Abstract
It is often difficult for instructors teaching laboratory courses in behavioral neuroscience to find appropriate experiments that can ethically examine biological parameters in human participants. In most instances, the default experiments that allow students to act as both experimenter and subject tend to be electrophysiological in nature (e.g., EEG, GSR, etc.). We report here the use of an experiment module that utilizes an easily-obtained enzyme immunoassay (EIA) kit to measure human salivary cortisol. Cortisol is a hormone of the adrenal cortex that can be used as a peripheral indicator of hypothalamic neural activity. Plasma (and salivary) cortisol levels rise due to circadian influences as well as perturbations in the organism’s environment (i.e., stressors). The involvement of the hypothalamic-pituitary-adrenal (HPA) axis in the pathophysiology of depression makes this an appealing module to students in behavioral neuroscience laboratories. Measurement of salivary cortisol takes advantage of a simple, painless, non-invasive sampling procedure. The assay can be performed successfully by anyone with access to a plate reader, a shaker or rotary mixer, and a few commonly used pipettors. A single plate assay can be completed in two to three hours. Students in our behavioral neuroscience laboratory class have utilized this kit successfully to examine the circadian cortisol rhythm as well as the effect of stress/relaxation on cortisol levels.
Keywords: neuroscience education, teaching methods, cortisol, glucocorticoids, stress, circadian rhythm, depression, anxiety
As instructors who teach laboratory courses in biological psychology/behavioral neuroscience, we have often been at a loss to find appropriate experiments where students are able to play both the role of experimenter and subject. The difficulty arises because there are few biological parameters representing CNS activity that can ethically be examined in human participants. As a result, the go-to experiments that allow students to act as both experimenter and subject tend to be electrophysiological in nature (e.g., EEG, GSR, etc.). It was our desire to create a laboratory module that would allow students to collect and analyze a biochemical measure of human neural activity. We report here the development of an experiment module that utilizes an easily obtainable enzyme immunoassay (EIA) kit (nearly identical to the ELISA) to measure human salivary cortisol.
Cortisol is a hormone of the adrenal cortex that can be used as a peripheral indicator of hypothalamic neural activity. Plasma (and salivary) cortisol levels rise due to circadian influences as well as perturbations in the organism’s environment (i.e., stressors) that make it possible to detect rather robust experimental effects. Also, there has been much debate on the role of cortisol and hypothalamic-pituitary-adrenal axis dysregulation in the pathophysiology of depression making for a clinically relevant extension to the lecture portion dealing with the “stress axis” (hypothalamic-pituitary-adrenal or HPA axis).
Collection of salivary cortisol is simple, painless, and non-invasive and can be performed at any time the subject desires. Sample storage is convenient as the samples can be kept in a home freezer. Repeated freeze-thaws do not adversely affect the determination of cortisol levels, so the students can just bring them in on the day of the assay without need of in-transport refrigeration or instructor/student coordination. The assay can be performed successfully by anyone with access to a plate reader and a few commonly-used laboratory items. A single plate assay can be completed in two hours (two to three hours by an inexperienced group of students under supervision).
With the available cortisol kit, our students have examined both circadian effects and stressor/relaxation effects on salivary cortisol levels in a laboratory class setting. The module has been employed twice and we intend to include it in each semester that the course is taught. One further impact of the module is that students have available another avenue of research to pursue as individual studies or honors thesis projects.
What is the “stress axis”?
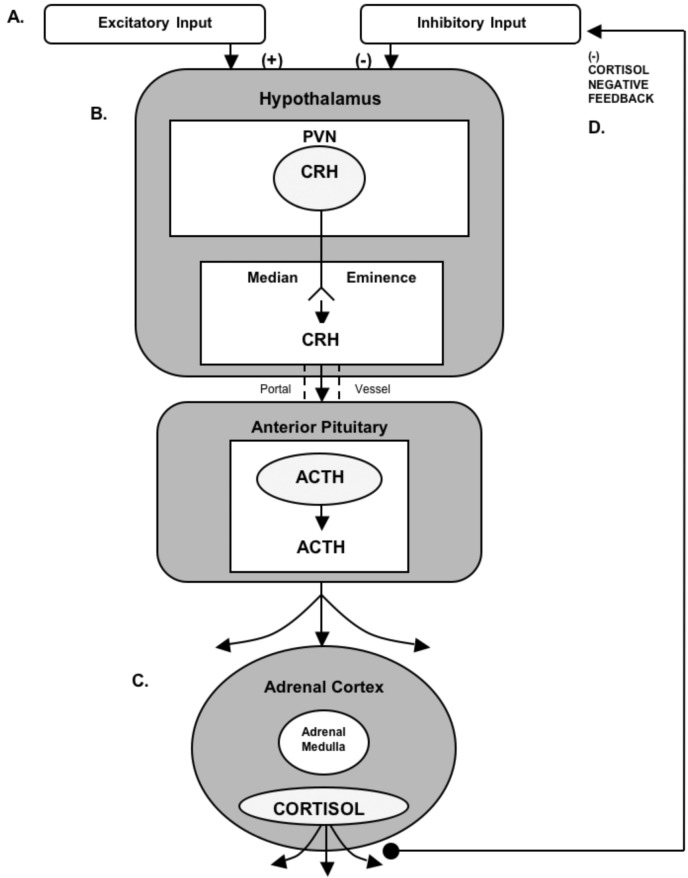
The chief components of the “stress axis” (the hypothalamic-pituitary-adrenal or HPA axis) are the paraventricular nucleus of the hypothalamus (PVN), the anterior portion of the pituitary, and the cortex of the adrenal glands. Cells in the PVN release corticotrophin releasing hormone (CRH) in response to circadian drive, a variety of pharmacological agents, trauma, or psychosocial perturbations (i.e., stressors; Fig. 1). CRH, traveling in a portal vascular system, binds to corticotrophs in the anterior pituitary causing the synthesis/release of adrenal corticotrophin hormone (ACTH) into the general circulation. In turn, circulating ACTH binds to receptors on adrenocortical cells resulting in the synthesis/release of cortisol into the bloodstream (reviewed by Miller and O’Callaghan, 2002). The typical circadian pattern of cortisol secretion shows an increase in the early morning hours that peaks at or slightly before the time of waking. However, depending on the strength of the stimulus (e.g., stressor), cortisol levels in the afternoon and evening can be elevated above those of the circadian peak. Cortisol exerts its effects throughout the brain and periphery primarily through binding to two known types of corticosteroid receptors—the glucocorticoid receptor and the mineralocorticoid receptor.
