Abstract
The continually renewing epithelium of the intestinal tract arises from the visceral endoderm by a series of complex developmental transitions. The mechanisms that establish and maintain the processes of cellular renewal, cell lineage allocation, and tissue restriction and spatial assignment of gene expression in this epithelium are unknown. An understanding of the regulation of intestine-specific gene regulation may provide information on the molecular mechanisms that direct these processes. In this regard, we show that intestine-specific transcription of sucrase-isomaltase, a gene that is expressed exclusively in differentiated enterocytes, is dependent on binding of a tissue-specific homeodomain protein (mouse Cdx-2) to an evolutionarily conserved promoter element in the sucrase-isomaltase gene. This protein is a member of the caudal family of homeodomain genes which appear to function in early developmental events in Drosophila melanogaster, during gastrulation in many species, and in intestinal endoderm. Unique for this homeodomain gene family, we show that mouse Cdx-2 binds as a dimer to its regulatory element and that dimerization in vitro is dependent on redox potential. These characteristics of the interaction of Cdx-2 with its regulatory element provide for a number of potential mechanisms for transcriptional regulation. Taken together, these findings suggest that members of the Cdx gene family play a fundamental role both in the establishment of the intestinal phenotype during development and in maintenance of this phenotype via transcriptional activation of differentiated intestinal genes.
Full text
PDF



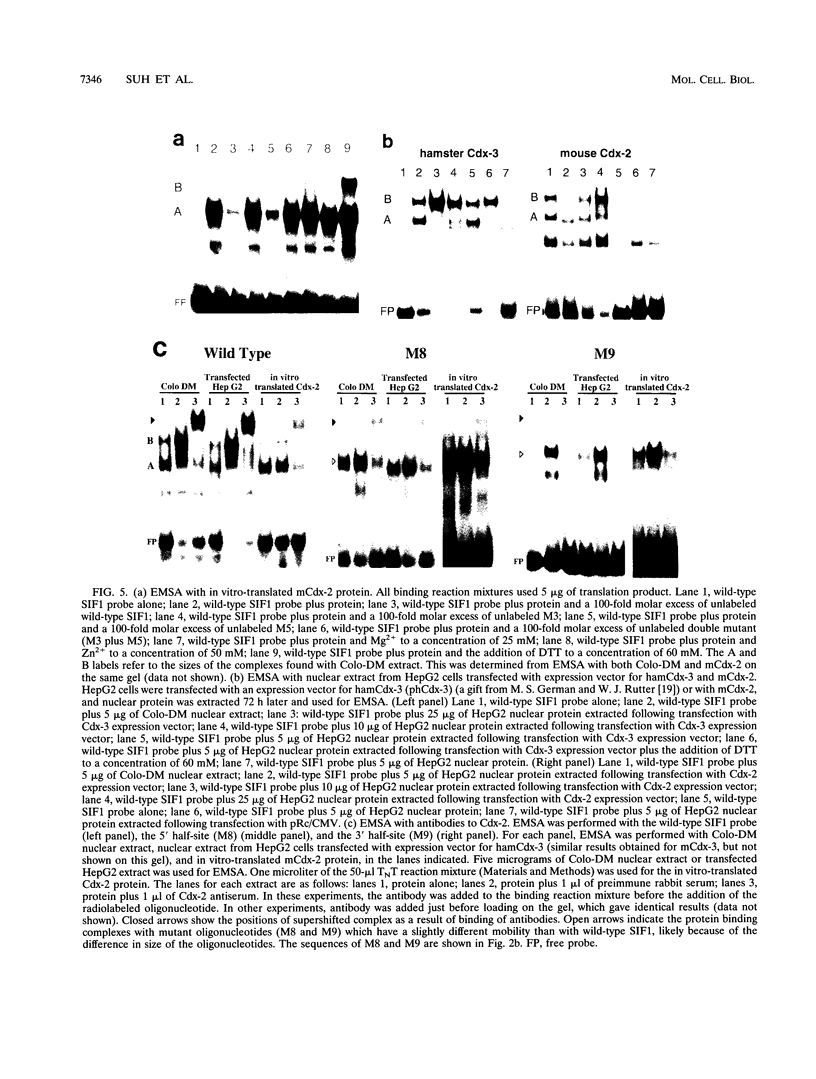
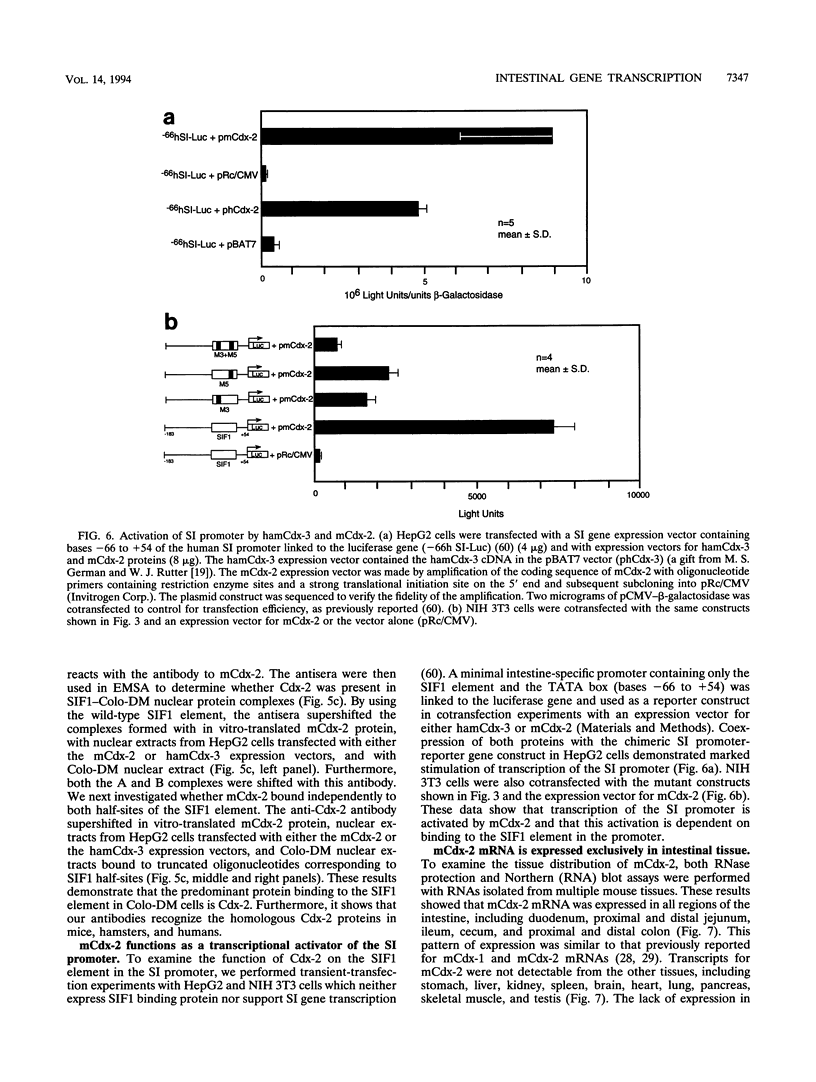

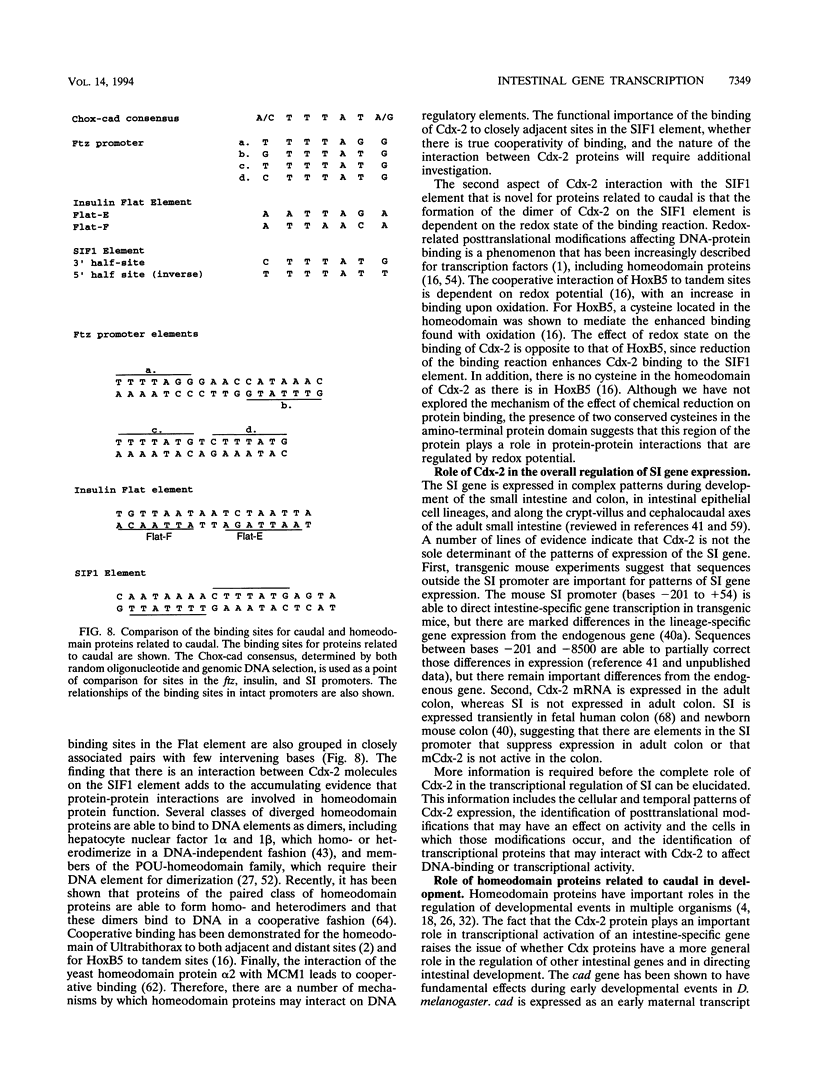


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. F. Redox control of transcription: sensors, response regulators, activators and repressors. FEBS Lett. 1993 Oct 18;332(3):203–207. doi: 10.1016/0014-5793(93)80631-4. [DOI] [PubMed] [Google Scholar]
- Beachy P. A., Varkey J., Young K. E., von Kessler D. P., Sun B. I., Ekker S. C. Cooperative binding of an Ultrabithorax homeodomain protein to nearby and distant DNA sites. Mol Cell Biol. 1993 Nov;13(11):6941–6956. doi: 10.1128/mcb.13.11.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B., Wright C. V., De Robertis E. M., Cho K. W. Organizer-specific homeobox genes in Xenopus laevis embryos. Science. 1991 Jul 12;253(5016):194–196. doi: 10.1126/science.1677215. [DOI] [PubMed] [Google Scholar]
