Abstract
Decades ago, classic experiments established the phenomenon of “neural induction” (Spemann and Mangold, 1924; Holtfreter, 1933). It appeared clear that amphibian ectoderm was pre-programmed to form epidermis, and that the neural phenotype was induced by a chemical signal from mesoderm. The “ectoderm makes skin, unless induced to make nervous system” model appeared in many textbooks.
This interpretation, however, was not simply incorrect but 180 degrees out of alignment with the actual situation. As subsequently demonstrated, the default state of amphibian ectoderm is neuronal, and the expression of the epidermal phenotype requires cell signaling (Hemmati-Brivanlou and Melton, 1992; 1994; 1997).
In this activity, students are presented with key experiments in a stepwise fashion. At several points, they work in groups to devise models that explain particular experimental results. The stepwise presentation of results mirrors the history of discoveries in this experimental system. Eventually, faced with seemingly contradictory data, students must revise their models substantially and in doing so, experience the paradigm shift.
The lesson also examines the history of this paradigm shift. Data inconsistent with the “epidermal default” model were published years before the “neural default” model was proposed, but the significance of the surprising new data was underemphasized by the scientists who made the discovery. Discussing this situation provides insight into how science works and highlights the possibility that working scientists may become entrenched in prevailing paradigms. Such “nature of science” discussions emphasize research as a human activity, and help to dispel student misconceptions about science and scientists.
Keywords: teaching, paradigm shift, ectoderm, neurons, epidermis, induction, bone morphogenetic protein (BMP), evolution, models, undergraduate
Controversy is a part of science, but conflicting models or data rarely are presented realistically in science textbooks (Seethaler, 2005; Oulton and Grace, 2004). In addition, modeling phenomena, devising hypotheses, and designing experiments are important activities of many working scientists, but textbooks usually present only the final, established hypotheses, models, and/or “classic” experiments. Much of science involves experimental tests that rule out particular models, refute hypotheses, or reveal gaps in understanding that require reconsideration of the situation at hand. If, per the recommendations of science reform documents, biology teaching should reflect the way research is carried out, (Siebert and McIntosh, 2001; NRC 2003), then students should routinely experience scientific controversy and develop, test, and reject hypotheses or models. Yet many undergraduates still experience biology as a textbook-based smooth path to deeper understanding. Since so many textbooks focus primarily or exclusively on “biological truths,” students may develop the misconception that discovery in science is a linear process with few digressions, blind alleys, or faulty models. Such a view may make biology appear less creative than it is, and contribute to a negative perception of research careers. Indeed, the college biology major has for years had a high dropout rate nationwide, due not to the difficulty of the material but to the perception that biology is “overwhelming” and/or “boring” (Seymour and Hewett, 1997; see also Cech and Kennedy, 2005).
Working researchers realize that in contrast to the “step-by-step accumulation of knowledge” viewpoint espoused by most textbooks, actual progress in science is not necessarily linear. New findings may emerge from unexpected places, and lead to rapid progress in previously unexpected directions (e.g. the discovery of the PCR method and its rapid application in fields well beyond bacterial nucleotide synthesis, where it originated). From time to time new data that simply cannot be made to fit a well-established model lead to paradigm shifts – the overturning of long-held and virtually universally accepted ideas (Kuhn, 1970). The Copernican revolution is probably the best-known example, but not all paradigm shifts are so global in scope. I suggest that basing a neuroscience class on a paradigm shift in developmental biology provides an opportunity to teach on two levels; science and the nature of science. In the lesson outlined below, I propose a paradigm shift lesson built on principles derived from our tested approach to primary literature, CREATE.
CREATE (Consider, Read, Elucidate hypotheses, Analyze and interpret data, and Think of the next Experiment; Hoskins et al., 2007; Hoskins 2008) focuses on primary literature as an inroad into the workings of science labs. CREATE students use a unique combination of pedagogical tools to facilitate their reading of sets of papers produced sequentially from the same lab, examining how a research project evolves over a period of years. Multiple class sessions focus on the same papers, as each figure or table is analyzed in depth. Assessments of the CREATE method as tested in an upper-level elective indicate that it both demystified science and humanized science and scientists for students in the course (Hoskins et al., 2007).
CREATE students urged adaptation of the approach to freshman or sophomore-level classes as well, as they felt that critical reading skills they developed in the CREATE semester subsequently improved their performance in other classes. I present here a modification of CREATE for a briefer lesson that can be used with freshmen or sophomores and covered in one or two class periods. This “neuroscience paradigm shift” example has proven useful both for biology majors and general-education students, as the lesson is taught on two levels: (1) the science behind the shifting paradigms, and (2) the prevailing ‘scientific culture” -- why was the old paradigm so hard to change?
Focusing on a paradigm shift in developmental neurobiology, I use repeated modeling of the system under study, rather than the reading of a series of linked papers, as the central approach. The question “What is the role of ectoderm in development of the nervous system?” is now understood quite differently than it was when most current Biology professors were in college or graduate school. For much of the 20th century, the story was “mesoderm induces ectoderm to form nervous system. If ectoderm does not receive an inducing signal from mesoderm, it differentiates according to its pre-programmed epidermal phenotype.” A variety of data appeared to support this model, whose corollary, “the default state of ectoderm is skin” was certainly written on many a final exam, receiving full credit.
The experiments that led to the overturning and reversal of this long-standing idea are my focal point. In addition to teaching fundamentals of vertebrate development, the classroom activities emphasize that much of our understanding of biology is built on experiments, that interpretation of experiments is not always straightforward, and that new findings may necessitate dramatic reinterpretation or reevaluation of older ones. This reality can contrast sharply with many students’ sense that biology is predictable, lacking in controversy, and/or that everything important is already known (Steitz, 2003; Seethaler, 2005).
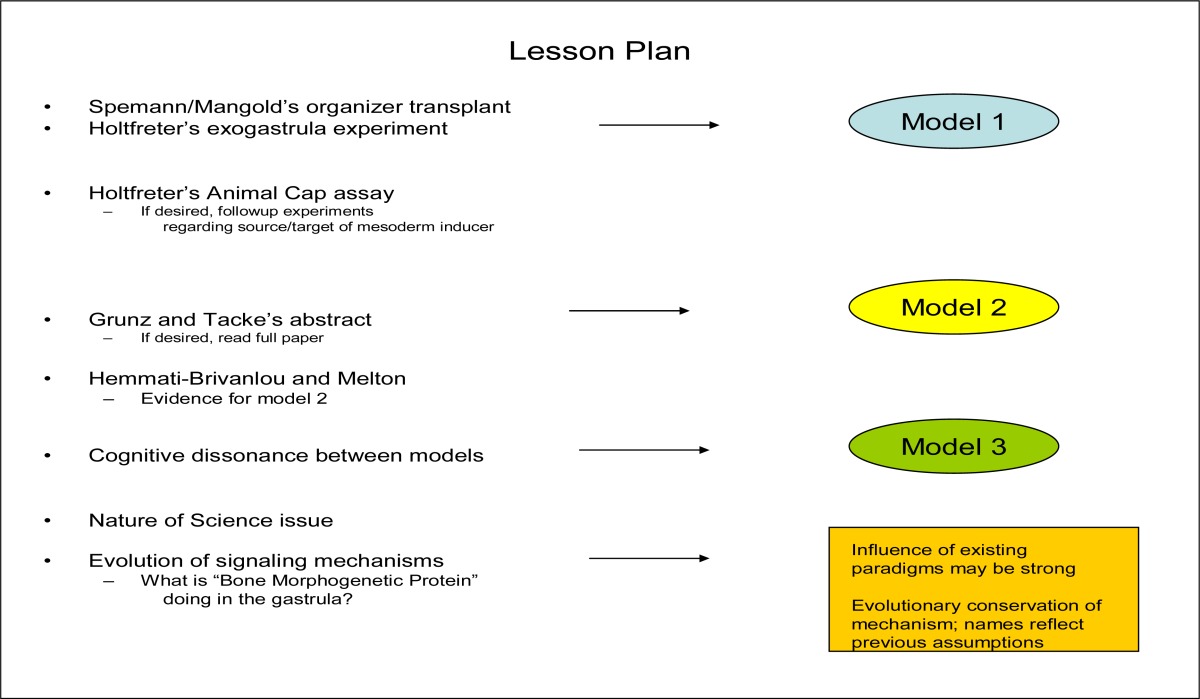
I have used this approach with Introductory Biology students (mainly Biology majors planning for medical school), general-education students taking a required science course, and junior/senior level Developmental Biology students. I hope that other faculty interested in teaching “how biological understanding develops” as well as “how the nervous system develops” will find this activity useful. In the course of the lesson I address five main issues, which can be taught together or divided among different class sessions.
