Abstract
Background
Studies in both human and mouse indicate that mediators released by mast cells can lead to bronchoconstriction, and thus these are important effector cells in life threatening anaphylaxis. Much of our understanding of the various functions of mast cells emanates from the study of mice lacking theses cells, particularly mice carrying mutations in the tyrosine kinase gene Kit. Definitive evidence for the role of mast cells in the altered immune response requires the demonstration that this response can be normalized by reconstitution of the mice with cultured bone marrow-derived mast cells (BMMCs). While many mast cell niches can be restored with BMMCs, this has not been demonstrated for mast cells present in the airways of the lung, cells poised to mediate bronchoconstriction during allergic responses.
Objective
To determine if mast cell deficient KitWsh/Wsh reconstituted lines are an appropriate model for the study of the role of these cells in bronchoconstriction associated with allergic responses.
Methods
KitWsh/Wsh mice were reconstituted with either whole bone marrow (WBM) or BMMCs and responses to IgE-mediated mast cell activation determined; including systemic hypothermia, mediator release, and bronchoconstriction in anesthetized, mechanically ventilated animals.
Results
Engraftment of KitWsh/Wsh mice with WBM and BMMCs results in reconstitution of the central airways with mast cells. While the treatment of the two groups of animals resulted in systemic changes when challenged with IgE/Ag in a model of passive anaphylaxis, bronchoconstriction was observed only in KitWsh/Wsh animal which had received a bone marrow transplant.
Conclusions
While BMMCs can populate the lung, they cannot restore IgE/Ag-mediated bronchoconstriction to mast cell-deficient animals. This suggests that the mast cell population which mediates this function may be unique, and to fill this niche in the lung cells must undergo a specific developmental program, one that is no longer available to cultured mast cells.
Keywords: Mast cell, lung, bronchoconstriction, reconstitution
Introduction
Mast cells arise as precursors in the bone marrow. This precursor cell is released into the blood stream and then migrates to various tissues where it undergoes its final differentiation, a process which is believed to be influenced by the local environment. At all stages of maturation mast cells express the receptor tyrosine kinase (CD117, c-KIT) and require the Kit ligand, stem cell factor (SCF), for survival. In the mouse, c-KIT and SCF are encoded at the white spotting (W) and steel (Sl) loci, respectively. Mutations in the W and/or Sl loci results in deficiencies in the production of melanocytes, germ cells, and hematopoietic cells (reviewed in [1]). While several mutations at this locus have been described, the most common mutations used for studying mast cells in mice are Sl/Sld, W/Wv, and Wsh/Wsh. The KitW/Wv (Wv) mouse, is a compound heterozygous animal, with the mutation deleting segments of the Kit coding region. In contrast, analysis of Kit in KitWsh/Wsh (Wsh) mice revealed no alteration in either the sequence or organization of the gene, but rather an inversion in the regulatory region [2, 3]. While all these mutations lead to profound mast cell deficiencies, as well as absence of coat pigment, Sl/Sld and W/Wv mutant mice are also anemic and sterile, phenotypes which are not seen in Wsh/Wsh mice. Therefore, the Wsh mouse has increasingly been used for study of mast cell function: the fertility of these mice simplifies the generation of these animals and their intercross with lines carrying other mutations.
Due to the lack of mast cells in the Wsh and Wv mice, alterations in the response of these lines to various pathogens and in models of autoimmune disease has been broadly used to support a role for the mast cells in these immune responses [4–7]. However, because the phenotype of lines lacking mast cells is not limited to this cell type, confirmation of mast cell function is dependant on the demonstration that the deficit in these mice can be corrected by restoration of the mast cell population. This can be done in one of two ways. The mice can be reconstituted with whole bone marrow (WBM) isolated from a wild type congenic animal. Alternatively, mast cell cultures can be established from bone marrow of wild type animals. Once the purity of these cultures is verified, these cells can be introduced into the Wsh or Wv mouse. The primary advantage of this later approach is that only the mast cell compartment is of donor origin: when total bone marrow is used, other hematopoetic compartments are also restored. The use of Wsh and Wv mice reconstituted with BMMCs has become increasingly common with the availability of mast cell cultures derived from mice carrying mutations generated by homologous recombination. This has allowed the identification of the role of specific pathways within mast cells, as well as assignment of mast cell mediators to specific pathophysiological changes during the immune response.
Mucosal type mast cells in the lung are intimately involved in allergic immune responses and are known to contribute to airway reactivity and hyperresponsiveness in models of anaphylaxis and asthma. In normal mice, mast cells are located throughout the main airways including within the trachea and bronchus, with few mast cells being found within the parenchyma [8]. Histological analysis has demonstrated the presence of mast cells in the lung of Wsh mice reconstituted with both WBM and BMMCs [9–11]. In the latter case, however, there has been disagreement on the extent to which the BMMCs can reconstitute various regions of the lung. Although it is generally accepted that BMMCs cannot reconstitute the trachea, histological studies by Wolters and colleagues [11] showed mast cells close to or within the smooth muscle layer of the airways of reconstituted Wsh mice. Conversely, studies by Grimbaldeston et al. [10] only noted reconstitution of mast cells in the lung parenchyma of Wsh mice engrafted with BMMCs.
Immunological studies also indicate that BMMC reconstitution can restore the immune response of Wsh mice, including their response in ovalbumin-induced models of asthma [12]. Some studies have also evaluated airway hyperresponsiveness (AHR) in the reconstituted mice [12–14]. In these instances the response of the mice to inhaled methacholine, a stable derivative of Ach which acts directly on the smooth muscle wall to elicit airway constriction, was evaluated and shown to be restored in the reconstituted animals. However, it is not unlikely that restoration of AHR in the Wsh mice that received BMMCs was the indirect consequence of normalization of the immune response. The possibility remains that, while some mast cell functions are restored in the Wsh mice, the mechanism(s) by which these cells contribute to pathophysiology during inflammation may not completely mimic that of a normal animal.
Recently we have shown that mast cells interacted with the parasympathetic pathways in the lung to mediate bronchoconstriction during anaphylaxis: the response was absent in Wsh mice but also absent in mice in which the parasympathetic pathways are inhibited [15]. This suggests that interactions between mast cells and neurons regulate airway caliber during inflammatory responses and raises the possibility that a unique subpopulation of mast cells may be required for this function. To begin to address this question we evaluated the ability of culture bone marrow derived mast cells to restore this response in the Wsh mice.
Methods
Experimental animals
Mast cell deficient C57BL/6 KitW-sh/W-sh mice and their wild-type C57BL/6J controls were purchased from Jackson Laboratory. All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) guidelines of the University of North Carolina at Chapel Hill.
