in this study, this immune response to CF can be considered as a protective response against Mtb challenge. IL-2 plays a major role in activation and proliferation of cytotoxic (CD8+) T cells that develop later in the acquired immune response.38–40 Studies have also suggested a significant role for CD8+ cells in protective immune responses against Mtb.41,42 The increased levels of IL-2 against CFP-10 and CF of Mtb also suggest a tilting of immune response in a Th1-dependent manner. TNF on its own is another effector cytokine that can synergize with IFN-γ to eliminate intracellular pathogens.22,43,44 The higher secretion of TNF in G5 upon stimulation with CFP-10 points to the fact that FVLP was able to induce a protective type of immune response. Compared to all other mice groups, the minimal production of IL-5 and IL-10 in G5 on stimulation with CFP-10 also emphasizes that a Th1 response is actually predominant when Mtb antigen is delivered as an FVLP.
Proliferation analysis indicated that mycobacterial antigen CFP-10 generated significant cell proliferation in mouse splenocytes when antigen was delivered as an FVLP. The ELISPOT analysis also supports the cytokine and ELISA results, indicating the generation of a Th1 immune response in G5 mice.
Adjuvants play a significant role in the nature of immune responses generated by any vaccine and they can direct the immune system in favor of a Th1- or Th2-type response.45,46 The Th1 response is essential for protective immunity against intracellular infectious agents and presumably against Mtb also.47 The currently available adjuvants, such as water/ oil emulsions and alum, mainly elicit Th2 immunity and are therefore frequently ineffective against intracellular pathogens, such as Mtb, that require a Th1 response.48,49 A few of the available adjuvants, such as lipid A and its derivatives, are capable of modulating cytokine and IgG isotype profiles characteristic of Th1 immunity. However, they are unable to mount a good T-cell response against soluble or exogenous antigens, a feature that is essential for the development of an effective subunit vaccine against infectious agents like Mtb.45,46,50 Functional T cells, especially from a Th1 response, are necessary for generating a protective immune response to Mtb,41,51 and therefore for developing an effective TB vaccine, it is essential to identify more vaccine candidates and validate these in animal models.
Recently a study showed the generation of 6kDa early secretory antigenic target (ESAT-6) specific immune responses in mice after immunization with hepatitis B core VLPs on Al(OH)3 adjuvant.52 This study showed increased antibody responses to ESAT-6 along with an increase in INF-γ-secreting cells, and our results corroborate their finding that hepatitis B core VLPs can be an efficient antigen carrier. Further, our results strongly suggest that antigen presentation on HBc-VLP obviates the need for an adjuvant and is able to generate Th1 immune responses.
HBc-VLPs are noninfectious. Unlike other VLPs they are not involved in virus receptor interactions and are not a target of neutralizing antibodies, and therefore carrier suppression would not be a major challenge.53,54 One of the major constraints in using HBc-VLPs as a vaccine delivery system is the inhibition of the assembly of the VLP by the inserted sequence. Therefore, in a modification of an earlier reported work,55 we altered the core gene with a Gly-rich linker sequence and inserted restriction sites in the MIR coding region such that foreign protein sequences could be inserted into the modified part. This significantly reduced the problem associated with assembly. HBc-VLPs are generally known to be extremely stable as they are resistant to denaturing agents and variations in pH and temperature, and these VLPs can be produced in all known homologous and heterologous expression systems.56 The capacity to cross present antigens is an added advantage of HBc-VLPs as a vaccine delivery platform against TB.57,58
In conclusion, our results show that the self-adjuvant and immunoenhancing properties of nano-sized HBc-VLPs could be an excellent platform for vaccine delivery against TB.