1) The use of experimental data to devise explanatory models, and the designing of new experiments based on the models.
Modeling is common practice in research science, but not often illustrated in the undergraduate classroom.
2) How scientists cope with data that don’t fit a model — are models “set in stone” or guidelines for further analysis?
Many students believe that “if something [even a hypothetical model] is published, it must be true.” This misconception makes it difficult to recognize the reality that science is ever-changing, a concept that can also be hard to glean from textbooks where everything appears to be both true and permanent.
3) Paradigms and their influence
Even unbiased researchers may be strongly affected by prevailing paradigms.
4) Research science as a “transparent” activity involving interactions even among groups that do not collaborate directly.
Illustration of the self-correcting nature of the research enterprise — because data are presented and shared, they are available for reinterpretation by scientists from other labs.
5) Evolution as a key underlying principle in biology.
Discussion of the inductive signal BMP-4, and why “bone” morphogenetic protein, is present in an early embryo, underscores the “recycling” of key signaling molecules, and provides insight into the relationship of evolution and development.
SUGGESTED CLASSROOM APPROACH
Origins of the Nervous System
Fate mapping of amphibian embryos showed early in the 20th century that early embryonic ectoderm gives rise to both skin and nervous system. Subsequent grafting and manipulation experiments led to the conclusion that “the default state of ectoderm is epidermal--to form nervous system, the ectoderm must be induced by a chemical signal.” This concept persisted for nearly 50 years, yet it was incorrect. Recent analyses have established instead that the default state of amphibian ectoderm is neural (Hemmati-Brivanlou and Melton, 1997). While one way to approach this fact is simply to tell the students the old and the new models, I suggest that it is more effective to let them make this discovery themselves, based on the data that were available as the story developed. In this way, students experience the process of building a model based on a series of experimental results, then experience cognitive dissonance related to new experimental results that “don’t add up,” and ultimately get the chance to devise a model to reconcile both sets of data. This process challenges students to think like scientists by designing explanatory models and integrating new data with old. The ease with which students adapt their models contrasts with the slowness with which the paradigm shift happened among developmental neurobiologists, underscoring the influence of prevailing paradigms.
The suggested activities can be divided among several classes, combined in one long class or lab session, or distributed between in-class activities and homework assignments. I have used this lesson in Developmental Biology classes (mainly Biology majors), and also in Biology-for-general-education-student courses, as it requires little in the way of background knowledge. Individual instructors of course can modify the suggested activities based on their own class’s level of preparation and on the time available.
I. Basics of early development in amphibians
I briefly describe fertilization, cleavage, blastula formation and gastrulation in amphibians, along with the methods of fate-mapping and tissue transplantation.
Working in small groups, students concept-map (Novak and Gowin, 1984; Novak, 1990) their understanding of these key issues. The group concept maps (on transparencies) are compared before the lesson moves forward. This step allows the instructor to ascertain what the students know and encourages the students to relate new information with information they may have learned previously, a key step in learning (Novak and Gowin, 1984; Novak, 1990; Fink, 2003; NRC 2003). These processes are the core of the Consider step of CREATE.
II. Fundamental experiments underlying the “mesoderm induces the nervous system” model
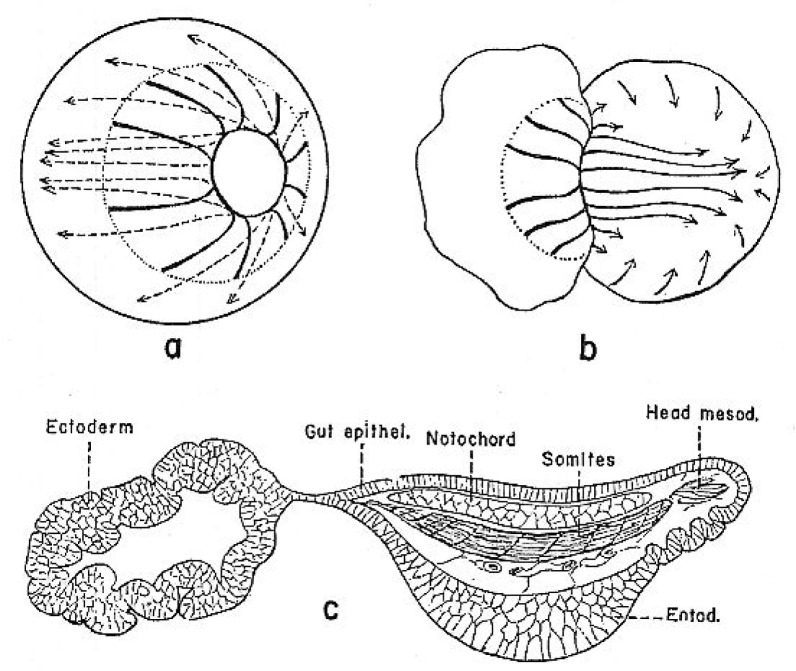
I begin with two key experiments performed early in the 20th century. Results of these studies strongly influenced scientists’ understanding of the formation of nervous system. First, I describe Hilde Mangold’s graft of the dorsal lip of the blastopore to a host embryo, resulting in a secondary embryo with well-organized brain, retinas, and spinal cord (Figure 1) (Spemann and Mangold, 1924). This experiment revealed the “organizing” ability of the dorsal lip. I continue with Johannes Holtfreter’s placing of embryos just starting gastrulation into high concentrations of salt solutions, blocking involution of mesoderm. In the “exogastrulae” that formed, the extruded mesoderm differentiated into disorganized muscle, blood, and connective tissue, associated with gut-like structures, while the remainder of the gastrula, the ectoderm, differentiated as skin (Figure 2; Holtfreter, 1933). No neuronal tissue was seen in the exogastrulae.
Figure 1.
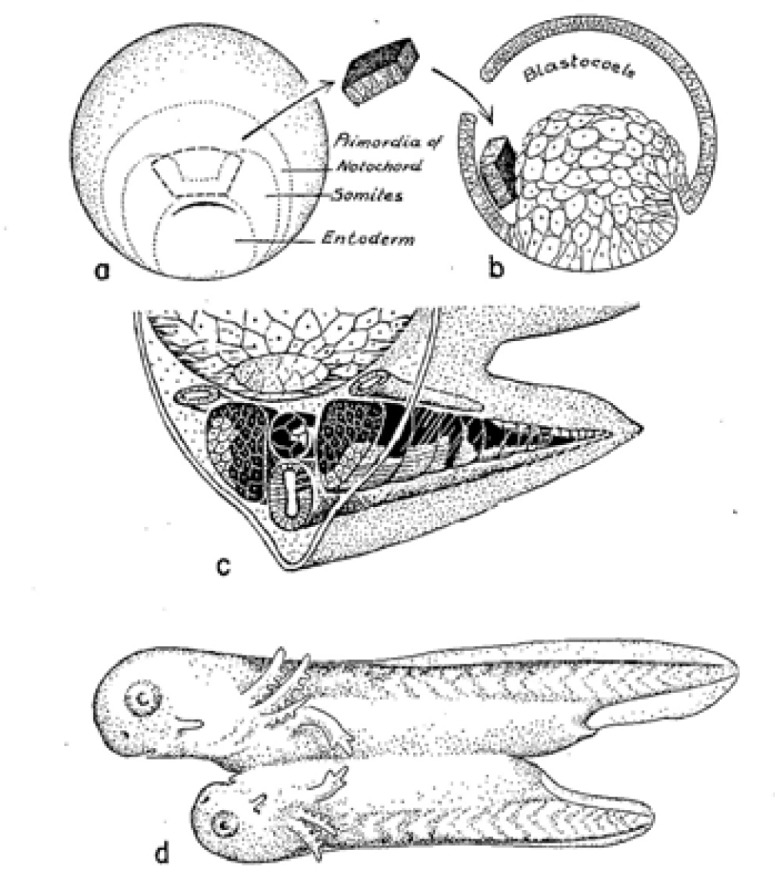
Summary of Hilde Mangold’s dorsal lip transplantation experiment (Spemann and Mangold, 1924). A graft of dorsal lip tissue from a pigmented embryo (a) placed in the blastocoele of an albino embryo (b) gives rise to conjoined embryos (d). Organs of the ‘secondary embryo’ contain a mix of pigmented and nonpigmented tissues, (c) indicating that the graft exerted an “organizing” effect on the host. Figure from Holtfreter and Hamburger, 1955.