Reconstitution of mast cell-deficient mice
Whole bone marrow
Bone marrow cells were collected from femurs of wild type C57BL/6J or C57BL/6 KitW-sh/W-sh mice. Whole bone marrow (WBM) cells were centrifuged at 1200 rpm for 5 min and resuspended in PBS (200µl per animal of bone marrow). Recipient mice were irradiated before WBM injection with a split dose of 10 Gy (two 5-Gy doses 3 h apart). WBM was injected i.v. via the tail vein into 4–8 weeks old C57BL/6 KitW-sh/W-sh recipient mice. Animals were analyzed after 12 weeks. In order to distinguish the effect of mast cells in bone marrow reconstituted animals, we compared C57BL/6 KitW-sh/W-sh animals reconstituted with WBM from C57BL/6J mice with C57BL/6J and C57BL/6 KitW-sh/W-sh animals reconstituted with WBM from C57BL/6 KitW-sh/W-sh mice.
BMMCs
Bone marrow was harvested from C57BL/6J mice and cultured in the presence of 5 ng/ml recombinant IL-3 and 10 ng/ml recombinant stem cell factor (SCF). Cells were cultured for 5 weeks, when cell populations consisted of >95% mast cells as assessed by expression of c-Kit and FcεRI on cell surface by fluorescence activated cell sort analysis (FACS). Ten million BMMC in 200 µl of PBS were injected i.v. via the tail vein into 3–9 week old C57BL/6 KitW-sh/W-sh mice. Mice were analyzed 12–16 weeks after injection.
Anaphylaxis protocols
Passive systemic anaphylaxis (PSA)
Mice were sensitized with 20 µg mouse monoclonal anti-DNP IgE (1.0 mg/ml, Sigma Chemical; 200 µl total volume in PBS) or an equal volume of PBS i.v. through the tail vein. Approximately 19–24 hours after anti-DNP IgE injection, mice were injected with 250 µl of 5 mg/ml DNP and rectal temperature monitored every 10 min for 40 min.
IgE-dependent passive anaphylaxis
Mice were sensitized with 20 µg mouse monoclonal anti-DNP IgE or an equal volume PBS i.v. through the tail vein, as described above. Approximately 19–24 hours after anti-DNP IgE injection, mice were anesthetized, catheterized, tracheostomized and mechanically ventilated as previously described [16]. Immediately following the baseline measurements, 250 µl of 5 mg/ml DNP (or an equal volume of PBS) was injected into the jugular vein. After administration, airway mechanics were determined using the Forced Oscillatory Technique (FOT) every 10 s for 3 min. Bar graphs represent the area under the curve.
Following removal from the ventilator, 0.5–1.0 ml of blood collected by cardiac puncture with a syringe containing 20 µl 100 U/ml heparin. The blood was then centrifuged at 12000 rpm for 5 min to collect plasma. Histamine levels were measured in the plasma by ELISA (Beckman Coulter). Bronchoalveolar lavage was performed five times with 1.0 ml of sterile HBSS each time. The recovered fluid (BALF) was centrifuged to remove cells. Histamine levels were measured in the BALF cell-free supernatant by ELISA (Beckman Coulter).
Histology
For histopathologic examination, the lungs were fixed by inflation (20 cm pressure) and immersion in 10% formalin. After paraffin embedding, 5-µm sections of the left lobe were cut longitudinally and stained with toluidine blue. To ensure that similar regions of the lung were used in the analysis of different groups of animals sections which included the entire length of the main bronchi were used for enumeration of mast cells. The entire lobe of the lung was scanned and the number of toluidine blue stained cells counted at 20× magnification. The area of the lung was determined using ImageJ software. Data were expressed according to number of mast cells per area (mm2) of the lung slice.
Receptor expression analysis of BMMCs
RNA was collected from 1 million BMMCs using RNAbee reagent (Qiagen) and converted to cDNA using a High Capacity cDNA RT kit (Applied Biosystems). FcεRI-α, FcεRI-β, and tryptophan hydroxylase (Tph1) expression was assessed by real-time PCR using commercially available probes (Applied Biosystems). Results were standardized to GAPDH expression.
Statistical analysis
Data are represented as means ± SEM. Analysis Of Variance (ANOVA) followed by Tukey-Kramer Honestly Significant Difference for multiple comparisons was performed on complex data sets. Statistical significance for single data points was assessed by Student’s two-tailed t-test. A P value of <0.05 was considered statistically significant.
Results
Engraftment with WBM and BMMCs can reconstitute peripheral responses to passive systemic anaphylaxis (PSA)
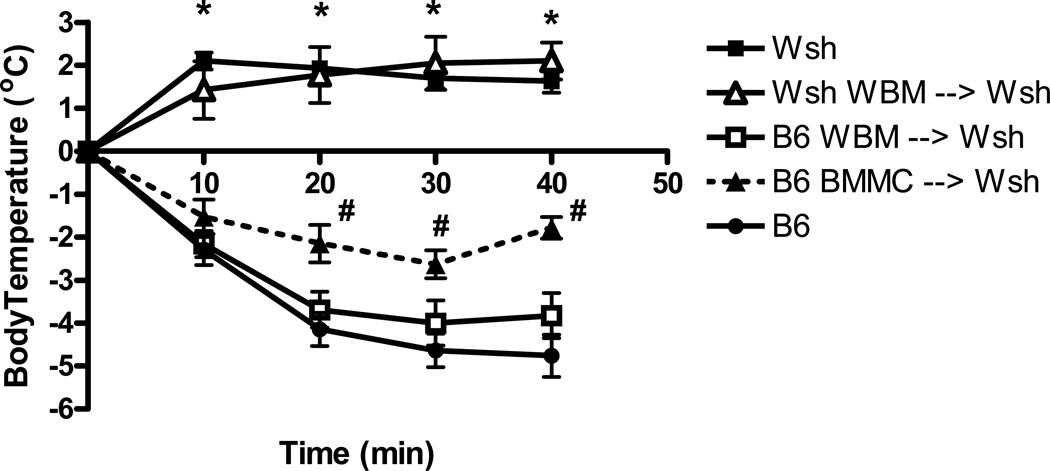
Cohorts of Wsh mice were reconstituted with either cultured isogenic C57BL/6J BMMCs or with WBM cells isolated from C57BL/6J mice. Mice receiving bone marrow transplantation were exposed to low doses of irradiation. In addition, Wsh mice were irradiated and reconstituted with Wsh bone marrow to control for possible changes in immune response related primarily to the experimental manipulations. Eight weeks after reconstitution we examined the response of the groups in a model of IgE-mediated anaphylaxis which previously has been shown to be mast cell dependant. All mice received i.v. monoclonal IgE and after 24 hours were exposed to antigen. This treatment results in systemic anaphylaxis, characterized by lethargy, hypotension, airway obstruction, and hypothermia [17, 18]. To quantitate these changes, we monitored the drop in body temperature associated with this response. No drop in temperature was observed in the Wsh mice or in the Wsh mice reconstituted with synergeneic marrow, consistent with the mast cell deficiency of these animals. In fact a small increase in temperature was observed in both groups, likely reflecting the stress/excitement associated with handling the animals during administration of antigen. Conversely, a rapid and sustained drop in body temperature was observed in Wsh mice reconstituted with whole bone marrow cells. This response was similar in magnitude and duration to that observed in wild type mice (Figure 1), indicating that these animals experienced systemic shock in response to antigen challenge. Mice reconstituted with cultured BMMCs displayed a significant drop in temperature compared to control animals; however, the hypothermia was not as pronounced as that observed in either wildtype B6 mice or WBM reconstituted Wsh animals. At three of the four time points examined the temperature of the Wsh mice reconstituted with the BMMC was significantly higher than that of the bone marrow chimeras.