Figure 1.
Components and Secretagogues of the HPA Axis. A. With sufficient input, neurons in the paraventricular nucleus (PVN) of the hypothalamus release corticotrophin releasing hormone (CRH) into the portal system connecting the anterior pituitary (B.), causing adrenal corticotrophin hormone (ACTH) to be released into the general circulation. C. Adrenal cortical cells respond to ACTH by producing and releasing the steroid cortisol, which is distributed throughout the body via the general circulation. D. One of cortisol’s many functions is to provide negative feedback through receptors located in the hypothalamus and pituitary, thus keeping HPA axis activity in check.
The HPA Axis and Depression
For some time, it has been known that a significantly higher percentage of depressed patients suffer from hypercortisolism than the general population and many other depressed patients who do not show classic hypercortisolism respond poorly to clinical challenges to this system such as the dexamethasone suppression test (DST). The increased incidence of hypercortisolism or abnormal response to a glucocorticoid suppression test observed in depressed patients has lead to hypotheses suggesting that depressed patients have a decreased sensitivity to the negative feedback effects of cortisol brought about by a decrease in responsiveness or number of corticosteroid receptors (reviewed by Holsboer, 2000).
What is the Dexamethasone Suppression Test?
Dexamethasone is a synthetic corticosteroid that has similar activity/effects to those of cortisol but is structurally unique enough that it does not confound the measurement of plasma/salivary cortisol levels when administered to patients. In the dexamethasone suppression test (DST), a small dose of dexamethasone is taken at bedtime. In a normal individual, the morning plasma concentration of cortisol (which would normally be high—remember this is the circadian peak) will be diminished due to the negative feedback effects exerted on the axis by the exogenously administered dexamethasone. In a subpopulation of depressed patients (and patients with certain other medical disorders), morning cortisol levels remain elevated. This finding suggests that these patients have a dysfunctional HPA axis negative feedback mechanism. More recently, the DST has been combined with a CRH challenge further increasing the sensitivity of the test to HPA axis.
What Does the Assay Kit Measure?
Researchers have argued over whether the HPA axis dysfunction observed in depression is simply an epiphenomenon or is causally implicated in the pathophysiology of the disorder (Krieg, 1994). Those arguing for a causal role of hypothalamic-pituitary-adrenal disturbance in depression may point to many lines of evidence including: 1) hypothalamic-pituitary-adrenal axis disturbance often precedes other depressive symptoms or resolves with antidepressant treatment days or weeks prior to relief of depressive symptomology; 2) “at risk” first degree relatives of depressed patients who have not yet had a depressive episode have an increased incidence of hypothalamic-pituitary-adrenal axis dysregulation; 3) individuals on steroid therapy for other disorders often suffer from “steroid psychosis” and 4) in a few studies, direct steroid manipulation in depressed patients has alleviated symptomology (reviewed by Holsboer, 2000; Holsboer and Barden, 1995). Whether the association between HPA axis and depression is causal, epiphenomenal, or both, it is known that processes altered in depressed patients (e.g., mood, cognitive ability, sleep patterns, eating behavior with weight changes, immune function, and activity levels) are known to be affected by administration or removal of corticosteroids.
Why Measure Cortisol as a Laboratory Exercise?
The primary interest in systemic cortisol levels is that they provide an indirect readout of CNS function/activity. Working backwards, an increase in plasma (or salivary) cortisol levels logically implies that circulating ACTH levels have increased as a result of the increase in activity of CRH-containing neurons in the hypothalamus.
The sampling procedure is simple, non-invasive, and can be done easily outside the laboratory at the convenience of the students and under naturalistic conditions.
The HPA axis is highly responsive. The effects are robust enough that both circadian effects and stressor effects should be observable in the laboratory classroom setting.
The relationship between psychological disorders (e.g., depression) and increased cortisol levels or HPA axis dysfunction has been hotly debated (i.e., cause or epiphenomenon). This adds texture to the lecture portion of the class dealing with HPA axis function and we have found that this aspect is of great interest to the students (many of whom will go on to clinical pursuits).