Figure 2.
Holtfreter’s exogastrulation experiment (1933). A, b show morphogenetic movements in a normal (a) and exogastrulating (b) amphibian embryo. C shows differentiated cell types seen in an exogastrulated embryo several days later. Note lack of identifiable neural tissue. Illustration from Holtfreter and Hamburger, 1955.
Based on these two experiments, I work with the class to jointly devise and sketch a model for “how the nervous system develops.” This sort of “cartooning” step is a key feature of the Read step of CREATE. Visual representations facilitate learning, especially for students who are not primarily verbal learners (Chickering and Gamson, 1987; Foertsch, 2000).
After some discussion, students typically suggest (as did developmental biologists of the mid-20th century) that Mangold’s experiment indicated “the grafted dorsal lip mesoderm induces the secondary embryonic axis, including the nervous system.” Holtfreter’s findings are interpreted to suggest that during normal development, the involuted mesoderm signals overlying ectoderm to differentiate into nervous system. The instructor diagrams this model on the board, with coaching from students (Model 1; Table 1). While the design of the first model could be done by students themselves, I find that drawing a simple diagrammatic cartoon on the board, illustrating student suggestions, is a useful way to get over the “fear of modeling” issues (e.g. “What is a model? I can’t draw! Do I have to draw it by hand? Can’t I use a computer?”) that can hold some students back.
Table 1.
Summary of steps in teaching this lesson. Model 1 is sketched on the blackboard by the instructor and remains on the board for the duration of class. Models 2 and 3 are devised by students working in small groups in class. As noted in the text, some of the steps could be assigned as homework rather than being carried out in class.
Model 1 shows involuted mesoderm inducing nervous system from overlying ectoderm, consistent with the mid-20th century interpretation of the two early experiments. The model illustrates the “ectoderm is programmed to form skin; needs a chemical signal from underlying mesoderm in order to form nervous system” interpretation. In this view, Mangold’s graft of the dorsal lip (composed of mesoderm and endoderm) to a new place in the embryo caused nervous system development, along with a new body axis, in a new location, because the mesoderm “told the ectoderm what to do”. Viewed by the same paradigm, Holtfreter’s exogastrula had no nervous system because the mesodermal signal could not reach the ectoderm and similarly “tell it what to do”. Ectoderm thus differentiated according to its ‘default” condition and formed skin. The Model 1s typically show isolated pieces of ectoderm forming skin, and mesoderm/ectoderm combinations forming neurons, via some action of the mesoderm. I draw Model 1 on the board and leave it up for the duration of the lesson.
III. Experiments on the origin of mesoderm
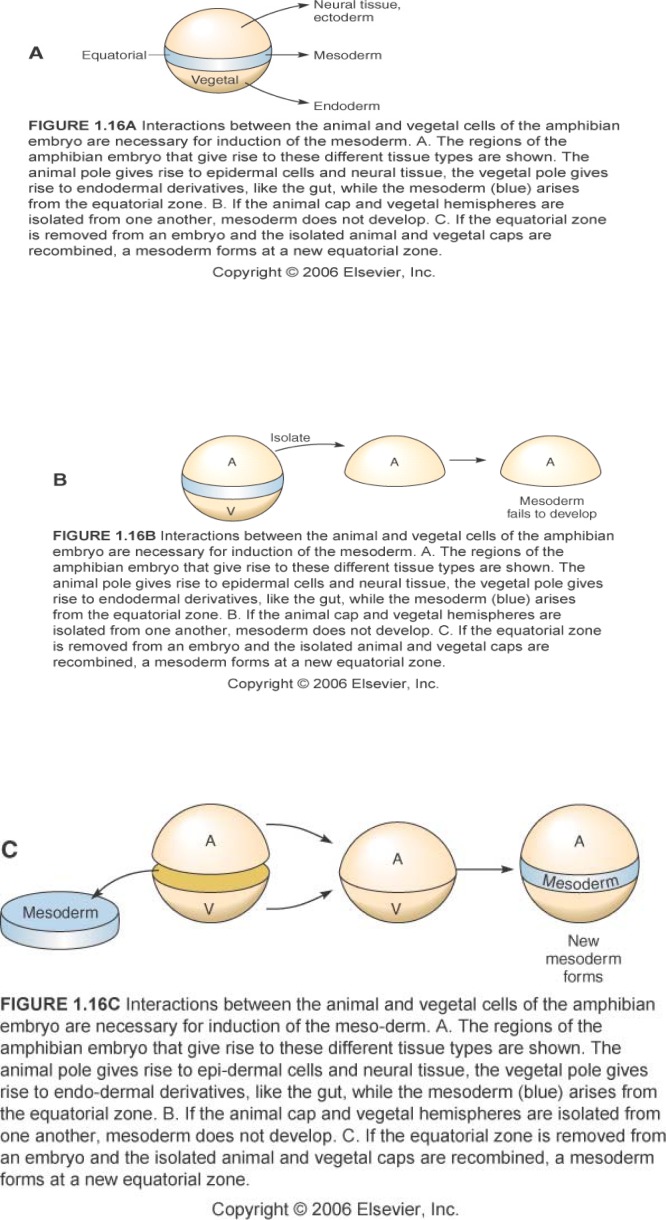
Model 1 highlights the importance of mesoderm in nervous system development. I continue the lesson with a consideration of the origin of mesoderm, the apparent “neuronal inducer tissue.” Given that mesoderm could so profoundly influence the development of other tissues in the gastrula, investigators wondered how, during development, mesoderm itself was established. Moving to the blastula, Nieuwkoop developed the “animal cap assay” (Nieuwkoop, 1969) to use in examining this question. The animal cap assay is a key feature of the paradigm-breaking experiments to be examined in the next phase of the lesson, so it is helpful to introduce it at this point.
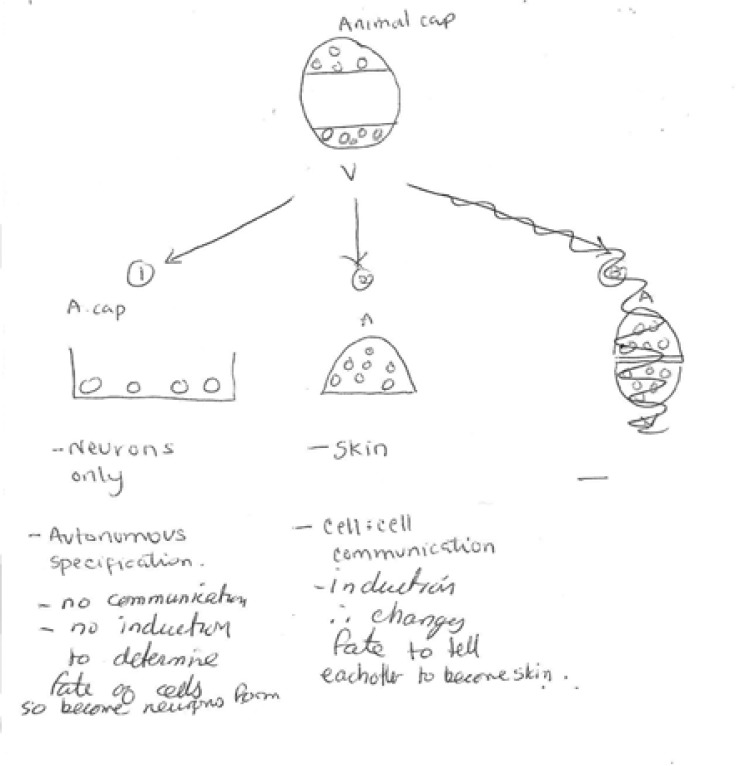
I present the animal cap story: If a blastula is cut into thirds perpendicular to the animal-vegetal axis, and the pieces isolated and cultured, the uppermost animal piece forms epidermis, and the vegetal-most piece forms yolky gut. If the animal and vegetal thirds are joined, mesoderm results (Figure 3a–c). This “animal cap assay” has been employed since the 1970s to study how mesoderm is induced (See Sanes et al., 2006 for review). For practice in model design, and as a basis for use of the animal cap assay in upcoming experiments, I challenge students to consider, in the animal cap assay: which tissue is the signaler and which is the signalee?