Figure 1. Body temperature after induction of passive systemic anaphylaxis.
Mice were sensitized with anti-DNP IgE. Following antigen challenge, rectal temperature was recorded every 10 min for up to 40 min. Wild-type (B6, n=8) mice exhibited significant reduction in rectal temperature after antigen challenge, whereas the temperature of mast cell-deficient (Wsh, n=3) and reconstitution control (Wsh→Wsh, n=4) mice increased slightly. Reconstitution of Wsh mice with C57BL/6 WBM (WBM→Wsh, n=10) or BMMCs (BMMC→Wsh, n=7) resulted in temperature drop after antigen challenge. *P<0.001 compared to all other groups; #P<0.05 compared to B6.
WBM and BMMCs transfer results in reconstitution of mast cell populations in the central airways
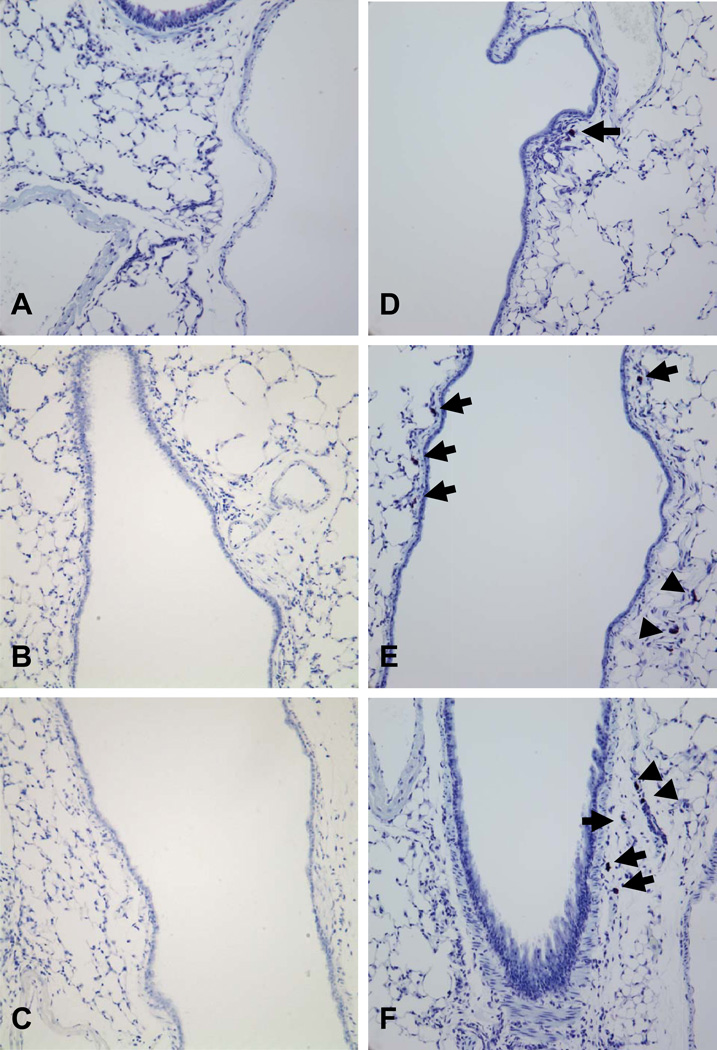
The systemic shock leading to drop in body temperature in response to IgE and antigen is mediated by the release of mast cell histamine in the blood stream [19], which is thought to act in the hypothalamus to trigger this response [20], and therefore would not be dependent on reconstitution of various mast cell populations in the lung. To address this, we therefore first carried out histological examination of the lungs, comparing the number and distribution of mast cells in wild type mice, mice reconstituted with BMMCs and mice reconstituted with WBM and their controls (Figure 2A-F). Consistent with previous studies of the Wv mast cell deficient line [9], no mast cells were detected in the Wsh mice, either along the main bronchi, the locations of the majority of the mast cells in the C57BL/6 mice, or in the parenchyma. Not surprisingly, this was also true of lungs from Wsh mice reconstituted with synergeneic bone marrow (Wsh→Wsh). In contrast, reconstitution of Wsh mice with B6 WBM or BMMCs resulted in a remarkable increase in numbers of mast cells in the lung. Similar to the B6 mice, mast cells could be detected along the central airways. However, mast cells were also observed in the lung parenchyma of these animals, a region in which few mast cells were observed in the C57BL/6 mice.
Figure 2. Airway mast cells.
Lungs of mast cell-deficient Wsh (A, n=10), Wsh WBM reconstituted Wsh (B, n=4), and Wsh WBM reconstituted B6 (C, n=5), B6 (D, n=10), B6 WBM reconstituted Wsh (E, n=8), BMMC reconstituted Wsh (F, n=8), were fixed in 10% formalin and stained with toluidine blue for mast cell identification and viewed at 20× magnification. Mast cells were undetected in Wsh and Wsh WBM reconstituted airways (A-C). Mast cells were visualized in close proximity to the central airway in B6 and the central airways (arrows) and parenchyma (arrow heads) in both WBM and BMMC reconstituted animals (D-F).