The assay kit measures the amount of free cortisol present in saliva. Circulating cortisol is largely bound by globulins (cortisol is generally not thought to interact with corticosteroid receptors while in this bound state). With some assays, total cortisol is measured but because large molecules (e.g., binding globulins) cannot penetrate the acinar cells of the salivary gland, all cortisol in the saliva is thought to be in a free state. Correlation (r) between free salivary cortisol and free plasma cortisol levels are widely reported to be approximately 0.90 (Kirschbaum and Hellhammer, 1989; 1994).
MATERIALS AND METHODS
Materials Needed
Table 1 shows the materials needed/suggested to perform this assay. The left-most column illustrates the best-case scenario (including requirements listed by DSL Laboratories). We have included other less-desirable options, some of which we actually used in our own class demonstration (in italics), and other options that we believe will work but have not been tested. Instructors should arrange to perform a pilot study with one of the kits to make sure that your equipment/supplies are compatible with the kit.
Table 1.
Materials Needed
| Most Desirable | Least Desirable | |
|---|---|---|
| Dual Wavelength Plate Reader (450 nm and 600 or 620 nm correction) | Single Wavelength Plate Reader (450 nm) | |
| Automatic Plate Washer | Hand Vacuum-Type Plate Washer | P 1000 Adjustable Pipetter or squeeze bottle |
| Pipette to deliver 25 μl | ||
| Multichannel Repeater to deliver 100 μl | Single Tip Repeater to deliver 100 μl | P 200 or P 250 Adjustable Pipette |
| Microtitration Plate Shaker | Rotary Mixer | Agitate by hand |
| Absorbent Bench Paper | Paper Towels | |
| Deionized Water | ||
| Vortex Mixer | ||
| Salivettes | Microfuge Tubes and Cotton Balls | |
| Centrifuge Capable of Spinning Salivettes | No centrifuge needed if cotton is employed | |
| Cortisol Kit (1 plate per 2–8 students | ||
| Disposable Latex Gloves |
The Mechanics of Sample Collection
If using microfuge tube and cotton
We have found that using a standard microfuge tube and cotton ball (we utilized real cotton rather than synthetic puffs) works well, is significantly cheaper than using the Salivettes™ (Sarstedt, Newton, NC; www.sarstedt.com), does not require centrifuging, and the cotton balls actually have a less objectionable taste than the standard Salivette™ gauze plug (they do offer flavored plugs but we believe these add to the already-substantial cost of the Salivette™). At the point the student wishes to collect the sample, he/she should simply pop the cotton ball into his/her mouth. The students should roll the cotton ball around with their tongue and gently chew to stimulate salivation. The goal is to completely saturate the cotton with saliva. This will take about one minute. If a student has a particularly dry mouth that will make it difficult to obtain a sample, he/she can chew on an inert substance (like Parafilm™ squares) for a few seconds to stimulate salivation (do not use gum or any other food-type product).
When the cotton is saturated, the student can pop open the lid to the microfuge tube and, with clean hands or while wearing latex gloves, squeeze contents of the cotton ball into the microfuge tube. Any mucus should stay trapped in the cotton and the saliva sample should look clear with no wispy precipitate. Two hundred μl is sufficient.
The cortisol in saliva is remarkably stable and would probably survive a month or more at room temperature. However, the saliva will grow mold and acquire a disgusting smell within a few days. So, given that repeated freeze-thaw cycles are not a problem with this molecule, students should get their samples (in their microfuge tubes) into a freezer (theirs or yours) at the earliest opportunity (within three days, maximum).
Unless part of the experimental manipulation (e.g., hungry vs. sated), it’s probably best if students do not eat or drink an hour or two before sampling. Students should absolutely not drink an acidic beverage (like fruit juice) just prior to sampling. The reduction in pH will give an artificially high reading for cortisol in this assay. If they do drink something acidic, they must wait at least a half-hour before sampling. Even water should not be drunk immediately prior to sampling as it may dilute the sample.
If using the Salivette™
After saturating the gauze cylinder it is placed into the upper chamber of the tube and the cap is snapped in place. Samples in these tubes can also be frozen as is. On the day of the assay, Salivette™ samples can be thawed and then spun at low speed to separate the saliva sample from the gauze.
