Abstract
The giant alga Chara corallina generates action potentials (APs) in response to mechanical stimulation, injury, or direct electrical stimulation. Students examine the waveform characteristics of these APs using standard intracellular recording techniques. Intracellular recording is easier than with neurons because of the large size of the Chara cell. Students observe very negative resting potentials (up to −250 mV), large AP amplitudes with depolarizing peaks approaching 0 mV, AP durations of seconds, and refractory periods up to several minutes. Students calculate Nernst potentials for the ions distributed across the Chara cell membrane to hypothesize the ions responsible for the resting potential and for the depolarizing phase of the AP. These calculations suggest that K+ is responsible for the resting potential and that Ca2+ influx and Ca2+-activated Cl− efflux are responsible for depolarizing phases of the AP, which they are. Comparison of the Chara AP characteristics with animal neuron and muscle APs reinforces understanding of mechanisms of excitability in animals, demonstrates that multiple solutions exist for action potential generation, and leads to discussion of the evolution of ion channels and excitability.
Keywords: action potential, Chara, excitability, evolution
Our present understanding of membrane excitability is based primarily on studies of action potentials (APs) and their generation in neuron and muscle cells of animals. However, until the discovery of the squid giant axon as a model preparation in the 1930s, advances in the understanding of excitability came from plant preparations (Cole, 1968). These early studies played a pivotal role in the development of ideas for the mechanisms of cellular excitability (Wayne, 1994). In plants, as well as animals, an adaptive response to environmental conditions and disturbances can be mediated by changes in the flow of ions across cell membranes (Wayne, 1993; Buchanan et al., 2000). In some plants, as in animals, this takes the form of an AP. For example, the folding of a Mimosa leaf after being touched and the closing of a Venus flytrap when an insect stimulates a sensory hair are mediated by APs (Pickard, 1973; Williams, 1976; Simons, 1992).
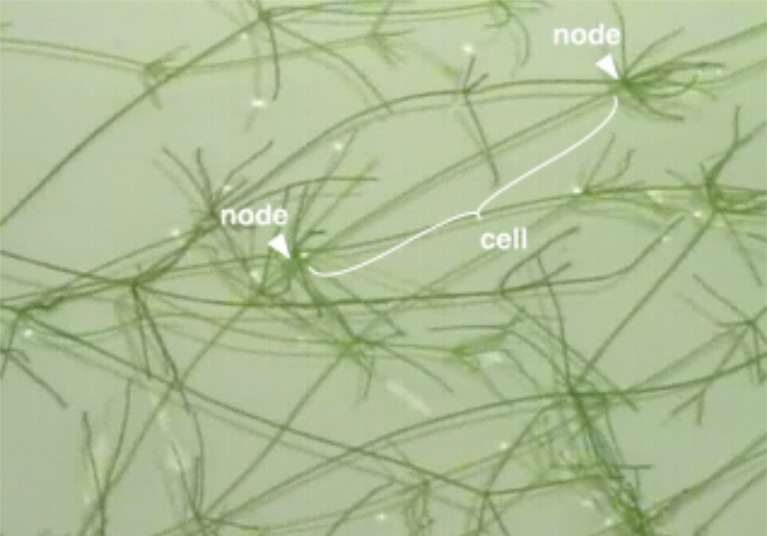
Chara corallina is a freshwater plant that inhabits temperate zone ponds and lakes. It consists of alternating nodes and internodes (Fig. 1). Each internodal segment is a single large cell, up to 10 cm in length. Because the cell is so large, it uses cytoplasmic streaming to distribute organelles and nutrients throughout the cytoplasm, which surrounds a large central vacuole (Fig. 2). This movement of intracellular materials is accomplished by a motility system based on actin and myosin (Kikuyama, 2001; Buchanan et al., 2000). This plant normally generates APs in response to deformation of the cell membrane (Shimmen, 2002). The AP spreads a signal that stops cytoplasmic streaming throughout the entire cell. Internal Ca2+ increases with the AP, activating a protein kinase. The kinase phosphorylates myosin to inhibit its interaction with actin, and thus terminates streaming (Wayne, 1993). This allows wound healing following a disruption of the cell wall and cell membrane, perhaps from an insect bite, without leakage of the pressurized cytoplasmic contents.
Figure 1.
Strands of Chara corallina. Each strand consists of large cells connected at nodes. The marked cell is approximately 7 cm long.
Figure 2.