Figure 3.
Induction of mesoderm in amphibian embryos. The three experiments illustrated provide essential background for the “animal cap assay.” Figures from Sanes et al., 2006; used with permission.
From this point on, all cartooning and model design is done by students working in groups of 3–4. Cartooning is an effective way to get students to engage with the material, visualize experiments performed, and clearly conceptualize their own ideas (Mathewson, 1999; Hoskins et al., 2007). Each group is provided a transparency, markers, and 10 minutes to come up with a clearly stated hypothesis, cartooned model showing “how you think the system works,” and a diagrammed “proposed experimental test” of the model (CREATE steps: Elucidate the hypothesis, Think of the next Experiment). I use the overhead projector to compare and discuss the models and experiments proposed by each group, considering the range of options devised, their similarities and differences, and whether any models or experimental designs have logical inconsistencies.
Depending on the level of sophistication of the class, discussion of the experiments proposed is a good place for 15-minute content reviews (e.g. in discussing how to use in situ hybridization or antibody staining to determine which piece was expressing a mesodermal phenotype, a good deal of cell/molecular biology can be re-examined.) As an alternative, for an introductory level class or for general-education students, it can be sufficient to simply take as given that there are ways of using markers to tell which piece is mesodermal, and focus chiefly on the logic of the experimental design.
To encourage wide-ranging thinking and model real science, when time permits I challenge groups to come up with two distinct experimental tests of their models. Alternatively, the design-a-second experiment assignment could be given as homework, so students have more time to develop their ideas. When the additional experiments are examined in class, students see that individuals working from the same model still come up with a variety of different experimental approaches, and their preconceptions about science as rigid and predictable are shaken (Hoskins et al., 2007).
The animal cap discussion concludes with the instructor presenting the actual experiment that was done — one that matches many students’ cartoons — animal and vegetal pieces marked differently are combined, mesoderm induction takes place, and the experimenter then determines which marker is expressed by the induced mesoderm. The actual experiment showed that the signal for mesoderm formation comes from the vegetal piece (endoderm) and the induced mesoderm is formed from animal cap tissues (Gurdon et al., 1985). Understanding the animal cap is key to understanding the experiments that overturned the amphibian neural induction paradigm. I next return to the neural induction question, with new findings.
IV. Further investigations of animal cap -- anomalous data
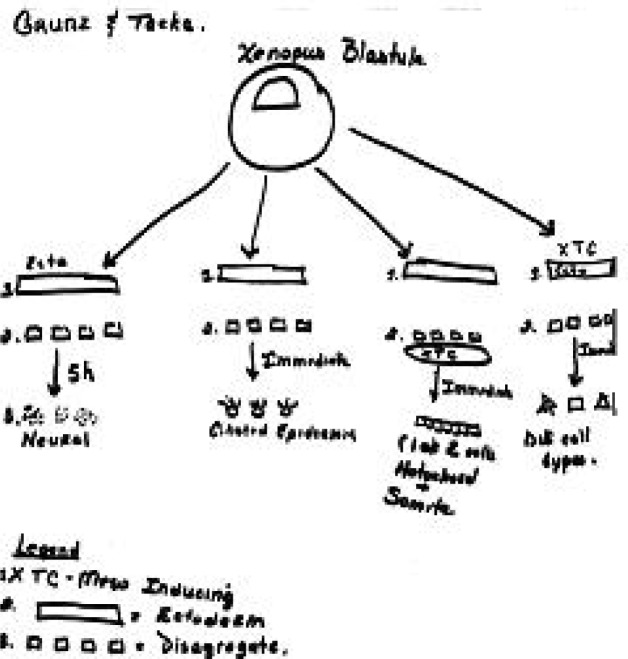
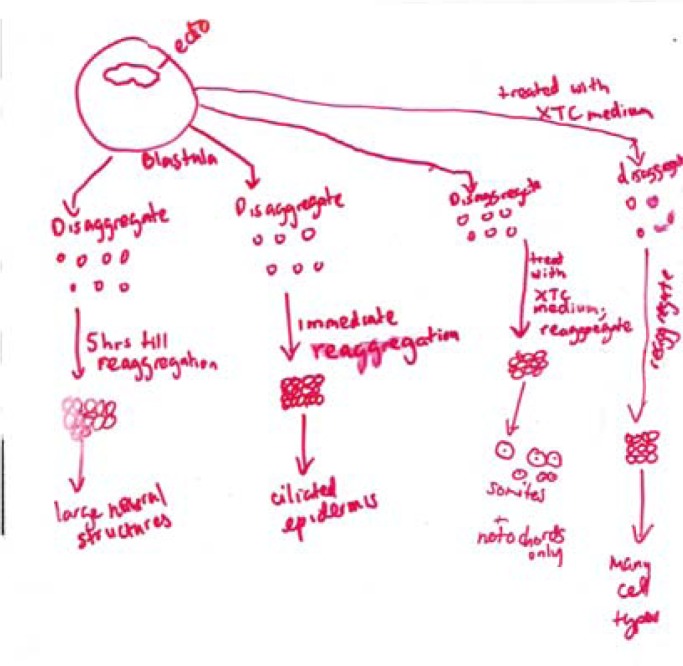
I explain that once the animal cap assay was established, numerous variations on the assay were performed to address a variety of questions. I focus on work of Grunz and Tacke (Grunz and Tacke, 1989), who carried out a series of experiments on animal caps cultured under various conditions.
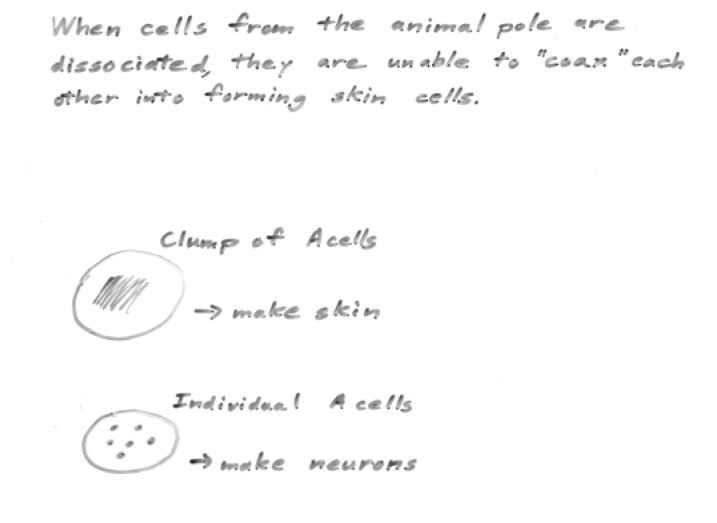
I hand out the abstract of Grunz and Tacke’s paper, and give the students 20 minutes to read it, look up any unfamiliar words, and again working in groups, to cartoon the basic results presented. (CREATE step: Read; here applied to the abstract only.) I compare cartoons created by different groups (e.g. Figure 4a, b) to ensure that all students understand Grunz and Tacke’s approach and findings. Notably, in one experiment, animal caps were dissociated, cultured five hours, and re-associated. Neural tissue differentiated. Yet according to Model 1, neurons cannot form without induction from mesoderm. This paper thus presents key paradigm-breaking information, but I do not make a point of this. Part of the value of the lesson for students is the experience of “discovering” the contradictory data themselves.
Figure 4a.
Sample Group work—student diagrams of the Grunz and Tacke experiments, based on reading the abstract in class.
Figure 4b.
Grunz and Tacke experiments as interpreted by a second small group (compare with Figure. 4a, to the left).
Once it is clear that students understand what Grunz and Tacke did, I challenge them first to interpret the findings described in the abstract (CREATE step; Analyze and interpret the data), and then to construct a model for neural and epidermal development consistent with Grunz and Tacke’s results (Model 2—see Figure 5, Figure 6, Table 1). I do not point out that these new findings (neurons can form from isolated animal cap cells) do not fit with the previously-designed Model 1, which is still up on the blackboard. After groups have drawn their models on transparencies, I screen each one and lead discussion both of the “majority model (e.g. Figure 5a) and of models that reveal misconceptions held by some students (see for example, Figure 5b).
Figure 5a.
Sample Student “Model 2” incorporating Grunz and Tacke’s findings.