Acknowledgments
We thank Dr E Sreekumar for valuable suggestions and Sanjay Dharmaseelan and Bindu Asokan for assistance in electron microscopy. DD acknowledges the University Grants Commission for the research fellowship.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dye C, Williams BG. The population dynamics and control of tuberculosis. Science. 2010;328(5980):856–861. doi: 10.1126/science.1185449. [DOI] [PubMed] [Google Scholar]
- 2.Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271(9):698–702. [PubMed] [Google Scholar]
- 3.Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22(6):1154–1158. doi: 10.1093/ije/22.6.1154. [DOI] [PubMed] [Google Scholar]
- 4.Jouanguy E, Altare F, Lamhamedi S, et al. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette–Guerin infection. N Engl J Med. 1996;335(26):1956–1961. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 5.Skwarczynski M, Toth I. Peptide-based subunit nanovaccines. Curr Drug Deliv. 2011;8(3):282–289. doi: 10.2174/156720111795256192. [DOI] [PubMed] [Google Scholar]
- 6.van Pinxteren LA, Cassidy JP, Smedegaard BH, Agger EM, Andersen P. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur J Immunol. 2000;30(12):3689–3698. doi: 10.1002/1521-4141(200012)30:12<3689::AID-IMMU3689>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Andersen P, Askgaard D, Ljungqvist L, Bentzon MW, Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun. 1991;59(4):1558–1563. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agger EM, Andersen P. Tuberculosis subunit vaccine development: on the role of interferon-gamma. Vaccine. 2001;19(17–19):2298–2302. doi: 10.1016/s0264-410x(00)00519-3. [DOI] [PubMed] [Google Scholar]
- 9.Weinrich Olsen A, van Pinxteren LA, Meng Okkels L, Birk Rasmussen P, Andersen P. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85b and esat-6. Infect Immun. 2001;69(5):2773–2778. doi: 10.1128/IAI.69.5.2773-2778.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62(6):2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horwitz MA, Lee BW, Dillon BJ, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1995;92(5):1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Wen K, Shen J, et al. Characterization of immune responses following intranasal immunization with the Mycobacterium bovis CFP-10 protein expressed by attenuated Salmonella typhimurium. Scand J Immunol. 2010;72(4):277–283. doi: 10.1111/j.1365-3083.2010.02421.x. [DOI] [PubMed] [Google Scholar]
- 13.Christy AJ, Dharman K, Dhandapaani G, et al. Epitope based recombinant BCG vaccine elicits specific Th1 polarized immune responses in BALB/c mice. Vaccine. 2012;30(7):1364–1370. doi: 10.1016/j.vaccine.2011.12.059. [DOI] [PubMed] [Google Scholar]
- 14.Arend SM, Geluk A, van Meijgaarden KE, et al. Antigenic equivalence of human T-cell responses to Mycobacterium tuberculosis-specific RD1-encoded protein antigens ESAT-6 and culture filtrate protein 10 and to mixtures of synthetic peptides. Infect Immun. 2000;68(6):3314–3321. doi: 10.1128/iai.68.6.3314-3321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiao D, Li L, Guo J, et al. Mycobacterium tuberculosis culture filtrate protein 10-specific effector/memory CD4(+) and CD8(+) T cells in tubercular pleural fluid, with biased usage of T cell receptor Vbeta chains. Infect Immun. 2011;79(8):3358–3365. doi: 10.1128/IAI.00014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguilar JC, Rodriguez EG. Vaccine adjuvants revisited. Vaccine. 2007;25(19):3752–3762. doi: 10.1016/j.vaccine.2007.01.111. [DOI] [PubMed] [Google Scholar]
- 17.Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462(7272):449–460. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 18.Rice–Ficht AC, Arenas–Gamboa AM, Kahl–McDonagh MM, Ficht TA. Polymeric particles in vaccine delivery. Curr Opin Microbiol. 2010;13(1):106–112. doi: 10.1016/j.mib.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Grgacic EV, Anderson DA. Virus-like particles: passport to immune recognition. Methods. 2006;40(1):60–65. doi: 10.1016/j.ymeth.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10(11):787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 21.Fifis T, Gamvrellis A, Crimeen–Irwin B, et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004;173(5):3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 22.Bertholet S, Ireton GC, Kahn M, et al. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J Immunol. 