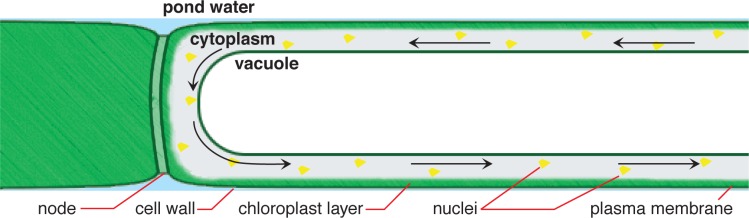
Cutaway diagram of a Chara cell. Organelles, mainly nuclei, circulate around the vacuole by cytoplasmic streaming. The vacuole is actually larger, and the cytoplasm and chloroplast layer much thinner than shown.
In this laboratory exercise, students examine APs in Chara corallina. Because the Chara cells are so large, recording action potentials with intracellular micro-electrodes is technically much easier than recording them from animal neurons or muscle fibers. Students determine that the Chara AP is an all-or-none response like the animal AP, and they quantify such descriptive features of APs as the amplitude, duration, and relative and absolute refractory periods. They will note that the plant AP is very different from animal APs. Chara APs are initiated from very hyperpolarized membrane potentials and are several seconds long. By calculating the Nernst potentials for the concentrations of K+, Na+, Ca2+ and Cl− across the cytoplasm-external interface (Table 1), students determine the ions most likely to be responsible for the resting and action potentials. In our experience, bringing attention to the occurrence of APs in plants and to the differences in AP characteristics between animals and plants promotes understanding of major concepts in cellular and molecular neurobiology. For example, by comparing the different mechanisms of depolarization in the plant and animal APs, students better understand the details of animal APs. In addition, this exercise leads to discussions of the evolution of nervous system excitability and to the realization that the different types of ion channels that contribute to this excitability have an ancient evolutionary origin (Hille, 2001).
Table 1.
Ion concentrations (mM). These concentrations give rise to the following Nernst potentials (mV) between cytoplasm and extracellular fluid: EK = −177, ENa = −99, ECa = +58, ECl = +100.
| [K+] | [Na+] | [Ca2+] | [Cl−] | |
|---|---|---|---|---|
| Pond water | 0.1 | 0.1 | 0.1 | 0.4 |
| Cytoplasm | 110.0 | 5.0 | 0.001 | 22.0 |
| Vacuole | 34.0 | 103.0 | 12.0 | 162.0 |
MATERIALS AND METHODS
Preparation
Chara can be obtained from biological supply houses. It is kept short-term near a window in an aquarium filled with artificial pond water (APW, 0.1 mM each NaCl, KCl and CaCl2). Nitella can also be used, but is more fragile. Chara is only available from many supply houses from May through October, while Nitella is available year round. You can grow your own Chara and Nitella by planting the algae in the bottom of a 10-gallon aquarium in 5 cm of autoclaved yard soil, covered with distilled or deionized water.
We recommend using bright green, relatively translucent cells. Older cells have thicker cell walls (4.0 μm, vs. 0.8 μm in young cells) and more starch grains, which makes them less translucent. The older cells are harder to penetrate and require blunter electrodes. Younger cells allow use of sharper electrodes (higher resistance), which will damage the cells less and lead to more satisfying results with fewer preparations. In addition, it is easier to see cytoplasmic streaming under a dissecting microscope in the younger cells. Rapid cytoplasmic streaming is a good indication of a healthy cell. Students can determine the streaming velocity using a calibrated ocular micrometer and a stopwatch to measure the time a particle moves a certain distance. Alternatively, a stopwatch and a ruler under the microscope will work; some dissecting microscopes label the field of view on the ocular (a label of 20 means that the field of view is 2 mm with a 10× objective, 1 mm at 20×, and so on).