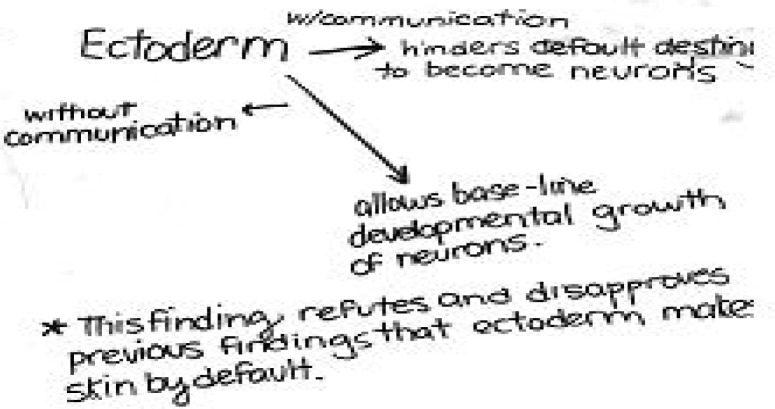
Figure 6a.
Sample model developed in a non-majors class. While this group did not clarify their model with a diagram, they did make an important prediction regarding cell-cell communication.
Figure 5b.
Group models can also be used to diagnose confused ideas and address them in class. In the upper cartoon from group of Bio majors, reference is made to “pregerminal” cells of the gastrula. The diagram also suggests that cell communication is handled exclusively by cell adhesion molecules, and proposes a “new experiment” (basically, culturing an isolated animal cap) whose outcome is already known. The lower diagram from a non-majors class illustrates confusion about which cells generate axons. Using diagrams like these as foci for class discussion allows misconceptions to be caught and clarified.
V. Testing a new model for ectoderm differentiation: What really is the default state?
Students are next challenged to design experiments to test their Model 2. (CREATE step: Think of the next Experiment). Depending on time available and the instructors’ goals, students can return to their groups and write out a hypothesis and cartoon outlining the experiments. Alternatively, experiments can be devised collaboratively in a “whole class” discussion. Typical proposed experiments use molecular approaches and tissue culture -- for example, the devising of assay systems in which neural-specific or epidermal-specific gene expression in ectoderm cells can be investigated under various conditions.
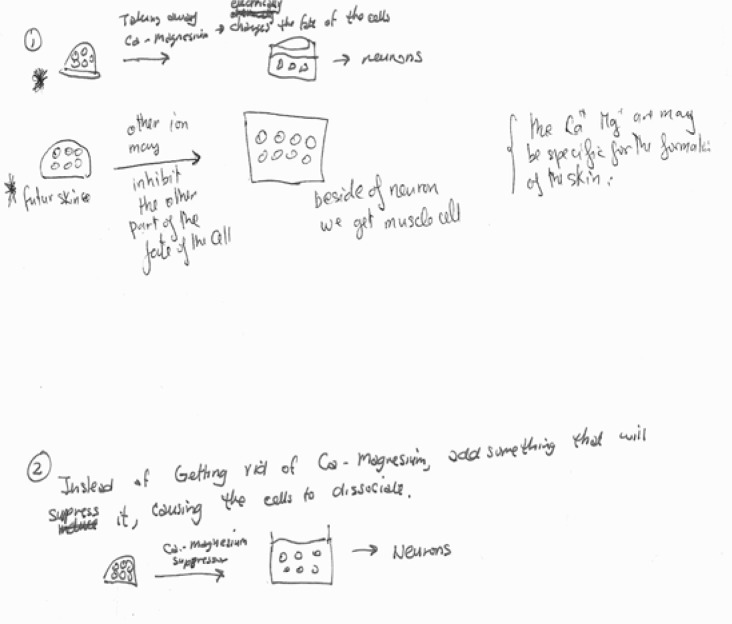
Experiments illustrated (Figure 7a, b) focus on the possible role of Ca+/Mg2+. Other typical experiments focus on timing issues—how long cells must be isolated in order to become neurons -- or on attempts to define or block the “signaling substance” responsible for the epidermal phenotype. Depending on my goals for the class, I may run a grant panel activity (Hoskins et al., 2007) to compare the different student-generated experiments.
Figure 7a.
Sample “next experiment” generated in a small group. This group of non-majors focused on the dissociation mechanism used by Grunz and Tacke, and the possibility that individual ions might have different effects on cell differentiation. Their experiment, (which lacks controls), proposes looking at what differentiates from dissociated cells that were separated without depleting Ca+/Mg2+.
Figure 7b.
upper. This group’s modification of Model 2 — “maybe mesoderm causes a similar mechanism of Ca+, Mg+ reduction and subsequent differentiation of cells into neurons” leads directly to their proposed next experiment (7b, lower) in which they attempt to isolate individual effects of Ca or Mg, in a controlled situation. Even hypotheses that are off the mark are useful.
I next move to a new study -- Hemmati-Brivanlou and Melton (1992), studying the role of activin, a member of the TGFß family in mesoderm induction and the patterning of the amphibian body axis. The investigators examined the role of activin-mediated signaling in this process, using a truncated activin receptor developed for this purpose (Hemmati-Brivanlou and Melton, 1992, 1994). Even for students who have not taken cell/molecular biology, the logic of this approach can be made clear. I describe the truncated receptor as “broken” and for introductory classes or general-education students take its existence and ability to interfere with signaling as given. For more advanced classes, discussing the genetic engineering of such a modified receptor, as well as considering why the modified receptor disrupts cell signaling, is an opportunity to review a good deal of cell/molecular biology, in context. (Alternatively, advanced students can be assigned to review this material as homework). For all levels of students, I discuss how introducing such a receptor into the system interferes with signal transduction.
Students begin to appreciate that much is learned in science from experiments that rule out particular explanations. Such a realization can counter student misconceptions that science is linear and predictable, and “you always know what to do next” (see Hoskins et al., 2007 for further discussion).
Depending on the level of sophistication of the class, the instructor could initiate a discussion about why the authors chose this approach to deplete activin activity rather than performing gene knockouts (not feasible in tetraploid Xenopus) or using RNAi (not an available technology in 1992). Students in advanced classes could also be challenged to consider (either in class or as a homework assignment) how such a problem might be approached in a genetic model system such as Drosophila.
The surprising result in the disruption-of-signaling experiments is that when animal caps from embryos expressing the truncated activin receptor were explanted, they expressed neural genes (Hemmati-Brivanlou and Melton, 1992; 1994), despite the absence of mesoderm. The authors suggested that activin was involved in some way in the neural/epidermal choice. Further studies showed that the truncated activin receptor affected signaling not only mediated by activin, but also signaling mediated by other TGFß ligands, including Bone Morphogenetic Protein 4 (Hemmati-Brivanlou and Melton, 1997). I explain to the students that additional experiments showed that while the truncated activin receptor disrupted signaling through both BMP-4 and activin, the key player for neural/epidermal choice appears to be BMP-4 (Fainsod et al., 1994; Hemmati-Brivanlou and Thomsen, 1995; see Hemmati-Brivanlou and Melton, 1997, for discussion).
At this point students are aware of two experimental situations in which blastula ectoderm forms neurons: (1) dissociation of an animal cap (Grunz and Tacke, 1989); or (2) decreased BMP-4 signaling between cells (Hemmati-Brivanlou and Melton, 1992). I challenge the students to again work in small groups and integrate the new information with their previous Model 2. Students quickly realize that the new findings are consistent with the phenomenon discovered previously by Grunz and Tacke, and suggest a molecular mechanism for Grunz and Tacke’s results. That is, in dissociating animal caps, you disrupt cells’ ability to “talk to their neighbors.” Dissociated caps produce neurons. If you block BMP, you bring about the same outcome, suggesting that the cellular conversation is likely to involve this molecule.
I ask students for a new hypothesis consistent with all of the findings. Typically they propose: Cells that signal each other with BMP 4 become epidermal; cells that don’t, or can’t, become neural. In these new models, the intrinsic programming (default state) of ectodermal cells is suggested to be neural--! Often by this point, a student has raised the issue of incompatible Model 1 and Model 2. If not, I now ask the students to take another look at Model 1. Clearly the prediction of their new modified Model 2 is in direct conflict with the experiments that began the class (organizer transplant, exogastrula) and formed the basis for Model 1.
VI: New model encompassing old and new findings: how does ‘natural’ neural differentiation occur in an intact embryo?