The Salivary Cortisol Assay
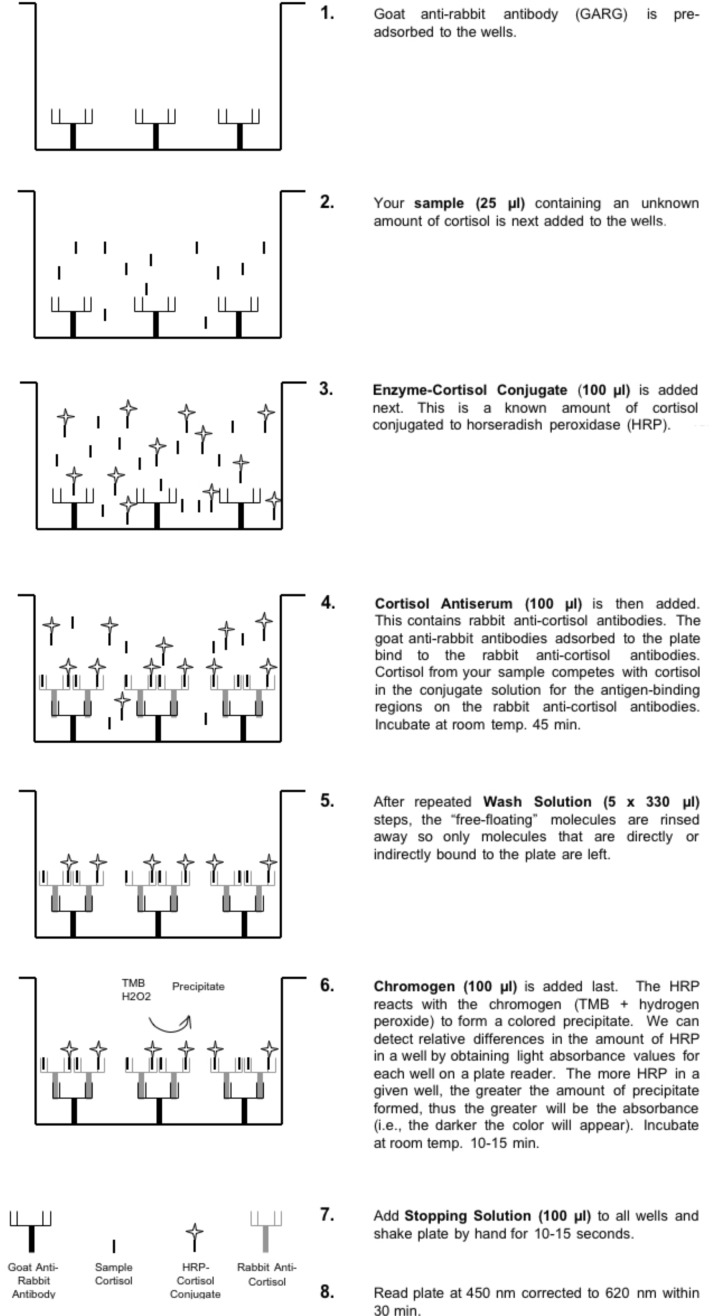
Figure 2 shows the steps involved and the reagents used in the salivary cortisol kit produced by Diagnostics Systems Laboratories, Inc. (DSL, Webster, Texas; www.dslabs.com). The assay includes all necessary reagents including known cortisol concentrations for creating the standard curve. The assay takes about two hours to complete (a little longer for inexperienced students under direct supervision). A single, one-time-use, 96-well plate (12 individual strips of eight wells) costs about a hundred dollars at the time of this writing. A similar kit for a similar price is made by Salimetrics (State College, PA; www.salimetrics.com), but we have no direct experience with the Salimetrics kit.
Figure 2.
Steps of the salivary cortisol enzyme immunoassay (EIA). The kit obtained from DSL contains 96 wells to which solutions are addeds as described for each step (1–8) in the figure.
Experimental Design
There are essentially two types of studies that can be performed. The first examines cortisol levels with respect to circadian periodicity. It compares samples that the students take in the early morning to those taken later in the day. The second type is a planned experiment where the students can play a role in determining what the hypothesis and independent variable will be. With eight students per plate (what we feel is the maximum), each student can run four separate samples (in duplicate). The design that we employed was to have students collect two samples for each type of study. Each student was provided with a “sampling kit,” a plastic bag containing labeled microfuge tubes, small cotton balls, latex gloves, and a coded data sheet (for maintaining privacy).
Study Type 1: Examining Circadian Periodicity Using The Whole Class
For this type of study, the student should obtain one sample around the time of the expected circadian peak (generally thought to be upon—or just prior to—waking). Again, a college student may be different than other members of the population in that a given student may set their alarm for 7:00 AM on Monday, Wednesday, and Friday, 9:00 AM on Tuesday and Thursday, and, after being relatively sleep-deprived during the course of the week, wake up at noon without the aid of an alarm on the weekend. For these reasons, the best one can do is to have students take the AM sample upon waking (6:00 – 9:00 AM). Students should keep the sample kit on their nightstand so they can do it immediately upon awakening. Participants will likely take this sample at different times based on their schedule so there will probably be representation at several time points around a theoretical peak.
Students should obtain a second sample at some point within a noon - 10:00 PM window (the exact time should be left to the student; it does not matter what the rest of the group is doing and it does not have to be taken on the same day as the AM sample). It should be taken when they feel pretty relaxed and have not been going at a hectic pace for several hours. There should be no alcohol in their system, etc. They should be clearly instructed to not, for instance, take this sample while they are bustling through classes at school. If there is an evening when they plan to watch three hours of television to unwind and have no pressing engagements the next day (such as having to give a presentation), this would be a good time to obtain the PM baseline sample.
Study Type 2: Small Group Experiment
Individual groups of students (four to eight) can conduct a within-subjects experiment of their choosing (within appropriate limits). Students can design it as a group and write a proposal with specific methods of collection, concise description of the independent variable, and instructions to participants. For instance, a group may wish to see if there is a difference between their at-class cortisol levels and their away-from-class cortisol levels. The samples should be taken on different days but at a similar time-point within each day. Thus, a student may take a 1:00 PM sample at school on Thursday, then take a 1:00 PM sample at home the following Saturday. For examples of the types of manipulations that have produced changes in cortisol, Kirschbaum and Hellhammer (1994) provide a comprehensive table of published experimental manipulations and their effect on cortisol levels.