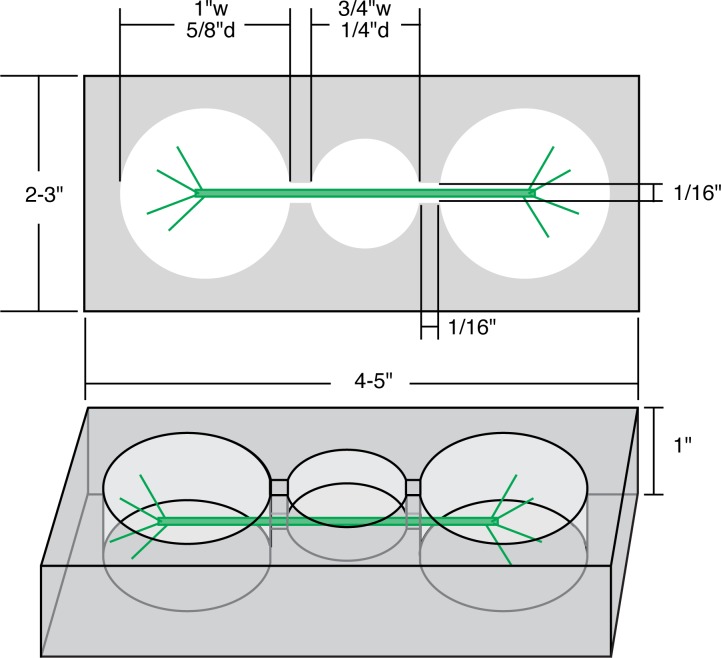
Our design for a Plexiglas recording chamber is shown in Figure 3. The chamber dimensions are not critical. We add metal screws tapped into the floor of the outer wells as poles for connecting the positive and negative leads from a stimulus isolation unit, and a Sylgard layer on the floor of the shallower, center recording well. A cell that fits into the recording chamber is trimmed, dried gently, and placed in the chamber, with care taken not to bend the cell. If a single cell is too short for the chamber, a string of cells can be placed in the chamber and the one in the middle recorded from (the cells are electrically connected, allowing the stimulus to pass between them). The cell is placed flat on the bottom of the recording well so that it does not roll when penetrated with the electrode. The three wells of the chamber are then electrically isolated with Vaseline from a syringe and, finally, filled with APW. A ground electrode consisting of a chloride-plated silver wire or a commercial sliver-chloride pellet is placed in the center well and secured with a thumb screw or dental wax. One can also use a 1.5 V AAA battery to pass current between the outer wells and stimulate APs and the cessation of cytoplasmic streaming (Reiss, 1994). A less elaborate preparation chamber can be made in a Petri dish with separate chambers created using silicone grease or Vaseline (Reiss, 1994). We found that it was harder to control leakage between the chambers with this method, which prompted us to design our Plexiglas chamber.
Figure 3.
Recording chamber. The two outer wells are for the stimulating wires; the shallower center well is layered with Sylgard and supports the cell for recording. The narrow slits connecting the compartments are filled with Vaseline after the cell is positioned. This prevents the stimulus current from traveling to ground through the saline. The dimensions are only suggested, and not critical.
Action Potential Recording
Standard intracellular recording and stimulation techniques are used to record the Chara resting potential and AP. Action potentials are initiated by stimulating across the metal screws in the two outer chamber wells (Figure 3). Intracellular recording from the Chara cell is accomplished much like recording from neurons and muscles except that the procedure for penetrating the cell wall is less delicate and electrodes are of lower resistance (1–10 MΩ, filled with 1.5 M KCl) and should have short shanks (Video 1). A gentle tap on the electrode manipulator is sometimes necessary to penetrate the cell. A thin band of Vaseline under the cell helps prevent the electrode from merely rolling the cell over. If a hole is torn in the cell wall and particles come streaming out, the students should start over with a new cell. A good electrode penetration will not stop cytoplasmic streaming (Video 1). If streaming does stop after penetration, allow the cell to resume streaming before continuing; this takes about four minutes. To see cytoplasmic streaming, we adjust the lighting from below and view under the highest magnification of the dissecting microscope. Slightly offsetting the magnification turret on the microscope gives a pseudo-relief image, which may make the streaming nuclei more visible.
Video 1.
Recording and cytoplasmic streaming. This is one frame of a video showing the recording procedure and results. The electrode comes from the top; the arrow indicates a nucleus. See http://crawdad.cornell.edu/chara.video.html for the full video.
A healthy cell will have a resting potential around −200 mV, but we find that resting potentials of around −100 mV, due to membrane damage caused by the electrode, will still allow AP generation. A 5 V, 100 to 200 ms duration stimulus pulse is used to initiate APs at first. If AP generation fails with this stimulus, small, incremental increases in stimulus amplitude should eventually elicit one. The voltage required to stimulate an AP can widely between student groups, depending mainly on the quality of electrical isolation between the wells of the recording chamber. Once the cell fires an AP, decrease the stimulus duration to 10 ms and increase the voltage. The shorter pulse reduces the stimulus artifact, making it possible to see the rise of the AP more clearly. Repeated stimuli should be at intervals of several minutes. The external solution should be changed occasionally to refresh the ionic gradients.