- Botas J. Control of morphogenesis and differentiation by HOM/Hox genes. Curr Opin Cell Biol. 1993 Dec;5(6):1015–1022. doi: 10.1016/0955-0674(93)90086-6. [DOI] [PubMed] [Google Scholar]
- Bürglin T. R., Finney M., Coulson A., Ruvkun G. Caenorhabditis elegans has scores of homoeobox-containing genes. Nature. 1989 Sep 21;341(6239):239–243. doi: 10.1038/341239a0. [DOI] [PubMed] [Google Scholar]
- Candia A. F., Hu J., Crosby J., Lalley P. A., Noden D., Nadeau J. H., Wright C. V. Mox-1 and Mox-2 define a novel homeobox gene subfamily and are differentially expressed during early mesodermal patterning in mouse embryos. Development. 1992 Dec;116(4):1123–1136. doi: 10.1242/dev.116.4.1123. [DOI] [PubMed] [Google Scholar]
- Cavener D. R., Ray S. C. Eukaryotic start and stop translation sites. Nucleic Acids Res. 1991 Jun 25;19(12):3185–3192. doi: 10.1093/nar/19.12.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. L., Santiago N. A., Zhu J. S., Gray G. M. Differential regulation of intestinal amino-oligopeptidase gene expression in neonatal and adult rats. Am J Physiol. 1991 Nov;261(5 Pt 1):G866–G871. doi: 10.1152/ajpgi.1991.261.5.G866. [DOI] [PubMed] [Google Scholar]
- Dearolf C. R., Topol J., Parker C. S. The caudal gene product is a direct activator of fushi tarazu transcription during Drosophila embryogenesis. Nature. 1989 Sep 28;341(6240):340–343. doi: 10.1038/341340a0. [DOI] [PubMed] [Google Scholar]
- Doll U., Niessing J. Continued expression of the chicken caudal homologue in endodermally derived organs. Dev Biol. 1993 Mar;156(1):155–163. doi: 10.1006/dbio.1993.1066. [DOI] [PubMed] [Google Scholar]
- Duprey P., Chowdhury K., Dressler G. R., Balling R., Simon D., Guenet J. L., Gruss P. A mouse gene homologous to the Drosophila gene caudal is expressed in epithelial cells from the embryonic intestine. Genes Dev. 1988 Dec;2(12A):1647–1654. doi: 10.1101/gad.2.12a.1647. [DOI] [PubMed] [Google Scholar]
- Freund J. N., Boukamel R., Benazzouz A. Gradient expression of Cdx along the rat intestine throughout postnatal development. FEBS Lett. 1992 Dec 14;314(2):163–166. doi: 10.1016/0014-5793(92)80965-j. [DOI] [PubMed] [Google Scholar]
- Frumkin A., Pillemer G., Haffner R., Tarcic N., Gruenbaum Y., Fainsod A. A role for CdxA in gut closure and intestinal epithelia differentiation. Development. 1994 Feb;120(2):253–263. doi: 10.1242/dev.120.2.253. [DOI] [PubMed] [Google Scholar]
- Frumkin A., Rangini Z., Ben-Yehuda A., Gruenbaum Y., Fainsod A. A chicken caudal homologue, CHox-cad, is expressed in the epiblast with posterior localization and in the early endodermal lineage. Development. 1991 May;112(1):207–219. doi: 10.1242/dev.112.1.207. [DOI] [PubMed] [Google Scholar]