2008;181(11):7948–7957. doi: 10.4049/jimmunol.181.11.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millington KA, Fortune SM, Low J, et al. Rv3615c is a highly immunodominant RD1 (Region of Difference 1)-dependent secreted antigen specific for Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A. 2011;108(14):5730–5735. doi: 10.1073/pnas.1015153108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu F, Wang J, Dou J, et al. Nanoparticle-based adjuvant for enhanced protective efficacy of DNA vaccine Ag85A-ESAT-6- IL-21 against Mycobacterium tuberculosis infection. Nanomedicine. 2012;8(8):1337–1344. doi: 10.1016/j.nano.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Griffiths G, Nystrom B, Sable SB, Khuller GK. Nanobead-based interventions for the treatment and prevention of tuberculosis. Nat Rev Microbiol. 2010;8(11):827–834. doi: 10.1038/nrmicro2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bivas–Benita M, Lin MY, Bal SM, et al. Pulmonary delivery of DNA encoding Mycobacterium tuberculosis latency antigen Rv1733c associated to PLGA–PEI nanoparticles enhances T cell responses in a DNA prime/protein boost vaccination regimen in mice. Vaccine. 2009;27(30):4010–4017. doi: 10.1016/j.vaccine.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 27.Wizemann H, von Brunn A. Purification of E. coli-expressed HIS-tagged hepatitis B core antigen by Ni2+-chelate affinity chromatography. J Virol Methods. 1999;77(2):189–197. doi: 10.1016/s0166-0934(98)00152-9. [DOI] [PubMed] [Google Scholar]
- 28.Vogel M, Diez M, Eisfeld J, Nassal M. In vitro assembly of mosaic hepatitis B virus capsid-like particles (CLPs): rescue into CLPs of assembly-deficient core protein fusions and FRET-suited CLPs. FEBS Lett. 2005;579(23):5211–5216. doi: 10.1016/j.febslet.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 29.Hubbard RD, Flory CM, Collins FM. Immunization of mice with mycobacterial culture filtrate proteins. Clin Exp Immunol. 1992;87(1):94–98. doi: 10.1111/j.1365-2249.1992.tb06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Y, Woodworth JS, Shin DS, Morris S, Behar SM. Vaccine-elicited 10-kilodalton culture filtrate protein-specific CD8+ T cells are sufficient to mediate protection against Mycobacterium tuberculosis infection. Infect Immun. 2008;76(5):2249–2255. doi: 10.1128/IAI.00024-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stukova MA, Sereinig S, Zabolotnyh NV, et al. Vaccine potential of influenza vectors expressing Mycobacterium tuberculosis ESAT-6 protein. Tuberculosis (Edinb) 2006;86(3–4):236–246. doi: 10.1016/j.tube.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Orme IM, Andersen P, Boom WH. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167(6):1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 33.Casadevall A. Antibody-mediated immunity against intracellular pathogens: two-dimensional thinking comes full circle. Infect Immun. 2003;71(8):4225–4228. doi: 10.1128/IAI.71.8.4225-4228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams A, Reljic R, Naylor I, et al. Passive protection with immunoglobulin A antibodies against tuberculous early infection of the lungs. Immunology. 2004;111(3):328–333. doi: 10.1111/j.1365-2567.2004.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finkelman FD, Holmes J, Katona IM, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 36.Natsuume–Sakai S, Motonishi K, Migita S. Quantitative estimations of five classes of immunoglobulin in inbred mouse strains. Immunology. 1977;32(6):861–866. [PMC free article] [PubMed] [Google Scholar]
- 37.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178(6):2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 39.Zhang M, Lin Y, Iyer DV, Gong J, Abrams JS, Barnes PF. T-cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect Immun. 1995;63(8):3231–3234. doi: 10.1128/iai.63.8.3231-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young S, O’Donnell M, Lockhart E, et al. Manipulation of immune responses to Mycobacterium bovis by vaccination with IL-2- and IL-18-secreting recombinant bacillus Calmette Guerin. Immunol Cell Biol. 2002;80(3):209–215. doi: 10.1046/j.1440-1711.2002.01078.x. [DOI] [PubMed] [Google Scholar]