Students characterize the Chara AP by quantifying its amplitude and its duration at half-amplitude. They examine AP variability by comparing these values from 5 to 10 action potentials elicited at least five min apart. The all-or-none nature of the AP is demonstrated by increasing the stimulus voltage and noting changes in AP waveform. Relative and absolute refractory periods are determined by repeating suprathreshold stimuli at defined intervals and noting any changes in the waveform, especially amplitude. Finally, students calculate the Nernst potentials for K+, Na+, Ca2+, and Cl− across the cytoplasm-external interface (Table 1) to suggest which ions could be responsible for the resting potential and the depolarizing phase of the AP.
A complete listing of materials and sources for this exercise can be found at http://crawdad.cornell.edu. More detail of the procedures, with suggestions for further student exploration, are found in Wyttenbach et al. (1999).
RESULTS
Cytoplasmic Streaming
In a healthy Chara cell, visible particles, mostly nuclei, will move relatively quickly (100 μm/s, as compared to vertebrate skeletal muscle filaments which slide at 5 μm/s) along the length of the cell just below the focal plane of the cell wall. This can be seen in Video 1 before the stimulus.
Action Potential Generation
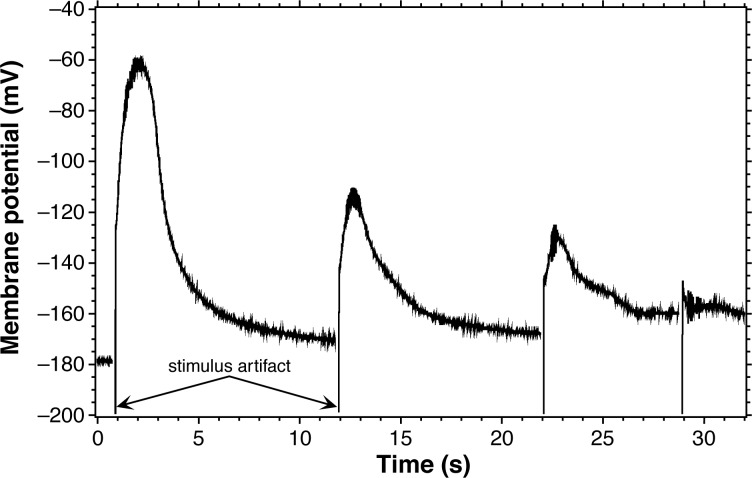
Video 1 shows recording from and AP generation in Chara, with cessation and recovery of cytoplasmic streaming. The AP rises from a resting potential of −170 mV, reaches its peak near 0 mV, and has a half-amplitude duration of about three seconds. Another example AP from a different cell is shown in Figure 4 (first AP). This AP had a lower amplitude (around 140 mV) and a shorter duration (around 2 s) than the one in Video 1. If ion concentrations remain constant inside the cell, the characteristics of APs repeated in the same cell should also remain fairly constant as long as sufficient time elapses between stimuli (see below).
Figure 4.
Repeated stimulation. Note the long relative refractory period between the first two APs. With the fourth stimulus, only a sub-threshold membrane response is seen.
Refractory Periods
Refractory periods of Chara APs are quite long compared to animal APs. In the example student data of Figure 4, the second AP was dramatically reduced in amplitude even with 11 seconds between stimuli. Students often find relative refractory periods up to 90 seconds and absolute refractory periods from 2 to 10 s. There can be a range of relative and absolute refractory periods between Chara cells, but they are always much longer than the refractory periods of animal cells.
Students may also find that the AP threshold stimulation voltage, waveform shape, and refractory periods between and within preparations vary over time. One source of variability in the AP waveform properties is the leakage of ions from the vacuole into the cytoplasm that will change the ion gradients. Since the vacuole occupies most of the cell space (Figure 2), the electrode is probably pushed through the cytoplasm and into the vacuole, facilitating compartment mixing. Ion concentrations in the cytoplasm, vacuole, and external solution can also change with repeated stimulation. This is why we suggest replacing the external solution periodically during the experiment.