- Galand G. Brush border membrane sucrase-isomaltase, maltase-glucoamylase and trehalase in mammals. Comparative development, effects of glucocorticoids, molecular mechanisms, and phylogenetic implications. Comp Biochem Physiol B. 1989;94(1):1–11. doi: 10.1016/0305-0491(89)90002-3. [DOI] [PubMed] [Google Scholar]
- Galang C. K., Hauser C. A. Cooperative DNA binding of the human HoxB5 (Hox-2.1) protein is under redox regulation in vitro. Mol Cell Biol. 1993 Aug;13(8):4609–4617. doi: 10.1128/mcb.13.8.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer L. W., Wright C. V. Murine Cdx-4 bears striking similarities to the Drosophila caudal gene in its homeodomain sequence and early expression pattern. Mech Dev. 1993 Sep;43(1):71–81. doi: 10.1016/0925-4773(93)90024-r. [DOI] [PubMed] [Google Scholar]
- Gehring W. J. Homeo boxes in the study of development. Science. 1987 Jun 5;236(4806):1245–1252. doi: 10.1126/science.2884726. [DOI] [PubMed] [Google Scholar]
- German M. S., Wang J., Chadwick R. B., Rutter W. J. Synergistic activation of the insulin gene by a LIM-homeo domain protein and a basic helix-loop-helix protein: building a functional insulin minienhancer complex. Genes Dev. 1992 Nov;6(11):2165–2176. doi: 10.1101/gad.6.11.2165. [DOI] [PubMed] [Google Scholar]
- Gordon J. I. Intestinal epithelial differentiation: new insights from chimeric and transgenic mice. J Cell Biol. 1989 Apr;108(4):1187–1194. doi: 10.1083/jcb.108.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. I. Understanding gastrointestinal epithelial cell biology: lessons from mice with help from worms and flies. Gastroenterology. 1993 Aug;105(2):315–324. doi: 10.1016/0016-5085(93)90703-f. [DOI] [PubMed] [Google Scholar]
- Gumucio D. L., Rood K. L., Gray T. A., Riordan M. F., Sartor C. I., Collins F. S. Nuclear proteins that bind the human gamma-globin gene promoter: alterations in binding produced by point mutations associated with hereditary persistence of fetal hemoglobin. Mol Cell Biol. 1988 Dec;8(12):5310–5322. doi: 10.1128/mcb.8.12.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffen K., Kedinger M., Simon-Assmann P. Mesenchyme-dependent differentiation of epithelial progenitor cells in the gut. J Pediatr Gastroenterol Nutr. 1987 Jan-Feb;6(1):14–23. doi: 10.1097/00005176-198701000-00005. [DOI] [PubMed] [Google Scholar]