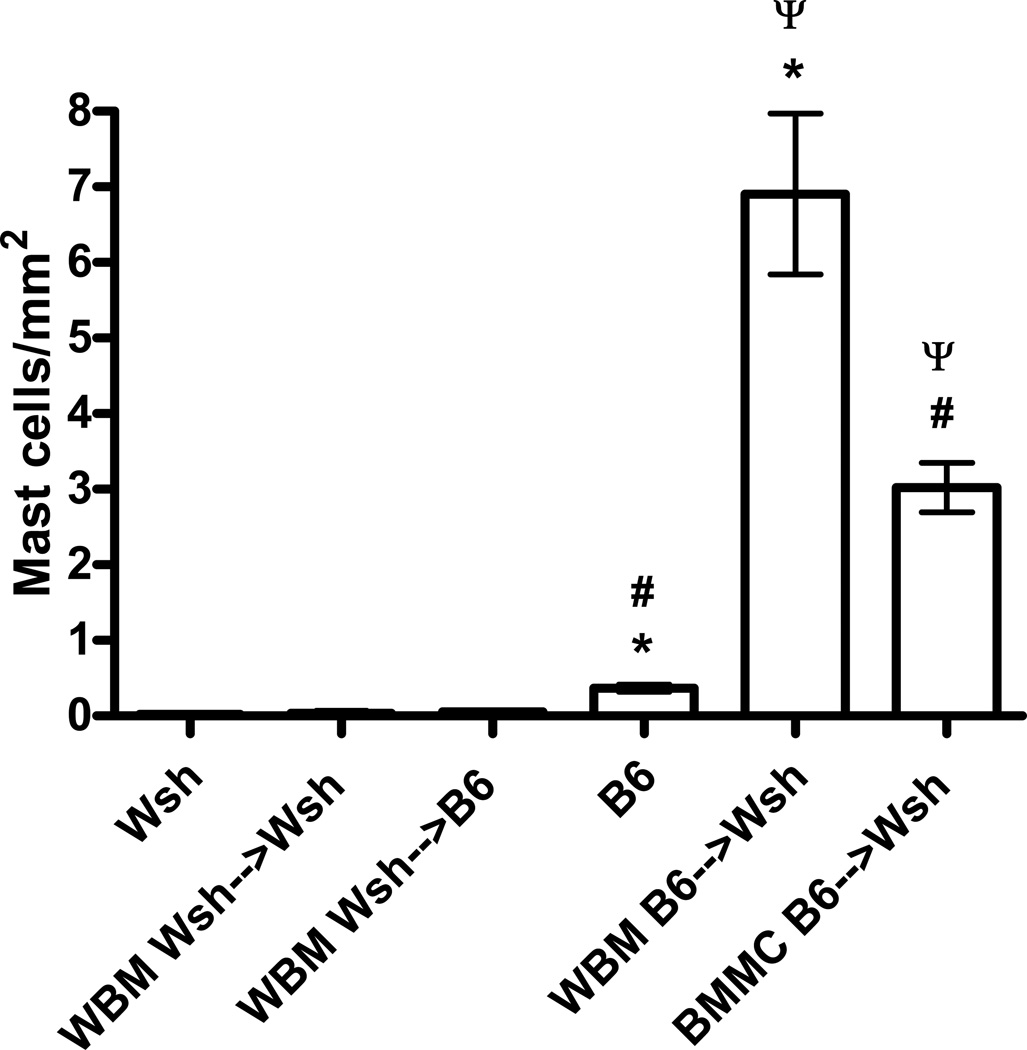
The number of mast cells in animals reconstituted with wild-type WBM or BMMCs was several fold higher than that seen in the wild-type animals (Figure 3). This increase in mast cell number was even more pronounced in the mice reconstituted with WBM, which had significantly more mast cells than mice reconstituted with BMMCs. No apparent difference was noted in the location of the mast cells surrounding the main airways of the wild type, BMMC, and WBM reconstituted animals.
Figure 3. Lung mast cells following reconstitution.
The number of mast cells per mm2 was quanitated by counting toluidine blue stained cells. Mast cells were undetected in Wsh (n=10), Wsh→Wsh (n=4), and Wsh→B6 (n=5) mice. Few mast cells were detected in wild-type B6 airways (n=10), while significantly higher numbers of mast cells were detected in both WBM (n=8) and BMMC (n=8) reconstituted animals. *P<0.001, #P<0.001, ψP<0.01.
Allergic bronchoconstriction is recapitulated in Wsh mice after reconstitution with WBM but not with BMMCs
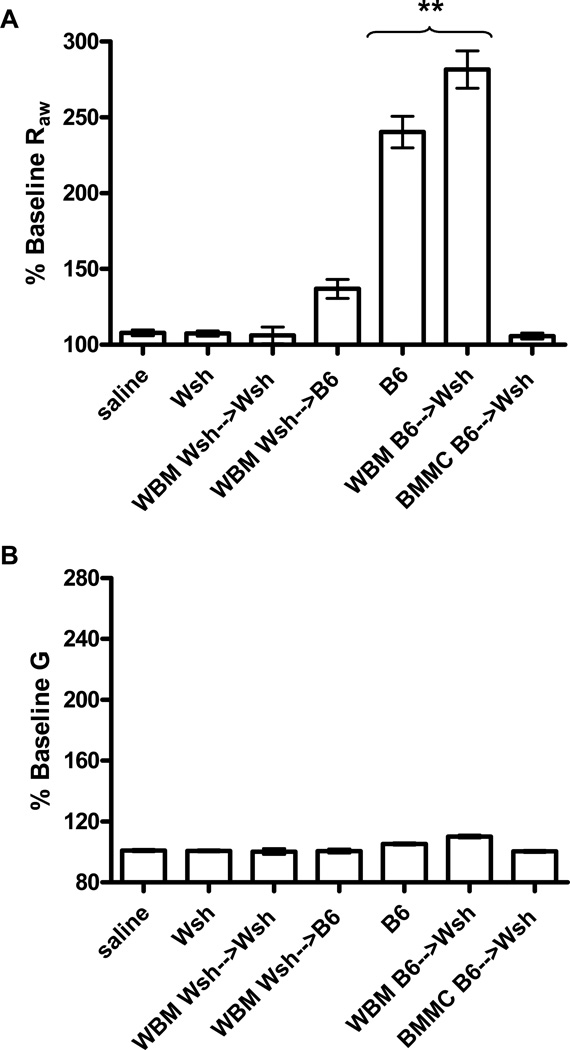
As both BMMC and WBM reconstitution of Wsh mice resulted in mast cells present in elevated numbers in both the airways and parenchyma, we next asked whether the reconstituted mice would respond to allergen and IgE with a comparable increase in the level of bronchoconstriction. To address this question we again treated mice with monoclonal IgE. After 24 hour mice were anesthetized, tracheotostimized and mechanically ventilated. Antigen was then delivered via a jugular catheter and the changes in airway resistance determined using the Forced Oscillation Technique to measure respiratory system input impedance and evaluate the Constant Phase Model. This technique allows for unique distinction between central and peripheral lung mechanics. As expected, treatment of the C57BL/6J mice that had received anti-DNP IgE showed a significant central airway constriction (Raw), with little to no change in peripheral airway mechanics (tissue damping, G) after challenge with DNP when compared with sensitized animals that received a PBS challenge (Figure 4A-B). This Raw response was absent in antigen challenged Wsh mice and reconstitution control mice (Wsh→Wsh and Wsh→B6): no bronchoconstriction was observed and their airway mechanics did not differ from the PBS-challenged controls.