DISCUSSION
Differences between Chara and animal APs are dramatic. They focus attention on the important characteristics of the AP as the mode of signal transmission in the nervous system. First, the resting potential from which the AP rises is very hyperpolarized in Chara cells compared to animal cells. Nernst potential calculations from the ion concentrations across the cytoplasm-external interface (Table 1) make it clear that K+ is mainly responsible for the resting potential, just as in animal cells. There is also an electrogenic, proton-pumping ATPase that may make some Chara RPs approach −250 mV (Wayne, 1994), but it can be ignored for this exercise.
Second, Nernst-potential calculations will also show that Ca2+ and Cl− have positive Nernst potentials (Table 1), and thus could both support the depolarizing phase of the Chara AP. This demonstrates that, in contrast to animal APs, which use the cations Na+ or Ca2+, a negative ion can leave the cell to create the depolarizing phase of an AP. In fact, both Ca2+ and Cl− contribute to the depolarizing phase of the Chara AP (Kikuyama, 2001). Details of the initiation and waveform of the Chara AP are complex: the AP actually consists of two membrane potential changes, the first occurring across the plasma membrane, and the second across the vacuolar membrane. An intracellular recording from the vacuole, where the electrode will most likely be placed, shows only one AP, but it usually has a fast and a slow depolarizing component. The initial fast component is due to influx of Ca2+ across the plasma membrane; the second, slower component is due to efflux of Cl− through Ca2+-activated Cl− channels, across the vacuolar and plasma membranes (Wayne, 1994). The large AP amplitude (up to several hundred mV in Chara compared to 100 mV in neurons) is due to the large driving force for the ions carrying the depolarizing current. The falling phase of the AP is due to an increase in K+ permeability (Kikuyama, 2001), again similar to animal cells. In a simplified summary, some external stimuli open mechano-sensitive ion channels, causing a depolarizing receptor potential. The Ca2+ ions carrying this depolarization spread into the cytoplasm to activate Ca2+-dependent Cl− channels in the plasma and vacuolar membranes to further depolarize the cell, and finally, K+ ions move across the membranes for the AP repolarization (Wayne, 1993).
The duration of the Chara AP and the long refractory periods will also draw attention from students used to observing animal APs. The long-duration (several seconds) Chara AP compares to the 2 to 10 ms (Na+-based) to up to hundreds of ms (Ca2+-based) APs seen in animal neurons and muscle cells like in the heart (Hammond, 2001; Hille, 2001). Faster kinetics of activation and inactivation of AP channels in animal cells probably account for this difference. Chara AP refractory periods are also much longer (seconds, rather than ms) than those found in animal cells. This could be due to slower channel kinetics for recovery from inactivation and/or slower mechanisms to reset the ionic gradients after an AP. Again this emphasizes the high speed of ion channel events required for signal transmission in animals compared to plants. This all translates into conduction velocities of 0.01–0.04 m/s for plant APs compared to 0.4 to 200 m/s for animal APs (Wayne, 1993).
Students may be frustrated by variability in the waveform of the Chara AP. This variability may be due to changes in ion concentrations in the external, cytoplasmic, and vacuolar compartments. The added complexity of the vacuole is one reason why electrophysiologists switched to the squid giant axon in the 1930s (Wayne, 1993). Variability is reduced when data are gathered quickly in a fresh preparation, when the external fluid is replaced periodically, and when young cells are recorded from with higher resistance electrodes. Note too, that some of the variability may be real rather than due to experimental technique. There may have been little evolutionary pressure for stereotypy of Chara APs, unlike animal APs. The timing of an animal AP is, of course, often critical to signal transmission. However, if the Chara AP is essentially a signal for a clotting mechanism (see Introduction and below), then precise AP waveform characteristics may be less critical.
We ask students to hypothesize the function of the Chara AP. They will see cytoplasmic streaming stop in less than 1 s after the AP and then slowly recover over about 250 s (Video 1; Staves and Wayne, 1993). The correlation between AP occurrence and streaming cessation should suggest a causal link between the two events. Students should understand that normally the AP is not elicited by electrical stimulation, but by some insult to the cell membrane. If the membrane’s integrity is compromised and the streaming continues, the circulating organelles would exit the cell. As mentioned in the Introduction, cessation of streaming by the AP spread may allow the cells time for damage control, for example, closing the wound. Action potentials recorded in higher plants like the Mimosa (Reiss, 1994) and the carnivorous plants play different roles. They are part of a signaling system that responds to mechanical stimulation by changing cell turgor, which leads to relatively rapid movements (Wayne, 1993; Shimmen, 2001).