The Assignment
After a general explanation of the lab module, HPA axis background, and sample collection instructions, students can divide up into small groups (four to eight students). Each group can meet and decide on an experiment and write a small proposal. This can be handed in to be approved and/or modified by the instructor. Allow a minimum of several weeks between the handing out of sampling kits and the actual running of samples in the laboratory. During the actual laboratory meeting, we used the downtime during incubation steps to show students what they would be doing with their raw data once the assay was completed. Students can be given a worksheet and graph paper (such as those generated using Graph Paper Printer™ software obtained from www.hotdownloads.com) and instructed in their use during this downtime. Once the assay is completed and the plate for a particular group has been read (we had groups sign up for specific assay times staggered throughout two days), students can be asked to do the following:
Take the raw optical density values from the plate reader printout and manually transform standards and sample optical density values to “proportion of zero absorbance” using the worksheet (logit values cannot be ≥ 1 so are best presented as the percent of some standard). For instance, if the absorbance for the zero standard had an optical density of 1.82, a sample having a raw absorbance of 1.43 would have a proportion of zero absorbance of: 1.43 ÷ 1.82 = 0.79.
- Manually plot the standard curve (using the proportion-of-zero values and omitting the zero point from the curve) and estimate the level of cortisol in your samples on three types of graphs by visual interpolation:
- Linear: shows the student what an exponential dose-response function looks like and the inherent difficulty in predicting sample levels based on a curved line.
- Log-Linear: shows how exponential data can be transformed into a relatively straight line utilizing a concept (common logarithms) with which the student is already familiar.
- Log-Logit: shows another type of straight-line transformation and allows students to visualize what our spreadsheet calculates.
Although students were responsible for hand-calculating cortisol values for their own samples, because of time and resource constraints, we entered all student raw data into the spreadsheet ourselves and gave students a copy of all data at the next class meeting. The spreadsheet we created for data reduction utilized a log-logit transformation but any curve-fitting program will do and even manual data reduction via the graph printouts should provide usable data. Once students have been given the data for the entire class, they can be asked to:
Create a scatter plot of each student’s (i.e., the entire class) AM value and PM baseline value (or the lowest of their PM values). Cortisol levels should be plotted against time of day. Alternatively, a categorical column graph of AM vs. PM could be generated.
Perform a regression analysis on the circadian data or (alternatively, based on level of sophistication) divide the data into discrete groups (i.e., AM vs. PM) and perform the proper t-test on the group means.
Create at least one figure and perform at least one analysis on their experimental “group” data.
Write an APA style paper (including figures and analyses above) with Study #1 being the “Circadian Periodicity” part of the lab and Study #2 being the “Group Experiment” aspect. The three graphs (linear, log-linear, and log-logit) and hand-calculated cortisol estimates using each graph type can be attached to the paper as an appendix.
RESULTS AND DISCUSSION
Professors’ Pilots of Circadian Periodicity (within-subjects, N=2)
When the kits were first received, we ran a pilot to identify any potential glitches with the assay and to make certain that we could obtain quality results with the equipment and supplies that we had on hand. Our first pilot (Table 2) showed a reasonable time-of-day effect (values shown below are means of duplicates expressed as μg/dl of free cortisol) with the AM samples (near the time of the suspected circadian peak) clearly elevated.
Table 2.
Professors’ Pilot #1
| Subject | AM Sample At Home | PM At Home |
|---|---|---|
| #1 | 0.63 | 0.47 |
| #2 | 0.66 | 0.17 |
A month later (long after the saliva collection kits and collection instructions had been handed out to students), we thawed and re-assayed the original samples to check for between-assay precision after a freeze/thaw cycle but we also collected impromptu PM samples while at school. We ran these along with the original samples. Data from this assay are shown in Table 3.
Table 3.
Professors’ Pilot #2
| Subject | AM Sample At Home | PM At Home | PM At School |
|---|---|---|---|
| #1 | 0.68 | 0.43 | 0.81 |
| #2 | 0.67 | 0.19 | 0.83 |
In addition to observing high assay precision in samples that had undergone freeze-thaw (0.68 vs. 0.63 for Subject #1 and 0.67 vs. 0.66 μg/dl for Subject #2), we also observed (somewhat surprisingly) a clear “effect of school” on cortisol levels. Cortisol levels for subjects #1 and #2 were two and four times higher respectively in those samples collected at school vs. the samples taken at a similar time of day at home. In fact, they were higher than either AM sample. This was our first suspicion that students might have difficulty obtaining low PM baseline samples.
Class Data
The data shown below are examples of actual data gathered by students in our Behavioral Neuroscience class. Students were given their sampling kits and instructions for sampling three to four weeks before we were scheduled to run the samples in lab. Students were allowed to divide up into groups of four to eight and design an experiment. Each student was allowed to collect and assay four different samples. Two of these samples were used for the circadian periodicity portion of the assignment and data for the entire class was pooled. Two were used for an experimental manipulation of each group’s choosing/design. In some cases, the PM circadian sample was used as the experimental control.