Figure 4. Anaphylactic bronchoconstriction after mast cell reconstitution of Wsh mice.
For reconstitution, WBM was harvested from either wild-type B6 or mast cell-deficient Wsh mice and mast cells were cultured from B6 bone marrow. B6 and Wsh mice were evaluated for comparison. Mice were sensitized with anti-DNP IgE, anesthetized, paralyzed, and mechanically ventilated. Mice were then challenged with antigen (DNP) and airway mechanics measured. Airway mechanics are expresses as airway resistance (Raw, A) and tissue damping (G, B). Control animals (B6, n=5) were challenged with PBS. Significant airway resistance was seen in B6 mice (n=12) and in Wsh animals reconstituted with B6 WBM (B6→Wsh, n=8) after antigen challenge. There was no response to antigen in the BMMC-reconstituted animals (BMMC→Wsh; n=20). The response of the Wsh and Wsh animals reconstituted with Wsh WBM (Wsh→Wsh, n=4) and those of B6 mice reconstituted with Wsh WBM (Wsh→B6, n=5) did not differ from the response of control animals treated with saline. **P<0.001 compared to all other groups.
As expected, an increase in airway resistance following antigen challenge was observed in the Wsh mice in which the mast cell population had been reconstituted by a bone marrow transplant (Figure 4A). Furthermore, the increase in airway resistance in these reconstituted animals did not differ significantly from the wild type B6 animals. Despite the increased number of mast cells in the parenchyma of these mice, airway constriction was still confined to the central airways; there was no change in the peripheral airway mechanics in these mice following antigen challenge (Figure 4B). To verify that the response observed in these animals was in fact due to reconstitution of mast cells and not the indirect consequences of the bone marrow transplant we examined cohorts of Wsh reconstituted with Wsh bone marrow and irradiated wild-type mice reconstituted with Wsh bone marrow. As expected, the airways of either wild-type or Wsh mice reconstituted with Wsh WBM did not constrict following antigen challenge.
We next evaluated the response of Wsh mice in which the mast cell population had been reconstituted with BMMCs. Again mice were treated with IgE, ventilated, and changes in airway resistance monitored during and after delivery of antigen. No changes in Raw (Figure 4A) or G (Figure 4B) were observed in these animals. This was surprising given the high number of mast cells in the lungs of these animals and the hypothermia, albeit attenuated, secondary to PSA observed on treatment of similar groups with IgE and antigen.
Release of mast cell mediators in Wsh reconstituted populations
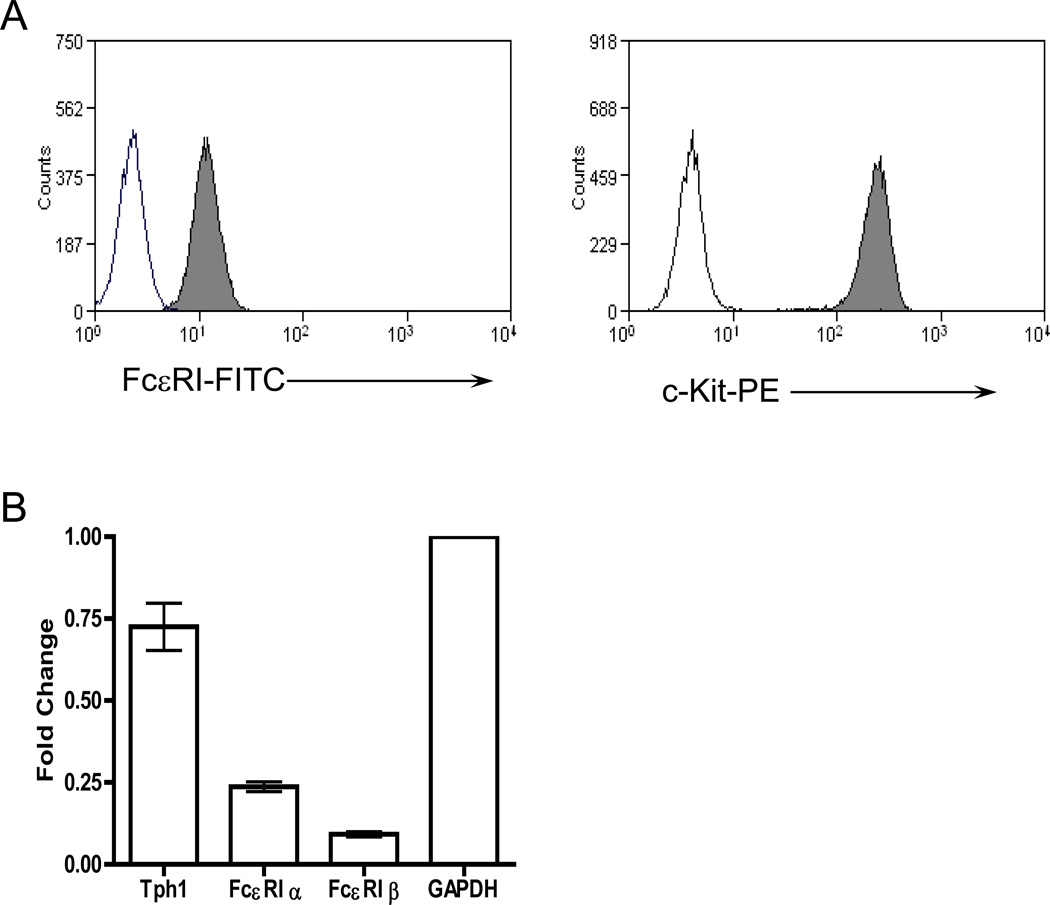
It is possible that lack of airway constriction in BMMC engrafted Wsh mice was due to improper receptor and/or mediator expression; therefore we characterized the BMMCs in vitro and analyzed their receptor expression and ability to degranulate. FACs analysis revealed that BMMCs expressed both c-kit and the FcεRI receptor (Figure 5A), two markers synonymous with mast cells. Real-time PCR analysis of BMMCs also indicated the presence of both α and β chains of FcεRI (Figure 5B), and IgE-dependent activation of BMMCs in vitro was confirmed by measuring hexosaminidase release after antigen challenge (data not shown). It has been shown that mast cell-derived serotonin is responsible for antigen-mediated central airway constriction [15]. Tryptophan hydroxylase, the enzyme required for serotonin synthesis, was also abundantly expressed in BMMCs (Figure 5B).