- 41.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A. 1992;89(24):12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen CY, Huang D, Wang RC, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 2009;5(4):e1000392. doi: 10.1371/journal.ppat.1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bogdan C, Moll H, Solbach W, Rollinghoff M. Tumor necrosis factor-alpha in combination with interferon-gamma, but not with interleukin 4 activates murine macrophages for elimination of Leishmania major amastigotes. Eur J Immunol. 1990;20(5):1131–1135. doi: 10.1002/eji.1830200528. [DOI] [PubMed] [Google Scholar]
- 44.Bekker LG, Freeman S, Murray PJ, Ryffel B, Kaplan G. TNF-alpha controls intracellular mycobacterial growth by both inducible nitric oxide synthase-dependent and inducible nitric oxide synthase-independent pathways. J Immunol. 2001;166(11):6728–6734. doi: 10.4049/jimmunol.166.11.6728. [DOI] [PubMed] [Google Scholar]
- 45.Gupta RK. Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev. 1998;32(3):155–172. doi: 10.1016/s0169-409x(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 46.Sun HX, Qin F, Ye YP. Relationship between haemolytic and adjuvant activity and structure of protopanaxadiol-type saponins from the roots of Panax notoginseng. Vaccine. 2005;23(48–49):5533–5542. doi: 10.1016/j.vaccine.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 47.McMurray DN. Determinants of vaccine-induced resistance in animal models of pulmonary tuberculosis. Scand J Infect Dis. 2001;33(3):175–178. doi: 10.1080/00365540151060743. [DOI] [PubMed] [Google Scholar]
- 48.Petrovsky N. Novel human polysaccharide adjuvants with dual Th1 and Th2 potentiating activity. Vaccine. 2006;24(Suppl 2):S2-26–29. doi: 10.1016/j.vaccine.2005.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang X, Bao L, Deng Y. A novel recombinant Mycobacterium bovis bacillus Calmette–Guerin strain expressing human granulocyte macrophage colony-stimulating factor and Mycobacterium tuberculosis early secretory antigenic target 6 complex augments Th1 immunity. Acta Biochim Biophys Sin (Shanghai) 2011;43(7):511–518. doi: 10.1093/abbs/gmr045. [DOI] [PubMed] [Google Scholar]
- 50.Soltysik S, Wu JY, Recchia J, et al. Structure/function studies of QS-21 adjuvant: assessment of triterpene aldehyde and glucuronic acid roles in adjuvant function. Vaccine. 1995;13(15):1403–1410. doi: 10.1016/0264-410x(95)00077-e. [DOI] [PubMed] [Google Scholar]
- 51.Wangoo A, Sparer T, Brown IN, et al. Contribution of Th1 and Th2 cells to protection and pathology in experimental models of granulomatous lung disease. J Immunol. 2001;166(5):3432–3439. doi: 10.4049/jimmunol.166.5.3432. [DOI] [PubMed] [Google Scholar]
- 52.Yin Y, Li H, Wu S, et al. Hepatitis B virus core particles displaying Mycobacterium tuberculosis antigen ESAT-6 enhance ESAT-6-specific immune responses. Vaccine. 2011;29(34):5645–5651. doi: 10.1016/j.vaccine.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 53.Pumpens P, Grens E. Hepatitis B core particles as a universal display model: a structure-function basis for development. FEBS Lett. 1999;442(1):1–6. doi: 10.1016/s0014-5793(98)01599-3. [DOI] [PubMed] [Google Scholar]
- 54.Ruedl C, Schwarz K, Jegerlehner A, Storni T, Manolova V, Bachmann MF. Virus-like particles as carriers for T-cell epitopes: limited inhibition of T-cell priming by carrier-specific antibodies. J Virol. 2005;79(2):717–724. doi: 10.1128/JVI.79.2.717-724.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kratz PA, Bottcher B, Nassal M. Native display of complete foreign protein domains on the surface of hepatitis B virus capsids. Proc Natl Acad Sci U S A. 1999;96(5):1915–1920. doi: 10.1073/pnas.96.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Newman M, Suk FM, Cajimat M, Chua PK, Shih C. Stability and morphology comparisons of self-assembled virus-like particles from wild-type and mutant human hepatitis B virus capsid proteins. J Virol. 2003;77(24):12950–12960. doi: 10.1128/JVI.77.24.12950-12960.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruedl C, Storni T, Lechner F, Bachi T, Bachmann MF. Cross-presentation of virus-like particles by skin-derived CD8(-) dendritic cells: a dispensable role for TAP. Eur J Immunol. 2002;32(3):818–825. doi: 10.1002/1521-4141(200203)32:3<818::AID-IMMU818>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 58.Jegerlehner A, Tissot A, Lechner F, et al. A molecular assembly system that renders antigens of choice highly repetitive for induction of protective B cell responses. Vaccine. 2002;20(25–26):3104–3112. doi: 10.1016/s0264-410x(02)00266-9. [DOI] [PubMed] [Google Scholar]