At this point I recap the puzzling situation. Based on the activin/BMP results we have modified Model 2 and predicted that the neuronal phenotype is “default” for ectoderm. However, this model directly contradicts our original model based on Spemann-Mangold and Holtfreter’s results (Model 1), where the ectoderm only differentiated as neuronal if it received a chemical signal from mesoderm. Isolated animal caps were thought, by that model, to “default” to skin. Having summarized this bit of cognitive dissonance, I point out that in a normal embryo, fate maps show that the nervous system forms from a sheet of contiguous cells, not from cells that have been dissociated. But in Model 2, ectodermal cells in a contiguous sheet (control animal caps) form epidermis (and they do the same in the exogastrula). How can these conflicting phenomena be reconciled?
We review Mangold’s experiment, which initiated our sense that mesoderm does play a role in the development of the nervous system. Only embryos with grafts of mesodermal “organizer” tissue developed secondary body axes including well-organized nervous systems. Yet, no mesoderm is present in either of the scenarios (dissociated animal cap cells or animal cap cells with dysfunctional BMP signaling) that can produce neurons. I list each of these seemingly contradictory points on the board.
I next challenge the students to devise a model that takes all the existing information into account, and “explains how the nervous system develops in an intact embryo.” Students recognize that in a normal embryo neurons don’t form via a mechanism involving dissociation, and recognize the conflict between Model 1 and the modified Model 2. Some students are frustrated at this point, because the carefully designed models have turned out to be contradictory, while other students are intrigued that there is more to the puzzle than they realized. Some of the modified Model 2’s (see Figure 7a, for example) have already attempted to reconcile the in vivo results (‘mesoderm induces the nervous system’) with Grunz and Tacke’s findings (‘dissociation of cells results in differentiation of nervous system). The model illustrated, however, is limited by its focus on the use of Ca-Mg free solutions, rather than the physical separation of animal cap cells, as the key variable.
Arguing about such issues and defending their ideas, or designing experiments to test them, helps students experience some of the activities undertaken by scientists in their own labs. Often there is a long period of brainstorming and planning before scientists actually start working at the bench. Participating in such activities can help students understand how science is really done, in contrast to how it may appear from their textbooks (AAAS, 1990; Ryder et al., 1999; Driver et al., 2000).
Groups design new models (Model 3, Table 1). It takes some time for students to work through the seeming contradictions, but eventually typical Model 3s incorporate an inhibitory influence from the dorsal lip. That is, students suggest that as they proposed originally (Model 1), the dorsal lip does indeed secrete something. However the students revise their suggestion about the role of the secretions by proposing that rather than “telling the cells to become neurons,” the effect of the lip is in fact inhibitory. “It tells them NOT to become skin.” Models suggest that the dorsal lip molecules block the BMP signal, which itself is blocking the expression of the neuronal default state. By inhibiting an inhibitor, the dorsal lip allows the “natural” baseline programming (neural) to be expressed.
In devising and discussing Model 3, students realize that they had previously leaped to two wrong conclusions when considering the initial Spemann/Mangold and Holtfreter data. First they had assumed that any signal emanating from the dorsal lip must be “positive” — a signal that turned genes on or “made something new happen.” Inhibitory signals were never considered. Students noted that they had also assumed that cells in a sheet of isolated ectoderm are somehow “inert” rather than potentially involved in active communication with each other.
In working through this problem, students realize that over a period of some 50 years, the model for “neural induction” a fundamental early event in vertebrate development, essentially shifted 180 degrees. (Interestingly, this is an example where Occam’s razor leads to an incorrect model, as the simplest explanation for Spemann and Mangold’s original finding was not, ultimately, the best.)
The completion of Model 3 design/discussion is a reasonable stopping point for some classes; however, I suggest that looking more closely at the presentation of some of the first data that didn’t fit with the original paradigm can provide interesting additional insights. I have extended this lesson as outlined below, delving beyond the data into the nature of science.
VII. Why didn’t Grunz and Tacke write the “neural is the default state” article?
Hemmati-Brivanlou and Melton’s discovery was made in experiments on mesoderm induction, where NCAM expression by “induced” ectoderm was being used as a positive control. The idea (based on the prevailing paradigm) was to use expression of NCAM in isolated animal caps as a sign that particular experimental treatments had induced mesoderm in the cap. Based on the prevailing “mesoderm induces ectoderm to make nervous system” paradigm, it was expected that caps with induced mesoderm would in turn induce neural markers. When control animal caps in which mesoderm had not been induced (due to inhibition of activin signaling) expressed NCAM independently, the authors recognized this as a paradigm-shifting finding. They followed up, after writing their research article, and discussed the new data in an additional mini-review in Cell, aimed at alerting a broader (beyond neuroscience) audience to this fundamental rethinking of how the vertebrate nervous system develops (Hemmati-Brivanlou and Melton, 1997).
Grunz and Tacke’s finding was not similarly highlighted in their 1989 paper in Cell Differentiation and Development. These investigators included the data as one part of a bigger study focused on other aspects of mesoderm differentiation. How can it be that these authors have “not noticed” or “explained away” their paradigm-breaking results, which were only brought to the fore by their being cited by the later authors in a more widely-read journal? In part, the answer is that Grunz and Tacke did not present their findings as paradigm-breaking. An advanced class might find it interesting to read the discussion section of the Grunz and Tacke paper, and model the explanations suggested for the seemingly anomalous differentiation of neurons from dissociated ectodermal cells. Ultimately, these authors explained their results from a perspective solidly within the Model 1 “mesoderm induces ectoderm to make nervous system” paradigm.
Examination of such issues allows students to consider human aspects of science, for example how focus on one set of ideas might make it difficult to see alternatives (none of the students suggested a Model 3 mechanism early in the lesson when the class designed Model 1, although in principle they could have), or how the existing paradigm can influence one’s interpretation of new results in unanticipated ways. Students consider, perhaps for the first time, that research scientists must guard against becoming overly entrenched in existing paradigms.
Textbooks rarely discuss the nature of science or focus on paradigms, and I find this added discussion to be a useful way to begin to wrap up the story and give students something to think about beyond what they learned from considering the experimental evidence and building the models.
VIII. Evolutionary considerations: Why is a “bone morphogen” in the gastrula?
Finally, I ask the students one more question: What is Bone Morphogenetic Protein doing in the gastrula? This question can be assigned as homework or worked on in small groups. In the latter case, I pose the question and then give the groups 1–15 minutes to come up with two different suggestions. Our goal in requiring more than one proposed explanation is to underscore the idea that explanations in Biology often call for multiple answers or hypotheses, some of which will be rejected later. Brainstorming can range widely and I emphasize that students are “allowed” to propose something that turns out to be incorrect, in an effort to underscore the open-ended nature of scientific investigation.
After all groups have generated ideas, the class reviews and discusses the proposed explanations. Typically, some groups suggest that BMP is expressed in cells of the embryo that are “turning into bone.” In subsequent discussion however, students realize that no bone will form in the tadpole until significantly later in development, and that in any case bone ultimately is derived from mesodermal, not ectodermal tissue. Yet the BMP in question is definitely acting on ectodermal animal caps isolated from a blastula. Other groups propose that ectoderm is bi-potential early in development and later “loses” its ability to generate mesodermal derivatives. Proposals of this type are debunked by other students who review the data on mesoderm induction. As the discussion progresses, students again experience cognitive dissonance as their “BMP induces bone; so if there is BMP in animal caps, there must be bone there” interpretation fails to explain the actual experimental observations.
I point out that the confusion the students are experiencing is typical of what happens in research labs when findings from a carefully-designed experiment fail to fall along the lines defined by the experimenter’s initial hypothesis. That is, in real-life research situations, it is not uncommon to predict that your experimental results will be either “A” or “B,” only to have the critical experiment produce result “Q.” Students begin to recognize that reacting to unexpected data is a part of science, as much as (or perhaps more than) the officially sanctioned approach of “read all the background information and then formulate a hypothesis and design an experiment” method which is more typical of their previous classroom laboratory experiences.
Eventually (or with prompting via the questions: What does BMP stand for? Why was that molecule named BMP?) a student or group looks at the problem from a different angle, and realizes that the molecule in question was named based on its function in a particular experimental assay. That is, the molecule is named BMP for historical reasons, based on the bone-growth scenario in which it was first detected. The name should not, however, be assumed to be an all-encompassing explanation of function. Students recognize that BMP plays a key role in the ectoderm’s skin/nervous system decision early in development, and then “returns” to play a distinct role in the development of a mesodermal derivative, bone, later on. I ask them what the molecule might have been named had it first been discovered in the blastula. Typical suggestions include “EIP,” Epidermal Inducer Protein, or NIP, Neural Inhibition Protein. Students also recognize that had the molecule first been discovered in the embryo, the question for biologists later on might have been “what is Neural Inhibition Protein doing in developing bone?”