Figure 5. Characterization of BMMCs.
A) Cell surface expression of FcεRI and c-Kit was verified by fluorescence activated cell sort analysis (FACS). Mast cells were exposed to IgE for 2 h and stained with FITC-labeled anti-IgE antibody (left panel gray), PE-labeled anti-c-Kit antibody (right panel gray) or rat IgG1-FITC (left panel transparent) and rat IgG2b-PE (right panel transparent) as isotype-matched controls. B) Expression of the FcεRI receptor and tryptophan hydroxylase was verified using real-time RT-PCR from RNA collected from 1 million BMMCs (n=3). Relative expression was standardized using GAPDH as a control.
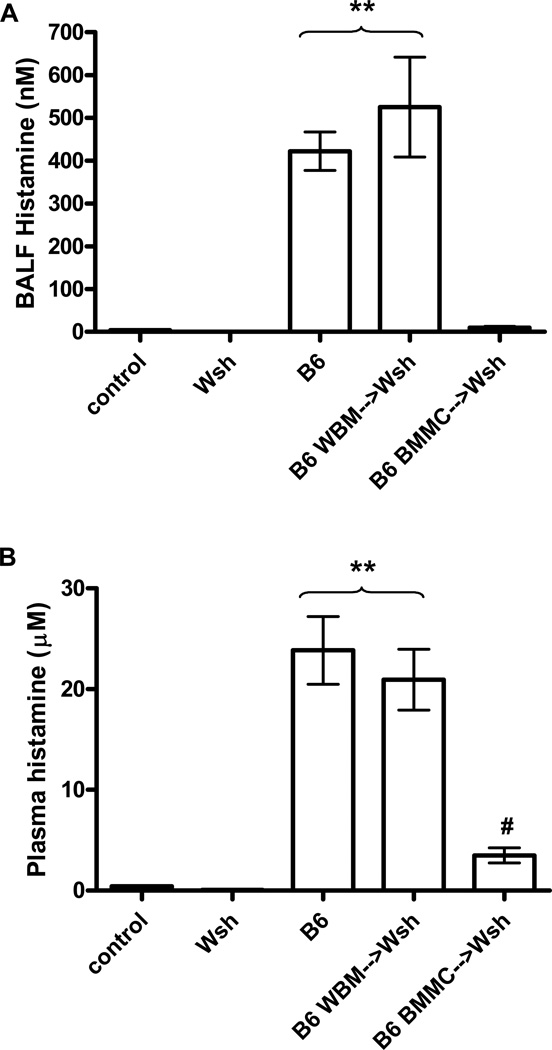
One possible explanation for the differences between the phenotype of the Wsh reconstituted by WBM versus BMMCs is that the BMMC-derived cells do not produce or release mast cell mediators necessary for bronchoconstriction in quantities similar to those released by mast cells differentiated from hematopoetic precursors. To begin to address this we used histamine as a surrogate marker for mast cell degranulation, comparing histamine release in both the bloodstream and lungs of WBM and BMMC reconstituted Wsh mice after sensitization and challenge with antigen. Antigen challenge of wild-type B6 mice resulted in elevated levels of histamine in both BALF and blood, while histamine was undetected in both PBS challenged controls and mast cell-deficient Wsh mice (Figure 6A-B). Following antigen challenge, histamine levels in both BALF and blood of WBM reconstituted Wsh mice were comparable to similarly challenged B6 mice (Figure 6A-B). While increased histamine levels were easily detected in the plasma from Wsh mice reconstituted with BMMCs, the levels were significantly lower than those observed in with wild type mice and Wsh mice reconstituted with bone marrow, consistent with the attenuated systemic shock observed in these animals. However, histamine could not be detected in the BALF from Wsh mice reconstituted with BMMCs, despite the high levels of mast cells present in the lung (Figure 6A-B). This was surprising given the fact that the mast cells appeared similar in morphology to those present in both the bone marrow reconstituted Wsh mice and those in wild type B6 mice (Figure 7A-C).
Figure 6. Histamine release following IgE-dependent passive anaphylaxis.
Mice sensitized with anti-DNP IgE. 24 hours following sensitization, mice were challenged with either DNP or PBS. Following challenge, animals were sacrificed and BALF and plasma was collected for analysis of histamine release. (A) Significant levels of histamine was measured in the BALF of wild-type (n=14) and WBM reconstituted Wsh (n=11) mice. Some histamine was detected in the BALF of BMMC reconstituted Wsh (n=20) mice, although the level was not significantly greater than that seen in control saline-treated animals (n=5) or Wsh animals (n=12). **P<0.001 compared to all other groups. (B) Large quantities of histamine were present in the plasma of wild-type mice (n=7) and Wsh animals reconstituted with WBM (n=8). Plasma histamine levels were significantly attenuated in BMMC reconstituted Wsh mice (n=10). Although some histamine was detected, levels were not significantly greater than Wsh (n=5) and PBS treated B6 (n=3) controls. **P<0.001 compared to all other groups, #P>0.05 compared to controls and Wsh.
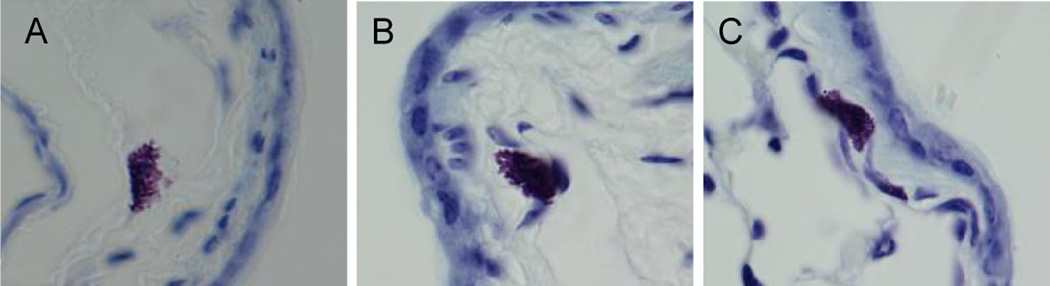
Figure 7. Mast cell granules.