- Holland P. W., Hogan B. L. Expression of homeo box genes during mouse development: a review. Genes Dev. 1988 Jul;2(7):773–782. doi: 10.1101/gad.2.7.773. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Flynn S. E., Voss J. W., Albert V. R., Kapiloff M. S., Wilson L., Rosenfeld M. G. The POU-specific domain of Pit-1 is essential for sequence-specific, high affinity DNA binding and DNA-dependent Pit-1-Pit-1 interactions. Cell. 1990 Jun 15;61(6):1021–1033. doi: 10.1016/0092-8674(90)90067-o. [DOI] [PubMed] [Google Scholar]
- James R., Erler T., Kazenwadel J. Structure of the murine homeobox gene cdx-2. Expression in embryonic and adult intestinal epithelium. J Biol Chem. 1994 May 27;269(21):15229–15237. [PubMed] [Google Scholar]
- James R., Kazenwadel J. Homeobox gene expression in the intestinal epithelium of adult mice. J Biol Chem. 1991 Feb 15;266(5):3246–3251. [PubMed] [Google Scholar]
- Joly J. S., Maury M., Joly C., Duprey P., Boulekbache H., Condamine H. Expression of a zebrafish caudal homeobox gene correlates with the establishment of posterior cell lineages at gastrulation. Differentiation. 1992 Jun;50(2):75–87. doi: 10.1111/j.1432-0436.1992.tb00488.x. [DOI] [PubMed] [Google Scholar]
- Karpinski B. A., Morle G. D., Huggenvik J., Uhler M. D., Leiden J. M. Molecular cloning of human CREB-2: an ATF/CREB transcription factor that can negatively regulate transcription from the cAMP response element. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4820–4824. doi: 10.1073/pnas.89.11.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Murine developmental control genes. Science. 1990 Jul 27;249(4967):374–379. doi: 10.1126/science.1974085. [DOI] [PubMed] [Google Scholar]
- Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol. 1987 Aug 20;196(4):947–950. doi: 10.1016/0022-2836(87)90418-9. [DOI] [PubMed] [Google Scholar]
- Kuwabara M. D., Sigman D. S. Footprinting DNA-protein complexes in situ following gel retardation assays using 1,10-phenanthroline-copper ion: Escherichia coli RNA polymerase-lac promoter complexes. Biochemistry. 1987 Nov 17;26(23):7234–7238. doi: 10.1021/bi00397a006. [DOI] [PubMed] [Google Scholar]
- Macdonald P. M., Struhl G. A molecular gradient in early Drosophila embryos and its role in specifying the body pattern. Nature. 1986 Dec 11;324(6097):537–545. doi: 10.1038/324537a0. [DOI] [PubMed] [Google Scholar]
- Margalit Y., Yarus S., Shapira E., Gruenbaum Y., Fainsod A. Isolation and characterization of target sequences of the chicken CdxA homeobox gene. Nucleic Acids Res. 1993 Oct 25;21(21):4915–4922. doi: 10.1093/nar/21.21.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz A. J., Wu G. D., Birkenmeier E. H., Traber P. G. The human sucrase-isomaltase gene directs complex patterns of gene expression in transgenic mice. Am J Physiol. 1993 Sep;265(3 Pt 1):G526–G539. doi: 10.1152/ajpgi.1993.265.3.G526. [DOI] [PubMed] [Google Scholar]