1. Circadian Periodicity Study (within-subjects design, N=20, entire class)
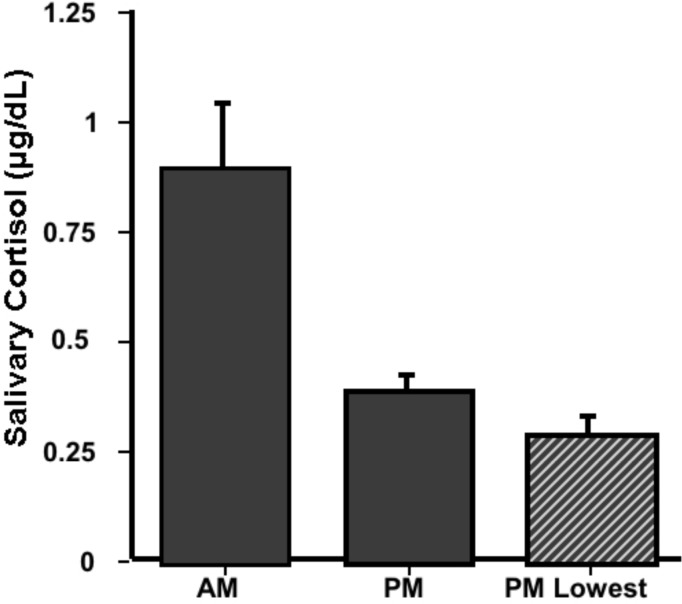
All students were told to obtain one sample immediately upon waking (AM Sample; 6:00 – 9:00 AM) while the other sample (supposed no-stress PM control; Noon - 10:00 PM) was to be taken while they were relaxed, had not been going at a hectic pace for a couple of hours, and had no pressing engagement (such as a class presentation) in the near future. Figure 3 shows that a circadian rhythm was observed, with AM cortisol levels significantly greater than PM levels (t(19)= 2.97, p < 0.01).
Figure 3.
Circadian Periodicity Study. AM and PM cortisol samples were obtained by the entire class (N=21). One morning sample was collected between 6 and 9 in the morning (AM) immediately upon waking.
The magnitude difference between AM and PM cortisol levels in a previous class (data not shown) was not as robust as the present data. We had observed that students’ chosen PM control often did not represent subjects’ lowest PM value taken (i.e., there was a large difference between mean PM control and mean of their lowest PM sample of all samples taken). We have included in Figure 3 the mean lowest PM value of the current group of subjects for comparison. The very negligible difference illustrates that this group of students did a much better job of obtaining PM samples under “basal” conditions.
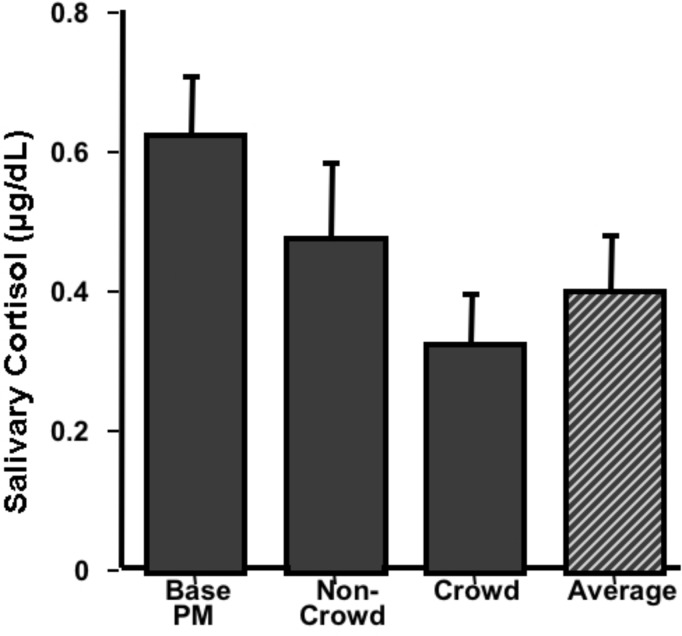
2. Group Experiment: Effect of Exercise in a Crowded Athletic Center (within-subjects, N=4)
This group tried to determine the effect of exercising in a crowded athletic center on cortisol levels. They collected saliva samples under two exercise conditions: once when the athletic center was crowded and once when it was relatively uncrowded. Their hypothesis was that exercise itself would increase cortisol levels and that the crowded condition would be more stressful than without the crowd, adding to the exercise-induced cortisol level. They collected the samples immediately after completing a rotation of four exercises and compared the cortisol levels from that to their PM circadian sample. As shown in Figure 4, their hypothesis was not supported. Not only did exercise seem to reduce cortisol levels, but the crowded condition produced the lowest levels. The group offered the following interpretation: the low levels observed in the crowded condition were due to the social support that the group members offered each other which was not present in the uncrowded condition.
Figure 4.
Effect of Exercise. Cortisol samples were collected by four students who were interested in the effect of exercise in crowded and uncrowded conditions on cortisol levels. They collected samples immediately after completing four weight-lifting exercises, once when the athletic center was crowded and once when it was uncrowded. Their PM circadian sample was used as a baseline. Data are presented as mean μg/dL + SEM.
Perhaps a more parsimonious explanation is that any exercise regime is really part of an unrealized “decompression” strategy and cortisol samples taken during this time tend to reflect a point at which cortisol levels tend to be lower (Average bar represents a single mean exercise level for each subject rather than separate crowded and non-crowded levels and when compared to PM Base t(3)=9.09, p < 0.01.) Notice the relatively high basal PM levels for subjects in this group.