Discussions of this sort are, in my view, invaluable in that they provide insight both into the history of signaling systems and into the way science is done. In this case, students begin to recognize the need to guard against being too easily led by assumptions about function (e.g. it’s called Bone Morphogenetic Protein, so wherever we find it there must be bone). Students also recognize the importance of being open to unexpected implications of one’s own data, and, to the extent possible, of maintaining awareness of prevailing paradigms that influence their thinking.
Equally important, students figure out for themselves an important lesson regarding evolutionary conservation of developmental mechanism (Salie et al., 2005). Embryos recycle key signaling molecules. The same extra-cellular signals and signaling systems may be used and re-used at different developmental time points for quite distinct functional/morphological purposes. As evolution becomes an ever-more influential paradigm in developmental biology, underscoring this theme aids student understanding.
As a final step in helping students integrate their understanding of different aspects of the lesson, I assign a final concept map, in which students add new concepts to those on their original map, noting in particular the links that needed to be changed, relabeled, or omitted in light of the new information. Students typically add a section based on “nature of science issues” as well. Depending on time available and the instructor’s goals, more advanced classes can follow up the story, for example reading subsequent work on the role of FGF in modulating the action of BMP-4 (see for example; Kudoh et al., 2004), the mechanisms by which the Spemann organizer signals interact with BMP-4 (Hemmati-Brivanlou and Thomsen, 1995; Zimmerman et al., 1996; Piccolo et al., 1996), or considering whether this model for amphibians is broadly applicable in other vertebrates (Stern, 2001).
DISCUSSION
This exercise (summarized in Tables 1 and 2) helps students (1) to see biology as an extended process of investigation, with models being developed, modified, or possibly even discarded as new findings emerge and are integrated with old. The exercise also (2) provides numerous insights, not often present in textbooks, into how science works. The idea that (3) prevailing paradigms can influence understanding in subtle ways, and the idea that paradigm shifts are possible, i.e. that “not everything is known, and even what we ‘know’ might change” provides students with a new vantage point from which to consider experimental findings in any system. Finally, (4) the recycling of the same signaling molecules in distinct tissues at different times in development provides an example of evolutionary conservation of developmental mechanism.
Table 2.
The CREATE approach as adapted for the paradigm-shift lesson. Pedagogical tools of the CREATE method, including concept mapping, cartooning, hypothesis development, modeling, close analysis of data, and experimental design, are active-learning approaches that encourage student participation and creative thinking. See Hoskins et al. 2007 for a more comprehensive study of the effects of CREATE on students’ critical thinking and on their attitudes toward science research and researchers.
| The CREATE approach, and related student activities | |
|---|---|
| C.R.E.A.T.E. step | Student Activity |
| Consider | Construct concept maps based on the central issues outlined in introduction and first model. Begin to examine relationships between variables. Design a group model consistent with early work in the system (Model 1). |
| Read | Draw cartoon models consistent with results of animal cap experiments and (later) results of other experimenters. Read Grunz and Tacke abstract and cartoon the findings of the series of experiments. |
| Elucidate hypotheses | Propose a testable hypothesis based on your model. |
| Analyze and interpret the data | Examine discrepancies between models (Model 1 and Model 2); try to resolve this controversy in new Model 3. Why is a paradigm shift necessary? |
| Think of the next Experiment | What experiment or study should be done to follow up on a given model and test the hypothesis you proposed? Cartoon your follow-up study on a transparency for in-class discussion and/or grant panels. Draw a second concept map that integrates key points from the three models, as well as “nature of science” issues |
As students participate in the series of class sessions and homework assignments outlined above, they recognize that long-standing models about fundamental issues may be subject to substantial revision (or in the present case, a 180° reversal), thus Take-home message 1: Science constantly changes, and old models may not stand up to new data. Even though something is published, it is still open for criticism and re-evaluation.
When students realize that the data refuting the “skin as default state” model were in fact available, yet not accurately interpreted, years before the model changed, they begin to see how entrenched paradigms can influence scientific thinking, even for experimenters who consider themselves unbiased. Thus Take-home message 2: Having the data is not enough. If data are not interpreted accurately, their significance may be missed. Even though scientists are, in principle, “objective,” the dominant paradigm of the time may influence interpretations in ways of which the experimenters themselves are unaware.
The late 20th-century model defines BMP as a key player in the differentiation of epidermis. Beyond the question of how ectoderm becomes epidermis or nervous system, the question of why “bone” morphogenetic protein is present in an embryo well in advance of any bone morphogenesis, leads to Take-home message 3: Key signaling molecules have been “recycled” during evolution. The naming of molecules is in some cases simply a reflection of the context in which they were discovered. Thus, this lesson offers an opportunity to reinforce students’ understanding of evolution as well as how science works (e.g. you find it, you name it).
In this lesson, “less is more.” The class can be taught based on minimal background information, all of which can be provided in a 15–20 minute lecture. The students spend the bulk of their class time devising and discussing models that reflect their understanding of data presented, and reading, cartooning, and discussing the abstract of a related paper. Active learning approaches and modeling/visualization methods used in the class are well-established aids to understanding (Chickering and Gamson, 1987; Brooks and Brooks, 1993; Bransford et al., 2000; Weimer, 2002). I suggest that because so many of the issues raised in the class are applicable to any scientific investigation, that limiting content in this situation nevertheless leads to learning gains.
It is also notable that this whole series of findings did not start with someone saying; “Let’s find out whether BMP-4 has a role in neural development” and then designing an experiment based on “The Scientific Method” often presented in textbooks. Instead, one finding led to another in a somewhat indirect way. The key discovery (that inhibition of BMP signaling led to neuralization of animal caps) was made in an experiment actually aimed at analyzing mesoderm induction; and in fact, focused originally on a different molecule, activin. It was critical to follow up this surprising side road, especially since this finding contradicted decades of “mesoderm induces the nervous system” dogma.
While it takes time for students to make these discoveries themselves, through discussion in their small groups, I feel this is time well-spent. The process of integrating new findings with old, modifying models to fit novel data, or perhaps throwing out old models altogether is integral to the practice of research science, but often not a part of prescribed courses in the Biology major. I suggest that by purposely exposing students to cognitive dissonance, and challenging them to creatively solve problems in biology, teachers help them experience some of the excitement of scientific discovery that may be lacking in “programmed” laboratory experiences or content-heavy lecture courses. Such an approach is in alignment with “best practices” in science pedagogy (Bransford et al., 2000) as well as with recent recommendations of scientific reform documents, that science teaching more accurately reflect the science research process (NRC 2003). I have taught this lesson in the context of a Developmental Neurobiology course for Biology majors, as well as in a general Biology course required for non-science majors. I have not rigorously assessed learning gains specific to this lesson, but in each course, student self-assessed understanding of and appreciation for the process of science increased during the semester (S. Hoskins, in preparation). I cannot attribute the positive changes in attitude specifically to this paradigm-shift lesson, however, as it is only one of a series of active learning activities carried out during the semester in each course. Faculty interested in assessing possible learning gains as a result of this approach may wish to adapt one of the assessments in our more comprehensive CREATE study (Hoskins et al., 2007), or consider the Field-Tested Learning Assessment toolkit designed for science instructors and available online at www.flaguide.org.
A recurring concern in teaching is “content coverage.” I recognize that in place of a lesson like this one, which could consume several class periods, an instructor could rapidly tell the entire story in a single traditional lecture. A significant body of literature in science education, however, argues against such an approach, because of the passivity it encourages in students, and the difficulties students have in learning when they are simply told information (see for example, Chickering and Gamson, 1987; Brooks and Brooks, 1993; Bransford et al., 2000; Siebert and McIntosh, 2001). In addition, allowing students to make some of the discoveries themselves, through devising their own mechanisms to explain new results, models scientific thinking in a fairly realistic way. By challenging students to generate their own ideas, we cast them in the role of scientists and show science as an ever-evolving set of concepts, rather than a set of textbook truths to be absorbed via lecture and memorized for an exam.