- Mendel D. B., Hansen L. P., Graves M. K., Conley P. B., Crabtree G. R. HNF-1 alpha and HNF-1 beta (vHNF-1) share dimerization and homeo domains, but not activation domains, and form heterodimers in vitro. Genes Dev. 1991 Jun;5(6):1042–1056. doi: 10.1101/gad.5.6.1042. [DOI] [PubMed] [Google Scholar]
- Meyer B. I., Gruss P. Mouse Cdx-1 expression during gastrulation. Development. 1993 Jan;117(1):191–203. doi: 10.1242/dev.117.1.191. [DOI] [PubMed] [Google Scholar]
- Mlodzik M., Fjose A., Gehring W. J. Isolation of caudal, a Drosophila homeo box-containing gene with maternal expression, whose transcripts form a concentration gradient at the pre-blastoderm stage. EMBO J. 1985 Nov;4(11):2961–2969. doi: 10.1002/j.1460-2075.1985.tb04030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M., Gehring W. J. Expression of the caudal gene in the germ line of Drosophila: formation of an RNA and protein gradient during early embryogenesis. Cell. 1987 Feb 13;48(3):465–478. doi: 10.1016/0092-8674(87)90197-8. [DOI] [PubMed] [Google Scholar]
- Mlodzik M., Gibson G., Gehring W. J. Effects of ectopic expression of caudal during Drosophila development. Development. 1990 Jun;109(2):271–277. doi: 10.1242/dev.109.2.271. [DOI] [PubMed] [Google Scholar]
- Pevny L., Simon M. C., Robertson E., Klein W. H., Tsai S. F., D'Agati V., Orkin S. H., Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991 Jan 17;349(6306):257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990 Dec;110(4):1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Quinn L. A., Moore G. E., Morgan R. T., Woods L. K. Cell lines from human colon carcinoma with unusual cell products, double minutes, and homogeneously staining regions. Cancer Res. 1979 Dec;39(12):4914–4924. [PubMed] [Google Scholar]
- Ruvkun G., Finney M. Regulation of transcription and cell identity by POU domain proteins. Cell. 1991 Feb 8;64(3):475–478. doi: 10.1016/0092-8674(91)90227-p. [DOI] [PubMed] [Google Scholar]
- Serrano J., Scavo L., Roth J., de la Rosa E. J., de Pablo F. A novel chicken homeobox-containing gene expressed in neurulating embryos. Biochem Biophys Res Commun. 1993 Jan 15;190(1):270–276. doi: 10.1006/bbrc.1993.1041. [DOI] [PubMed] [Google Scholar]
- Singh H., Clerc R. G., LeBowitz J. H. Molecular cloning of sequence-specific DNA binding proteins using recognition site probes. Biotechniques. 1989 Mar;7(3):252–261. [PubMed] [Google Scholar]
- Singh H., LeBowitz J. H., Baldwin A. S., Jr, Sharp P. A. Molecular cloning of an enhancer binding protein: isolation by screening of an expression library with a recognition site DNA. Cell. 1988 Feb 12;52(3):415–423. doi: 10.1016/s0092-8674(88)80034-5. [DOI] [PubMed] [Google Scholar]