Lungs of B6 (A, n=10), WBM reconstituted Wsh (B, n=8), and BMMC reconstituted Wsh (C, n=8) mice were fixed in 10% formalin and stained with toluidine blue for mast cell identification and viewed at 100× magnification under oil immersion. Histologically, quantity of granules in lung mast cells appears similar in all groups.
Discussion
Antigen mediated degranulation of mast cells in the airways can lead to life threatening bronchoconstriction, presumably through the actions of chemical mediators stored in mast cell granules on airway smooth muscle. Similarly in mice, an increase in airway resistance can be measured in response to treatment with IgE and antigen. The dependence of this response on mast cells is supported by the failure to observe bronchoconstriction in Wsh mice [15]. The lack of mast cells in Wsh mice and the ability to restore many mast cell functions by reconstitution of this mouse line with cultured bone marrow derived mast cells suggests that this strategy could also be used to investigate the mechanism(s) by which mast cells alter smooth muscle physiology in the lung and mediate bronchoconstriction in response to antigen challenge. However, we report here that, while mast cells are observed in Wsh mice reconstituted with BMMCs there are important qualitative differences between these populations and those in both wild type mice and mice reconstituted with hemaotopoetic stem cells from whole bone marrow. A critical mast cell function in the lung, the ability to alter smooth muscle tone, is not restored in these animals.
There are a number of possible reasons for the lack of response in reconstituted Wsh mice. Wsh mice carry a mutation in the regulatory region upstream of the ckit promoter which negatively effects the expression of ckit in a tissue-specific manner. This limits the development of mast cells. However, altered expression of ckit has additional consequences including cardiac hypertrophy, splenomegaly with expanded myeloid and megakaryocyte populations, and bone marrow abnormalities such as neutrophilia and thrombocytosis [21]. In addition, the Wsh mutation also results in alteration in the development of interstitial cells of cajal (ICC). ICCs in the gut serve as “pacemaker” cells that trigger gut contraction and have also been shown to modulate neurotransmission [22, 23]. Other smooth muscle containing tissues are also known to contain these cells, although their function in other tissues is still being investigated. Therefore it is possible that the lack of ability of BMMCs to restore function may reflect the fact that attenuated bronchoconstriction is not due to the loss of mast cell function, but rather other physiological consequences of altered ckit expression, such as alteration of the smooth muscle physiology or innervation of the airways. This is not supported by our demonstration that the response can be restored by treatment of mice with total bone marrow. However, we cannot formally rule out the possibility that a population of cells of hematopoietic origin, in addition to mast cells, is restored under these experimental conditions.
It is also possible that the cultured mast cells cannot efficiently reconstitute the lung or migrate to specific compartment of lung. However, similar to previous studies [10, 11], we report that resident mast cells are observed in Wsh mice reconstituted with either whole bone marrow or cultures of bone marrow-derived mast cells. In fact, in our study, the number of mast cells was dramatically increased in the reconstituted animals compared to wild type B6 mice. Because mast cell-mediated airway constriction occurs exclusively within the central airways of wild-type B6 mice [15], mast cell location after reconstitution may be of particular importance when studying this reaction. Mast cells in wild-type B6 mice are extremely rare in the lung parenchyma but are commonly found within the trachea and around the bronchi, specifically within close proximity to bronchial smooth muscle [8]. Wolters et al. [11] reported that 12 weeks after i.v. injection of 107 BMMCs, the lungs, but not trachea, of reconstituted Wsh mice contained mast cells, and histologically the majority of these mast cells were seen in close proximity of the large airways. Conversely, the study by Grimbaldeston et al. [10], while supporting the inability of the trachea to reconstitute mast cells, reported that in the reconstituted animals the majority of the mast cells were localized to the parenchyma. We observed higher total number of mast cells in the animals reconstituted with both WBM and BMMCs. Mast cells were observed in the submucosa of the conducting airways of the BMMC reconstituted mice in locations that appeared similar to those observed in both WBM reconstituted animals and wild type B6 animals. However, we cannot rule out the possibility that there are subtle but important differences, not apparent using the methods employed in this study, that distinguish the location of the mast cells in BMMC and WBM reconstituted animals. For example, it is possible that the location of the mast cells vis a vie the neurons present in the airways is not equivalent, and we have recently reported interaction between these cell types is essential for bronchoconstriction [15].
A second possibility is that limited differentiation pathways are available to cultured BMMCs, and these alter the spectrum of physiological activities in which these mast cells can participate. We did however verify that the BMMCs used for the reconstitution experiments expressed c-kit and the FcεRI receptor, as well as tryptophan hydroxylase for serotonin synthesis, which is the mediator required for antigen-mediated bronchoconstriction [15]. In addition, BMMCs are able to degranulate following IgE-mediated activation in vitro, as measured by hexosiminidase release (data not shown). The normal differentiation of the BMMC after reconstitution of Wsh mice is supported by our demonstration that these animals displayed other pathophysiological changes characteristic of passive anaphylaxis. Treatment of these animals with monoclonal IgE and antigen resulted in a rapid drop in body temperature, although the response was not as dramatic as that observed in wild type mice or bone marrow chimeras. This response is attributed to the action of mast cell histamine in the brain, specifically through binding to histamine receptors on the hypothalamus [20]. Consistent with the reduced PSA response in these animals, blood histamine levels following antigen challenge of sensitized mice were reduced compared to both WBM reconstituted and wild-type mice. However, the ability to observe a PSA response and to measure an increase in plasma histamine indicates the presence of some functional mast cells capable of responding to IgE in the BMMC reconstituted animals. In contrast, histamine could not be detected in the BALF of the BMMC reconstituted animals despite the high number of mast cells present in both the airways and parenchyma of these animals. This was interesting considering that histamine was easily detected in the B6 BALF regardless of the low numbers of mast cells present in the wild-type lung. The similar levels of histamine detected in the BALF of the bone marrow reconstituted animals and the wild type animals was also surprising given the large difference in the number of total mast cells in the lung of the former. This suggests a model in which not all mast cells in the lung are capable of releasing a given mediator, but rather a limited number of mast cells in a particular niche in the lung develop precise characteristics through specific interaction with surrounding cells. We cannot however rule out the possibility that additional cell types of bone marrow origin are present in abnormally high levels in the lungs of these mice and that these cells can respond to IgE+antigen. For example, basophils are known to express the FcεRI receptor and contain mediators such as histamine and serotonin in their granules similarly to mast cells.