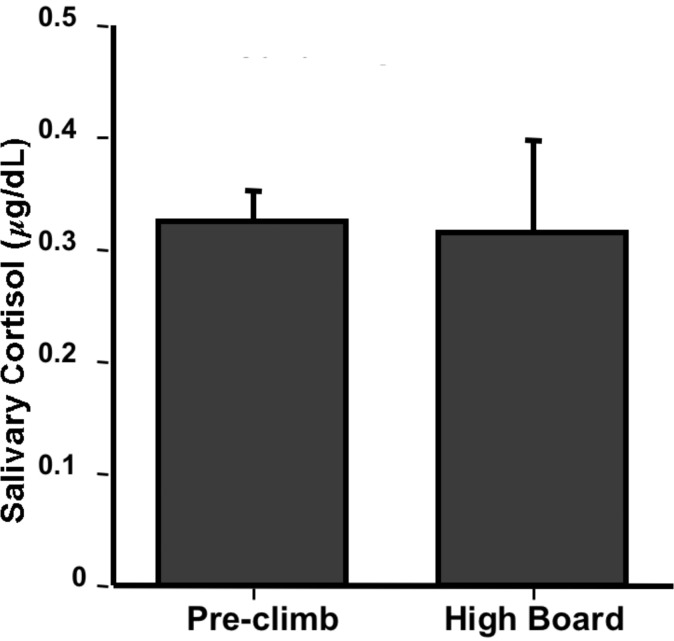
3. Group Experiment: Effect of High Dive Exposure (within-subjects, N=4)
Although no significant effects were observed by this group, this experiment illustrates the free creative process that students really seem to enjoy with this paradigm. This group was interested in examining the effect of being on the high dive on cortisol levels. They hypothesized that exposure to the high dive would serve as an acute stressor and would produce a significant increase in salivary cortisol, which would be especially noticeable in the group members who did not have experience on the high dive (two were experienced, two did their best to avoid heights and had never been on the high dive). They went to the athletic center as a group and collected a sample at the base of the high dive (pre-climb). Then each participant individually ascended the ladder and went to the end of the diving board, remaining there for two minutes. Another saliva sample was collected two minutes after climbing down from the board. As Figure 5 shows, there was no effect of high dive exposure. The group members were surprised by these data, as heart rate data collected at the same time showed a marked increase. One possibility that they offered was that the anticipation of doing the experiment may have been a stressor itself, stimulating the negative feedback system and producing low cortisol levels. This is certainly a feasible contributor toward lower cortisol levels. Another possibility is that their sampling schedule did not allow for cortisol to reach peak levels in the saliva, and that the sample should have been taken 10–20 minutes after the diving board exposure.
Figure 5.
Effect of High Dive Exposure. Four students collected saliva samples before and after a two minute exposure to the high dive. Data are presented as mean μg/dL + SEM.
Overall Precision of Sample Duplicates in Different Lab Groups
One way of assessing “believability” of the student data is to examine the overall precision of their assay duplicates. Table 4 shows the mean differences between sample duplicates (standard curve not included as these were initially loaded onto the plate by the instructor) for each of 10 groups (each group ran their own samples on their own plate) from two different classes.
Table 4.
Assay Precision of Two Classes
| Mean % difference between sample duplicates | Number of duplicate sample sets run by each group | Number of duplicates that had ≥ 40% difference |
|---|---|---|
| 5% | 24 | 0 |
| 7% | 16 | 0 |
| 7% | 20 | 1 |
| 8% | 15 | 0 |
| 10% | 11 | 0 |
| 12% | 24 | 3 |
| 14% | 24 | 3 |
| 15% | 14 | 2 |
| 15% | 15 | 1 |
| 53% | 24 | 11 |
Assay error (by each group of four to six students) ranged from an extraordinary 5% mean difference with no large (defined by us as ≥ 40%) difference observed in any duplicate to an unacceptable 53% mean difference with almost half of the sample duplicates containing large errors. Had we had an extra kit on hand, we would have made these students re-run their samples. Generally, results will not be this poor and the few samples that have a really large percentage difference can be re-run with spare reagents and strips or excluded from analysis without significantly impacting the study. With the exception of one group, these data clearly illustrate that, with supervision and rudimentary pipette training (we consider it essential that students get to practice pipetting, perhaps during previous lab meetings that do not take the full class period), students are capable of obtaining usable salivary cortisol data with this kit.
CONCLUSIONS
Measurement of salivary cortisol, with the aid of an easily obtainable kit, is an ideal human subject project (and a terrific alternative to electrophysiological recording) for a course in Physiological Psychology or Behavioral Neuroscience. It can also be utilized as a demonstration in a Behavioral Endocrinology lecture or seminar course where students can self-sample under a variety of conditions. The professor can then easily run the samples by him/herself in a couple of hours and report the data to the class.
One important issue that must be addressed before adopting this laboratory exercise is the proper procedure for ensuring both anonymity during data collection and the proper context for interpreting cortisol levels. This is especially important because of the relationship between cortisol and depression. Although we have made a point in our lectures to students that cortisol levels are not diagnostic alone, and that college students in particular have wide day-to-day variations, it is likely that a student will attempt to interpret how their own level relates to their mental health. Our procedure to ensure privacy was to code microfuge tubes with numbers such that only the student would be aware of his/her own set of numbers. In addition, the data was discussed with regard to means and not by individual data points.
Another ethical issue is whether to seek institutional review board (IRB) approval for this project. The first time that we utilized this paradigm an IRB representative advised us that we did not need IRB approval because this fell under the rubric of “classroom demonstration.” Only later did we decide that it was successful enough as a laboratory exercise to share with other instructors via publication. Thus, prior to our second time utilizing this project as part of a Behavioral Neuroscience course, we obtained IRB approval with publication in mind. We leave it to the independent instructors/institutions as to whether or not approval from their IRB is necessary.