This laboratory exercise leads naturally to discussions of ion channel evolution and the evolution of excitability. Excellent background for these discussions can be found in Hille (2001; see also Harris-Warrick, 2000). Ion channels are of ancient origin. For example, bacteria have stretch-sensitive ion channels (Brehm et al., 1991), and a gene necessary for a virus’s life cycle codes for a potassium channel (Plugge et al., 2000)! Ion channels were important innovations that appeared early in evolution, before the plant and animal kingdoms separated, and well before the advent of nervous systems (Hille, 2001). In all organisms, ion channels serve important cellular functions unrelated to excitability, and these basic functions laid the foundation for excitability. These functions include osmotic balance, controlling intracellular Ca2+ concentrations for cellular processes, and sensory-motor responses, whether for changing ciliary orientation in Paramecium, manipulating turgor in plants for movement, or for activating muscles groups in animals. Students should realize that most identified ion channel types are present in both plants and animals, and can even be part of the same gene families (Hille, 2001; Buchanan et al., 2000). Subtle gene mutations and gene duplications created the vast array of ion channels held in common by protozoans, plants, invertebrates, and vertebrates (Hille, 2001; Harris-Warrick, 2000).
Acknowledgments
We thank Lin Davidson for introducing us to the Chara preparation, and the reviewers for helpful comments. Development of this exercise was supported by National Science Foundation grant 955095, the Howard Hughes Medical Institute, and the Department of Neurobiology and Behavior at Cornell University.
REFERENCES
- Buchanan BB, Gruissem W, Jones RL. Biochemistry and Molecular Biology of Plants. Rockville, Maryland: American Society of Plant Physiologists; 2000. [Google Scholar]
- Brehm P, Okamura Y, Mandel G. Ion channel evolution. Sem Neurosci. 1991;3:355–367. [Google Scholar]
- Cole KC. Membranes, Ions, and Impulses: a Chapter of Classical Biophysics. Berkeley, CA: University of California Press; 1968. [Google Scholar]
- Hammond C. Cellular and Molecular Neurobiology. San Diego, CA: Academic Press; 2001. [Google Scholar]
- Harris-Warrick RM. Ion channels and receptors: molecular targets for behavioral evolution. J Comp Physiol A. 2000;186:605–616. doi: 10.1007/s003590000133. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates; 2001. Chapter 22. [Google Scholar]
- Kikuyama M. Role of Ca2+ in membrane excitation and cell motility in characean cells as a model system. Int Rev Cytol. 2001;201:85–114. doi: 10.1016/s0074-7696(01)01002-6. [DOI] [PubMed] [Google Scholar]
- Pickard BG. Action potentials in higher plants. Bot Rev. 1973;39:172–201. [Google Scholar]
- Plugge B, Gazzarrini S, Nelson M, Cerana R, Van Etten JL, Derst C, DiFrancesco D, Moroni A, Thiel G. A potassium channel protein encoded by chlorella virus PBCV-1. Science. 2000;287:1641–1644. doi: 10.1126/science.287.5458.1641. [DOI] [PubMed] [Google Scholar]
- Reiss C. Experiments in Plant Physiology. Saddle River, NJ: Prentice Hall, Inc; 1994. [Google Scholar]
- Shimmen T. Involvement of receptor potentials and action potentials in mechano-perception in plants. Aust J Plant Physiol. 2001;28:567–576. [Google Scholar]
- Simons PJ. The Action Plant. Oxford, UK: Blackwell; 1992. [Google Scholar]
- Staves M P, Wayne R. The touch-induced action potential in Chara: Inquiry into the ionic basis and the mechanoreceptor. Aust J Plant Physiol. 1993;20:471–488. [Google Scholar]
- Wayne R. Excitability in plant cells. Am Scientist. 1993;81:140–151. [Google Scholar]
- Wayne R. The excitability of plant cells: With a special emphasis on Characean internodal cells. Bot Rev. 1994;60:265–367. doi: 10.1007/BF02960261. [DOI] [PubMed] [Google Scholar]
- Williams SE. Comparative sensory physiology of the Droseraceae—the evolution of a plant sensory system. Proc Am Phil Soc. 1976;120:187–204. [Google Scholar]
- Wyttenbach RA, Johnson BR, Hoy RR. Crawdad: A CD-ROM Lab Manual for Neurophysiology. Sunderland, MA: Sinauer Associates; 1999. [Google Scholar]