In closing, I wish to underscore the success of this lesson with general-education students as well as biology majors. Textbooks for general-education students tend to focus on content, and to present the standard basics of introductory biology, albeit with more colorful metaphors and flashier graphics than may appear in the majors’ books. Given that many general-education students have been assiduously avoiding biology since high school, such a presentation may still not be as effective as would be ideal. I suggest that it is critically important that general-education students, whose votes on key issues such as stem cell legislation and national research funding levels, will play a significant role in the scientific future of the U.S., have a deeper understanding of “what scientists do” and how they do it. My experience teaching this neural development lesson to general-education students suggests that the creativity and “design” aspects of biological research are quite attractive and understandable to this group of students, many of whom are artists or designers themselves. Thus, I propose that lessons with a “nature of science” component could be helpful, in addition to providing students practice in data analysis and model design, in conveying to nonscientists the beauty and creativity inherent in research science.
Figure 6b.
Model from a non-majors class. Even without significant background in developmental biology, students can work their way to the idea that cell-cell communication (here termed ‘coaxing’ plays an important role in determining ectodermal cell fate.
Acknowledgments
This work was supported by the National Science Foundation (CCLI program #0618536). I thank the National Science Foundation for support, Dr. Leslie Stevens for comments on the manuscript, and CCNY students of Science 10001, Biology 101 and Biology 375 for participation in and feedback on the exercise.
Figure 3 from Sanes et al., Development of the Nervous System, Second Edition, Copyright 2006, Elsevier, Inc., used with permission. Figure 1, 2 from Willier et al., 1955; out of print.
REFERENCES
- American Association for the Advancement of Science . Science for all Americans. New York: Oxford University Press; 1990. The nature of science; pp. 1–14. [Google Scholar]
- Bransford J, Brown A, Cocking RR. How people learn: brain, mind, experience, and school. Expanded Edition. Washington, DC: National Academies Press; 2000. [Google Scholar]
- Brooks JG, Brooks MG. The case for constructivist classrooms. Alexandria, VA: Association for Supervision and Curriculum Development; 1993. [Google Scholar]
- Cech T, Kennedy D. Doing more for Kate. Science. 2005;310:1741. doi: 10.1126/science.1123580. [DOI] [PubMed] [Google Scholar]
- Chickering AW, Gamson ZF. Seven principles for good practice. AAHE Bulletin. 1987;39:3–7. [Google Scholar]
- Driver R, Newton P, Osborne J. Establishing the norms of scientific argumentation in classrooms. Science Ed. 2000;84:287–312. [Google Scholar]
- Fainsod A, Steinbeisser H, De Robertis EM. On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. EMBO J. 1994;13:5015–5025. doi: 10.1002/j.1460-2075.1994.tb06830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink LD. Creating significant learning experiences. San Francisco, CA: John Wiley & Sons; 2003. A taxonomy of significant learning; pp. 27–59. [Google Scholar]
- Foertsch J. Models for undergraduate instruction: the potential of modeling and visualization technology in science and math education. Targeting curricular change: reform in undergraduate education in science, math, engineering, and technology; Report of the 1996 AAHE Conference on Institutional Change; plus four commissioned papers on pedagogical reform in the SMET disciplines; Washington, DC: American Association of Higher Education; 2000. pp. 37–40. [Google Scholar]
- Grunz H, Tacke H. Neural differentiation of Xenopus laevis ectoderm takes place after disaggregation and delayed reaggregation without inducer. Cell Differ Dev. 1989;28:211–217. doi: 10.1016/0922-3371(89)90006-3. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Fairman S, Mohun TJ, Brennan S. Activation of muscle specific actin genes in Xenopus development by an induction between animal and vegetal cells of a blastula. Cell. 1985;41:913–922. doi: 10.1016/s0092-8674(85)80072-6. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton D. A truncated activin receptor inhibits mesoderm induction and formation of axial structures in Xenopus embryos. Nature. 1992;359:609–614. doi: 10.1038/359609a0. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton D. Inhibition of activin receptor signaling promotes neuralization in Xenopus. Cell. 1994;77:273–281. doi: 10.1016/0092-8674(94)90319-0. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton D. Vertebrate embryonic cells will become nerve cells unless told otherwise. Cell. 1997;88:13–17. doi: 10.1016/s0092-8674(00)81853-x. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Thomsen GH. Ventral mesodermal patterning in Xenopus embryos: expression patterns and activities of BMP-2 and BMP-4. Dev Genet. 1995;17:78–89. doi: 10.1002/dvg.1020170109. [DOI] [PubMed] [Google Scholar]
- Holtfreter J. Die totale Exogastrulation, eine Selbstablosung des Ektoderms vom Entomesoderm. Wilhelm Roux’ Arch Entwickl Mech Org. 1933;129:669–793. doi: 10.1007/BF00656583. [DOI] [PubMed] [Google Scholar]
- Holtfreter J, Hamburger V. Embryogenesis: progressive differentiation—amphibians. In: Willier BH, Weiss PA, Hamburger V, editors. Analysis of development. Philadelphia, PA: W.B. Saunders; 1955. pp. 230–296. [Google Scholar]
- Hoskins S. “But if it’s in the newspaper, doesn’t that mean it’s true?”. 2008. Developing critical reading and analysis skills by analyzing newspaper science using C.R.E.A.T.E. In press, Am Biol Teach.
- Hoskins S, Stevens L, Nehm R. Selective use of the primary literature transforms the classroom into a virtual laboratory. Genetics. 2007;176:1381–1389. doi: 10.1534/genetics.107.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh T, Concha ML, Houart C, Dawid IB, Wilson SW. Combinatorial Fgf and Bmp signaling patterns the gastrula ectoderm into prospective neural and epidermal domains. Development. 2004;131:3581–3592. doi: 10.1242/dev.01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn TS. The structure of scientific revolutions. 2nd ed. Chicago, IL: The University of Chicago Press; 1970. [Google Scholar]
- Mathewson JH. Visual-spatial thinking: an aspect of science overlooked by educators. Sci Educ. 1999;83:33–54. [Google Scholar]
- National Research Council . Evaluating and improving undergraduate teaching in science, technology, engineering and mathematics. Washington, DC: National Academies Press; 2003. Seven principles of learning; pp. 20–22. [Google Scholar]
- Nieuwkoop PD. The formation of mesoderm in urodelan amphibians. Paper I. Induction by the endoderm. Rouxs Arch Dev Biol. 1969;162:341–373. [Google Scholar]
- Novak JD, Gowin DB. Learning how to learn. New York and Cambridge, UK: Cambridge University Press; 1984. Concept mapping for meaningful learning; pp. 15–54. [Google Scholar]
- Novak JD. Concept mapping: a useful tool for science education. J Res Sci Teach. 1990;27:937–949. [Google Scholar]
- Oulton C, Dillon J, Grace MM. Reconceptualizing the teaching of controversial issues. Int J Sci Educ. 2004;26:411–423. [Google Scholar]
- Piccolo S, Sasai YI, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder J, Leach J, Driver R. Undergraduate students’ images of science. J Res Sci Teach. 1999;36:201–209. [Google Scholar]
- Salie R, Nierderofler V, Arber S. Patterning molecules: multitasking in the nervous system. Neuron. 2005;45:189–192. doi: 10.1016/j.neuron.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Sanes D, Reh T, Harris W. Development of the nervous system. 2nd ed. London, UK: Elsevier Academic Press; 2006. [Google Scholar]
- Seethaler S. Helping students make links through science controversy. Am Biol Teach. 2005;67:265–274. [Google Scholar]
- Seymour E, Hewett N. Talking about leaving: why undergraduates leave the sciences. Boulder, CO: Westview Press; 1997. [Google Scholar]
- Siebert ED, McIntosh WJ. College pathways to the science education standards. Arlington, VA: National Science Teachers Association; 2001. [Google Scholar]
- Spemann H, Mangold H. Uber Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. Arch f mikr Anat u Entw Mech. 1924;100:599–638. [Google Scholar]
- Steitz J. Commentary: Bio 2010 — new challenges for biology educators. Cell Biol Educ. 2003;2:87–91. doi: 10.1187/cbe.03-02-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CD. Initial patterning of the central nervous system: how many organizers? Nat Rev Neurosci. 2001;2:92–98. doi: 10.1038/35053563. [DOI] [PubMed] [Google Scholar]
- Weimer M. Learner-centered teaching. San Francisco, CA: John Wiley and Sons; 2002. [Google Scholar]
- Willier BH, Weiss PA, Hamburger V, editors. Analysis of Development. Philadelphia, PA: W.B. Saunders; 1955. [Google Scholar]
- Zimmerman L, De Jesus-Escobar J, Harland R. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]