- Sánchez-García I., Rabbitts T. H. Redox regulation of in vitro DNA-binding activity by the homeodomain of the Isl-1 protein. J Mol Biol. 1993 Jun 20;231(4):945–949. doi: 10.1006/jmbi.1993.1343. [DOI] [PubMed] [Google Scholar]
- Traber P. G. Differentiation of intestinal epithelial cells: lessons from the study of intestine-specific gene expression. J Lab Clin Med. 1994 Apr;123(4):467–477. [PubMed] [Google Scholar]
- Traber P. G. Regulation of sucrase-isomaltase gene expression along the crypt-villus axis of rat small intestine. Biochem Biophys Res Commun. 1990 Dec 31;173(3):765–773. doi: 10.1016/s0006-291x(05)80853-8. [DOI] [PubMed] [Google Scholar]
- Traber P. G., Wu G. D., Wang W. Novel DNA-binding proteins regulate intestine-specific transcription of the sucrase-isomaltase gene. Mol Cell Biol. 1992 Aug;12(8):3614–3627. doi: 10.1128/mcb.12.8.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber P. G., Yu L., Wu G. D., Judge T. A. Sucrase-isomaltase gene expression along crypt-villus axis of human small intestine is regulated at level of mRNA abundance. Am J Physiol. 1992 Jan;262(1 Pt 1):G123–G130. doi: 10.1152/ajpgi.1992.262.1.G123. [DOI] [PubMed] [Google Scholar]
- Vershon A. K., Johnson A. D. A short, disordered protein region mediates interactions between the homeodomain of the yeast alpha 2 protein and the MCM1 protein. Cell. 1993 Jan 15;72(1):105–112. doi: 10.1016/0092-8674(93)90054-t. [DOI] [PubMed] [Google Scholar]
- Vinson C. R., LaMarco K. L., Johnson P. F., Landschulz W. H., McKnight S. L. In situ detection of sequence-specific DNA binding activity specified by a recombinant bacteriophage. Genes Dev. 1988 Jul;2(7):801–806. doi: 10.1101/gad.2.7.801. [DOI] [PubMed] [Google Scholar]
- Wilson D., Sheng G., Lecuit T., Dostatni N., Desplan C. Cooperative dimerization of paired class homeo domains on DNA. Genes Dev. 1993 Nov;7(11):2120–2134. doi: 10.1101/gad.7.11.2120. [DOI] [PubMed] [Google Scholar]
- Wu G. D., Wang W., Traber P. G. Isolation and characterization of the human sucrase-isomaltase gene and demonstration of intestine-specific transcriptional elements. J Biol Chem. 1992 Apr 15;267(11):7863–7870. [PubMed] [Google Scholar]
- Zweibaum A., Triadou N., Kedinger M., Augeron C., Robine-Léon S., Pinto M., Rousset M., Haffen K. Sucrase-isomaltase: a marker of foetal and malignant epithelial cells of the human colon. Int J Cancer. 1983 Oct 15;32(4):407–412. doi: 10.1002/ijc.2910320403. [DOI] [PubMed] [Google Scholar]