Several studies have used Wsh mice to analyze mast cell function in allergic inflammation and AHR to methacholine [13, 14]. In many of these studies the attenuation of AHR in the Wsh mice was restored in mice constituted with wild type BMMCs. However, in these studies antigen-mediated airway constriction was not assessed. It is quite likely that the ability of the BMMCs to restore AHR to methacholine is the result of the production of inflammatory mediators by lung mast cells and the impact of these mediators and/or recruited inflammatory cells on smooth muscle physiology. This likely reflects a different and additional role for the mast cell in airway physiology, one that is independent from the ability of preformed mediators in mast cell granules to initiate rapid constriction of smooth muscle upon mast cell degranulation by IgE/antigen.
In summary, our study suggests that while functions of mast cells can be studied in vivo using the Wsh reconstitution model, this does not currently include the study of antigen/IgE mediated bronchoconstriction. A further study of this model, however, may lead to the identification of the mechanism(s) by which maturing mast cells or their precursor cells develop the ability to populate very specific niches in the lung and in other organs. It is interesting to speculate that differences in the propensity of the mast cell to populate these specific sites may profoundly alter the susceptibility of individuals to allergic disease and or the manifestations of various symptoms of these diseases once they are acquired.
Acknowledgements
The authors would like to thank Kim Burns for assistance with histology and staining and Dr. Bob Bagnell for help with data analysis.
This work was supported by NIH Grants NIH/NHLBI R01 HL068141 to B.H.K.
Footnotes
The authors have no conflicting financial interests.
References
- 1.Heib V, Becker M, Taube C, Stassen M. Advances in the understanding of mast cell function. Br J Haematol. 2008;142:683–694. doi: 10.1111/j.1365-2141.2008.07244.x. [DOI] [PubMed] [Google Scholar]
- 2.Duttlinger R, Manova K, Chu TY, Gyssler C, Zelenetz AD, Bachvarova RF, Besmer P. W-sash affects positive and negative elements controlling c-kit expression: ectopic c-kit expression at sites of kit-ligand expression affects melanogenesis. Development. 1993;118:705–717. doi: 10.1242/dev.118.3.705. [DOI] [PubMed] [Google Scholar]
- 3.Nagle DL, Kozak CA, Mano H, Chapman VM, Bucan M. Physical mapping of the Tec and Gabrb1 loci reveals that the Wsh mutation on mouse chromosome 5 is associated with an inversion. Hum Mol Genet. 1995;4:2073–2079. doi: 10.1093/hmg/4.11.2073. [DOI] [PubMed] [Google Scholar]
- 4.Lin L, Gerth AJ, Peng SL. Susceptibility of mast cell-deficient W/Wv mice to pristane-induced experimental lupus nephritis. Immunol Lett. 2004;91:93–97. doi: 10.1016/j.imlet.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Xu X, Zhang D, Lyubynska N, Wolters PJ, Killeen NP, Baluk P, McDonald DM, Hawgood S, Caughey GH. Mast cells protect mice from Mycoplasma pneumonia. Am J Respir Crit Care Med. 2006;173:219–225. doi: 10.1164/rccm.200507-1034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 7.Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000;191:813–822. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gersch C, Dewald O, Zoerlein M, Michael LH, Entman ML, Frangogiannis NG. Mast cells and macrophages in normal C57/BL/6 mice. Histochem Cell Biol. 2002;118:41–49. doi: 10.1007/s00418-002-0425-z. [DOI] [PubMed] [Google Scholar]
- 9.Du T, Friend DS, Austen KF, Katz HR. Tissue-dependent differences in the asynchronous appearance of mast cells in normal mice and in congenic mast cell-deficient mice after infusion of normal bone marrow cells. Clin Exp Immunol. 1996;103:316–321. doi: 10.1046/j.1365-2249.1996.d01-610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, Caughey GH. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient Kit(W-sh)/Kit(W-sh) sash mice. Clin Exp Allergy. 2005;35:82–88. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med. 2000;192:455–462. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hua X, Chason KD, Fredholm BB, Deshpande DA, Penn RB, Tilley SL. Adenosine induces airway hyperresponsiveness through activation of A3 receptors on mast cells. J Allergy Clin Immunol. 2008;122:107–113. 113, e101–e107. doi: 10.1016/j.jaci.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakae S, Ho LH, Yu M, Monteforte R, Iikura M, Suto H, Galli SJ. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice. J Allergy Clin Immunol. 2007;120:48–55. doi: 10.1016/j.jaci.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 15.Cyphert JM, Kovarova M, Allen IC, Hartney JM, Murphy DL, Wess J, Koller BH. Cooperation between mast cells and neurons is essential for antigen-mediated bronchoconstriction. J Immunol. 2009;182:7430–7439. doi: 10.4049/jimmunol.0900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen IC, Pace AJ, Jania LA, Ledford JG, Latour AM, Snouwaert JN, Bernier V, Stocco R, Therien AG, Koller BH. Expression and function of NPSR1/GPRA in the lung before and after induction of asthma-like disease. Am J Physiol Lung Cell Mol Physiol. 2006 doi: 10.1152/ajplung.00174.2006. [DOI] [PubMed] [Google Scholar]
- 17.Martin TR, Ando A, Takeishi T, Katona IM, Drazen JM, Galli SJ. Mast cells contribute to the changes in heart rate, but not hypotension or death, associated with active anaphylaxis in mice. J Immunol. 1993;151:367–376. [PubMed] [Google Scholar]
- 18.Ujike A, Ishikawa Y, Ono M, Yuasa T, Yoshino T, Fukumoto M, Ravetch JV, Takai T. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J Exp Med. 1999;189:1573–1579. doi: 10.1084/jem.189.10.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makabe-Kobayashi Y, Hori Y, Adachi T, Ishigaki-Suzuki S, Kikuchi Y, Kagaya Y, Shirato K, Nagy A, Ujike A, Takai T, Watanabe T, Ohtsu H. The control effect of histamine on body temperature and respiratory function in IgE-dependent systemic anaphylaxis. J Allergy Clin Immunol. 2002;110:298–303. doi: 10.1067/mai.2002.125977. [DOI] [PubMed] [Google Scholar]
- 20.Sakata T, Yoshimatsu H, Kurokawa M. Thermoregulation modulated by hypothalamic histamine in rats. Inflamm Res. 1997;46(Suppl 1):S35–S36. [PubMed] [Google Scholar]
- 21.Nigrovic PA, Gray DH, Jones T, Hallgren J, Kuo FC, Chaletzky B, Gurish M, Mathis D, Benoist C, Lee DM. Genetic inversion in mast cell-deficient (W(sh)) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol. 2008;173:1693–1701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- 23.Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]