A methodological concern that we have regarding the quality of data generated using this laboratory exercise is the potential difficulty in having active college students collect “no-stress” PM samples that are to serve as a basis for comparing both circadian peak samples as well as samples collected under times of stress. With a very hectic campus life, some students may find it difficult to obtain a true no-stress sample. Kirschbaum and Hellhammer (1989) report that in a study with 48 students and 54 young mothers (as opposed to older subjects), only the early morning values showed stability over three days. They found considerable intra-subject variability across days at other time-points throughout the day.
Getting good samples can be maximized by passing out the sample kits/instructions early in the semester and not running them until late in the semester so that students aren’t rushed into taking samples at undesirable times. Also, since microfuge tubes and cotton are inexpensive, a good strategy may be to have each student obtain several samples during the PM or AM on different days and then combine the samples in equal volume prior to assaying (thus assaying a single “Average AM” or “Average PM” sample derived from four or five samples taken on different days). While we did not encounter a widespread problem obtaining relatively low PM cortisol samples in the data presented here, it was an issue in our prior experience. We wonder if emphasizing this problem while providing students with sample collection instructions was instrumental in achieving the excellent circadian rhythm data in the present study.
While we are happy with the circadian data this semester, only one group was able to show some type of effect in the small group experiment. The data for the exercise experiment (Figure 4) are typical of results that we have found in a previous class. That is, the data make sense ex post facto but do not support the original hypothesis of the student researchers. In a group from a previous class (data not shown), it was hypothesized that watching a scary movie would produce higher cortisol levels than watching a comedy. No such difference was observed but, similar to the exercise effect, they found that watching any movie resulted in lower cortisol levels than their alleged “no-stress” control PM sample. Periods like the two movie nights, where the students just sat with friends for a couple of hours and did nothing, probably occur very rarely for many students.
These two examples bring up an important point about the experiments designed by students. For our first time using this module, we discouraged students from utilizing relaxation manipulations in order to observe an experimental difference in baseline cortisol values. Our thinking was that PM no-stress cortisol levels would be low enough that a floor effect would obscure any effect of relaxation. The expected circadian cortisol rhythm, however, is not as clear-cut in the college student population where baseline cortisol levels may often be elevated. As a result, we now are more open to including relaxation manipulations in addition to stressor manipulations in the laboratory module, where appropriate “relaxation control” conditions are not as important.
As a final example (and one which illustrates a true stressor effect) one group (data not shown) had the intent of showing that cortisol levels would be higher on a day where they were taking an examination than on a normal school day where there was no exam given. The results showed no difference in cortisol levels between the exam day and the non-exam school day, but did show that samples taken during either time were higher than their PM baseline sample, which were taken while not at school. This group’s observation mirrors the effect observed in our small N professor pilot study (Table 3); Did anyone think school was not a stressor?
We feel that the combination of experimental failures (all students think that they have a slam dunk of a hypothesis) and “unexpected” significant effects provides a very good education. It gives the students a glimpse, perhaps their first, of what “real research” often produces. And it makes them think--which is really the goal.
Following the first use of this module in a Behavioral Neuroscience course, several students approached us about the desire to do an independent study project examining the effects of some variable on salivary cortisol. Since that time we have supervised two honors thesis projects, one in which spirituality and religiousness was examined as a modulator of the stress response, and the other examining the effect of yoga training on basal and exercise-induced cortisol levels. In fact, some students have moved beyond cortisol, collecting salivary samples for testosterone and dihydroepiandrosterone (DHEA) utilizing kits similar to the cortisol kit used in the above studies. For obvious reasons, this is an attractive paradigm for students. Also, in the case where a small department may employ a single neuroscience professor who utilizes animal models exclusively, measurement of salivary cortisol (or other hormones) provides a simple alternative for a student who wishes to perform an independent study or honors research project, but does not desire to work with non-human subjects.
Another impact of using this module has been to generate discussion about the type of stress associated with being in college. We and some of the students are considering what we might do to better understand stress in our school environment. Thus, we have found the salivary cortisol module to be a wonderful learning experience—for us as well as for our students.
Acknowledgments
This work was supported by The Howard Hughes Medical Institute and The Keck Foundation. The authors wish to thank members of PSY/ZOO Behavioral Neuroscience courses taught in Spring 2002 and 2004 for their enthusiasm and feedback regarding this module.
Footnotes
A free CD-ROM containing instructions, lecture notes, presentation materials, and a data-handling spreadsheet is available. Please contact Marc Zimmer at mzim@conncoll.edu to obtain the CD-ROM or view our website at http://www.conncoll.edu/is/k-hhmi/k-hhmi-fellows.html.
REFERENCES
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendo. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharm. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Barden N. Antidepressants and hypothalamic – pituitary - adrenocortical regulation. Endocr Rev. 1996;2:187–205. doi: 10.1210/edrv-17-2-187. [DOI] [PubMed] [Google Scholar]
- Miller DB, O’Callahaghan JP. Neuroendocrine aspects of the response to stress. Metabolism. 2002;51(Suppl 1):5–10. doi: 10.1053/meta.2002.33184. [DOI] [PubMed] [Google Scholar]
- Kreig JC. Laboratory tests in depression: Is it worth the effort? J Psych Res. 1994;28:337–339. doi: 10.1016/0022-3956(94)90016-7. [DOI] [PubMed] [Google Scholar]